Levitra enthält Vardenafil, das eine kürzere Wirkdauer als Tadalafil hat, dafür aber schnell einsetzt. Männer, die diskret bestellen möchten, suchen häufig nach levitra kaufen ohne rezept. Dabei spielt die rechtliche Lage in der Schweiz eine wichtige Rolle.
Diabesity.eu2
Journal of Neuroendocrinology, 2004, Vol. 16, 589–604
Cellular Localization of GABAA Receptor a SubunitImmunoreactivity in the Rat Hypothalamus: Relationship WithNeurones Containing Orexigenic or Anorexigenic Peptides
M. Ba¨ckberg,* C. Ultenius,* J.-M. Fritschy† and B. Meister**Department of Neuroscience, The Retzius Laboratory, Karolinska Institutet, Stockholm, Sweden.
†Institute of Pharmacology and Toxicology, University of Zu¨rich, Zu¨rich, Switzerland.
Key words: GABA, hypothalamus, food intake, body weight, immunohistochemistry.
c-Aminobutyric acid (GABA), the major inhibitory neurotransmitter in the brain, acts via two different typeof GABA receptors. GABAA receptors are composed of five subunits that belong to eight different classes.
Depending on their subunit composition, distinct pharmacological and electrophysiological properties areobtained. GABA is produced in certain hypothalamic neurones known to be involved in control of feedingbehaviour. We report the detailed immunohistochemical localization of four GABAAR a subunits inhypothalamic regions associated with the regulation of feeding behaviour. Immunoreactive structures for allstudied GABAAR a subunits were observed in the hypothalamus, but with subunit-specific staining patterns.
GABAAR a1 immunoreactivity was most prominent in the dorsomedial hypothalamic nucleus and in thelateral hypothalamic area (LHA), whereas GABAAR a2, a3 and a5 subunits exhibited particularly strongimmunoreactivity in the ventromedial hypothalamic nucleus. In comparison, GABAAR a subunit immu-noreactivities were generally weak in the arcuate nucleus. In the ventromedial part of the arcuate nucleus,neuropeptide Y- and agouti-related peptide-containing cell bodies, which also are known to be GABAergic,were immunoreactive for only the GABAAR a3 subunit, whereas pro-opiomelanocortin- and cocaine- andamphetamine-regulated transcript- containing cell bodies located in the ventrolateral subdivision of thearcuate nucleus, showed GABAAR a1, a2 and a3 subunit immunoreactivity. In the LHA, GABAAR a3 subunitimmunoreactivity was demonstrated in both melanin-concentrating hormone (MCH) and orexin-containingneurones. In addition, MCH neurones contained GABAAR a2 immunoreactivity. In neurones of thetuberomammillary nucleus, GABAAR a2 and a5 subunits were colocalized with histidine decarboxylase, amarker for histamine-containing neurones.
complexes (3, 6). Even assuming that a functioning GABA
requires a combination of at least one a, one b and one c
The c-aminobutyric acid (GABA)ergic system is the major
subunit (7), the number of different subunits renders the
contributor of the inhibitory tone throughout the mammalian
possibility of the constitution of a large number of pentameric
central nervous system (1). GABA mediates its effects by
GABAAR combinations. However, experiments using sub-
activating two types of receptors; the GABAA receptor
unit-specific antibodies to immunoprecipitate native receptor
(GABAAR) and the GABAB receptor (GABABR). GA-
molecules, together with experiments expressing combina-
BAARs are chloride ion channels that mediate fast synaptic
tions of subunit cDNAs in mammalian cells, suggest that a
transmission and belong to a superfamily of pentameric
finite number of GABAAR subtypes exists in the brain (8, 9).
ligand-gated ion channels (2, 3), whereas GABABRs belong
In situ hybridization and immunohistochemical studies show
to the family of seven-transmembrane receptors (4, 5).
that functionally distinct neurones express different GA-
GABAAR represents one of the most complex receptor
BAAR subunits (10–12). Among the GABAAR a subunits,
systems due to the heterologous assembly of receptors from a
the a1 subunit is most widely distributed (10–12) and is
repertoire of subunits (a1)6, b1)4, c1)3, d, e, p, h and q1)3),
practically present in all brain regions. However, in the
encoded by at least 20 genes into distinct heteromeric receptor
hypothalamus, the GABAAR a2 subunit is the predominant
Correspondence to: Professor Bjo¨rn Meister, Department of Neuroscience, Karolinska Institutet, The Retzius Laboratory, Retzius va¨g 8, SE-171 77Stockholm, Sweden (e-mail:
[email protected]).
Ó 2004 Blackwell Publishing Ltd
GABAA receptors in hypothalamus
Ó 2004 Blackwell Publishing Ltd, Journal of Neuroendocrinology, 16, 589–604
GABAA receptors in hypothalamus 591
To reveal the chemical identity of GABA
AR a subunit variant (10, 12). The GABAAR a1, a3
AR-immunoreactive (ir) neu-
rones, direct double-labelling was performed by combining rabbit polyclonal
5 subunit mRNAs are also found in hypothalamic
nuclei, but in lower amounts (12).
AR a1 antiserum with mouse monoclonal antibodies to adrenocortic-
otrophin (ACTH) (diluted 1 : 2000; Peninsula Laboratories, Belmont, CA,
The hypothalamus is a vital centre which serves to maintain
USA), a marker for pro-opiomelanocortin (POMC)-containing neurones),
homeostasis, including the control of food intake and body
neuropeptide Y (NPY) (diluted 1 : 400) (31), chicken polyclonal antiserum to
weight (13, 14). There is a dense network of GABAergic
cocaine- and amphetamine-regulated transcript (CART) (diluted 1 : 200;
terminals within the entire hypothalamus, and GABA is
AB5340P, Chemicon International, Temecula, CA, USA) or guinea-pigpolyclonal antiserum to GABA
synthesized in neurones of several hypothalamic nuclei (1),
BR1 (diluted 1 : 1500; AB1531; Chemicon
International). Guinea-pig polyclonal GABAAR a2, a3 or a5 antiserum was
which are known to be involved in control of feeding
combined with mouse monoclonal antibodies to ACTH (Peninsula Laborat-
behaviour (15). GABA has been described as an orexigenic
ories), rabbit polyclonal antiserum to NPY (diluted 1 : 1600; Peninsula
neurotransmitter (15), acting through both GABA
Laboratories), agouti-related peptide (AGRP) (diluted 1 : 400; Phoenix
Pharmaceuticals, Belmont, CA, USA), glutamic acid decarboxylase 65
GABABR (16–21). To increase our understanding of the
(GAD65) (diluted 1 : 2000; AB5082, Chemicon International), melanin-
mechanism by which GABA affects body weight via
concentrating hormone (MCH) (diluted 1 : 800; kind gift from Dr W. Vale),
GABAAR, we studied the detailed cellular localization of
orexin (diluted 1 : 800; kind gift from Drs L. de Lecea and G. Sutcliffe),
histidine decarboxylase (HDC), a marker for histamine-containing neurones
AR a1, a2, a3 and a5 subunits in the hypothalamus
using immunohistochemistry. To define the chemical identity
(diluted 1 : 2000) (32), chicken polyclonal antiserum to CART (ChemiconInternational) or goat polyclonal antiserum to GABA
of hypothalamic GABA
BR1 (diluted 1 : 1000;
sc-7338; Santa Cruz Biotechnology, Santa Cruz, CA, USA). The combina-
employed a direct double-labelling technique, combined with
tions were visualized with Cy3-conjugated donkey anti-rabbit IgG combined
confocal microscopy, to study colocalization of GABAAR a
with Cy5-conjugated donkey antimouse IgG, donkey anti-guinea-pig IgG,
subunits with hypothalamic peptides, which have been
donkey anti-chicken IgG or with Cy3-conjugated donkey anti-guinea-pig IgG
implicated as important regulators of food intake.
combined with Cy5-conjugated donkey anti-goat IgG, donkey anti-mouseIgG and donkey anti-rabbit IgG or with Cy5-conjugated donkey anti-guinea-pig IgG combined with Cy3-conjugated donkey anti-chicken secondary
Materials and methods
antibodies (all diluted 1 : 250 and purchased from Jackson Immuno-Research). After rinsing in PBS, all sections were mounted in a mixture ofglycerol containing 2.5% 1,4-diazabicyclo[2.2.2]octane (DABCO; Sigma) to
Animals and tissue preparations
prevent fading of immunofluorescence. Sections were visualized and imageswere obtained using a Bio-Rad RadiancePlus laser confocal scanning system
Males Sprague-Dawley rats (weighint 150–200 g; B & K Universal, Stock-
(Bio-Rad, Hercules, CA, USA). The excitation wavelength was 543 nm for
holm, Sweden) were used. The rats were kept for at least 1 week under a
Cy3- and 638 nm for Cy5-induced fluorescence. Images were processed using
12 : 12 h light/dark cycle (lights on at 06.00 h) in a temperature-controlled
the Adobe PhotoshopTM 6.0.1 software (Adobe Systems Inc., San Jose, CA,
room and had free access to food pellets and tap water. The experiments were
approved by the local ethical committee for animal experiments, Stockholm,Sweden. The rats were anaesthetized with sodium pentobarbitone (injectedintraperitoneally, 40 mg/kg; Apoteket Produktion & Laboratorier, Umea˚,
Sweden) and perfused via the ascending aorta with 50 ml of Ca2+-freeTyrode's solution (37 °C), followed by 50 ml of formalin-picric acid fixative(37 °C) (4% paraformaldehyde and 0.4% picric acid in 0.16 M phosphate
buffer, pH 6.9). Perfusions were thereafter continued for 6 min with ice-coldfixative. Some rats received an injection of colchicine(120 lg in 20 ll 0.9%
Incubation with antisera to GABAAR subunits a1, a2, a3 and
NaCl; Sigma, St Louis, MO, USA) into the lateral ventricle 24 h before
a5 revealed immunoreactivity in many areas of the hypotha-
perfusion. Colchicine arrests axonal transport, thereby increasing levels of
lamus (Figs 1A–I and 2A–I). However, there were several
transmitters, enzymes and peptides/proteins in the cell soma (22).
differences in the staining patterns obtained with the
The brains were removed and postfixed in the same fixative for 90 min at
4 °C and rinsed for at least 24 h in 0.1
phosphate buffer (pH 7.4)
AR a subunit-specific antisera. Within the paraven-
containing 10% sucrose, 0.02% bacitracin (Sigma) and 0.01% sodium azide
tricular nucleus (PVN), only weak to moderate fluorescence
(Riedel-de Haen, Seeize, Germany). The brains were frozen and 10 lm
intensity was demonstrated for all the analysed subunits
coronal sections were cut in a cryostat (Microm HM560, Walldorf, Germany).
(Fig. 1A–D). There were several GABAAR a1-, a2-, a3- and a5-ir cell bodies in the magnocellular division of the PVN
(Fig. 1E–H). In the arcuate nucleus, and in its ventromedial
Sections were incubated overnight at 4 °C with rabbit polyclonal antiserum to
aspect in particular, GABAAR a1, a2, a3 and a5 immunore-
GABAAR a1 subunit (diluted 1 : 20 000), guinea pig polyclonal antisera to
activity exhibited a weak staining pattern in comparison with
GABAAR a2 subunit (diluted 1 : 2500), GABAAR a3 subunit (diluted
other areas at mid-hypothalamic levels (Fig. 2A–D). In the
1 : 2500) or GABAAR a5 subunit (diluted 1 : 2500). All GABAAR subunit
ventromedial hypothalamic nucleus (VMH), immunopositive
specific antisera have been extensively characterized (23–27) and theirsuitability for immunohistochemistry has been documented previously (10,
cell bodies were found for all GABAAR a subunits (Fig. 2E–H);
28–30). The antisera were diluted in 0.3% Triton X-100, 0.01% sodium azide
however, GABAAR a1 immunoreactivity was weaker com-
(Riedel-de Haen), 0.02% bacitracin (Sigma) in phosphate-buffered saline
pared with GABAAR subunits a2, a3 and a5 (Fig. 2A–D).
(PBS; 0.1 M phosphate buffer; pH 7.4; 0.15 M NaCl). The sections were rinsed
in PBS and incubated with Cy3-conjugated donkey anti-rabbit or antiguinea
AR a1 immunoreactivity was especially prominent in
pig secondary antibodies (diluted 1 : 250; Jackson ImmunoResearch, West
the dorsomedial hypothalamic nucleus (DMH) and in the
Grove, PA, USA) for 1 h at room temperature.
lateral hypothalamic (LHA) and perifornical areas (Fig. 2A)
(A–E) Images of the sections from a hypothalamic level of paraventricular nucleus (PVN) obtained via confocal microscopy after incubation with
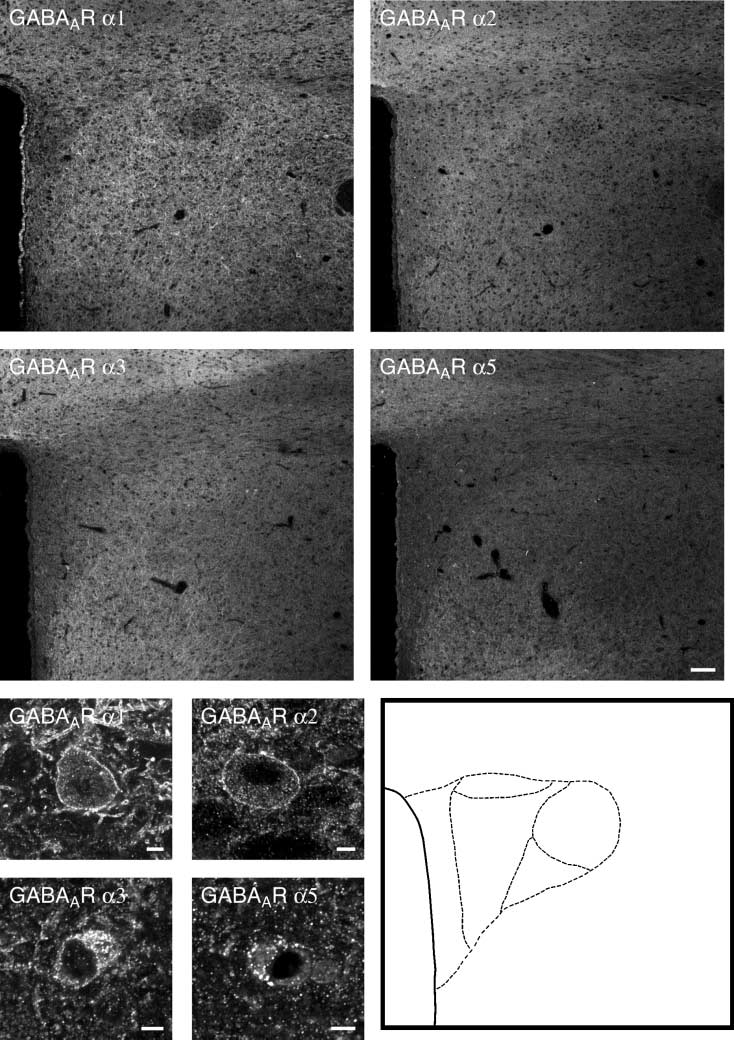
GABAAR a1 (A), a2 (B), a3 (C) and a5 (D) subunit antiserum. All studied subunits showa diffuse staining of the neuropil in the PVN. At the subcellular level,presence of scattered GABAAR a1- (E), a2- (F), a3- (G) and a5 (H)-positive neurones, presumably magnocellular neurones, are observed. Schematic drawingsbased on the images illustrate the location of landmarks (I). dp, Dorsal parvocellular part; mpd, medial parvocellular part, dorsal zone; mpv, medialparvocellular part, ventral zone; pml, posterior magnocellular part, lateral zone; pv, periventricular part; 3V, third ventricle. Scale bars ¼ 100 lm.
Ó 2004 Blackwell Publishing Ltd, Journal of Neuroendocrinology, 16, 589–604
GABAA receptors in hypothalamus
Ó 2004 Blackwell Publishing Ltd, Journal of Neuroendocrinology, 16, 589–604
GABAA receptors in hypothalamus 593
compared to the GABAAR subunits a2, a3 and a5, which
demonstrated to contain GABAAR a3 immunoreactivity
displayed a weak staining in these areas (Fig. 2B–D). How-
(Figs 3E,F and 4C,D), but lacked GABAAR a1, a2 and a5
ever, stronger GABAAR a2-ir was also present in ventral
immunoreactivity (Figs 3A–D,G–H and 4A,B,E,F). By contrast,
parts of the DMH (Fig. 2B). A distinct border was observed
most of the larger POMC- and CART-containing neurones
between the internal and external layers of the median
located in the ventrolateral part of the arcuate nucleus were
eminence, where all subtypes showed stronger immunoreac-
shown to contain many of the investigated GABAAR a
tivity in the internal layer (Fig. 2A–D).
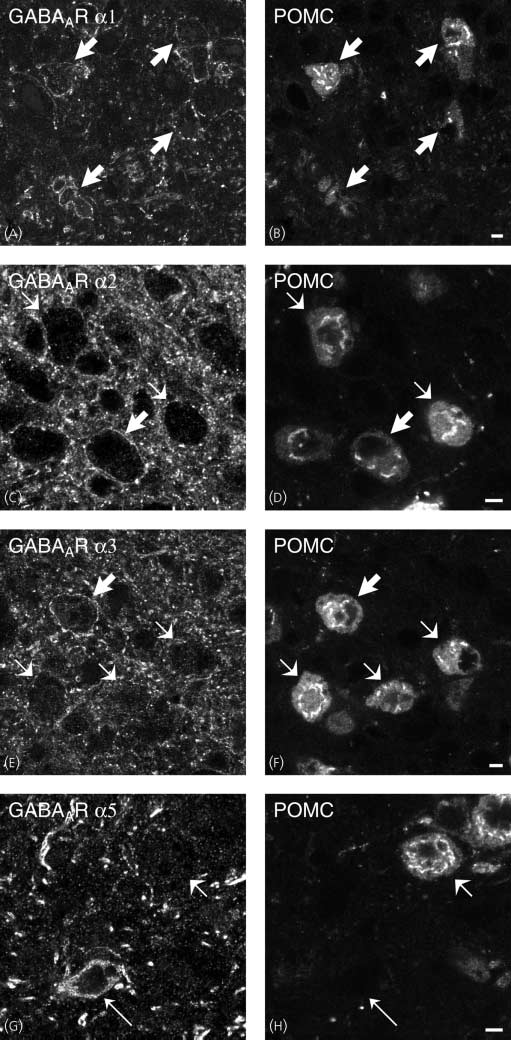
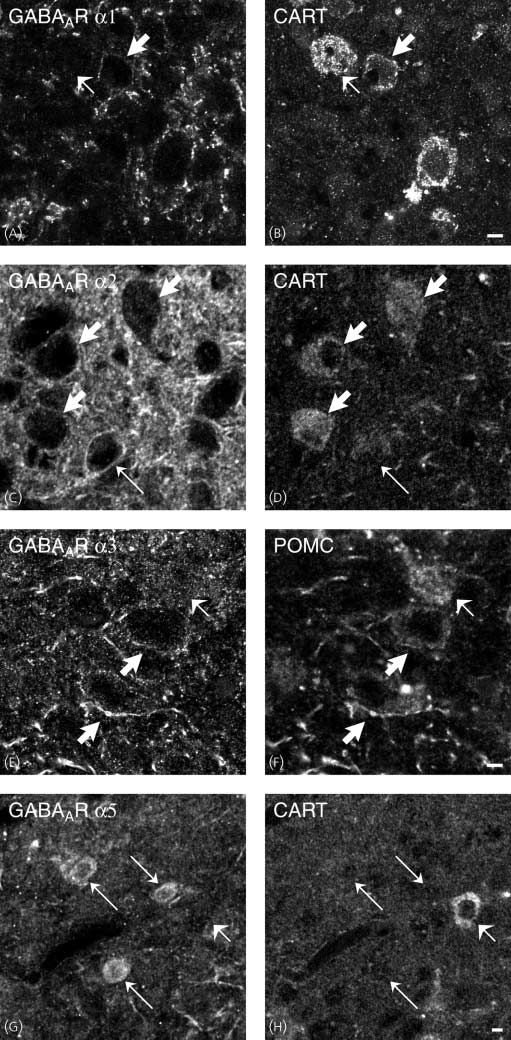
subunits (Figs 5A–H and 6A–H). Thus, GABAAR a1, a2 and a3
There were differences in the subcellular localization of
subtypes were all present in the periphery of individual
GABAAR immunoreactivity among different types of neu-
POMC/CART-positive neurones (Figs 5A–F and 6A–F),
rones. At high magnification, the GABAAR a subunit-specific
whereas GABAAR a5 immunoreactivity was not detected in
antisera labelled numerous, very fine elements throughout the
these neurones (Figs 5G,H and 6G,H). GABABR1-ir neurones
neuropil, but also individual soma and their dendrites. There
located in the arcuate nucleus exhibited immunoreactivity for
were patches of immunoreactive material in individual
all studied GABAAR a subtypes (data not shown). Many
GABAAR a subunit-positive neurones, presumably corres-
large MCH- or orexin-ir neurones in the LHA contained
ponding to receptor aggregates. In most GABAAR a subunit-
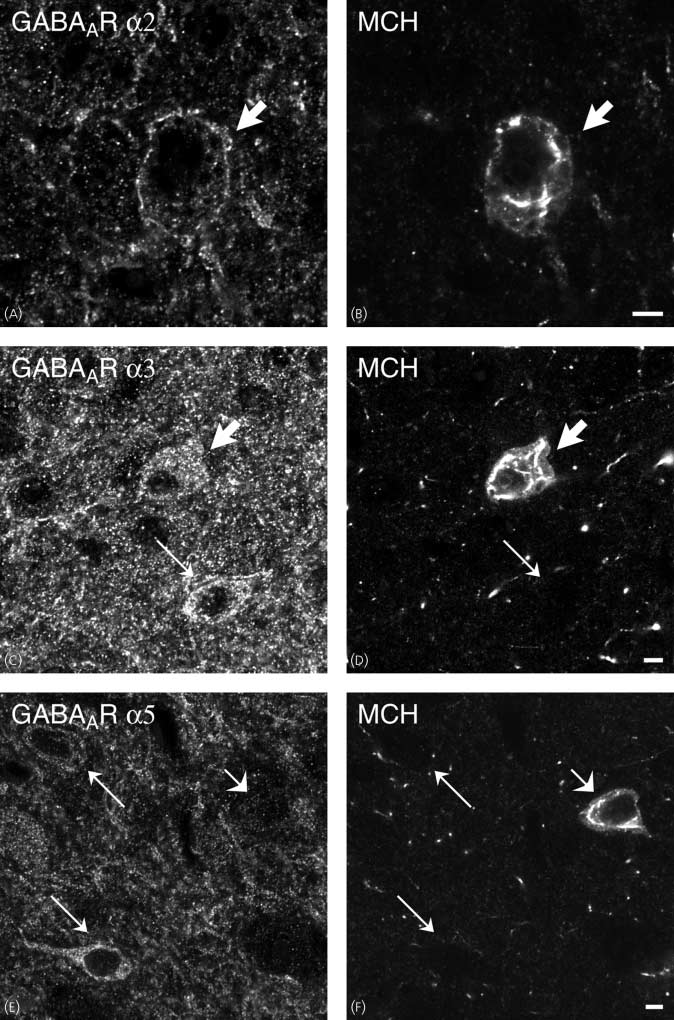
GABAAR a subunit immunoreactivity. Thus, GABAAR a2
ir neurones, the immunolabelled aggregates increased in
and a3, but not GABAAR a5 subtype immunoreactivity was
numbers to such an extent that the membrane appeared to be
present in most MCH-ir neurones in the LHA (Figs 7A,B,C–F).
continuously labelled; however, some aggregates were of
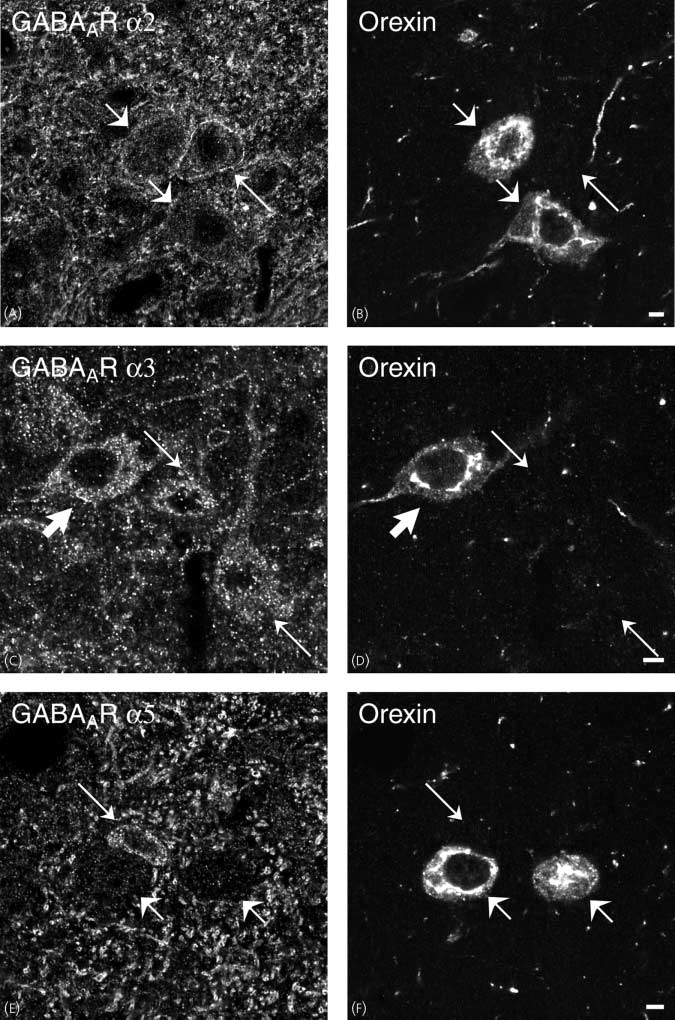
Orexin-containing cell bodies were GABAAR a3- (Fig. 8C–D),
higher intensity and size. Within the arcuate nucleus, staining
but not GABAAR a2- or a5-ir (Figs 8A,B,E,F). The fewGAD-
of the GABAAR a1 a2 and a3 subunits was predominantly
positive cell bodies seen in the LHA were not GABAAR a3-
observed in the periphery of individual cell bodies, presum-
or a5-ir (data not shown). However, GAD immunoreactivity
ably representing an association with the plasma membrane
was demonstrated in a few GABAAR a2-ir cell bodies in this
(Figs 3A,C,E, 4A,C, 5A,C,E and 6A,C,E), whereas the GABAAR
region (data not shown). The majority of the tuberomamm-
a5 subunit immunoreactivity in addition showed a cytoplas-
illary histaminergic cell bodies, identified with an antiserum
matic punctate staining (Figs 3G, 4E, 5G and 6G). The amount
to HDC, were shown to contain GABAAR a2 and a5
of cytoplasmatic staining obtained with the antisera to the
immunoreactivity (Figs 9A,B,E,F). GABAAR a3 immunoreac-
different GABAAR subunits varied greatly among different
tivity was weak and presumably located in nerve fibres
types of neurones and a subunits. Within the LHA,
projecting to tuberomammillary neurones and not localized
GABAAR a1 and a2 subunits were primarily localized to
to the cell soma (Figs 9C,D).
the plasma membrane, whereas both GABAAR a3 and a5subunit immunoreactivities showed a cytoplasmatic staining
(Figs 7A–F and 8A–F). GABAAR a2 subunit immunoreactiv-ity was also detected primarily in the periphery of individual
neurones in the tuberomammillary nucleus (TMN) (Fig. 9A),whereas the GABAAR a5 subunit was distributed in the
The present results showa w
idespread distribution of
cytoplasm (Fig. 9E). GABAAR a1- and GABAAR a3-postive
GABAAR a subunit immunoreactivity in the rat hypotha-
neurones were not detected in the TMN.
lamus in agreement with previously published data (10).
In untreated rats, direct double-labelling showed that
Because it has been reported that GABAAR a4 and a6
GAD65-ir nerve fibres and terminals surrounded and were
subunit mRNAs are not expressed in the hypothalamus,
present in close association with GABAAR a2, a3 and a5
these subunits were not included in this study (12). The
subunit-ir cell bodies located in the arcuate nucleus as well as
presence of different GABAAR a1, a2, a3 and a5 subunits in
in the LHA (data not shown).
neurones known to be involved in ingestive behavioursuggests a GABAergic influence via GABAARs on hy-pothalamic neuronal pathways regulating feeding behaviour
Chemical identity of GABAAR-ir neurones
(see below).
For the studies in which the GABAAR a-immunoreactiveneurones were chemically defined, an approximate number of
Cellular localization of GABA
15–10 sections were examined for each combination.
AR a subunit immunoreactivity
In colchicine-treated rats, NPY- and AGRP-containing cell
The GABAAR a1 subunit has been described as the most
bodies located in the ventromedial arcuate nucleus were
abundant a subunit in adult brain (23, 33–35). However, in
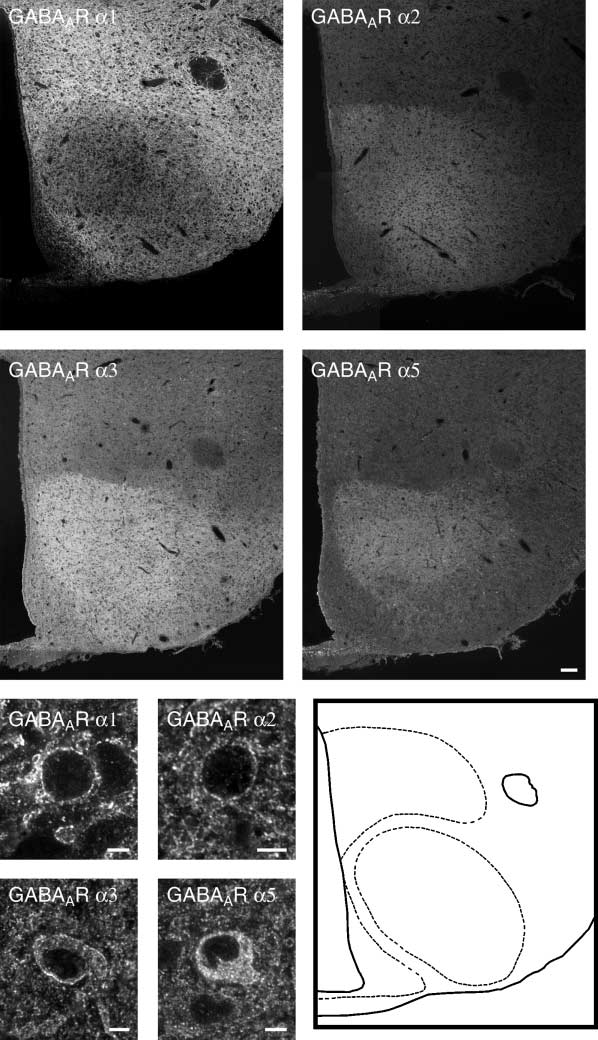
(A–E) Montage of images of a midhypothalamic level obtained via confocal microscopy after incubation with GABAAR a1 (A), a2 (B), a3 (C) and a5 (D)
antiserum. The GABAAR a1, a2, a3 and a5 subunits display differences in their staining patterns. All subunits showa diffuse staining of the external layer of themedian eminence, whereas the internal layer shows stronger labelling. The ventrolateral part of the arcuate nucleus (ARC) exhibit a more moderate level ofimmunoreactivity as compared with the low fluorescence intensity of GABAAR a1 observed in the ventromedial part of ARC. GABAAR a2, a3 and a5 subunitsexhibit a prominent staining in the ventromedial hypothalamic nucleus (VMH), which appears to be devoid of GABAAR a1 subunit immunoreactivity.
However, at high magnification, immunoreactivity is detected for all GABAAR a subunits in individual cell bodies (E–H). By contrast, GABAAR a1 appears tobe the dominant immunoreactivity in the dorsomedial hypothalamic nucleus (DMH) of the studied subunits, although parts of the DMH also showratherstrong GABAAR a2 fluorescence intensity. In the lateral hypothalamic area (LHA), the GABAAR a1 showa prominent staining pattern, whereas the otherstudied subunits are more moderately expressed. Schematic drawings based on the photomontages illustrate the location of landmarks (I). f, Fornix; 3V, thirdventricle. Scale bars ¼ 100 lm.
Ó 2004 Blackwell Publishing Ltd, Journal of Neuroendocrinology, 16, 589–604
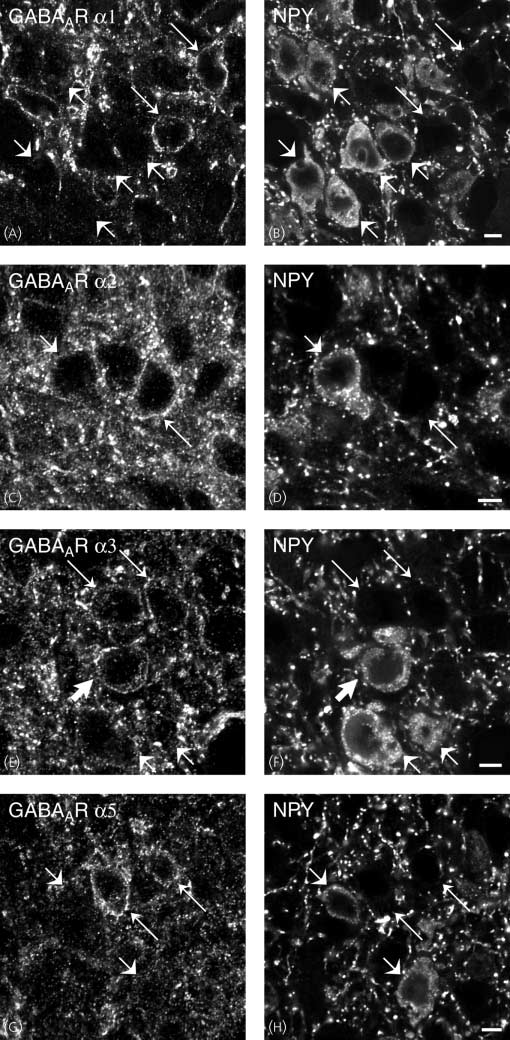
(A–H) Images obtained via confocal microscopy of sections of the rat arcuate nucleus after direct double-labelling combining rabbit antiserum to GABAAR
a1 (A) with mouse monoclonal antibodies to neuropeptide Y (NPY) (B) and guinea-pig antiserum to GABAAR a2, a3 and a5 (C,E,G) w ith rabbit antiserum to NPY(D,F,H). All studied subtypes are expressed in the ventromedial part of the arcuate nucleus (A,C,E,G). Comparison of (A,C,E,G) w ith (B,D,F,H), respectively, shows thatthere are GABAAR a3-ir neurones that contain NPY (thick arrows). There are also GABAAR a1, a2, a3 and a5-positive neurones that are NPY-negative (thinarrows) or GABAAR a1, a2, a3 and a5-negative neurones that are NPY-positive (short arrows). Scale bars ¼ 5 lm.
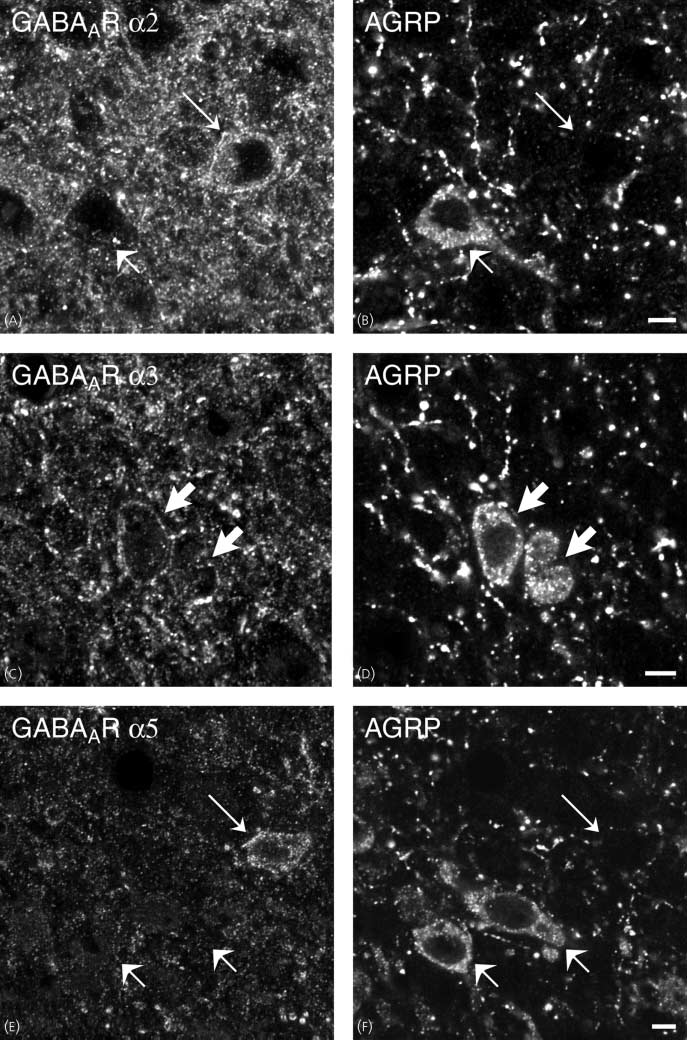
(A–F) Images obtained via confocal microscopy of sections of the rat arcuate nucleus after direct double-labelling combining guinea-pig antiserum to
GABAAR a2, a3 and a5 (A,C,E) with rabbit polyclonal antiserum to agouti-related peptide (AGRP) (B,D,F). All studied subtypes are expressed in theventromedial part of the arcuate nucleus (A,C,E). Comparison of (A,C,E) w ith (B,D,F), respectively, shows that there are GABAAR a3-ir neurones that containAGRP (thick arrows). There are also GABAAR a2, a3 and a5-positive neurones that are neuropeptide Y (NPY)-negative (thin arrows) or GABAAR a1, a2, a3and a5-negative neurones that are NPY-positive (short arrows). Scale bars ¼ 5 lm.
GABAA receptors in hypothalamus
Ó 2004 Blackwell Publishing Ltd, Journal of Neuroendocrinology, 16, 589–604
GABAA receptors in hypothalamus 597
the hypothalamus, the GABAAR a2 subunit appears to be the
a2, a3 and a5 subunit immunoreactivities were found in the
most dominating a subunit (10, 12). It has been described that
cell bodies of the arcuate nucleus. However, there were
the GABAAR a1 and GABAAR a2 subunits display an
obvious differences among two subpopulations of neurones
approximately complementary distributions; GABAAR a1
within the nucleus. NPY/AGRP-containing neurones were
subunit immunoreactivity is prominent in regions where
only positive for the GABAAR a3 subunit and thus lacked
GABAAR a2 subunit immunoreactivity is absent or weak
GABAAR a1, a2 and a5 expression. On the other hand,
(10). This observation is in agreement with the present
POMC/CART-containing neurones were positive for GA-
findings. In accordance with a previous study by Fritschy and
BAAR a1, a2 and a3 subunit immunoreactivity. These
Mo¨hler (10), the PVN exhibited only weak to moderate
results suggest that the ventromedial POMC/CART neu-
staining for all the subunits analysed. In this study, we
rones may be the main targets for GABA, whereas the
detected several GABAAR a2, a3 and a5 subunit-ir cell bodies
NPY/GABA neurones are not. In this context, it is
in the magnocellular part of the PVN.
important to point out that there is a clear difference
The GABAAR a1 subunit antiserum exhibited the strongest
between the two neuronal population in the arcuate nucleus
immunoreactivity of the studied subunits and the staining
with regard to the GABAergic expression pattern. NPY/
pattern was different as compared with GABAAR a2, a3 and
AGRP neurones are GABAergic, whereas POMC/CART
a5 subunits at the mid-hypothalamic level. GABAAR a1
neurones appear not to be GABAergic.
subunit exhibited strong immunoreactivity in the DMH and
In the LHA, there were GABAAR a1-, a2-, a3- and a5-ir
in the LHA, whereas GABAAR a2, a3 and a5 subunits
neurones. This region contains two food-stimulatory pep-
displayed strong immunoreactivity in the VMH. It is
tides, MCH and orexin, which are synthesized in two
important to note that there was only weak fluorescence
separate cell populations (37, 38). MCH was colocalized
intensity for all studied GABAAR a subunits in the arcuate
with GABAAR a2 and a3 subunits, whereas orexin-contain-
nucleus, although the immunoreactivity appeared to be
ing neurones only had GABAAR a3 subunit immuno reactiv-
stronger in the ventrolateral subdivision of the nucleus
ity. Whether or not GABAAR a1 subunit immunoreactivity
compared to the ventromedial part.
is present in these two cell populations could not be
The subcellular localization of the GABAAR a subunits
established because the antisera to the GABAAR a1 subunit,
studied varied between different GABAAR a subunit and
MCH and orexin, are all raised in rabbits. Consequently,
hypothalamic regions. These differences may suggest possible
direct double-labelling could not be performed. In colchi-
variations in receptor turnover and or reflect presence of a
cine-treated rats, a fewGAD65-positive neurones could be
pool of receptor proteins not being inserted into the plasma
detected in the lateral hypothalamus. We observed colocal-
membrane. In addition, it can not be excluded that colchicine
ization of GAD65 and GABAAR a2 subunit, but not with
treatment effects the cellular staining of GABAAR a1, a2, a3
GABAAR a3, a5 subunits. Furthermore, there were some
and a5 subunits because it has be shown that exposure of
GAD65-positive neurones that were GABAAR a2-negative.
cultured neurones to colchicine appears to produce a stronger
Presumably they may be GABAAR a1-positive because
cytoplasmatic immunostaining of the GABAAR a subunits
GABAAR a1 antiserum displayed strong immunoreactivity
without affecting the total cellular level of the proteins (36).
in the LHA. Unfortunately, we were not able to confirm
However, we did not detect any obvious differences in cellular
this assumption for the same reasons as described above.
staining for the different GABAAa subunits when comparing
The magnocellular HDC-containing neurones of the TMN
untreated and colchicine-treated rats.
were the only cells investigated that contained GABAAR a5
In accordance with earlier studies, we detected a regional
subunit immunoreactivity, although the subunit was seen in
codistribution of GABAAR a subunits in rat hypothalamic
all studied hypothalamic areas. In addition, GABAAR a2
neurones (10, 11). In spite of the fact that different GABAAR
subunit was detected in these neurones. However, histamine-
a subunits have been demonstrated to be colocalized in
containing cell bodies of the TMN did not exhibit GABAAR
neurones with histochemical methods (10), it cannot be
a1 or a3 subunit immunoreactivity, in accordance with earlier
concluded whether the neurone-specific colocalization dem-
studies (10, 12).
onstrates that the GABAAR a subunits are part of the samereceptor or, alternatively, are in different GABAAR com-
Functional considerations in relation to body weight regulation
GABA-synthesizing neurones located in the ventromedialsubdivision of the arcuate nucleus coexpress the orexigenic
Chemical identity of GABAAR a subunit-ir cell bodies
peptides, NPY and AGRP (39, 40). GABA is also colocalized
One or more of the studied GABAAR a subunit immu-
with NPY and some AGRP-ir nerve terminals within the
noreactivities were observed in neurones containing media-
PVN (41, 42). In agreement, microinjection of muscimol, a
tors that stimulates or inhibits food intake. GABAAR a1,
GABAAR agonist, into PVN stimulates feeding (17, 18, 41).
(A–H) Images obtained via confocal microscopy of sections of the rat arcuate nucleus after direct double-labelling combining rabbit antiserum to
GABAAR a1 (A) or guinea-pig antiserum to GABAAR a2, a3 and a5 (C,E,G) with mouse monoclonal antibodies to adrenocorticotropic hormone; a marker forpro-opiomelanocortin-(POMC)-containing neurones (B,D,F,H). All studied subtypes are expressed in the ventrolateral part of the arcuate nucleus (A,C,E,G).
Comparison of (A,C,E,G) w ith (B,D,F,H), respectively, shows that there are GABAAR a1-, a2-, a3-ir neurones that contain POMC (thick arrows). GABAAR a5-expressing neurones appears to be POMC-negative (G,H; thin arrows). Some POMC-positive cell bodies are GABAAR a2-, a3-negative neurones [compareshort arrows in (C) w ith (D) and (E) w ith (F)], whereas all POMC-containing neurones appear to be GABAAR a1-positive (A,B). Scale bars ¼ 5 lm.
Ó 2004 Blackwell Publishing Ltd, Journal of Neuroendocrinology, 16, 589–604
(A–H) Images obtained via confocal microscopy of sections of the rat arcuate nucleus after direct double-labelling combining rabbit antiserum to
GABAAR a1 (A) or guinea-pig antiserum to GABAAR a2, a3 and a5 (C,E,G) with chicken polyclonal antiserum to cocaine and amphetamine-regulatedtranscript (CART) (B,D,F,H). All studied subtypes are expressed in the ventrolateral part of the arcuate nucleus (A,C,E,G). Comparison of (A,C,E,G) w ith(B,D,F,H), respectively, shows that there are GABAAR a1-, a2-, a3-ir neurones that contain pro-opiomelanocortin-(POMC) (thick arrows). GABAAR a5-expressing neurones appears to be CART-negative (G,H; thin arrows). Some CART-positive cell bodies are GABAAR a2-, a3-negative neurones [compare shortarrows in (C) w ith (D) and (E) w ith (F)], whereas all POMC-containing neurones appear to be GABAAR a1-positive (A,B). Scale bars ¼ 5 lm.
GABAA receptors in hypothalamus 599
(A–F) Images obtained via confocal microscopy of sections of the lateral hypothalamic area (LHA) after direct double-labelling combining guinea-pig
antiserum to GABAAR a2, a3 and a5 (A,C,E) with rabbit polyclonal antiserum to melanin-concentrating hormone (MCH) (B,D,F). All studied subtypes areexpressed in the LHA (A,C,E). Comparison of (A,C,E) w ith (B,D,F), respectively, shows that there are GABAAR a2- and a3-ir neurones that contain MCH (thickarrows), whereas MCH-containing neurones lack GABAAR a5 [compare short arrows in (E) w ith (F)]. There are also GABAAR a3-positive neurones that areMCH-negative (thin arrows). Scale bars ¼ 5 lm.
Ó 2004 Blackwell Publishing Ltd, Journal of Neuroendocrinology, 16, 589–604
GABAA receptors in hypothalamus
(A–D) Images obtained via confocal microscopy of sections of the lateral hypothalamic area (LHA) after direct double-labelling combining guinea-pig
antiserum to GABAAR a2, a3 and a5 (A,C,E) with rabbit polyclonal antiserum to orexin (B,D,F). All studied subtypes are expressed in the LHA (A,C,E).
Comparison of (A,C,E) w ith (B,D,F), respectively, shows that there are GABAAR a3-ir neurones that contain orexin (thick arrows), whereas orexin-containingneurones lack GABAAR a2- and a5 immunoreactivity [compare short arrows in (A) w ith (B) and (E) w ith (F)]. There are also GABAAR a3-positive neuronesthat are orexin-negative (C,D; thin arrows). Scale bars ¼ 5 lm.
Ó 2004 Blackwell Publishing Ltd, Journal of Neuroendocrinology, 16, 589–604
GABAA receptors in hypothalamus 601
(A–F) Images obtained via confocal microscopy of sections of the tuberomammillary nucleus (TMN) after direct double-labelling combining guinea-pig
antiserum to GABAAR a2, a3 and a5 (A,C,E) with rabbit antiserum to histidine decarboxylase (HDC); a marker for histamine-containing neurones (B,D,F). Allstudied subtypes are expressed in the TMN (A,C,E) (GABAAR a1 not shown). Note that most GABAAR a2- and a5-ir neurones exhibit HDC immunoreactivity[compare thick arrows in (A) w ith (B) and (E) w ith (F)]. GABAAR a3-containing nerve terminals appear to be localized in close contact to HDC-positiveneurones (C,D). Scale bars ¼ 5 lm.
Ó 2004 Blackwell Publishing Ltd, Journal of Neuroendocrinology, 16, 589–604
GABAA receptors in hypothalamus
Furthermore, this hypothalamic site also gives an orexigenic
BAAR a1, whereas the signal for GABAAR a2 is greater in the
response to NPY microinjections (43). Because coadminis-
VMH in the adult animals (58).
tration of NPY and muscimol into the PVN enhanced the
In the LHA, MCH neurones were colocalized with the
feeding over that evoked by NPY or muscimol alone (41), it is
GABAAR a2 and a3 subunits, whereas orexin neurones only
likely that GABAARs and NPY nerve endings are connecting
expressed GABAAR a3 subunit immunoreactivity. By con-
to the same target cells in the PVN. Whereas neurones located
trast to the arcuate nucleus, the GABAAR a3 subunit
in the ventromedial subdivision of the arcuate nucleus contain
immunoreactivity was more cytoplasmic in the LHA. The
orexigenic mediators (44–47), the ventrolaterally located
function of tuberomammillary GABAARs composed of only
arcuate neurones contain the anorexigenic peptides a-MSH
the a5 subunit has been debated because pharmacological
and CART (15, 48). It has been demonstrated that
analysis of GABAergic currents in tuberomammillary neu-
GABAergic fibres form synaptic contacts with POMC-con-
rones does not support the presence of the GABAAR a5
taining neurones in the ventrolateral division of the arcuate
subunit or its dominant role in functional GABAAR (59). A
nucleus (49) and that the GABAAR b1 subunit is localized on
GABAAR that is composed of a GABAAR a5 subunit is
these neurones (50). When considered together with our
insensitive to zolpidem (a selective a- and b-subunit modu-
results, it appears that POMC-neurones contain GABAAR
lator) (60–62); however, it appears not to consist of any
receptor complexes composed of a1, a2 and/or a3 (either as a
zolpidem-insensitive tuberomammillary neurones (59). Hista-
duplicate or in combination) combined with at least one b1
minergic neurones of the TMN project to many brain regions,
subunit. Functional evidence for GABAARs on POMC
including the hypothalamus (63, 64) and have been suggested
neurones is provided by results showing that GABA and
to be involved in several brain functions (e.g. suppression of
muscimol inhibit a-MSH (an anorexigenic peptide derived
eating and arousal) (65). In agreement with earlier studies,
from POMC) release and POMC gene expression (51).
GABAAR a1 and a3 subunit-ir cell bodies were not detected
Presumably, the GABAAR complex located on POMC/
in the TMN (10, 11), but we observed a dense innervation to
CART neurones does not represent somatodendritic autor-
histaminergic neurones of terminals in which the GABAAR
eceptors because GABA has not been detected in POMC/
a3 subunit was expressed. GABAARs containing the a3
CART neurones (39, 40). The origin of GABAergic terminals
subunit located in nerve endings apposing TMN neurones
contacting POMC neurones has suggested to be GABA/NPY
may act as presynaptic autoreceptors.
neurones located in the ventromedial part of the arcuate
The extensive interconnectivity and prevalence of GAB-
nucleus (39, 40) because the POMC neurones also contain
Aergic neurones in the hypothalamus, combined with the
postsynaptic NPY Y1 receptors (52). Further evidence for
diverse structural assemblies afforded by the multiplicity of
projections from the ventromedial arcuate nucleus comes
possible GABAAR subtype combinations in hypothalamic
from experiments conducted with chemical lesions of the
nuclei, adds a daunting level of complexity to the GABAergic
arcuate nucleus. Parenteral treatment of rodents with mono-
influence on the control of feeding via GABAARs. The
sodium glutamate, which eliminates all cell bodies in the
restricted expression of the GABAAR a subunit on certain
ventromedial arcuate nucleus, results in a reduced plexus of
neurones may be essential to an understanding of the
GAD- and NPY-ir fibres in the ventrolateral part of the
GABAergic modulation of feeding. Our results provide a
arcuate nucleus (53).
morphological basis for the diversity of GABAAR on
We noticed strong immunoreactivity of GABAAR a2, a3,
hypothalamic neurones, which are presumably integrated in
and a5 subunits in the VMH, which is a region suggested to
the neuronal circuits regulating food intake.
act as a satiety centre (54). Infusion of GABAAR agonist intothe VMH increases food intake dose-dependently in lean rats
and the effect is blocked by local pretreatment with theGABAAR antagonist picrotoxin (17). These results suggest
This research was supported by support by EC FP6 funding (contract LSHM-
CT-2003-503041), the Swedish Research Council (72X-10358-10A), the
ARs located in the VMH are involved in the
feeding system, where activation of GABA
National Network in Neuroscience (NNN), A˚hle´n-stiftelsen, Dr P. Ha˚kans-
sons stiftelse (Druvan), Knut and Alice Wallenberg Foundation (confocal
satiety-related neurones. Such neurones may be glutamatergic
system), stiftelsen Elsa and Sigurd Goljes Minne, the Swedish Society for
because the VMH contains many neurones expressing the
Medical Research and funds from Karolinska Institutet. We wish to express
vesicular glutamate transporter 2 (55). It has also been
our sincere gratitude to Professor Tomas Ho¨kfelt for providing us with
suggested that GABA influences the development and
antisera for double-labelling.
organization of the VMH (56). GABAAR b3 knockout micedisplay an unusually large VMH. The GABA
Accepted 4 May 2004
are densely expressed in VMH (12) during development (57).
Whether these GABAAR a subunits are also important for
VMH development is unclear but, because high levels ofGABA
Mugnaini E, Oertel WH. An atlas of the distribution of GABAergic
AR a5 are found in the embryonic VMH (57), the
neurons and terminals in the rat CNS as revealed by GAD im-
GABAAR a5 and b3 subunits may be components of some of
munocytochemistry. In: Bjo¨rklund A, Ho¨kfelt T, eds. Handbook of
the GABAAR in neurones of the VMH. Colocalization of
Chemical Neuroanatomy, vol. 4. GABA and Neuropeptides in the
CNS, Part 1. Amsterdam: Elsevier, 1985: 436–608.
AR a2 and b3 subunit mRNA was apparent in the
hypothalamus of adult rats (12), but a developmental switch
Unwin N. Neurotransmitter action: opening of ligand-gated ionchannels. Cell 1993; 72 (Suppl. ): 31–41.
of GABAAR a2 expression in the VMH has been suggested.
Neonatal animals showhighest immunoreactivity for GA-
Ó 2004 Blackwell Publishing Ltd, Journal of Neuroendocrinology, 16, 589–604
GABAA receptors in hypothalamus 603
Barnard EA, Skolnick P, Olsen RW, Mo¨hler H, Sieghart W, Biggio
brain by a5- and d-subunit-specific immunopurification. J Biol Chem
G, Braestrup C, Bateson AN, Langer SZ, International Union of
1993; 268: 5965–5973.
Pharmacology. XV. Subtypes of c-aminobutyric acid A receptors:
Redecker C, Wang W, Fritschy JM, Witte OW. Widespread and
classification on the basis of subunit structure and receptor function.
long-lasting alterations in GABAA-receptor subtypes after focal
Pharmacol Rev 1998; 50: 291–313.
cortical infarcts in rats: mediation by NMDA-dependent processes.
Bowery NG. GABAB receptor pharmacology. Annu Rev Pharmacol
J Cereb Blood Flow Metab 2002; 22: 1463–1475.
Toxicol 1993; 33: 109–147.
Neumann-Haefelin T, Staiger JF, Redecker C, Zilles K, Fritschy
Kerr DI, Ong J. GABAB receptors. Pharmacol Ther 1995; 67: 187–
JM, Mo¨hler H, Witte OW. Immunohistochemical evidence for
dysregulation of the GABAergic system ipsilateral to photochemi-
Whiting PJ, Bonnert TP, McKernan RM, Farrar S, Le Bourdelles B,
cally induced cortical infarcts in rats. Neuroscience 1998; 87: 871–
Heavens RP, Smith DW, Hewson L, Rigby MR, Sirinathsinghji DJ,
Thompson SA, Wafford KA. Molecular and functional diversity of
Fritschy JM, Weinmann O, Wenzel A, Benke D. Synapse-specific
the expanding GABAA receptor gene family. Ann NY Acad Sci 1999;
localization of NMDA and GABAA receptor subunits revealed by
868: 645–653.
antigen-retrieval immunohistochemistry. J Comp Neurol 1998; 390:
Sieghart W, Fuchs K, Tretter V, Ebert V, Jechlinger M, Hoger H,
Adamiker D. Structure and subunit composition of GABAA recep-
Grouzmann E, Comoy E, Walker P, Burnier M, Bohuon C, Waeber
tors. Neurochem Int 1999; 34: 379–385.
B, Brunner H. Production and characterization of four anti-neuro-
Sieghart W, Sperk G. Subunit composition, distribution and func-
peptide Y monoclonal antibodies. Hybridoma 1992; 11: 409–424.
tion of GABAA receptor subtypes. Curr Top Med Chem 2002; 2:
Watanabe T, Taguchi Y, Shiosaka S, Tanaka J, Kubota H, Terano
Y, Tohyama M, Wada H. Distribution of the histaminergic neuron
Whiting PJ. GABAA receptor subtypes in the brain: a paradigm for
system in the central nervous system of rats: a fluorescent immu-
CNS drug discovery? Drug Discov Today 2003; 8: 445–450.
nohistochemical analysis with histidine decarboxylase as a marker.
Fritschy JM, Mo¨hler H. GABAA-receptor heterogeneity in the adult
Brain Res 1984; 295: 13–25.
rat brain: differential regional and cellular distribution of seven
Benke D, Fritschy JM, Trzeciak A, Bannwarth W, Mohler H. Dis-
major subunits. J Comp Neurol 1995; 359: 154–194.
tribution, prevalence, and drug binding profile of c-aminobutyric
Pirker S, Schwarzer C, Wieselthaler A, Sieghart W, Sperk G. GA-
acid type A receptor subtypes differing in the b-subunit variant.
BAA receptors. immunocytochemical distribution of 13 subunits in
J Biol Chem 1994; 269: 27100–27107.
the adult rat brain. Neuroscience 2000; 101: 815–850.
Duggan MJ, Pollard S, Stephenson FA. Quantitative immunopre-
Wisden W, Laurie DJ, Monyer H, Seeburg PH. The distribution of
cipitation studies with anti-c-aminobutyric acid A receptor c2 1–15
13 GABAA receptor subunit mRNAs in the rat brain. I. Telen-
Cys antibodies. J Neurochem 1992; 58: 72–77.
cephalon, diencephalon, mesencephalon. J Neurosci 1992; 12: 1040–
Ruano D, Araujo F, Machado A, de Blas AL, Vitorica J. Molecular
characterization of type I GABAA receptor complex from rat cer-
Anand BK, Brobeck JR. Localization of a Ôfeeding centerÕ in the
ebral cortex and hippocampus. Brain Res Mol Brain Res 1994; 25:
hypothalamus of the rat. J Biol Med 1951; 24: 123–140.
Hetherington AW, Ranson SW. Hypothalamic lesions and adiposity
Ho WH, Wang SM, Yin HS. Regulation of the subcellular distri-
in the rat. Anat Rec 1940; 78: 149–172.
bution and gene expression of GABAA receptor by microtubules and
Kalra SP, Dube MG, Pu S, Xu B, Horvath TL, Kalra PS. Inter-
microfilaments in cultured brain neurons. J Cell Biochem 2001; 83:
acting appetite-regulating pathways in the hypothalamic regulation
of body weight. Endocr Rev 1999; 20: 68–100.
Elias CF, Saper CB, Maratos-Flier E, Tritos NA, Lee C, Kelly J,
Grandison L, Guidotti A. Stimulation of food intake by muscimol
Tatro JB, Hoffman GE, Ollmann MM, Barsh GS, Sakurai T,
and beta endorphin. Neuropharmacology 1977; 16: 533–536.
Yanagisawa M, Elmquist JK. Chemically defined projections linking
Kelly J, Rothstein J, Grossman SP. GABA and hypothalamic
the mediobasal hypothalamus and the lateral hypothalamic area.
feeding systems. I. Topographic analysis of the effects of microin-
J Comp Neurol 1998; 402: 442–459.
jections of muscimol. Physiol Behav 1979; 23: 1123–1134.
Broberger C, De Lecea L, Sutcliffe JG, Ho¨kfelt T. Hypocretin/or-
Kelly J, Grossman SP. GABA and hypothalamic feeding systems. II.
exin- and melanin-concentrating hormone-expressing cells form
A comparison of GABA, glycine and actylcholine agonists and their
distinct populations in the rodent lateral hypothalamus: relationship
antagonists. Pharmacol Biochem Behav 1979; 11: 647–652.
to the neuropeptide Y and agouti gene-related protein systems.
Ebenezer IS. The effect of intracerebroventricular administration of
J Comp Neurol 1998; 402: 460–474.
baclofen on food intake in rats. Neuroreport 1990; 1: 73–76.
Ovesjo¨ ML, Gamstedt M, Collin M, Meister B. GABAergic nature
Ebenezer IS, Pringle AK. The effect of systemic administration of
of hypothalamic leptin target neurones in the ventromedial arcuate
baclofen on food intake in rats. Neuropharmacology 1992; 31: 39–
nucleus. J Neuroendocrinol 2001; 13: 505–516.
Horvath TL, Bechmann I, Naftolin F, Kalra SP, Leranth C. Het-
Ebenezer IS. Intraperitoneal administration of baclofen increases
erogenity in the neuropeptide Y-containing neurons of the rat
consumption of both solid and liquid diets in rats. Eur J Pharmacol
arcuate nucleus: GABAergic and non-GABAergic subpopulations.
1995; 273: 183–185.
Brain Res 1997; 756: 283–286.
Dahlstro¨m A. Influence of colchicine axoplasmic transport of amine
Pu S, Jain MR, Horvath TL, Diano S, Kalra PS, Kalra SP. Inter-
storage granules in rat sympathetic adrenergic nerves. Acta Physiol
actions between neuropeptide Y and c-aminobutyric acid in stimu-
Scand 1969; 76: 33A–34A.
lation of feeding: a morphological and pharmacological analysis.
Benke D, Mertens S, Trzeciak A, Gillessen D, Mo¨hler H. GABAA
Endocrinology 1999; 140: 933–940.
receptors display association of c2-subunit with a1- and b2/3-sub-
Ba¨ckberg M, Collin M, Ovesjo¨ ML, Meister B. Chemical coding of
units. J Biol Chem 1991; 266: 4478–4483.
GABAB receptor-immunoreactive neurones in hypothalamic regions
Benke D, Cicin-Sain A, Mertens S, Mo¨hler H. Immunochemical
regulating body weight. J Neuroendocrinol 2003; 15: 1–14.
identification of the a1- and a3-subunits of the GABAA-receptor in
Stanley BG, Leibowitz SF. Neuropeptide Y injected in the para-
rat brain. J Recept Res 1991; 11: 407–424.
ventricular hypothalamus: a powerful stimulant of feeding behavior.
Gao B, Fritschy JM, Benke D, Mo¨hler H. Neuron-specific expres-
Proc Natl Acad Sci USA 1985; 82: 3940–3943.
sion of GABAA-receptor subtypes: differential association of the a1-
Chronwall BM, DiMaggio DA, Massari VJ, Pickel VM, Ruggiero
and a3-subunits with serotonergic and GABAergic neurons. Neuro-
DA, O'Donohue TL. The anatomy of neuropeptide-Y-containing
science 1993; 54: 881–892.
neurons in rat brain. Neuroscience 1985; 15: 1159–1181.
Marksitzer R, Benke D, Fritschy JM, Trzeciak A, Bannwarth W,
Allen YS, Adrian TE, Allen JM, Tatemoto K, CrowTJ, Bloom SR,
Mo¨hler H. GABAA-receptors: drug binding profile and distribution
Polak JM. Neuropeptide Y distribution in the rat brain. Science
of receptors containing the a2-subunit in situ. J Recept Res 1993; 13:
1983; 221: 877–879.
Ollmann MM, Wilson BD, Yang YK, Kerns JA, Chen Y, Gantz I,
Mertens S, Benke D, Mo¨hler H. GABAA receptor populations with
Barsh GS. Antagonism of central melanocortin receptors in vitro and
novel subunit combinations and drug binding profiles identified in
in vivo by agouti-related protein. Science 1997; 278: 135–138.
Ó 2004 Blackwell Publishing Ltd, Journal of Neuroendocrinology, 16, 589–604
GABAA receptors in hypothalamus
Shutter JR, Graham M, Kinsey AC, Scully S, Luthy R, Stark KL.
Dellovade TL, Davis AM, Ferguson C, Sieghart W, Homanics
Hypothalamic expression of ART, a novel gene related to agouti, is
GE, Tobet SA. GABA influences the development of the ventro-
up-regulated in obese and diabetic mutant mice. Genes Dev 1997; 11:
medial nucleus of the hypothalamus. J Neurobiol 2001; 49: 264–
Marx J. Cellular warriors at the battle of the bulge. Science 2003;
Laurie DJ, Wisden W, Seeburg PH. The distribution of thirteen
299: 846–849.
GABAA receptor subunit mRNAs in the rat brain. III. Embryonic
Horvath TL, Naftolin F, Leranth C. GABAergic and catecholam-
and postnatal development. J Neurosci 1992; 12: 4151–4172.
inergic innervation of mediobasal hypothalamic b-endorphin cells
Davis AM, Penschuck S, Fritschy JM, McCarthy MM. Develop-
projecting to the medial preoptic area. Neuroscience 1992; 51: 391–
mental switch in the expression of GABA(A) receptor subunits a(1)
and a(2) in the hypothalamus and limbic system of the rat. Brain Res
Blasquez C, Jegou S, Feuilloley M, Rosier A, Vandesande F, Vaudry
Dev Brain Res 2000; 119: 127–138.
H. Visualization of c-aminobutyric acid A receptors on proopi-
Sergeeva OA, Eriksson KS, Sharonova IN, Vorobjev VS, Haas HL.
omelanocortin-producing neurons in the rat hypothalamus. Endo-
GABAA receptor heterogeneity in histaminergic neurons. Eur J
crinology 1994; 135: 2759–2764.
Neurosci 2002; 16: 1472–1482.
Jegou S, Blasquez C, Delbende C, Bunel DT, Vaudry H. Regulation
Korpi ER, Gru¨nder G, Lu¨ddens H. Drug interactions at GABAA
of a-melanocyte-stimulating hormone release from hypothalamic
receptors. Prog Neurobiol 2002; 67: 113–159.
neurons. Ann NY Acad Sci 1993; 680: 260–278.
Araujo F, Ruano D, Vitorica J. Native c-aminobutyric acid type A
Broberger C, Landry M, Wong H, Walsh JN, Ho¨kfelt T. Subtypes
receptors from rat hippocampus, containing both a1 and a5 subunits,
Y1 and Y2 of the neuropeptide Y receptor are respectively expressed
exhibit a single benzodiazepine binding site with a5 pharmacological
in pro-opiomelanocortin- and neuropeptide-Y-containing neurons
properties. J Pharmacol Exp Ther 1999; 290: 989–997.
of the rat hypothalamic arcuate nucleus. Neuroendocrinology 1997;
Lu¨ddens H, Seeburg PH, Korpi ER. Impact of beta and gamma
66: 393–408.
variants on ligand-binding properties of c-aminobutyric acid type A
Meister B, Ceccatelli S, Ho¨kfelt T, Anden NE, Anden M, Theo-
receptors. Mol Pharmacol 1994; 45: 810–814.
dorsson E. Neurotransmitters, neuropeptides and binding sites in the
Ko¨hler C, Swanson LW, Haglund L, Wu JY. The cytoarchitecture,
rat mediobasal hypothalamus: effects of monosodium glutamate
histochemistry and projections of the tuberomammillary nucleus in
(MSG) lesions. Exp Brain Res 1989; 76: 343–368.
the rat. Neuroscience 1985; 16: 85–110.
Nisbett RE. Hunger, obesity, and the ventromedial hypothalamus.
Ericson H, Watanabe T, Ko¨hler C. Morphological analysis of the
Psychol Rev 1972; 79: 433–453.
tuberomammillary nucleus in the rat brain: delineation of subgroups
Collin M, Backberg M, Ovesjo¨ ML, Fisone G, Edwards RH, Fu-
with antibody against 1-histidine decarboxylase as a marker. J Comp
jiyama F, Meister B. Plasma membrane and vesicular glutamate
Neurol 1987; 263: 1–24.
transporter mRNAs/proteins in hypothalamic neurons that regulate
Brown RE, Stevens DR, Haas HL. The physiology of brain hista-
body weight. Eur J Neurosci 2003; 18: 1265–1278.
mine. Prog Neurobiol 2001; 63: 637–672.
Ó 2004 Blackwell Publishing Ltd, Journal of Neuroendocrinology, 16, 589–604
Source: http://www.diabesity.eu/pubs/backbergultenius.pdf
Université de Bordeaux U.F.R DES SCIENCES MEDICALES Thèse pour l'obtention du DIPLÔME D'ETAT DE DOCTEUR EN MEDECINE Médecine générale Présentée et soutenue publiquement Le 10 décembre 2015 VAN OVERLOOP Romain Né le 06 mars 1987 à Marseille Etude de la consommation chronique d'inhibiteurs de la pompe à protons en EHPAD : indications documentées et médications associées pour 134
04/09/2009 Cellule interministérielle de communication QUESTIONS / REPONSES CONCERNANT LA GRIPPE A (H1N1) 2009 I) LE VIRUS A(H1N1) 2009, LES TRAITEMENTS, LA VACCINATION 9 Qu'est ce que la grippe ? Que sont les virus grippaux ? La grippe est une infection respiratoire aiguë, très contagieuse, due aux virus Influenzae. Les virus grippaux se répartissent entre différents types : A, B et C. Les virus A et B sont à l'origine des épidémies saisonnières mais seul le virus A peut être responsable de pandémies. Le virus C occasionne des cas sporadiques. Les virus grippaux se caractérisent par leurs fréquentes mutations.








