Levitra enthält Vardenafil, das eine kürzere Wirkdauer als Tadalafil hat, dafür aber schnell einsetzt. Männer, die diskret bestellen möchten, suchen häufig nach levitra kaufen ohne rezept. Dabei spielt die rechtliche Lage in der Schweiz eine wichtige Rolle.
Untitled
Continuous Infusion of Beta-Lactam Antibioticsin Severe Sepsis: A Multicenter Double-Blind,Randomized Controlled Trial
Joel M. Dulhunty,1 Jason A. Roberts,1 Joshua S. Davis,2 Steven A. R. Webb,3 Rinaldo Bellomo,4 Charles Gomersall,5Charudatt Shirwadkar,6 Glenn M. Eastwood,4 John Myburgh,7 David L. Paterson,8 and Jeffrey Lipman1
1Department of Intensive Care Medicine, Royal Brisbane and Women's Hospital, and Burns, Trauma and Critical Care Research Centre, University of
Queensland, Brisbane, 2Menzies School of Health Research, Charles Darwin University and Royal Darwin Hospital, 3Royal Perth Hospital, and Schoolof Medicine and Pharmacology, University of Western Australia, Perth, 4Department of Intensive Care, Austin Hospital, Melbourne, Australia; 5Princeof Wales Hospital and Chinese University of Hong Kong, Hong Kong; 6Blacktown Hospital, 7Critical Care and Trauma Division, George Institute forGlobal Health, Sydney, and 8Infectious Diseases Unit, Royal Brisbane and Women's Hospital, and University of Queensland Centre for ClinicalResearch, Brisbane, Australia
(See the Editorial Commentary by Drusano and Lodise, on pages 245–7, and the Invited Article by Falagas et al, onpages 272–82.)
Background. Beta-lactam antibiotics are a commonly used treatment for severe sepsis, with intermittent bolus
dosing standard therapy, despite a strong theoretical rationale for continuous administration. The aim of this trialwas to determine the clinical and pharmacokinetic differences between continuous and intermittent dosing inpatients with severe sepsis.
Methods. This was a prospective, double-blind, randomized controlled trial of continuous infusion versus inter-
mittent bolus dosing of piperacillin-tazobactam, meropenem, and ticarcillin-clavulanate conducted in 5 intensive care
units across Australia and Hong Kong. The primary pharmacokinetic outcome on treatment analysis was plasmaantibiotic concentration above the minimum inhibitory concentration (MIC) on days 3 and 4. The assessed clinicaloutcomes were clinical response 7–14 days after study drug cessation, ICU-free days at day 28 and hospital survival.
Results. Sixty patients were enrolled with 30 patients each allocated to the intervention and control groups.
Plasma antibiotic concentrations exceeded the MIC in 82% of patients (18 of 22) in the continuous arm versus 29%(6 of 21) in the intermittent arm (P = .001). Clinical cure was higher in the continuous group (70% vs 43%; P = .037),but ICU-free days (19.5 vs 17 days; P = .14) did not significantly differ between groups. Survival to hospital dischargewas 90% in the continuous group versus 80% in the intermittent group (P = .47).
Conclusions. Continuous administration of beta-lactam antibiotics achieved higher plasma antibiotic concentra-
tions than intermittent administration with improvement in clinical cure. This study provides a strong rationale forfurther multicenter trials with sufficient power to identify differences in patient-centered endpoints.
Keywords. pharmacokinetics; clinical outcome; meropenem; piperacillin-tazobactam; ticarcillin-clavulanate.
Severe sepsis is a major cause of mortality worldwide. In
(ICU) admissions are associated with severe sepsis (over
Australia and New Zealand, 11.8% of intensive care unit
17 000 episodes per annum) with in-hospital mortalityof 37.5% and a mortality burden 4 times the Australianannual road toll [, ]. This burden is evident globally [–
Received 4 March 2012; accepted 6 August 2012; electronically published 16
]. Early administration of antibiotics active against
October 2012.
Correspondence: Joel Dulhunty, MBBS, MTH, PhD, Dept of Intensive Care Med-
the infecting organism is a cornerstone of effective man-
icine, Royal Brisbane and Women's Hospital, Herston, QLD 4029, Australia
agement In a recent point prevalence study of ICU
antibiotic usage in Australia and New Zealand, 3 of the 4
Clinical Infectious Diseases
The Author 2012. Published by Oxford University Press on behalf of the Infectious
most commonly used antibiotics in treatment were
Diseases Society of America. All rights reserved. For Permissions, please e-mail:
beta-lactams, with ticarcillin-clavulanate, meropenem,
DOI: 10.1093/cid/cis856
and piperacillin-tazobactam accounting for 56% of all
236 • CID 2013:56 (15 January) • Dulhunty et al
antibiotics used . Given that subtherapeutic dosing is associat-
planned commencement or commencement within the previ-
ed with poorer clinical outcomes and increased incidence of drug
ous 24 hours of ticarcillin-clavulanate, piperacillin-tazobactam
resistance ], optimal dosing of beta-lactam antibiotics has
or meropenem; and (3) an expected or actual ICU stay greater
the potential to improve the outcome for critically ill patients
than 48 hours. Patients were excluded if they were <18 years
with severe sepsis.
of age, had an allergy to one or more of the study medications,
Beta-lactam antibiotics are administered almost exclusively
were receiving palliative or supportive treatment only, were re-
by intermittent bolus dosing However, there are strong
ceiving continuous renal replacement therapy, did not have
pharmacodynamic data suggesting that this mode of adminis-
central venous catheter access with at least 3 lumens (a dedi-
tration may be less effective than administration by continuous
cated lumen was required for study drug administration), or
infusion. Bacterial killing for beta-lactam antibiotics is related to
had received the study drug for >24 hours.
the duration of time that bacteria are exposed to a concentrationof antibiotic that exceeds the minimum inhibitory concentra-
tion (MIC), that is, T>MIC Administration of beta-lactam
Patients were randomized to receive either (1) active infusion
antibiotics by infusion produces higher blood and interstitial
and placebo boluses (intervention arm) or (2) placebo infusion
fluid concentrations with greater time above the MIC compared
and active boluses (control arm). The 24-hour dose was clini-
with intermittent dosing, particularly for bacteria with high
cian-chosen and unaffected by randomization. Ticarcillin-
MIC values, which are common in the ICU
clavulanate and piperacillin-tazobactam (or placebo) infusions
Although continuous infusion has been shown to be superior
were changed every 24 hours, while meropenem (or placebo)
to intermittent administration in animal and ex vivo models, 2
infusions were changed 8 hourly, as determined by antibiotic
meta-analyses of the human trials to date have not demonstrat-
stability at room temperature ]. Labeling was used to
ed differences in clinical cure or survival [, These human
conceal the syringe contents for bolus administration. Infusion
trials, however, have been primarily conducted in noncritically
contents were concealed by dilution of medication in 100–
ill patients and were underpowered, even when pooled, limiting
250 mL infusion bags. Both methods of administration were
their applicability to patients with severe sepsis. In addition, 13
used with the active treatment contained in only one adminis-
of the 14 studies included in a recent meta-analysis used non-
tration route. Clinical staff, data collectors, and patients were
equivalent dosing in the treatment arms limiting direct compar-
blinded to allocation status.
isons between the 2 delivery methods ]. The aim of this trial
was to determine the clinical and pharmacokinetic differences
Antibiotic Plasma Levels
between continuous and intermittent dosing in critically ill pa-
A maximum of 3 blood samples per patient were taken imme-
tients with severe sepsis to establish feasibility to proceed with a
diately prior to the active (or placebo) bolus dose during a 48-
larger multicenter trial.
hour window period on days 3 and 4 to determine plasmatrough levels. Blood samples were centrifuged at 3000 rpm for
10 minutes and the plasma stored at −80°C until batchedanalysis at a central laboratory; samples were stored at −20°C
Study Design and Setting
for <30 hours at one site until storage at −80°C. Antibiotic
This prospective, multicenter, double-blind, concealed, ran-
concentration was determined by validated high performance
domized controlled trial was conducted at Royal Brisbane and
liquid chromatography which included within-batch cali-
Women's Hospital, Austin Hospital, Blacktown Hospital, and
brators and quality controls Samples were prepared by
Royal Darwin Hospital, Australia, and Prince of Wales Hospi-
protein precipitation with a dichloromethane wash, and the
tal, Hong Kong. Recruitment occurred between April 2010
extracts separated on a C18 stationary phase and monitored
and November 2011. Institutional ethics approval for the
by ultraviolet. Accuracy and precision of the assays were vali-
study was obtained at each site. Consent was obtained from
dated at high, medium, and low concentrations of the calibra-
the patient or from a substitute decision maker prior to study
tion range. All results met the bioanalysis acceptance criteria
enrollment. The study was registered with the Australian New
of the US Food and Drug Administration []. Free
Zealand Clinical Trials Registry (ACTRN12610000238077).
(unbound) drug concentrations were determined using pub-lished protein binding values (2% for meropenem, 21% for pi-
Selection Criteria
peracillin, and 45% for ticarcillin) [
Patients were eligible if they met all of the following inclusioncriteria: (1) severe sepsis in the previous 48 hours, defined as
Outcomes and Measurements
confirmed or suspected infection with new organ dysfunction
The primary pharmacokinetic endpoint was plasma antibiotic
based on diagnostic criteria published elsewhere [(2)
concentration above MIC, scored as a dichotomous variable.
Continuous Infusion of Beta-Lactams • CID 2013:56 (15 January) • 237
MIC breakpoints for Pseudomonas aeruginosa (16 mg/L for
Adequacy of blinding was assessed by clinician survey. A
piperacillin and ticarcillin, and 2 mg/L for meropenem) were
nurse on day 1 or 2 and a medical officer at a later date during
used and scored as positive if all measured free plasma antibi-
study enrollment were asked whether they thought the patient
otic concentrations exceeded the breakpoint
was receiving continuous or intermittent treatment and the
Secondary endpoints included clinical response rated by
degree of certainty in this decision using a 5-point scale [
blinded clinicians at a test of cure date 7–14 days after studydrug cessation (Table ) Time to clinical resolution was
Statistical Analysis
defined as the number of days from randomization to the first
An on-treatment analysis of all patients with plasma antibiotic
identified date of clinical resolution; this was set at 28 days for
samples taken on days 3 and 4 was performed for the primary
patients who did not achieve clinical cure within a 28-day
pharmacokinetic endpoint (n = 22 and 21 for the intervention
period. Vital status at ICU and hospital discharge and ICU-
and control group, respectively). Free plasma antibiotic con-
free days at day 28 were also evaluated. "ICU-free days" was
centration differences were analyzed by Mann-Whitney U test
defined as the number of days alive and free of ICU admission
and expressed as box (median and interquartile range [IQR])
in the first 28 days postrandomization. Daily sequential organ
and whiskers (10–90 percentile). An intention-to-treat analysis
failure assessment (SOFA) scores were recorded [The
of all randomized patients was performed for clinical end-
focus of infection, concomitant antibiotic use, and duration of
points (n = 30 in each group). The primary outcome was eval-
therapy were recorded. Adverse events during treatment were
uated by Fisher exact test. Secondary outcomes were analyzed
evaluated as, almost certainly, probably, possibly, or unlikely
by Student t test or Mann-Whitney U test depending on
caused by study medications.
whether inspection of a normal Q-Q plot confirmed or reject-ed the normality assumption, respectively. A Kaplan-Meier
curve, with follow-up until hospital discharge, was plotted to
A sample of 60 patients was calculated to achieve a power of
show survival trend; a log-rank test was used to compare treat-
80% to detect a 15% absolute difference in the primary
ment groups. Mean ± standard deviation are reported for nor-
outcome at a significance level of 5%, with a target of 8–16
mally distributed variables and median [IQR] for nonnormal
participants per site.
variables. A 2-sided P value <.05 was considered statisticallysignificant. Statistical analysis was conducted using IBM SPSSStatistics 19 (IBM Corporation, Armonk, New York). James
Randomization and Masking
and Bang blinding indices were computed using Stata
Randomization was stratified by institution with 1:1 allocation
software (StataCorp LP, College Station, Texas). Box and
to treatment arm. Following study enrollment, an unblinded
whisker plots were generated in GraphPad Prism 5 (GraphPad
research nurse or pharmacist responsible for preparation of
Software, Inc, La Jolla, California).
the blinded medications determined allocation status byopening a sequentially numbered sealed envelope.
Clinician-Rated Outcome De
Recruitment and Baseline Characteristics
Sixty patients were enrolled; 16 at Royal Brisbane and Women's
Clinical response
Hospital, 14 at Austin Hospital, 12 at Blacktown Hospital, 10 atRoyal Darwin Hospital, and 8 at Prince of Wales Hospital.
Resolution—disappearance of all signs and symptoms
Forty-four patients (73%) completed 4 or more days of ran-
related to the infection
domized treatment, with equal distribution between treatment
Improvement—a marked or moderate reduction in theseverity and/or number of signs and symptoms of infection
arms (Figure Four patients were discharged from the ICU
Failure—insufficient lessening of the signs and symptoms of
within 48 hours of randomization, and 2 patients died during
infection to qualify as improvement, including death orindeterminate (no evaluation possible, for any reason)
this period. The 24-hour antibiotic dose for the interventionand control groups was comparable: 13.5 [13.5–13.5] g versus
13.5 [11.3–13.5] g for piperacillin-tazobactam, 3.0 [3.0–3.8] g
Resolution—as above
versus 3.0 [3.0–3.0] g for meropenem, and 12.4–13.5 g (2 partici-
All other findings (ie, sum of 2 and 3 above)
pants) versus 12.4 g (1 participant) for ticarcillin-clavulanate.
Clinical cure (treatment exclusions)
Fourteen patients in each group had a beta-lactam suscepti-
Participants where the study drug, excluding beta-lactam
ble organism identified as the primary causative organism
antibiotic de-escalation, was changed due to nonresolution ofinfection are defined as nonresolution (regardless of clinical
(Table Four patients in the intervention group had a non-
response at test of cure date)—otherwise as above
susceptible organism identified (Enterococcus species in 3
238 • CID 2013:56 (15 January) • Dulhunty et al
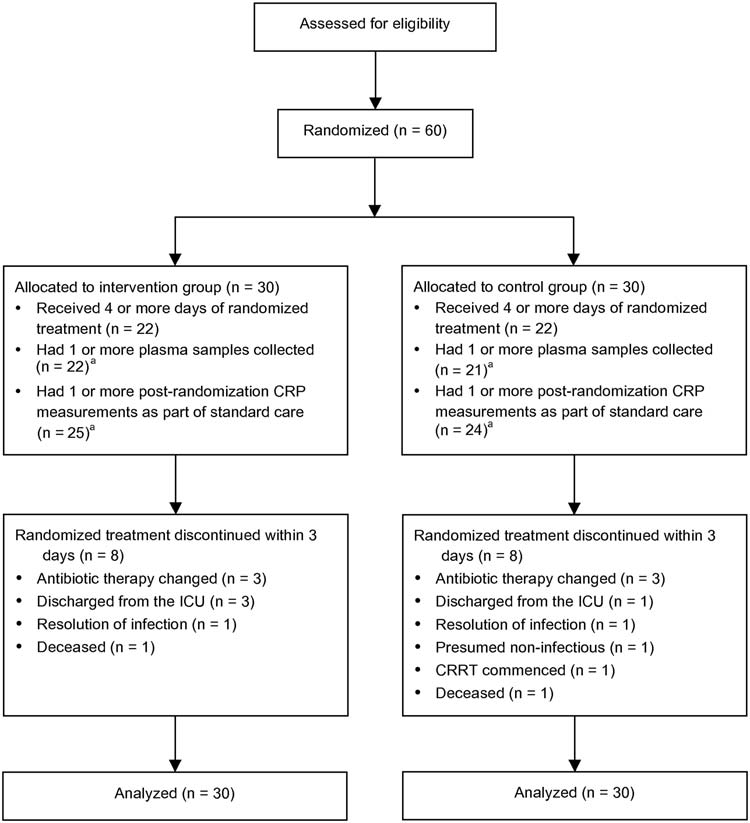
CONSORT flow diagram. Abbreviations: CRP, C-reactive protein; CRRT, continuous renal replacement therapy; CVC, central venous catheter;
ICU, intensive care unit. aSub-group analysis.
patients and human metapneumovirus in a fourth). Four pa-
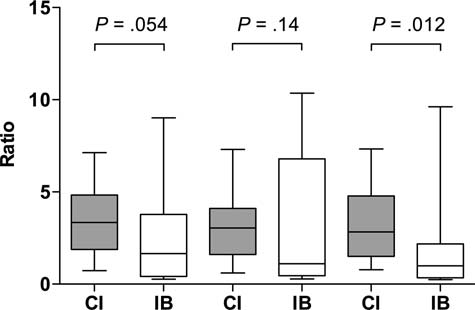
The ratio of plasma antibiotic concentration to MIC for the
tients in the control group had a nonsusceptible organism
intervention and control group is displayed in Figure for all
identified: methicillin-resistant Staphylococcus aureus in 2 pa-
3 samples: 3.3 [1.9–4.8] μg/mL vs 1.7 [0.4–3.8] μg/mL for
tients, Coxiella burnetii (Q fever) in one, and Stenotrophomo-
sample 1, 3.0 [1.6–4.1] μg/mL vs 1.1 [0.5–6.8] μg/mL for
nas maltophilia in a fourth. Baseline characteristics of the 2
sample 2, and 2.8 [1.5–4.8] μg/mL vs 1.0 [0.3–2.2] μg/mL
groups are reported in Table .
for sample 3, respectively.
Study endpoints are displayed in Table and survival analysis
is shown in Figure . For patients receiving meropenem, plasma
Plasma antibiotic concentration measured in the first sample
antibiotic concentration was greater than MIC for all samples in
was significantly higher in the intervention group compared
8 of 8 patients (100%) in the intervention group, compared with
with the control group for meropenem (9.2 [7.9–12.9] μg/mL
2 of 9 (22%) in the control group; for patients receiving pipera-
vs 3.3 [0.8–4.2] μg/mL), but not for piperacillin (35.6 [21.4–
cillin-tazobactam, group differences in plasma antibiotic concen-
52.0] μg/mL vs 36.4 [6.2–142.2] μg/mL) or ticarcillin (9.1 μg/
tration above MIC were 9 of 12 (75%) vs 4 of 11 (36%), and for
mL and 130.9 μg/mL vs 14.1 μg/mL, respectively; Figure
ticarcillin-clavulanate 1 of 2 (50%) vs 0 of 1, respectively.
Continuous Infusion of Beta-Lactams • CID 2013:56 (15 January) • 239
Organisms Identified on Blood Culture
Baseline and Study Characteristics
Intervention Group
Enterococcus sppa
Chronic health evaluation
Pseudomonas aeruginosa
Serratia marascens
Proteus mirabilis
Aeromonas hydrophilia
Burkholderia cepacia
Enterobacter cloacae
Haemophilus influenzae
Klebsiella oxytoca
Morganella morganii
Salmonella typhimurium
Duration of study treatment (days)
Organism identified
Streptococcus milleri
Site of infection
Streptococcus pneumonia
Streptococcus pyogenes
Vibrio vulnificus
Abbreviations: ABC, Acinetobacter baumanii-calcoaceticus complex; ESBL,
Skin or skin structure
extended-spectrum beta-lactamase; MRSA, methicillin-resistant Staphylococcusaureus; MSSA, methicillin-sensitive Staphylococcus aureus.
aEnterocococcus faecalis in 2 cases and Enterococcus spp (unidentified) in 1
Central nervous system
b–e Indicate multiple organisms identified in 4 cases.
Postrandomization CRP
Adequacy of Blinding
Nursing and medical staff completed a blinding questionnaire
for 56 (93.3%) and 51 study participants (85.0%), respectively.
ICU length of stay
Perceptions of randomization status are displayed in Table
Of the 33 respondents (30.8%) who believed they knew which
Abbreviations: APACHE, Acute Physiology and Chronic Health Evaluation;
treatment arm the participant was in, 13 made a judgment
CRP, C-reactive protein; ICU, intensive care unit.
based on physical characteristics of the infusion bag or
a Five participants in each group had an additional site of identified infection
syringe, and 9 made the judgment with reference to improve-
(lung, urinary tract, and intra-abdominal).
b One participant had an additional site of infection (lung).
ment or nonimprovement in the patients' condition, withvarious reasons provided for the remaining judgments. Blind-ing indices are reported in Table .
No adverse events occurred as a result of study participation.
Two patients died during study enrolment: one patient deteri-
This is the first multicenter ICU trial to our knowledge com-
orated following consent but prior to commencement of the
paring the effects of continuous and intermittent administra-
blinded medication with the cause of death septic shock due
tion of beta-lactam antibiotics. Our results showed that
to aspiration pneumonitis, and one patient with deteriorating
continuous infusion of beta-lactam antibiotics achieved signif-
respiratory failure and septic shock died 3 days after ICU
icant pharmacokinetic separation in T> MIC and higher rates
admission due to pneumonia. Both events were assessed as
of clinical cure compared with intermittent administration in
unlikely to be related to the study drug or intervention.
critically ill patients with severe sepsis. Our study is the only
240 • CID 2013:56 (15 January) • Dulhunty et al
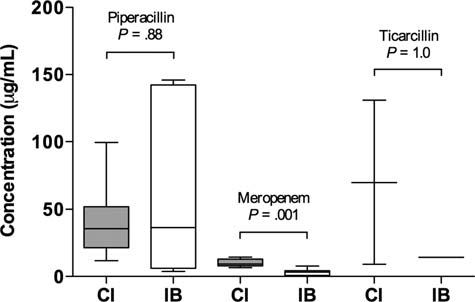
Study Endpoints by Treatment Group
Plasma antibiotic
concentration >MIC
Clinical cure (test of
Clinical cure (test of
cure date withtreatment exclusions)
Clinical cure (last day
11 (6.75–24.25)b 16.5 (7–28)b
resolution (days)
Free plasma antibiotic concentration between treatment
Time to resolution of
groups on the first sample. Abbreviations: CI, continuous infusion; IB,
intermittent bolus.
ICU length of stay
19.5 (12.75–24)
continuous vs intermittent beta-lactam dosing trial that has
18 (12.75–22)d .22
been conducted in a blinded fashion with allocation conceal-
ment [and the largest of a limited number of studies con-
Hospital survival
ducted exclusively in an ICU setting ]. This
Abbreviations: CRP, C-reactive protein; ICU, intensive care unit; MIC,
multicenter study demonstrated the feasibility of randomizing
minimum inhibitory concentration.
patients following commencement of 3 commonly prescribed
a Plasma samples were available for 22 and 21 patients in the intervention
beta-lactam antibiotics for severe sepsis and the ability to ad-
and control groups, respectively (subgroup analysis).
b Time to clinical resolution was set at 28 d for 7 and 13 patients in the
minister concealed medications in the ICU in a safe manner.
intervention and control groups, respectively, as clinical resolution did not
Continuous infusion has shown to produce higher blood
occur during this period.
and interstitial fluid concentrations and more rapid bacterial
c Postrandomization CRP levels were available for 25 and 24 patients in theintervention and control groups, respectively (subgroup analysis); time to
killing, particularly for bacteria with high MIC values in im-
resolution of CRP was set at 28 d for 6 patients in each group as CRP was
munodeficient ex vivo and animal models A ret-
not measured below 100 mg/L during this period.
d
rospective study by Lodise and colleagues in critically ill
Subgroup analysis (28 and 26 patients in intervention and control groups,
patients with P. aeruginosa found that using extended infu-sions of piperacillin-tazobactam to increase T>MIC resulted in
improved 14-day survival (12.2% vs 31.6%, P = .04) in a sub-population of patients with high levels of sickness severity(APACHE II score >17) compared with a historical cohort[Another retrospective review of 359 patients treated forgram-negative infections across 14 hospitals in the UnitedStates found that extended infusion of piperacillin-tazobactamprolonged survival by 2.8 days (P < .01) compared with nonex-tended infusion of beta-lactam antibiotics []. However,apart from a single center ICU study by Roberts and col-leagues, which observed a 27% higher cure rate with continu-ous infusion of ceftriaxone (P = .06) [our study is the onlytrial to our knowledge to report a significant difference in clin-ical cure rates for continuous versus intermittent administra-tion of beta-lactam antibiotics. This may in part by beexplained by a focus on patients with a higher acuity of illness
Free plasma antibiotic concentration to minimum inhibitory
concentration ratio for 3 samples. Abbreviations: CI, continuous infusion;
and dosing that was independent of treatment arm. Given pre-
IB, intermittent bolus.
vious data showing that, in critically ill patients in the ICU,
Continuous Infusion of Beta-Lactams • CID 2013:56 (15 January) • 241
.13 (−.011, .27)b
−.036 (−.11, .035)b
−.096 (−.026, .071)b
Abbreviations: BI, blinding index; CI, confidence interval.
James' BI reference range (0 to 1): 0 = complete unblinding, .5 = randomguessing, 1 = complete blinding. Bang's BI reference range (−1 to 1):
−1 = complete blinding, 0 = random guessing, 1 = complete unblinding.
a 95% CIs that are >.5 indicate adequate blinding.
b 95% CIs that include 0 indicate adequate blinding.
greater concentration range in the piperacillin-tazobactambolus group, including a greater number of patients with low
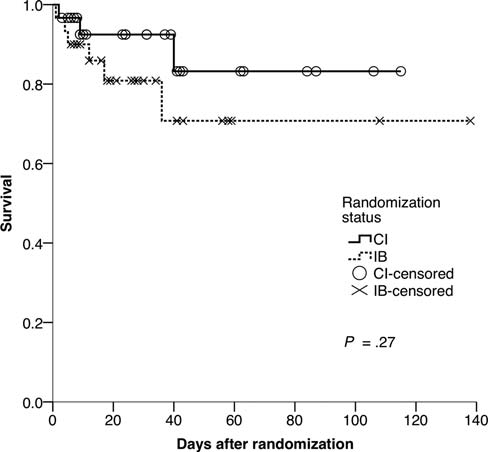
Survival curve for patients in both treatment groups (data
have been censored for patients discharged from hospital). Abbreviations:
The study was not powered to evaluate any effect on surviv-
CI, continuous infusion; IB, intermittent bolus.
al and suggests a clinical signal for the surrogate endpoint ofclinical cure at 7–14 days after study drug cessation (27%higher in the intervention group), even after adjusting for
maintaining 100% T>MIC for beta-lactam antibiotics is associ-
treatment changes. Additionally, a number of other surrogate
ated with greater clinical cure than dosing that results in any-
clinical endpoints, including ICU-free days at day 28 moved
thing <100% (82% vs 33%, P = .002) [the nonequivalent
in a favorable direction but did not achieve statistical signifi-
dosing between treatment arms (lower in the continuous arm)
cance. The progression to achieving a definitive clinical
in 13 of the 14 previous trials may be a significant confound-
answer via a stepwise research program is well described in
ing factor ]. Our study demonstrated that clinician-deter-
the literature [Our study provides an important step in
mined dosing by continuous infusion might alone be
establishing suitable endpoints for a large well-designed pro-
sufficient to improve clinical cure. Although differences in
spective phase II multicenter study of continuous administra-
plasma antibiotic concentration between groups were most
tion of beta-lactam antibiotics in critically ill patients with
prominent in patients receiving meropenem, higher rates of
severe sepsis.
100% T>MIC in measured samples were also present for pa-
The potential benefits to patients and the health system by
tients on piperacillin-tazobactam. This was evidenced by the
improved methods of antibiotic delivery of beta-lactam antibi-otics are considerable. If a 4% absolute reduction in hospital
Perception of Blinding Status
mortality is achievable (with point estimates of 6.6%–10.0%observed in this study), then this intervention has the poten-
tial to save over 800 lives each year in Australia and New
Zealand [and over 37 000 lives in the United Sates [In
addition, in an era of increasingly expensive therapies, admin-
istration of beta-lactam antibiotics via continuous infusion
compared with intermittent dosing represents greater cost-effi-
ciency in terms of workload and labor costs, while remaining
believe —continuous
cost neutral in terms of drug costs
This study has a number of limitations. Despite treatment
groups being largely well balanced, differences existed for
some baseline characteristics, such as 6 years younger mean
age, 13% more males, 13% higher comorbidity, and a 13%
higher proportion of pre-ICU infections in the intervention
group. A modest sample size in each group may have similarly
242 • CID 2013:56 (15 January) • Dulhunty et al
resulted in potential confounding by unmeasured variables. In
Potential conflicts of interest. J. A. R. has served as a consultant for
terms of plasma antibiotic concentrations, only trough con-
AstraZeneca, Pfizer, Gilead and Janssen-Cilag. S. A. R. W. has attendedAdvisory Boards and acted as a consultant to Janssen-Cilag and
centrations were measured. Therefore, concentrations at 40%–
AstraZeneca. C. G. has served as a consultant for Janssen-Cilag and
70% T>MIC could only be inferred to be greater than the MIC.
Pfizer. J. M. has received travel and speaker fees in relation to investigator-
A limited number of extreme concentration values in the in-
initiated research projects from Fresenius Kabi. D. L. P. has received re-search grants from AstraZeneca and has attended Advisory Boards, acted
termittent group suggested the presence of some sample
as a consultant to, or given lectures with honoraria from Three Rivers
timing error.
Pharmaceuticals, Merck, AstraZeneca, SanofiAventis, Pfizer, Johnson &
Clinician blinding is important for surrogate outcomes,
Johnson, and Leo Pharmaceuticals. J. L. has received research grants fromAstraZeneca and has attended Advisory Boards, acted as a consultant to,
such as ICU-free days, which can be influenced by discharge
or given lectures with honoraria from AstraZeneca, Janssen-Cilag, Merck
decisions and clinician ratings of clinical cure. Although a mi-
Sharp & Dohme, Pfizer, and Wyeth Australia. All other authors report no
nority of staff was able to determine treatment arm by subtle
physical indicators, we demonstrated that concealed adminis-
All authors have submitted the ICMJE Form for Disclosure of Potential
Conflicts of Interest. Conflicts that the editors consider relevant to the
tration achieved satisfactory levels of blinding in a multicenter
content of the manuscript have been disclosed.
context. In particular, compounding of antibiotic medicationsin infusion bags and labeling of syringes to obscure content
for intermittent dosing was sufficient to achieve blinding
without the need for more costly and labor-intensive mea-
1. Finfer S, Bellomo R, Lipman J, French C, Dobb G, Myburgh J. Adult-
sures, such as colored tubing and covered infusion bags. The
population incidence of severe sepsis in Australian and New Zealand
finding that medical staff identified the intermittent arm at a
intensive care units. Intensive Care Med 2004; 30:589–96.
2. Department of Infrastructure, Transport, Regional Development and
significantly higher rate than chance may relate to a smaller
Local Government. Road deaths Australia: 2008 statistical summary,
sample size, given that a similar identification rate for nursing
2009. Available at:
staff in the intermittent group was nonsignificant.
Accessed 11 October 2012.
3. Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J,
Pinsky MR. Epidemiology of severe sepsis in the United States: analy-
sis of incidence, outcome, and associated costs of care. Crit Care Med2001; 29:1303–10.
4. Vincent JL, Rello J, Marshall J, et al. International study of the preva-
This is the first multicenter ICU trial that we are aware of that
lence and outcomes of infection in intensive care units. JAMA 2009;
compares continuous and intermittent administration of beta-
5. Martin CM, Priestap F, Fisher H, et al. A prospective, observational
lactam antibiotics. The results provide evidence of the phar-
registry of patients with severe sepsis: the Canadian sepsis treatment
macokinetic separation of continuous infusions against bolus
and response registry. Crit Care Med 2009; 37:81–8.
dosing, higher rates of clinical cure associated with continuous
6. Dellinger RP, Levy MM, Carlet JM, et al. Surviving sepsis campaign:
international guidelines for management of severe sepsis and septic
infusion, and the feasibility of blinding study medications in a
shock: 2008. Intensive Care Med 2008; 34:17–60.
multicenter study. We believe evaluating continuous infusion
7. Dulhunty JM, Paterson DL, Webb SAR, Lipman J. Antimicrobial uti-
in a severe sepsis cohort via a phase II randomized controlled
lisation in 37 Australian and New Zealand intensive care units.
trial is both justified and feasible.
Anaesth Intensive Care 2011; 39:231–7.
8. Lodise TP Jr, Lomaestro B, Drusano GL. Piperacillin-tazobactam for
Pseudomonas aeruginosa infection: clinical implications of an extend-
ed-infusion dosing strategy. Clin Infect Dis 2007; 44:357–63.
9. McKinnon PS, Paladino JA, Schentag JJ. Evaluation of area under the
Associate Professor Graham Reece (Blacktown
inhibitory curve (AUIC) and time above the minimum inhibitory
Hospital) and Associate Professor Dianne Stephens (Royal Darwin Hospi-
concentration (T>MIC) as predictors of outcome for cefepime and
tal) provided site coordination as Associate Investigators on this study. We
ceftazidime in serious bacterial infections. Int J Antimicrob Agents
thank ICU research coordinators Leah Peck and Helen Young (Austin
2008; 31:345–51.
Hospital), Kiran Nand and Treena Sara (Blacktown Hospital), Patricia
Leung (Prince of Wales Hospital), Renae Deans, Paul Jarrett, Melissa
resistance–what's dosing got to do with it? Crit Care Med 2008; 36:
Lassig-Smith, Therese Starr and Janine Stuart (Royal Brisbane and
Women's Hospital), Jane Thomas (Royal Darwin Hospital); Steven
11. Roberts JA, Webb S, Paterson D, Ho KM, Lipman J. A systematic
Fowler, ICU pharmacist at Royal Darwin Hospital; Steven Wallis, Suzanne
review on clinical benefits of continuous administration of beta-lactam
Parker-Scott and Xin Liu at The Burns, Trauma and Critical Care Re-
antibiotics. Crit Care Med 2009; 37:2071–8.
search Centre, The University of Queensland, for laboratory analysis; and
12. Roberts JA, Kirkpatrick CM, Roberts MS, Robertson TA, Dalley AJ,
Lee Jones and Louise Marquart at the Queensland Institute of Medical
Lipman J. Meropenem dosing in critically ill patients with sepsis and
Research for statistical analysis of blinding.
without renal dysfunction: intermittent bolus versus continuous ad-
Financial support.
This work was supported by the Intensive Care
ministration? Monte Carlo dosing simulations and subcutaneous
Foundation (2010); a Royal Brisbane and Women's Hospital Foundation
tissue distribution. J Antimicrob Chemother 2009; 64:142–50.
grant to J. M. D. (2011); and National Health and Medical Research
13. Roberts JA, Paratz J, Paratz E, Krueger WA, Lipman J. Continuous
Council Training Research Fellowships to J. A. R. and J. S. D. (569917 and
infusion of beta-lactam antibiotics in severe infections: a review of its
role. Int J Antimicrob Agents 2007; 30:11–8.
Continuous Infusion of Beta-Lactams • CID 2013:56 (15 January) • 243
14. Kasiakou SK, Lawrence KR, Choulis N, Falagas ME. Continuous
26. MIMS Online (Australia). Full product information: Timentin, 2012.
versus intermittent intravenous administration of antibacterials with
Available at: . Accessed 11 October
time-dependent action: a systematic review of pharmacokinetic and
pharmacodynamic parameters. Drugs 2005; 65:2499–511.
27. European Committee on Antimicrobial Susceptibility Testing. Break-
15. Kasiakou SK, Sermaides GJ, Michalopoulos A, Soteriades ES, Falagas
point tables for interpretation of MICs and zone diameters, 2012. Avail-
ME. Continuous versus intermittent intravenous administration of an-
tibiotics: a meta-analysis of randomised controlled trials. Lancet Infect
Dis 2005; 5:581–9.
11 October 2012.
16. Bernard GR, Vincent JL, Laterre PF, et al. Efficacy and safety of re-
28. Roberts JA, Boots R, Rickard CM, et al. Is continuous infusion ceftri-
combinant human activated protein C for severe sepsis. N Engl J Med
axone better than once-a-day dosing in intensive care? A randomized
controlled pilot study. J Antimicrob Chemother 2007; 59:285–91.
17. Viaene E, Chanteux H, Servais H, Mingeot-Leclercq MP, Tulkens PM.
29. Vincent JL, Moreno R, Takala J, et al. The SOFA (Sepsis-related
Comparative stability studies of antipseudomonal beta-lactams for po-
Organ Failure Assessment) score to describe organ dysfunction/
tential administration through portable elastomeric pumps (home
failure. Intensive Care Med 1996; 22:707–10.
therapy for cystic fibrosis patients) and motor-operated syringes (in-
30. Kolahi J, Bang H, Park J. Towards a proposal for assessment of blind-
tensive care units). Antimicrob Agents Chemother 2002; 46:2327–32.
ing success in clinical trials: up-to-date review. Community Dent Oral
18. Arlicot N, Rochefort GY, Schlecht D, Lamoureux F, Marchand S,
Epidemiol 2009; 37:477–84.
Antier D. Stability of antibiotics in portable pumps used for bronchial
31. Bang H, Ni L, Davis CE. Assessment of blinding in clinical trials.
superinfection: guidelines for prescribers. Pediatrics 2007; 120:1255–9.
Control Clin Trials 2004; 25:143–56.
19. Zhang Y, Trissel LA. Stability of piperacillin and ticarcillin in Auto-
32. Nicolau DP, McNabb J, Lacy MK, Quintiliani R, Nightingale CH.
Dose infusion system bags. Ann Pharmacother 2001; 35:1360–3.
Continuous versus intermittent administration of ceftazidime in inten-
20. Smith DL, Bauer SM, Nicolau DP. Stability of meropenem in polyvi-
sive care unit patients with nosocomial pneumonia. Int J Antimicrob
nyl chloride bags and an elastomeric infusion device. Am J Health
Agents 2001; 17:497–504.
Syst Pharm 2004; 61:1682–5.
33. Rafati MR, Rouini MR, Mojtahedzadeh M, et al. Clinical efficacy of
21. Patel PR, Cook SE. Stability of meropenem in intravenous solutions.
continuous infusion of piperacillin compared with intermittent dosing
Am J Health Syst Pharm 1997; 54:412–21.
in septic critically ill patients. Int J Antimicrob Agents 2006;
22. McWhinney BC, Wallis SC, Hillister T, Roberts JA, Lipman J, Ungerer
JP. Analysis of 12 beta-lactam antibiotics in human plasma by HPLC
34. Sakka SG, Glauner AK, Bulitta JB, et al. Population pharmacokinetics
with ultraviolet detection. J Chromatogr B Analyt Technol Biomed
and pharmacodynamics of continuous versus short-term infusion of
Life Sci 2010; 878:2039–43.
imipenem-cilastatin in critically ill patients in a randomized, con-
23. US Department of Health and Human Services Food and Drug Ad-
trolled trial. Antimicrob Agents Chemother 2007; 51:3304–10.
ministration, Centre for Drug Evaluation and Research, and Center for
35. Georges B, Conil JM, Cougot P, et al. Cefepime in critically ill patients:
Veterinary Medicine. Guidance for industry: bioanalytical method val-
continuous infusion vs an intermittent dosing regimen. Int J Clin
idation, 2001. Available at:
Pharmacol Ther 2005; 43:360–9.
36. Mouton JW, Vinks AA. Continuous infusion of beta-lactams. Curr
11 October 2012.
Opin Crit Care 2007; 13:598–606.
24. MIMS Online (Australia). Full product information: Merrem IV,
37. Yost RJ, Cappelletty DM, group RS. The retrospective cohort of ex-
2012. Available at: . Accessed 11
tended-infusion piperacillin-tazobactam (RECEIPT) study: a multi-
October 2012.
center study. Pharmacotherapy 2011; 31:767–75.
25. MIMS Online (Australia). Full product information: Tazocin EF, 2012.
38. McAuley DF, O'Kane C, Griffiths MJD. A stepwise approach to justify
Available at: Accessed 11 October
phase III randomized clinical trials and enhance the likelihood of a
positive result. Crit Care Med 2010; 38:S523–27.
244 • CID 2013:56 (15 January) • Dulhunty et al
Source: http://criticalcare.queensu.ca/assets/CID_2012_DulhuntyContinuous_Infusion_of_Beta-Lactam_Antibiotics_in_Severe_Sepsis_A_Multicenter_Double-Blind_Randomized_Controlled_Trial.pdf
Venta de medicamentos en Internet: Consejo General de Colegios riesgo de falsificaciones Oficiales de Farmacéuticos La falsificación y posterior venta de bienes de consumo está constituyéndose como uno de los negocios más lucrativos que existe, incluso por encima del tráfico de estupefacientes. Los beneficios económicos que estas prácticas reportan, así como la ausencia casi total de castigo para los implicados, están aumentando cada vez más tanto la incidencia como la variedad de las falsificaciones.
Director DGAC, General José Huepe: "No podemos transar con la seguridad" Condiciones riesgosas Volar en invierno Vuelo de Cordillera Cuidado con el encierro en cajones Luis Montt, alcalde de La Reina: Por una comuna parque 1 . TOBALABAEREO • JULIO 2009 San Pedro de Atacama, II Región, Chile. Fotógrafo: Juan Oliver Núñez




