Levitra enthält Vardenafil, das eine kürzere Wirkdauer als Tadalafil hat, dafür aber schnell einsetzt. Männer, die diskret bestellen möchten, suchen häufig nach levitra kaufen ohne rezept. Dabei spielt die rechtliche Lage in der Schweiz eine wichtige Rolle.
ausgabe 55/2
Induction of Diploid Eggs With Colchicine During
Embryo Sac Development in Populus
By J. WANG1),2), X. Y. KANG1),2),*), D. L. LI2), H. W. CHEN2) and P. D. ZHANG1),2)
(Received 22nd August 2009)
induction of 2
n pollen, due to easy screening by their
Diploid (2
n) eggs were induced by treating developing
size. JOHNSSON and EKLUNDH (1940) first induced 2
n
embryo sacs of
Populus with colchicine solution, in order
pollen of
P. tremula and
P. tremuloides with colchicine,
to produce triploid plants. The optimal pollinated time
and produced triploid plants by pollinating to female
of female catkins was confirmed as timing point for each
catkins of diploid plants with the artificial 2
n pollen. So
treatment. When female catkins of
P. pseudo-simonii x
far, more triploids have been obtained by crossing with
P. nigra ‘Zheyin3#' had become 5.62 ± 0.13 cm long 84 h
induced 2
n pollen in
P. deltoides,
P. alba,
P. tomentosa
after they emerged from their bract scales and all stig-
and other white poplars (MASHKINA et al., 1989; ZHANG
mas were exposed, pistils all over the entire catkin had
and LI, 1992; KANG et al., 2000), and the pachytene
optimal stigma receptivity. Observation of paraffin sec-
stage of microsporogenesis is proved as the optimal
tions showed that embryo sac development of ‘Zheyin3#',which initiated 12 h before pollination and finished
stage for 2
n pollen induction with colchicine. Although
132 h after pollination, was a successive and asynchro-
the percentage of artificial 2
n pollen can be upward of
nous process. Generative cell division of pollen of the
80 % (ZHANG and LI, 1992; KANG et al., 1999), the effect
male parent
P. x
beijingensis took place 3–16 h after pol-
of triploid production is not good because of competition
lination. Catkins of 18–96 h after pollination of
from normal pollen (KANG and ZHU, 1997; KANG, 2002).
‘Zheyin3#' were treated with colchicine solution. In the
Compared with 2
n pollen, triploid production via 2
n
progeny, twenty three triploids were detected by chro-
eggs may be more suitable. However, reports on induc-
mosome counting and the highest rate of triploids was
tion of 2
n eggs are rare in
Populus, because it is difficult
66.7 % in one treatment. The rate of triploid yield waspositively correlated with the frequency of four-nucleate
to timely determine the exact stage of megasporogene-
embryo sacs (
r = 0.6721,
p = 0.0981) and was not sig-
sis, which progresses in the inside of ovules. Based on
nificantly correlated with the percentages of uni-, two-
the temporal relationship between megasporogenesis
and eight-nucleate embryo sac (
r = –0.1667,
p = 0.7210,
and microspore development under similar cultured con-
r = –0.3069,
p = 0.5031 and
r = 0.0189,
p = 0.9679,
ditions in
P. alba x
P. glandulosa, LI et al. (2008)
respectively), suggesting that the third mitotic division
obtained 12 triploids by inducing unreduced megaspores
of embryo sac may be the effective stage to induce 2
n
with a 0.5 % colchicine solution at the prophase of the
eggs. Through this approach, completely homozygous
first meiotic division, and the highest rate of produced
2
n eggs can be produced. Its significance for plant breed-
triploids in one treatment was 16.7 %. It is well known
ing is discussed.
that functional megaspore develops into mature female
Key words: 2
n egg, colchicine, embryo sac development,
Popu-
gametophyte after megasporogenesis, i.e. megagameto-
genesis (also called embryo sac development). In thisprocess, the megaspore undergoes at least one round of
mitosis without cytokinesis. For
Populus, the embryosac development complies with
Polygonum type, in
The genus
Populus, as an important source of fuel,
which a 7-celled mature embryo sac formation initiated
fibre and lumber, is widely distributed and cultivated
from a functional megaspore via three rounds of mitotic
over the northern hemisphere (RAE et al., 2007). Some
division (NAGARAJ, 1952; KIMURA, 1955, 1963; LI et al.,
triploid cultivars of
Populus have many desirable prop-
1982; LI and ZHU, 1988; ZHU and LI, 1989; DONG, 1984;
erties in growth and pulpwood characteristics compared
FAN, 1984; LI and MA, 2006). Potentially, these mitotic
to diploids (VAN BUIJTENEN et al., 1958; EINSPAHR et al.,
divisions offer us the possibility to induce 2
n eggs.
1972; ZHU et al., 1995). Thus, triploid breeding is one ofthe most powerful approaches for improvement of
Popu-
Colchicine is an alkaloid that contributes to the pre-
vention of tubulin polymerization, thereby arresting for-mation of spindle and restraining nuclear division at
Diploid (2
n) gametes are usually applied to produce
metaphase (JORDAN and WILSON, 1999). Consequently,
triploids of
Populus. There are many investigations on
colchicine has been widely used to induce polyploids inplants (EIGSTI and DUSTIN, 1955). In polyploid breeding
1) National Engineering Laboratory for Tree Breeding, Beijing
programs of the genus
Populus, colchicine is the most
Forestry University, 100083, Beijing, P. R. China.
commonly used reagent and has good effects (JOHNSSON
2) Key Laboratory of Genetics and Breeding in Forest Trees and
and EKLUNDH, 1940; EINSPAHR, 1965; KANG et al., 1999,
Ornamental Plants, Ministry of Education, Beijing Forestry
2004; LI et al., 2008). In our investigation, female
University, 100083, Beijing, P.R. China.
catkins of
P. pseudo-simonii x
P. nigra ‘Zheyin3#' under
*) Address correspondence to: P.O. Box 118, Beijing Forestry
embryo sac development were treated with colchicine
University, 100083, Beijing, P.R. China; Tel.: +86-10-62336104;E-Mail:
[email protected]
solution, in order to discuss the possibility of 2
n egg
Silvae Genetica 59, 1 (2010)
induction and to serve to triploid breeding programs of
The branches were cultured in a greenhouse (10–20 °C)
the genus Populus.
Determination of optimal stigma receptivity
Materials and Methods
Female catkins were selected to examine stigma
Plant materials
receptivity of ‘Zheyin3#' every 6 h, starting with the
Female floral branches of P. pseudo-simonii x P. nigra
catkins emerging from bract scales. Lengths of the
‘Zheyin3#' (female parent, 2n = 2x = 38) were collected
catkins and colour of their stigmas were recorded. For
from a plantation in Tongliao City (Inner Mongolia
each time, the lengths of 10 catkins were measured with
Autonomous Region, P. R. China). Male floral branches
a vernier caliper. The catkins were hand-pollinated with
of P. x beijingensis (male parent, 2n = 2x = 38) were col-
fresh pollen of P. x beijingensis. Four hours after pollina-
lected from the campus of Beijing Forestry University.
tion, the catkins were fixed in FAA (70 % ethanol: acetic
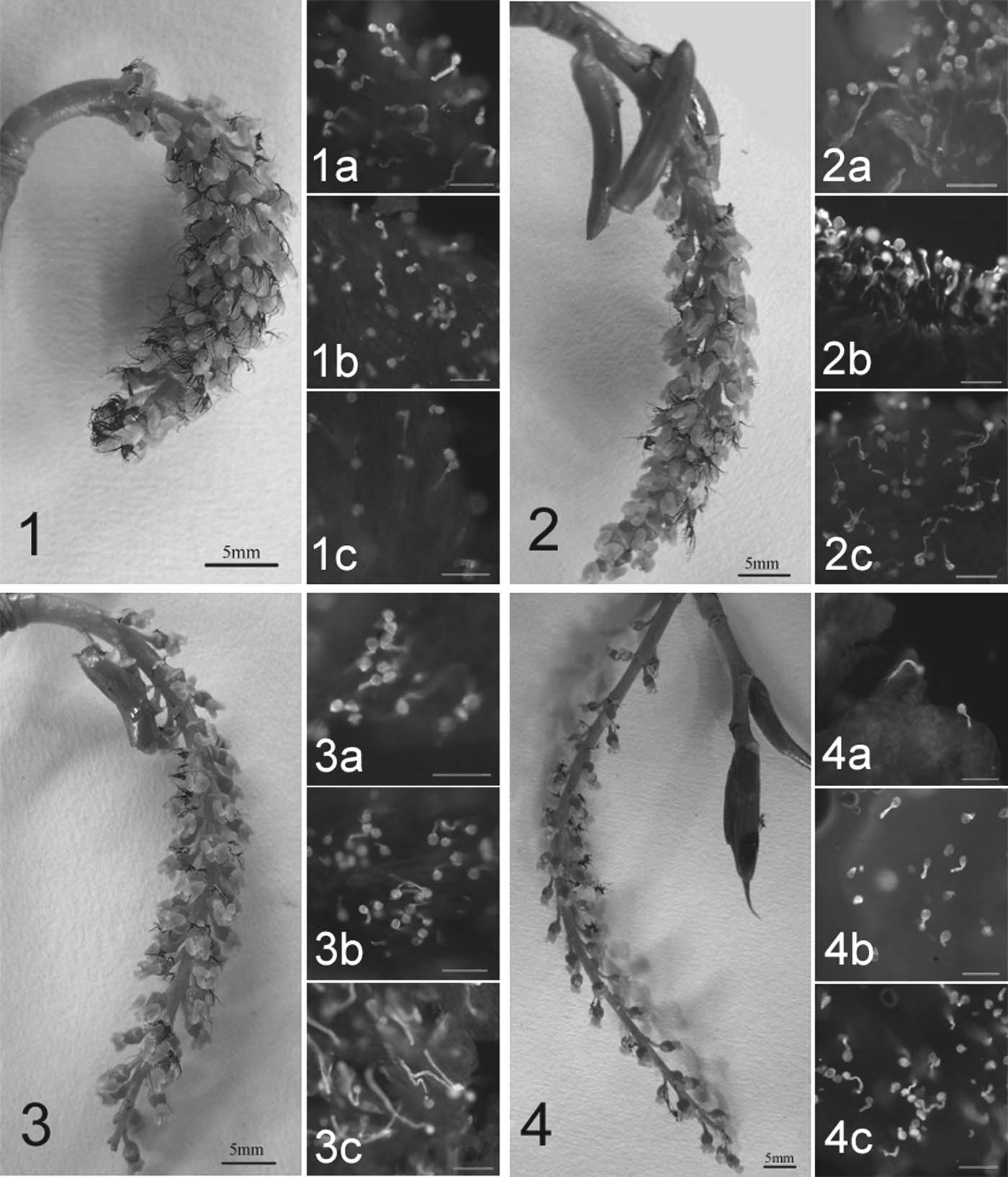
Figure 1. – Stigma receptivity of P. pseudo-simonii x P. nigra ‘Zheyin3#'. Lower-case let-ters a, b and c show pollen germination on stigmas at base, middle and top of catkinsrespectively. Unmarked bars are equal to 100 µm.
Fig. 1: Catkin before optimal pollinated time. Normal pollen germination on basal andmiddle stigmas (1a and 1b). Only a few pollen grains germinated on the top stigmas(1c).
Fig. 2: Catkin at optimal pollinated time. Vigorous pollen germination on stigmas at dif-ferent parts of the catkin.
Fig. 3: Catkin after optimal pollinated time. Pollen germination on the basal stigmas(3a) is not as good as that on the middle and top stigmas (3b and 3c).
Fig. 4: Catkin with retained receptivity. No receptivity on the basal stigmas (4a), tardygermination on the middle stigmas (4b) and relatively normal germination on the topstigmas (4c).
acid: 40 % formaldehyde, 90:5:5) for 24 h at 4 °C. After
opment of generative cells was examined under the
being washed with distilled water, the stigmas at the
above fluorescence microscope.
top, middle and base of each fixed catkin were respec-tively softened in 8 M NaOH for 2 h. Following further
Determination of developmental process of embryo sacs
washing in distilled water, the flowers were squashed in
The female buds and catkins of ‘Zheyin3#' were fixed
0.1% aniline blue and stained for 10 min. The prepara-
in FAA at 4 °C every 12 h after being cultured and every
tions were observed under a fluorescence microscope
6 h after pollination, until maturation of seeds. Ovaries
(Olympus BX51).
from each fixed buds and catkin were embedded withparaffin and sectioned at 8–10 µm. The sections were
Observation of generative cell division in pollen
stained with iron-hematoxylin and observed under the
The development of generative cells was studied in
microscope. During the embryo sac development, a total
vivo. Pollen grains of P. x beijingensis were pollinated to
of 1,470 sacs were analyzed in order to reveal the
stigmas with optimal receptivity of the female parent.
process of embryo sac development.
The stigmas were fixed in FAA every 2 h for 24 h at 4 °C,starting from being pollinated until 48 h after pollina-
Induction of 2n eggs
tion. Subsequently, they were softened in 8 M NaOH for
When female catkins acquired the optimal receptivity,
at least 12 h. After being washed in distilled water, the
they were pollinated with fresh collected pollen of
samples were squashed in one drop of 4',6-diamidino-2-
P. x beijingensis. Based on the process of the embryo sac
phenylindole (DAPI) and stained for 10 min. The devel-
development of ‘Zheyin3#', the female catkins 18–96 h
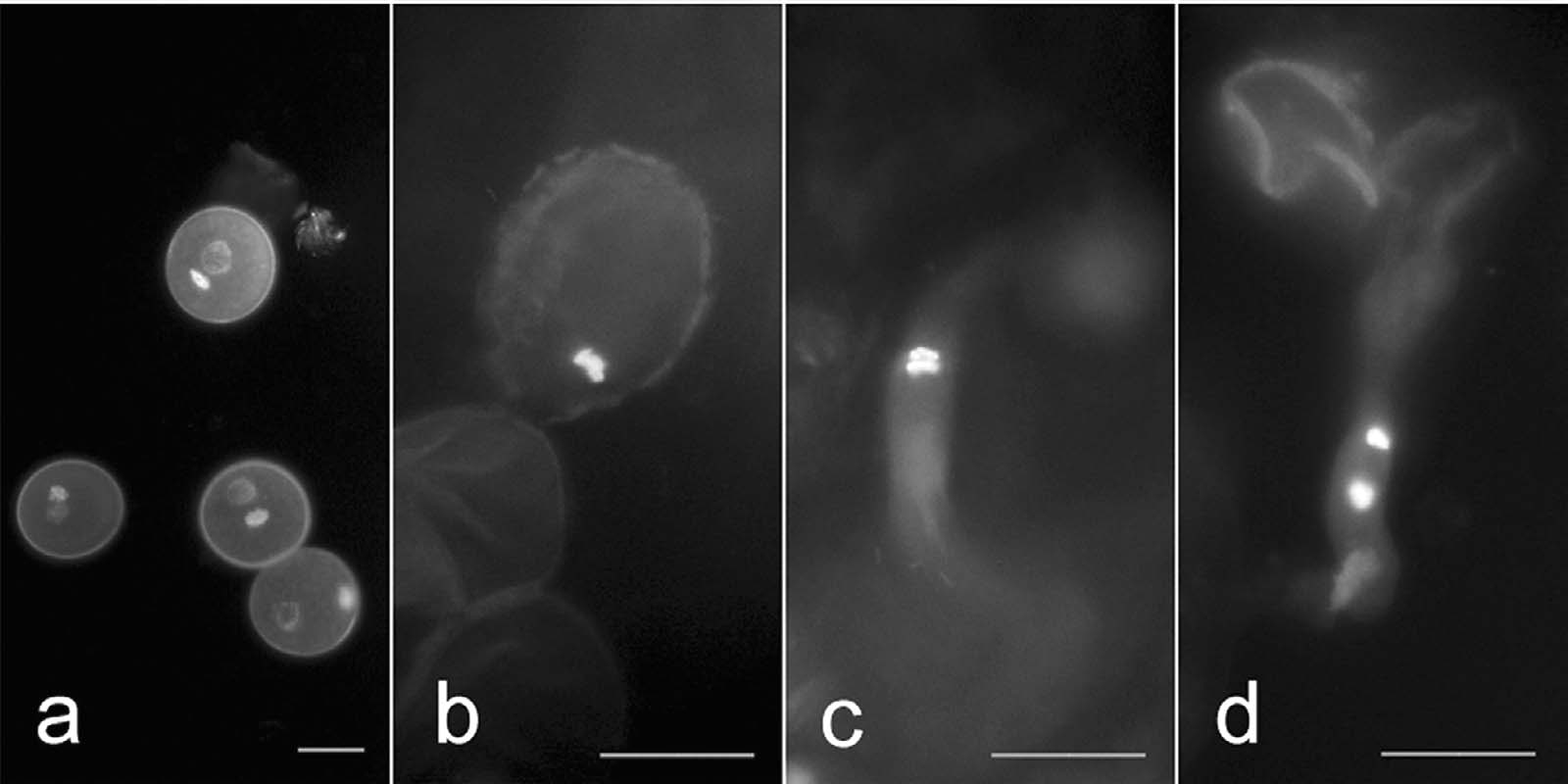
Figure 5. – Generative cell division of P. x beijingensis in vivo. Two-celled pollen (a), mitoticmetaphase (b), anaphase (c) and two formed sperms in tube (d). Bars are equal to 10 µm.
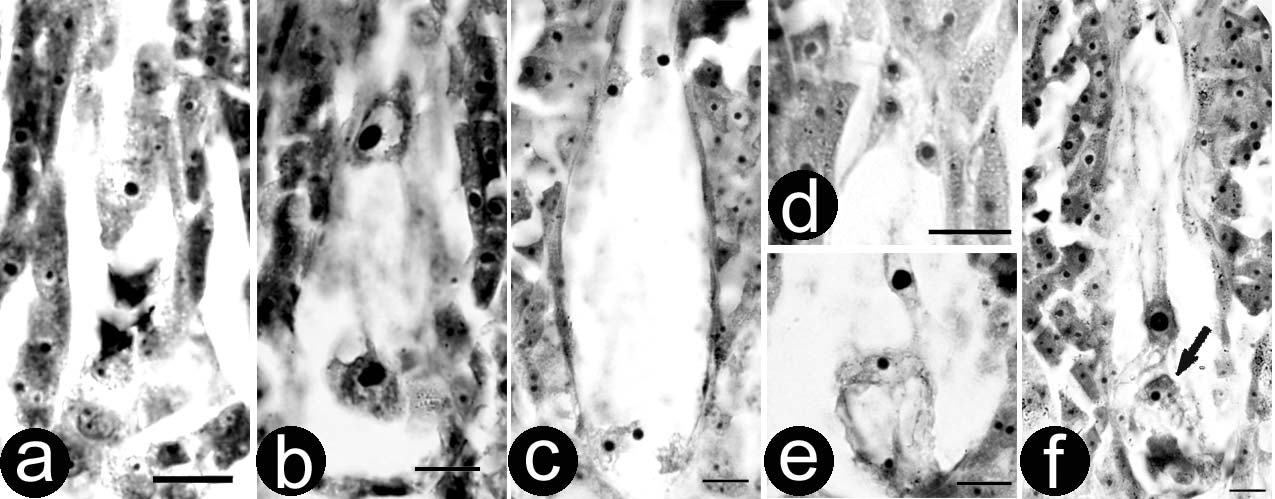
Figure 6. – Embryo sac development and fertilization of P. pseudo-simonii xP. nigra ‘Zheyin3#'. The chalazal end is at the top in all figures. a, Uni-nucleateembryo sac with three degenerated megaspores. b, Two-nucleate embryo sac. c,Four-nucleate embryo sac. d–e, Mature embryo sac including three antipodalcells (d), one secondary nucleus and one egg apparatus (e). One of the synergidcells is out of focus. f, Fertilization. The arrow shows one sperm is close to egg.
Bars are equal to 10 µm.
after pollination were immersed in 0.3–0.5 % colchicine
during 18 h to 42 h after pollination. After treatment,
solution for 18–30 h. In view of the weak colchicine-tol-
the colchicine was rinsed away under taps. The catkins
erance of catkins soon after pollination, both 0.3 % and
without treatment were set as the control group. After
0.5 % colchicine solution were used to treat the catkins
the catkins matured, seeds were collected and germinat-
Table 1. – Process of embryo sac development in P. pseudo-simonii x P. nigra ‘Zheyin3#'.
ed in soil. When the seedlings grew to about 30 cm in
4a). The middle and top stigmas were still green-yellow.
height, they were transplanted to the field.
Pollen germination on the middle and top stigmas wasbetter than on the basal stigmas, but not flourishing
Analysis of ploidy level
(Figs. 4b and c). Seven days after they emerged from
The determination of the ploidy level was conducted
their bract scales, all stigmas at the catkins became
by somatic chromosome counting. Stem tips were
brown and pollen no longer germinated.
removed from the seedlings and pretreated with a satu-rated solution of paradichlorobenzene for 4 h at 25 °C.
Division of generative cells in pollen of P. x beijingensis
Subsequently, the materials were fixed in a fresh
Mature pollen grains of P. x beijingensis were of the 2-
Carnoy solution (ethanol: acetic acid, 3:1) for 24 h at
celled type (Fig. 5a). In vivo, pollen grains started to
4 °C. The fixed materials were then hydrolyzed in a
germinate on the stigmas of ‘Zheyin3#' 2 h after pollina-
mixed fluid of 38 % HCl and ethanol (1:1) for 25 min at
tion. Generative cell division occurred 3–16 h after polli-
room temperature. After being washed in distilled water
nation (Figs. 5b and c). Two sperm cells were formed
three times for 15 min, the hydrolyzed materials were
12–18 h after pollination (Fig. 5d).
squashed heavily in a Carbol Fuchsin solution. Chromo-some counting was carried out under the Olympus
Development of embryo sacs
BX51. At least 20 cells with a well-spread metaphase
Embryo sac development of ‘Zheyin3#' was of the typi-
from each seedling were observed.
cal Polygonum type. Functional megaspore formed a 7-celled mature embryo sac via three rounds of mitotic
divisions (Fig. 6). The mature embryo sac consisted oftwo synergid cells, an egg cell, a central cell and three
Stigma receptivity of ‘Zheyin3#'
antipodal cells (Figs. 6d and e).
The stigma receptivity of ‘Zheyin3#' lasted 3–4 d. The
The embryo sac development was a successive and
receptivity among pistils in different parts of catkin var-
asynchronous process (Table 1). It was initiated 12 h
ied. The pistils at the base of the catkin were the first to
before pollination. Three micropylar megaspores of a
acquire stigma receptivity and were also the first to lose
tetrad began to degenerate and the enlarged functional
it; the pistils at the top of the catkin acquired it at last
megaspore at the chalazal end formed a uni-nucleate
but were also the last to lose it.
embryo sac (Fig. 6a). Until 24 h after pollination, the
Sixty hours after the catkins emerged from their bract
uni-nucleate embryo sac was dominant, although other
scales, they developed into 3.45 ± 0.06 cm long. All stig-
stages, such as the tetrad, the two-nucleate embryo sac
mas in the catkins were green-yellow in colour. Bracts of
(Fig. 6b), the four-nucleate sac (Fig. 6c) and even the
basal pistils evaginated and stigmas were exposed. After
eight-nucleate sac (Figs. 6d and e), were also observed.
pollination, pollen germination was observed on the
Thirty to forty-eight hours after pollination, the propor-
basal stigmas. However, stigmas at the middle and top
tion of the two-nucleate embryo sac was predominant.
of the catkins, covered under bracts, had not acquired
With the development of embryo sacs, the rate of the
receptivity. Twelve hours later, the catkins were
four-nucleate embryo sac increased gradually. Its per-
4.23 ± 0.13 cm in length. Except for the top stigmas, the
centage was greater than that of other stages 54–60 h
middle and basal stigmas had both emerged from their
after pollination. After that, all cells developed into
bracts (Fig. 1). Pollen germination tests showed that the
eight-nucleate and mature embryo sacs in succession.
stigmas at the middle and base of the catkins both had
Fertilization occurred between 84 h and 144 h after pol-
receptivity (Figs. 1a and b). Only a few pollen grains
lination (Fig. 6f).
with little germination adhered to the top stigmas(Fig. 1c). With their development, the catkins elongated
Production of triploids
to 5.62 ± 0.13 cm long 84 h after they emerged from their
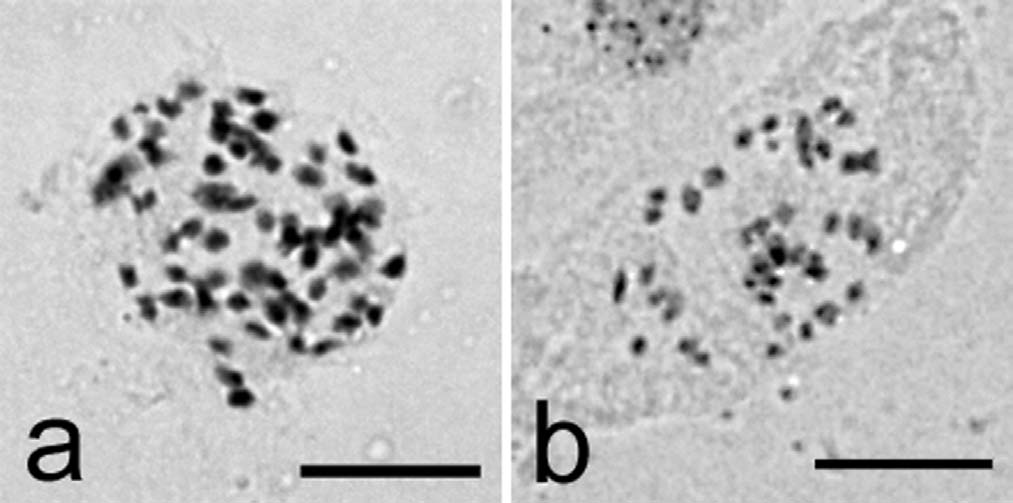
Table 2 shows that a total of 23 triploids (2n = 3x = 57,
bract scales. All bracts turned inside out, some even
Fig. 7a) were obtained by treating pollinated catkins of
were shed. At this stage, all stigmas were exposed
‘Zheyin3#', under embryo sac development. In some
(Fig. 2). Pollen germination tests showed that plenty ofpollen germinated vigorously on all stigmas at differentparts of the catkins (Figs. 2a, b and c), which indicatedthat the catkins acquired the optimal stigma receptivity.
Therefore, this time was the optimal pollinated time.
When the catkins developed to 6.27 ± 0.10 cm in length102 h after they emerged from their bract scales, theybegan to become less incompact (Fig. 3). Although pollengrains still germinated on all stigmas, the number ofgerminated pollen grains on the basal stigmas was lessthan that on the middle and top stigmas (Figs. 3a, band c). Five to six days after they emerged from theirbract scales, the catkins relaxed completely and were10.26 ± 0.18 cm long (Fig. 4). The basal stigmas became
Figure 7. – Number of Chromosomes of diploid and triploid
yellow-green in colour with a little brown. Only a few
progenies. Fifty seven chromosomes in triploid (a) and thirty
pollen grains clung to the stigmas and germinated (Fig.
eight in diploid (b). Bar is equal to 10 µm.
Table 2. – Triploid production through treating developing embryo sacs of P. pseudo-simonii x P. nigra‘Zheyin3#' with colchicine solution.
treatments, no seedlings survived. All seedlings in the
yield in progeny. Therefore, exact pollination should be
control group were confirmed as diploid (2n = 2x = 38,
emphasized. In the present study, the optimal stigma
Fig. 7b). Nineteen triploids were produced in the treat-
receptivity of catkin of ‘Zheyin3#' was identified, which
ments 54–66 h after pollination, representing 82.6 % of
insured successful hybridization and reliable seed set.
the total number of triploids. The highest rate of
Furthermore, treatment of 2n egg induction is based on
triploids in one treatment was 66.7 %, which occurred 60
timely determination of stage of embryo sac develop-
h after pollination.
ment, but it is difficult to detect because of its location
Correlation analyses were made between the efficien-
inside of ovule. In view of the result that the embryo sac
cy of triploid production and percentage of each develop-
development of ‘Zheyin3#' almost undertook after polli-
mental stage of embryo sac (Fig. 8). A moderate positive
nation, the optimal pollinated time was useful to be
correlation was found between the rate of triploids and
regarded as a reference point for each treatment in this
the percentage of four-nucleate embryo sacs (r = 0.6721,
p = 0.0981). However, there were no significant corre-
There are two types of mature pollen in plants, i.e. 2-
lations between the rate of triploids and the percent-
celled and 3-celled types. For 2-celled pollen, division of
ages of uni-, two- and eight-nucleate embryo sacs
generative cell occurs in pollen tube after germination.
(r = –0.1667, p = 0.7210, r = -0.3069, p = 0.5031 and
EINSPAHR (1965) unexpectedly obtained some putative
r = 0.0189, p = 0.9679, respectively).
triploids when he treated catkins in 6–30 h after polli-nation in order to induce tetraploids by treating newlyformed embryos of quaking aspen with colchicine. WIN-
TON (1968) explained that production of these triploids
Pollination at the right time is the basis for successful
was caused by an unreduced division of generative cell
hybridization. Stigma receptivity is an important factor
in pollen tube. In our investigation, however, the gener-
affecting effective pollination, which is related to seed
ative cell of pollen of male parent P. x beijingensis divid-
yield (EGEA et al., 1991; SANZOL and HERRERO, 2001).
ed 3–16 h after pollination, which did not coincide with
Estimation of 2n egg production relies on the polyploid
the effective period of triploid production. It suggests
Figure 8. – Correlation analyses between rate of triploids and percentage of each embryo sac developmental stage.
that the triploids in this study are not from fertilization
catkins under embryo sac development and the highest
of diploid generative cell.
rate was up to 66.7 % in one treatment, which suggests
In the genus Populus, induction of 2n gametes has, so
that a novel approach for 2n egg induction has been dis-
far, focused on the meiotic process. Prophase I is an effi-
covered and that this approach is more efficient for
cient stage to induce 2n gametes. For pollen chromo-
triploid production than both the 2n pollen and 2n
some doubling, the percentage of 2n pollen can be
megaspore approaches. Further more, correlation analy-
upward of 80% by treating with colchicine (Z
ses showed that the rate of triploid production was posi-
tively correlated with the frequency of four-nucleate
I, 1992; KANG et al., 1999). However, it is not compati-
ble between the high percentage of 2n pollen and the
embryo sac, but not significantly correlated with both
rate of triploid production by pollinating with them,
uni- and two-nucleate sacs, indicating that the third
owing to the weak competitive ability of 2n pollen com-
mitotic division during embryo sac development may be
pared with normal pollen in germination and fertiliza-
more effective for 2n egg induction through external
actions, but it can not exclude the possibility of 2n egg
ANG and ZHU, 1997; KANG, 2002). The highest
rate of triploid production by pollinating with 2n pollen
formation by mitotic inhibition at the first or second
was just 12.9%, even though the 2n pollen had been
treated by γ-irradiation to enhance their competitive
KANG et al. (2004) obtained 57.1% allotriploids of
ability (KANG et al., 2000). For female gamete chromo-
white poplar by treating female catkins with colchicine
some doubling, the effect just can be estimated by poly-
24–36 h after pollination. However, he did not give a
ploidy production in progeny. LI et al. (2008) obtained 12
reasonable explanation on origin of these triploids. Addi-
triploids by crossing with 2n megaspores induced by
tionally, when EINSPAHR
(1965) treated pollinated
colchicine at prophase I of megasporogenesis and the
catkins of quaking aspen to induce tetraploid, he unex-
highest rate of triploid production was 16.7 % in one
pectedly screened some putative triploids. Although
treatment. In the present investigation, twenty-three
chromosome doubling of sperm cells is possible (WINTON,
triploids were produced by treating pollinated female
1968), production of 2n eggs during embryo sac develop-
ment may be a better explanation for the results of the
earlier studies, in view of its high efficiency.
BASTIAANSSEN, H. J. M., P. M. M. M. VAN DEN BERG,
There are three modes for polyploid formation through
P. LINDHOUT, E. JACOBSEN and M. S. RAMANNA (1998):
2n gametes, i.e. 2n + n, n + 2n and 2n + 2n mode. The
Postmeiotic restitution in 2n-egg formation of diploid
usage of 2n pollen is restricted, because it does not com-
potato. Heredity 81: 20–27.
pete well with normal pollen (HARLAN and DEWET, 1975;
BREMER, G. (1959): Increase of chromosome number in
KANG and ZHU, 1997). Therefore, fertilization between
species hybrids of Saccharum in relation to the embryo-
2n egg and n pollen may be the most common mode for
sac development. Bibliographia Genetica 18: 1–99.
triploid origin (HARLAN and DEWET, 1975). In general,
BREMER, G. (1963): Problems in breeding and cytology of
formation of 2n eggs is attributed to meiotic division
sugar cane. Euphytica 12: 178–188.
restitution (PFEIFFER and BINGHAM, 1983; WERNER and
BRETAGNOLLE, F. and J. D. THOMPSON (1995): Gametes
PELOQUIN, 1987, 1990, 1991; CONICELLA et al., 1991;
with the somatic chromosome number: mechanisms of
their formation and role in the evolution of autopoly-
GBURIA et al., 2002), aberrant cytokinesis (WERNER and
ploid plants. New Phytol 129: 1–22.
ELOQUIN, 1990), postmeiotic restitution (PMR) (BAS-
BURSON, B. L., M. A. HUSSEY, J. M. ACTKINSON and G. S.
TIAANSSEN et al., 1998) and apospory (BURSON et al.,2002). Endo-duplication of chalazal megaspore after
SHAFER (2002): Effect of pollination time on the frequen-cy of 2n + n fertilization in apomictic buffelgrass. Crop
meiosis is one explanation for the PMR mechanism
Sci 42: 1075–1080.
(BREMER, 1959, 1963). After megasporogenesis, megas-
CONICELLA, C., A. BARONE, A. DEL GIUDICE, L. FRUSCIANTE
pores undergo embryo sac development to form mature
and L. M. MONTI (1991): Cytological evidences of SDR-
megagametophyte. YAMADA and TAO (2007) suggested
FDR mixture in the formation of 2n eggs in a potato
the possibility that 2n or 3n eggs could be initiated by
diploid clone. Theor Appl Genet 81: 59–63.
abnormal mitosis during embryo sac development in
DONG, Y. (1984): Some embryological observations on Pop-
Diospyros kaki ‘Fujiwaragosho'. Our work proved that
ulus tomentosa Carr. II. about the structures of ovule
2n eggs could be produced during embryo sac develop-
and embryo sac, the process of fertilization and the
ment, which is a strong supplementation of the PMR
development of embryo. J Beijing For College 1: 83–94.
EGEA, J., L. BURGOS, J. E. GARCIA and L. EGEA (1991):
Diploid gametes are of evolutionary importance in the
Stigma receptivity and style performance in several
origin of new polyploids (HARLAN and DEWET, 1975). The
apricot cultivars. J Hortic Sci 66: 19–25.
mechanism of 2n gamete production was reviewed by
EIGSTI, O. J. and P. J. DUSTIN (1955): Colchicine in agricul-
VEILLEUX (1985) and BRETAGNOLLE and THOMPSON
ture, medicine, biology, and chemistry. Iowa State Col-
(1995). Depending on the mechanism for 2n gamete for-
lege Press, Ames.
mation, the genetic consequences in the polyploid proge-
EINSPAHR, D. W. (1965): Colchicine treatment of newly
ny vary. The first division restitution type 2n gamete
formed embryos of quaking aspen. For Sci 11(4):
theoretically transmits approximately 80 % parental
heterozygosity to the progeny and the second division
EINSPAHR, D. W., M. K. BENSON and M. L. HARDER (1972):
restitution type can transmit about 40 % (M
Within-tree variation in specific gravity of young quak-
ing aspen. Genetics and Physiology Notes, Institute of
ELOQUIN, 1977). However, different from the for-
Paper Chemistry, U.S., 13: 8.
mer types of 2n gametes, completely homozygous 2ngametes can arise from the PMR mechanism (B
FAN, R. W. (1984): A comparison study of the development
of the ovule and embryo sac of clones of Aigeiros
TIAANSSEN et al., 1998). Originated from mitotic inhibi-
poplars. J Nanjing Inst For 3: 44–50.
tion, the 2n eggs induced in our investigation could be
HARLAN, J. R. and J. M. J. DEWET (1975): On Ö. Winge
deduced to be characterized by complete homozygosity,
and a prayer: the origins of polyploidy. Bot Rev 41:
which is promising in improvement and genetic research
of trees and crops. More plants will be able to benefit
JOHNSSON, H. and C. EKLUNDH (1940): Colchicine treat-
from this approach. Because nearly all trees and most
ment as a method in breeding hardwood species.
agricultural crops are highly heterozygous, induced
Svensk Papp Tidn 43: 373–377.
parthenogenesis from the completely homozygous 2n
JORDAN, M. A. and L. WILSON (1999): The use and action
eggs is able to produce homozygous progeny. Additional-
of drugs in analyzing mitosis. Method Cell Biol 61:
ly, when the 2n eggs are fertilized with normal pollen to
give rise to triploids, the homozygosity from the mater-
KANG, X. Y. (2002): Cytogenesis and triploid breeding of
nal genome will be helpful to analyze the dosage effect
Chinese white poplar. China Environmental Science
Press, Beijing.
KANG, X. Y. and Z. T. ZHU (1997): A study on the 2n pollen
vitality and germinant characteristics of white poplars.
Acta Botanica Yunnanica 19(4): 402–406.
The authors thank the Forestry Research Institute of
KANG, X. Y., Z. T. ZHU and H. B. LIN (1999): Study on the
Tongliao City, the Inner Mongolia Autonomous Region,
effective treating period for pollen chromosome dou-
P. R. China, for collecting the plant material and for
bling of Populus tomentosa x P. bolleana. Sci Silvae
additional help. The authors also thank Dr. G. HAZEN-
Sinicae 35(4): 21–24.
BERG for critical reading of the manuscript. This work
KANG, X. Y., Z. T. ZHU and H. B. LIN (2000): Radiosensitiv-
was supported by the National Natural Science Founda-
ity of different ploidy pollen in poplars and its applica-
tion of China (Grant No. 30671708).
tion. Acta Genet Sinica 27(1): 78–82.
KANG, X. Y., P. D. ZHANG, P. GAO and F. ZHAO (2004): Dis-
RAE, A. M., N. R. STREET and M. RODRÍGUEZ-ACOSTA
covery of a new way of poplar triploids induced with
(2007): Populus trees, pp. 1–28. In: Genome mapping
colchicine after pollination. J Beijing For Univ 26(1):
and molecular breeding in plants, vol. 7, Forest trees,
edited by C. KOLE, Springer-Verlag, Berlin, Heidelberg.
KIMURA, C. (1955): The embryo sac of Populus sieboldii
SANZOL, J. and M. HERRERO (2001): The "effective pollina-
Miquel. Sci Rep Tohoku Univ Biol 21: 122–125.
tion period" in fruit trees. Sci Hortic 90(1): 1–17.
KIMURA, C. (1963): On the embryo sac formation of some
VAN BUIJTENEN, J. P., P. N. JORANSON and D. W. EINSPAHR
members of the Salicaceae. Sci Rep Tohoku Univ Biol
(1958): Diploid versus triploid aspen as pulpwood
sources with reference to growth, chemical, physical
LI, W. T. and T. ZHU (1988): Development of pollen and
and pulping differences. Tappi 41(4): 170–175.
embryo sac in Populus euphratica Oliv. For Res 1(2):
VEILLEUX, R. E. (1985): Diploid and polyploid gametes in
crop plants: mechanisms of formation and utilization in
LI, W. D. and F. S. MA (2006): Reproductive biology of sex-
plant breeding. Plant Breeding Rev 3: 253–288.
ual hybridization in woody plants: an atlas. Science
WERNER, J. E. and S. J. PELOQUIN (1987): Frequency and
Press, Beijing.
mechanisms of 2n egg formation in haploid Tuberosum-
LI, W. T., R. W. FAN and X. L. MAI (1982): On the embry-
wild species F hybrids. Am Potato J 64: 641–654.
ological observations of the seed development of Popu-
WERNER, J. E. and S. J. PELOQUIN (1990): Inheritance and
lus simonii Carr. Sci Silvae Sinicae 18(2): 113–119.
two medchanisms of 2n egg formation in 2x potatoes. J
LI, Y. H., X. Y. KANG, S. D. WANG, Z. H. ZHANG and H. W.
Hered 81(5): 371–374.
CHEN (2008): Triploid induction in Populus alba x
WERNER, J. E. and S. J. PELOQUIN (1991): Occurrence and
P. glandulosa by chromosome doubling of female
mechanisms of 2n egg formation in 2x potato. Genome
gametes. Silvae Genet 57(1): 37–40.
MASHKINA, O. S., L. M. BURDAEVA, M. M. BELOZEROVA and
WINTON, L. L. (1968): Fertilization in forced quaking
L. N. VYUNOVA (1989): Method of obtaining diploid
aspen and cottonwood. Silvae Genet 17(1): 20–21.
pollen of woody species. Lesovedenie 1: 19–25.
YAMADA, A. and R. TAO (2007): Controlled pollination with
MENDIBURU, A. O. and S. J. PELOQUIN (1977): The signifi-
sorted reduced and unreduced pollen grains reveals
cance of 2n gametes in potato breeding. Theor Appl
unreduced embryo sac formation in Diospyros kaki
Genet 49: 53–61.
Thunb. ‘Fujiwaragosho'. J Japan Soc Hort Sci 76(2):
NAGARAJ, M. (1952): Floral morphology of Populus del-
toids and P. tremuloides. Botanical Gazette 114:
ZHANG, Z. Y. and F. L. LI (1992): The techniques of pollen
chromosome doubling of Populus tomentosa. J Beijing
OGBURIA, M. N., T. YABUYA and T. ADACHI (2002): A cytoge-
For Univ 14(Suppl. 3): 52–58.
netic study of bilateral sexual polyploidization in cassa-
ZHU, T. and W. T. LI (1989): Formation and development
va (Manihot esculenta Crantz). Plant Breeding 121:
of the ovule and embryo sac in Populus lasiocarpa
Oliver. J Wuhan Bot Res 7(1): 13–20.
PFEIFFER, T. W. and E. T. BINGHAM (1983): Abnormal meio-
ZHU, Z. T., H. B. LIN and X. Y. KANG (1995): Studies on
sis in alfalfa, Medicago sativa: cytology of 2n egg and 4n
allotriploid breeding of Populus tomentosa B301 clones.
pollen formation. Can J Genet Cytol 25: 107–112.
Sci Silvae Sinicae 31(6): 499–505.
Herausgeber: Johann Heinrich von Thünen-Institut. Bundesforschungsinstitut für Ländliche Räume, Wald und Fischerei.
Schriftleitung: Institut für Forstgenetik, Sieker Landstraße 2, D-22927 Großhansdorf
Verlag: J. D. Sauerländer's Verlag, Finkenhofstraße 21, D-60322 Frankfurt a. M.
Anzeigenverwaltung: J. D. Sauerländer's Verlag, Frankfurt am Main.
Satz und Druck: ADN Offsetdruck, Battenberg — Printed in Germany.
J. D. Sauerländer's Verlag, Frankfurt a. M., 2010
Source: http://germanjournalofforestresearch.de/fileadmin/content/dokument/archiv/silvaegenetica/59_2010/Heft1/_05__Wang_2364.pdf
CONTRATO DE LICENCIA DE SOLUCIÓN BLACKBERRY LE ROGAMOS LEA EL PRESENTE DOCUMENTO DETENIDAMENTE ANTES DE INSTALAR O UTILIZAR EL SOFTWARE. ESTE CONTRATO CONTIENE DISPOSICIONES QUE LIMITAN O EXCLUYEN LA RESPONSABILIDAD DE RIM FRENTE A USTED Y QUE DE LO CONTRARIO AFECTAN SUS DERECHOS LEGALES. SEGÚN SU JURISDICCIÓN, ESTE CONTRATO TAMBIÉN PUEDE REQUERIR QUE USTED RECURRA A ARBITRAJE SOBRE UNA BASE INDIVIDUAL A LOS FINES DE RESOLVER CONFLICTOS EN LUGAR DE JUICIOS POR JURADO O ACCIONES COLECTIVAS. EL PRESENTE CONTRATO NO AFECTA SUS DERECHOS LEGALES OBLIGATORIOS APLICABLES EN SU JURISDICCIÓN, EN LA MEDIDA QUE TENGA DERECHO A LOS DERECHOS LEGALES OBLIGATORIOS CORRESPONDIENTES. Este Contrato de Licencia de Solución BlackBerry (el "Contrato") es un contrato legal entre usted: individualmente si usted lo acepta en su propio carácter; o si usted está autorizado para adquirir el Software (según se define abajo) en nombre de su compañía u otra entidad, entre la entidad para cuyo beneficio usted actúa (en cualquier caso, "Usted"), y Research In Motion Limited ("RIM") con sede social en 295 Phillip Street, Waterloo, Ontario, Canadá N2L 3W8 (conjuntamente las "Partes" e individualmente una "Parte"). En el contexto de la distribución de Productos/Servicios (según se definen abajo), RIM significa Research In Motion E-Commerce S.a.r.l u otra afiliada de RIM identificada como distribuidor de productos/servicios en Su Jurisdicción en http://www.blackberry.com/legal/rime ("RIME"). Si usted está utilizando el Software junto con un Dispositivo de mano en su carácter personal y en nombre de su compañía u otra entidad, en ese caso, "Usted" significará usted en su carácter personal para algunos elementos del Software y los Servicios de RIM, y significará la entidad en cuyo nombre usted actúa para otro Software y los Servicios de RIM (por ej. si la compañía para la cual usted trabaja lo autoriza a celebrar este Contrato con respecto al uso por su parte de una cuenta de correo electrónico de Servidor de empresa de BlackBerry ("BES") y de aplicaciones de gestión de información personal de BlackBerry ("Aplicaciones PIM"), pero no lo autoriza ni asume responsabilidad por el uso por su parte de otro software o los servicios, tales como el Software de cliente Windows Live Messenger o una Tienda RIME, en ese caso, "Usted" significa su compañía para la cuenta de correo electrónico BES y las Aplicaciones PIM, y "Usted" significa usted personalmente en relación al uso por su parte del Software de cliente Windows Live Messenger y la Tienda RIME). En relación con la licencia y distribución del Software, RIM es un licenciatario directo o indirecto de: (a) cualquiera una o más de sus subsidiarias y afiliadas (las subsidiarias y afiliadas correspondientes junto con RIM se denominan en este Contrato "Compañías del Grupo RIM"); o (b) un tercero licenciante para cualquiera de las Compañías del Grupo RIM inclusive RIM.
Rational therapy for vomiting in dogs and cats Lauren A. Trepanier, DVM, PhD, Dip. ACVIM, Dip. ACVCP University of Wisconsin-Madison, School of Veterinary Medicine, Madison, Wisconsin Vomiting is a common problem in veterinary patients, and can lead to dehydration, weight loss, and reflux esophagitis. There are several clinically effective veterinary anti-emetic drugs. Choosing among these options depends on the likely cause of the vomiting and the mechanisms of action and side effects of each drug. The first step before considering an antiemetic in a dog or cat is a reasonable work-up to rule out serious underlying disease. Every acutely vomiting animal that is brought to a veterinary clinic deserves two view abdominal radiographs to rule out obstruction. Using antiemetics empirically in animals with unrecognized GI obstruction can delay the diagnosis and worsen the prognosis. If vomiting is severe or persistent, a CBC, biochemical panel, and pancreatic lipase test are indicated.




