Levitra enthält Vardenafil, das eine kürzere Wirkdauer als Tadalafil hat, dafür aber schnell einsetzt. Männer, die diskret bestellen möchten, suchen häufig nach levitra kaufen ohne rezept. Dabei spielt die rechtliche Lage in der Schweiz eine wichtige Rolle.
Ilipo.cl
OBES SURGDOI 10.1007/s11695-010-0126-y
CLINICAL RESEARCH
Efficacy of Low-Level Laser Therapy for Body Contouringand Spot Fat Reduction
Mary K. Caruso-Davis & Thomas S. Guillot & Vinod K. Podichetty & Nazar Mashtalir &Nikhil V. Dhurandhar & Olga Dubuisson & Ying Yu & Frank L. Greenway
# Springer Science+Business Media, LLC 2010
Results Data were analyzed for those with body weight
Background Low-level laser therapy (LLLT) is commonly
fluctuations within 1.5 kg during 4 weeks of the study.
used in medical applications, but scientific studies of its
Each treatment gave a 0.4–0.5 cm loss in waist girth.
efficacy and the mechanism by which it causes loss of fat
Cumulative girth loss after 4 weeks was −2.15 cm
from fat cells for body contouring are lacking. This study
(−0.78 ± 2.82 vs. 1.35 ± 2.64 cm for the control group,
examined the effectiveness and mechanism by which 635–
p < 0.05). A blinded evaluation of standardized pictures
680 nm LLLT acts as a non-invasive body contouring
showed statistically significant cosmetic improvement after
4 weeks of laser treatment. In vitro studies suggested that
Methods Forty healthy men and women ages 18–65 years
laser treatment increases fat loss from adipocytes by release
with a BMI <30 kg/m2 were randomized 1:1 to laser or
of triglycerides, without inducing lipolysis or cell lysis.
control treatment. Subject's waistlines were treated 30 min
Conclusions LLLT achieved safe and significant girth
twice a week for 4 weeks. Standardized waist circumfer-
loss sustained over repeated treatments and cumulative
ence measurements and photographs were taken before and
over 4 weeks of eight treatments. The girth loss from the
after treatments 1, 3, and 8. Subjects were asked not to
waist gave clinically and statistically significant cosmetic
change their diet or exercise habits. In vitro assays were
conducted to determine cell lysis, glycerol, and triglyceriderelease.
Keywords Cold laser . Fat reduction . Low-level lasertherapy . Non-invasive laser
This study was supported by Meridian Medical, Inc., Vancouver, BC,Canada V6K 4L9.
M. K. Caruso-DavisSchool of Human Ecology, Louisiana State University,Baton Rouge, LA 70803, USA
Laser-based devices are used in a broad array of medicaland surgical applications and their biological effects have
been documented for over 20 years. More recently low-
Plastic and Reconstructive Surgery,
level laser (LLL) devices have been used to facilitate tissue
Baton Rouge, LA 70808, USA
repair and healing processes. Although physiological
methods responsible for augmented cell proliferation and
Research Practice Partners,
pain relief are unknown, well-controlled clinical trials have
Miramar, FL 33027, USA
demonstrated that low-level lasers provide therapeutic relief
N. Mashtalir : N. V. Dhurandhar : O. Dubuisson : Y. Yu :
of pain. Low-level laser therapy is defined as management
F. L. Greenway (
*)
with a dose rate that causes no immediate demonstrable
Pennington Biomedical Research Center,
temperature rise of the treated tissue and no macroscopi-
Louisiana State University System,
cally visible change in tissue structure The dosage is a
Baton Rouge, LA 70808, USAe-mail:
[email protected]
magnitude used to define the laser beam energy applied to a
particular area of the body tissue measured in joules per
Randomization was created from random number tables
square centimeter.
and the treatment codes were stored in sealed envelopes
The Meridian LAPEX 2000 LipoLaser System is a semi-
during the study. Subjects could not be using light
conductor-based, low-level laser therapy device (LLLD).
sensitizing agents, diuretics, or undergoing photodynamic
The LAPEX 2000 LipoLaser was originally developed and
therapy. Subjects were required to have a stable weight,
approved for the treatment of pain due to carpel tunnel
gaining or losing no more than 2.5 kg in 6 months prior to
syndrome. The LAPEX 2000 LipoLaser has been modified
the trial. Subjects could not be on a weight reduction
and is now being rigorously evaluated for its effectiveness
regimen, and they were asked not to change their diet or
in reducing areas of local fat accumulation for cosmetic
exercise habits during the trial. This study was performed in
purposes. The LAPEX 2000 LipoLaser emits light at 635–
accordance with the Declaration of Helsinki and approved
680 nm. It is non-thermal and does not heat the tissues. As
by the Argus Institutional Review Board. Written informed
such, it is considered to be a non-invasive treatment.
consent was obtained from all participants prior to study
Neira et al. [] evaluated the effect of a 635–680 nm, 10-
mW diode laser radiation with exclusive energy optics on
The laser therapy device consisted of a console housing
treated fat cells in biopsy specimens. Fat cells were treated
most electronics, the controls for the device, and two
in vivo with 1.2–3.6 J/cm2 of energy from the laser for 2 to
multiprobes that housed four lasers emitting visible laser
6 min. The cells were then removed by lipectomy,
light at a wavelength of 635–680 nm. Each subject had two
examined by electron microscopy, and compared to cells
treatments per week for a total of eight treatments over
removed by lipectomy that were not treated with the laser.
4 weeks. Each treatment session lasted approximately
Fat cells that were not exposed to the laser treatment looked
30 min. The two multiprobes were placed over the waist
like round grapes. Eighty percent of the fat was released
bilaterally in three positions as well as two enhancement
from the fat cells after 4 min of laser light exposure and
probes that were placed to both sides of the inguinal region
99% was released after 6 min of exposure. After exposure
and the laser was activated for 10 min in each of these
to the laser light, pores in fat cells were visible by scanning
positions to encompass the waist from the back to the front.
electron microscope. It was presumed, but not demonstrat-
The control arm of the trial utilized the device, but the
ed, that the fat was released from these pores, taken up in
multiprobes of the device were inactivated during the
the lymphatics and reesterified in other tissues or metabo-
treatment session.
lized for energy
Two individuals conducted the study. One administered the
Several studies have recognized that LLL accelerates
treatment, and the other, who was blinded to treatment
repair processes, stimulates cell proliferation, and pro-
allocation, obtained measurements and photographs. The
motes vascularization in injured tissues [–However,
individual administering the treatment remained blinded to
clinical application to body fat reduction as a minimally
photographic and girth measurements. Each subject was
invasive option is an evolving field which is not well
advised about the rules of blinding, and the individual taking
studied. We conducted a blinded clinical trial to describe
photographs and measurements could not relay this informa-
the application of low-level laser therapy to local fat
tion to the subject. The individual administering the treatment
reduction for cosmetic purposes. As a secondary objec-
did not enter the room where the photographs and measure-
tive, we also investigated the mechanism by which the
ments were obtained. A case report form was used for each
laser causes fat loss from fat cells. The mechanistic study
measurement session and these forms were placed in a sealed
investigated whether the fat loss induced by the laser is
envelope until data was analyzed at the end of the study. Two
due to (1) the activation of the complement cascade lysing
separate people who were not involved in other aspects of the
adipocytes, (2) adipocyte death, or (3) release of intact
study did the blinded evaluations of the photographs.
triglycerides from cells vs. the release of glycerol and fatty
All subjects had photographs taken at a standardized
acids after lipolysis.
distance with a standard background and lighting. Girthmeasurements of the waist were obtained in the mannerrecommended by the United States National Institutes of
Health (NIH) guidance at the iliac crest using a tape measurewith standardized tension and oriented parallel to the floor
]. A reference point on the body for the pictures andmeasurements was relocated at each evaluation by measuring
Forty healthy men and women between the ages of 18–
a distance from the floor that was determined in the first
65 years, inclusive, and body mass index (BMI) no greater
measurement at baseline. The specified measured distance
than 29.9 kg/m2 were randomized in a 1:1 ratio to an
was used to ensure all measurements and photographs were
experimental laser treatment or to a control laser treatment.
obtained in the same location. The camera was placed on a
tripod at a fixed distance from the floor but was adjusted to
Table 1 Baseline demographic characteristics of study subjects in theLAPEX 2000 LipoLaser study
the specific height of each individual participant. Standard-ized waist measurements were taken at baseline, treatment 3,
and treatment 8. Standardized photographs were taken beforeand after the initial treatment, treatment 3, and treatment 8.
Weight was measured and BMI was calculated at baseline
and at treatment 8 (week 4). Blood pressure was measured at
baseline, treatment 3, and treatment 8. All adverse events
were recorded in the case report forms.
The waist circumference measurements were compared
between the control and laser-treated group using a t test.
The data were analyzed using completers and the more
Waist circumference (cm)
conservative intent to treat analysis. The blinded observers
judged improvement on a 0–3 scale. Zero on this qualitative
Body mass index (kg/m2)
scale represented no improvement, 1 represented mild
improvement, 2 represented moderate improvement, and 3
Systolic blood pressure
represented marked improvement. The results of the two
observers were averaged and compared by t test.
Diastolic blood pressure75.35
In Vitro Studies Using Human Fat Cells
Values are means and standard deviation (SD).
Experiment 1 Does the laser activate the complement
using a 10× (Zeiss Achroplan objective) and a 20× (LD plan
NeoFluor objective), and a Zeiss Axiocam HRc camera [
Human adipose-derived stem cells obtained from subcu-
taneous fat during abdominal surgery were plated and
Experiment 3 Does the laser increase triglyceride release or
differentiated to form adipocytes as described by Bunnel et
lipolysis from adipocytes?
al. [Human adipocytes were differentiated in 12-well
This experiment used human adipocytes in eight 6-well
plates. Three of the wells in the plates were left as a control.
plates. Two wells in each plate were used as a control with
Fresh plasma replaced one third of the cell culture media inanother three wells. The next three wells had one third ofthe media replaced with plasma that was heat-inactivated todestroy complement. The final three wells in each plate hadone third of the media replaced by a combination of freshhuman plasma and white blood cells. One experimentalplate was irradiated with the LAPEX 2000 LipoLaser for10 min and the other was left as a non-irradiated control.
The cells were then evaluated for evidence of lysis underthe microscope.
Experiment 2 Does the laser kill adipocytes?
To evaluate influence of LAPEX 2000 LipoLaser on
adipose cell death and viability, we used LIVE/DEAD® CellViability Assays (Invitrogen). Human adipocytes were differ-entiated in 96-well plates. The experimental plate was
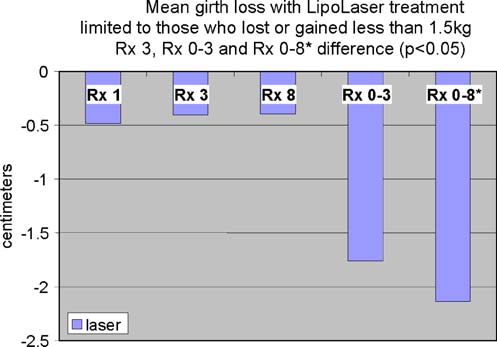
Fig. 1 The difference in girth loss between placebo and the LAPEX
irradiated with the LAPEX 2000 LipoLaser for 10 min and
2000 LipoLaser at treatments 1, 3, and 8 were all 0.4 to 0.5 cm, and
the other was left as a non-irradiated control. The cells were
the difference in girth loss at treatment 3 was statistically significant
then probed with cell viability assay reagent using the
(p < 0.05). The difference in cumulative girth loss compared fromtreatment 1 to 3 was statistically significant by LOCF or completer's
manufacturer's protocol. Calcein and propidium iodide
analysis (p < 0.05). The difference in cumulative girth loss at
emissions were then analyzed using a fluorescent plate
treatment 8 was significant in subjects who remained within 1.5 kg
reader. Images were acquired on a Zeiss Axiovert 40 CFL
of their baseline weights (p < 0.05)
scheduling conflicts. There were no adverse events in eithergroup during the trial. The groups were well balanced atbaseline, and the group characteristics are illustrated inTable . Mean weight and BMI did not change significantlyover the eight treatments and 4 weeks. Blood pressure didnot change significantly from baseline to treatment 3, fromtreatment 3 to treatment 8, or from baseline to treatment 8.
The mean placebo subtracted reductions in waist girth attreatments 1, 3, and 8 with the LAPEX 2000 LipoLaserwere 0.49, 0.41, and 0.40 cm, respectively. This singletreatment difference, 0.41 cm (laser −0.59 ± 0.71 cm vs.
placebo −0.19 ± 0.47 cm) (mean±SD), was significant(p < 0.05) on the third treatment done during week 2 in thecompleters analysis, but was not statistically significant by
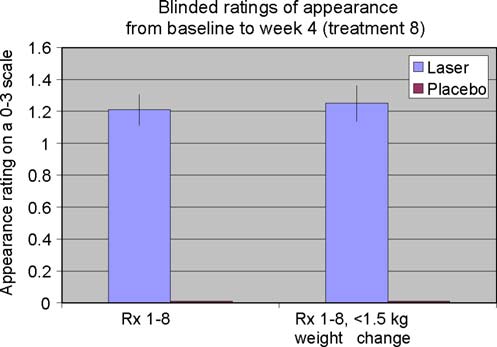
Fig. 2 Blinded appearance ratings on a 0–3 scale over 4 weeks and
the intent to treat analysis. The cumulative girth loss at
eight treatments favored the LAPEX 2000 LipoLaser treatment
treatment 3 on week 2 was a significant 1.74 cm
compared to the placebo treatment (p < 0.001)
(laser −1.89 ± 2.97 cm vs. placebo −0.16 ± 2.46 cm,
media containing 10% fetal bovine serum (FBS). Two other
p < 0.05) on both the completers' analysis and by intent to
wells had 25% of the media with 10% FBS replaced with
treat analysis. Cumulative girth loss at treatment 8 (4 weeks of
human serum with 10% FBS. The last two wells had 25%
treatment) was 2.15 cm with 15 subjects in the laser group and
of the media with 10% FBS replaced with heat-inactivated
16 subjects in the placebo group (laser −0.78 ± 2.82 cm vs.
human serum with 10% FBS. Four of the plates were
placebo 1.35 ± 2.64 cm) in those who maintained their weight
irradiated for 10 min with the laser and the other four plates
within 1.5 kg of their baseline weight (p < 0.05). Cumulative
served as a non-irradiated control. Media from the eight
girth loss at treatment 8 (4 weeks of treatment) was 1.33 cm
replicates of each of the three conditions in the laser
with 19 subjects completing in the placebo group and 20
irradiated plates and the non-irradiated control plates were
subjects completing in the laser group (laser −0.87 ± 2.65 cm
used for glycerol and triglyceride determination.
vs. placebo 0.47 ± 3.19 cm) regardless of weight change (p=NS). The standardized pictures of the participants showed asignificant 1.21 difference (laser 1.21 ± 0.42 vs. placebo0 ± 0) in appearance on a 0–3 scale favoring the LAPEX 2000
LipoLaser group comparing baseline to week 4 (treatment 8)pictures (p < 0.001). When only those participants that
remained within 1.5 kg of their baseline weight (N = 31)were considered, the improvement in appearance increased to
Forty subjects participated in the clinical trial. Twenty were
1.25 (laser 1.25 ± 0.45 vs. placebo 0 ± 0) on a 0–3 scale
treated with the LAPEX 2000 LipoLaser and 20 were
comparing baseline to week 4 (treatment 8) pictures
treated with an inactive version of the device. One subject
(p < 0.001). The girth difference in the laser group compared
in the treatment group did not complete the study due to
to the placebo group is illustrated in Fig. The differences in
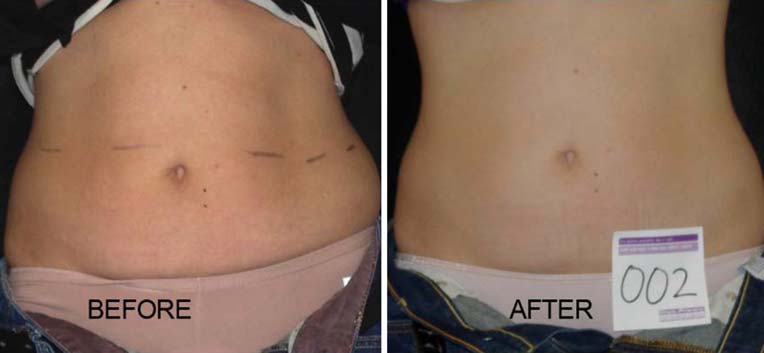
Fig. 3 Woman before and after4 weeks and eight treatmentswith the LAPEX 2000LipoLaser
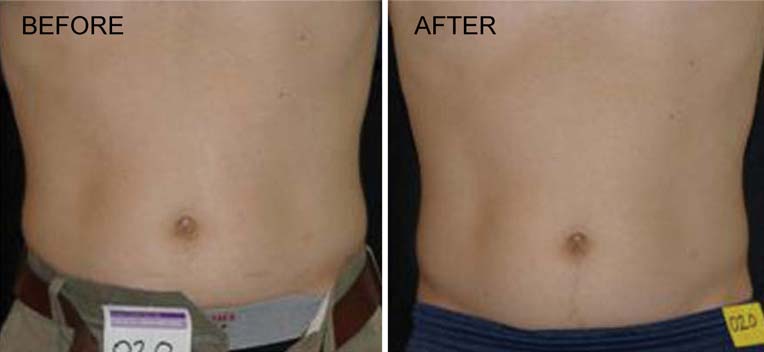
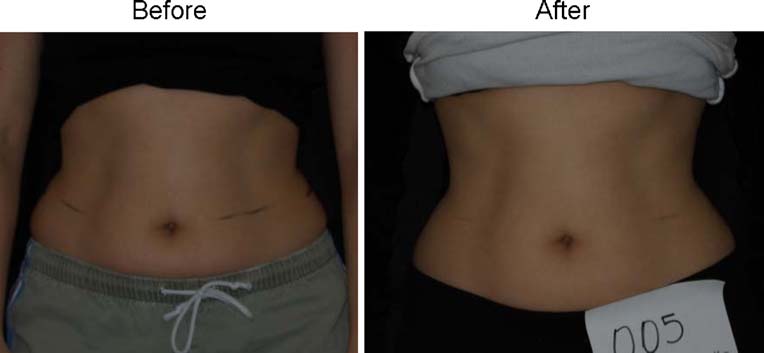
Fig. 4 Man before and after4 weeks and eight treatmentswith the LAPEX 2000LipoLaser
appearance from baseline to week 4 (treatment 8) in the whole
Experiment 2 The laser does not kill adipocytes.
group and the subjects who remained within 1.5 kg of their
The number of viable cells in the laser-treated or
baseline weight are illustrated in Figs. and . Fig.
untreated group as determined by the propidium iodide
shows a placebo subject at baseline and 4 weeks (treatment 8)
assay were similar, but calcein levels were lower in the
who remained within 1.5 kg of her baseline weight. Figs.
laser-treated cells (Fig. Calcein, a non-fluorescent dye,
and show a subject who lost 2.2 kg and 2.7 kg in the laser
gets transported through the cell membrane, becomes
and placebo treatment groups, respectively.
fluorescent due to cleavage with cellular esterases, and getstrapped intracellularly. Normally functioning cells canextrude the entrapped dye. Considering the equal cell
In Vitro Study Using Human Fat Cells
viability in the two groups, lower calcein levels in thelaser-treated group suggests either intact metabolic func-
Experiment 1 The laser does not activate the complement
tioning of cells and/or reduction of cell-trapped calcein,
perhaps by leakage.
The fat cells that came into contact with plasma or
These findings are also consistent with the studies by
plasma with white blood cells were lysed in both the laser-
Niera [] in which the laser-treated cells showed micro-
treated and the control plate, but cells in the control wells or
pores in the membrane, which presumably contributed to
in wells with heat-inactivated plasma were not lysed. This
the leakage of fat from those cells.
indicates that serum complement does lyse fat cells, but thatthe laser does not activate complement. This is consistent
Experiment 3 The laser increases triglyceride release, but
with the mechanism shown by Niera [] in which the laser
not lipolysis from adipocytes. Baseline triglycerides in the
created pores through which the fat leaked from the fat cells
control wells were undetectable and were increased, as
into the interstitial space.
expected, in the wells with serum. The control wells did not
Fig. 5 Woman before and after4 weeks and eight treatmentswith the placebo LAPEX 2000LipoLaser who maintained herweight within 1.5 kg of startingweight
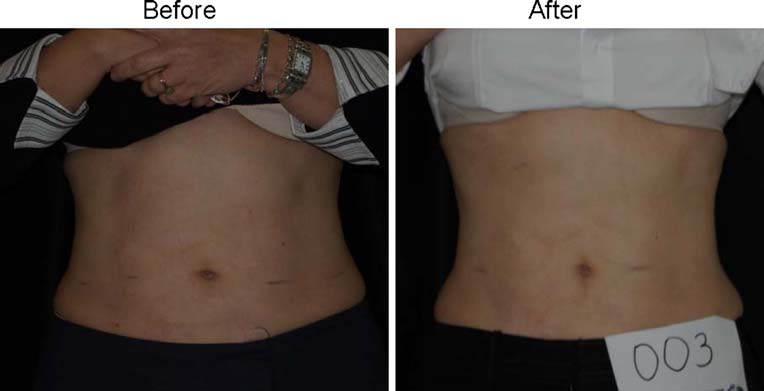
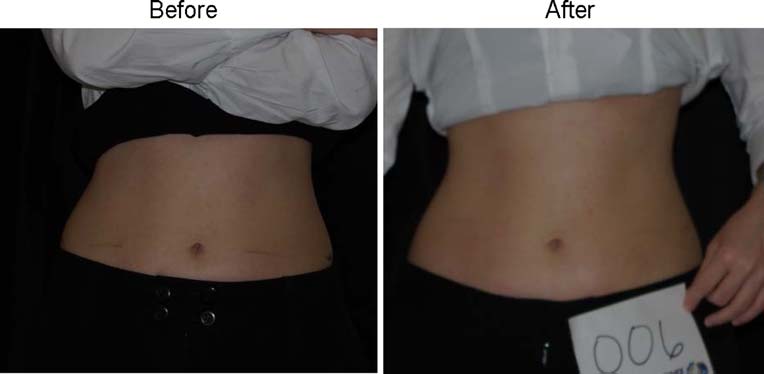
Fig. 6 Woman who lost 2.2 kgbefore and after 4 weeks andeight treatments with theLAPEX 2000 LipoLaser
increase triglycerides or glycerol in the media in response
media in the presence of heat-inactivated or normal serum
to laser irradiation. The laser-irradiated wells containing
suggesting that any fat loss from adipocytes in response to
serum had significantly greater increases in triglycerides
the laser treatment is not due to a stimulation of lipolysis.
than the non-irradiated wells containing serum (69 ± 1.7 vs.
On the other hand, the increase of triglyceride into the
66.7 ± 1.5 mg/dL, p = 0.004). The laser-irradiated wells
media in response to laser irradiation in the presence of
containing heat-inactivated serum had a significantly
normal or heat-inactivated plasma suggests leakage of
greater increase in triglycerides than the non-irradiated
intact triglycerides from cells, a possible mechanism to
wells containing heat-inactivated serum (72.6 ± 1.8 vs.
explain the observations of Niera [], which showed
70.1 ± 1.6 mg/dL, p = 0.008). Baseline glycerol levels were
reduction in lipid content and the appearance of micropores
not different in the laser-treated or the non-irradiated groups
in laser-treated adipocytes. These findings suggest that
(0.11 ± 0.01 vs. 011 ± 0.01 mmol/L, p = 0.44). The laser-
human serum is necessary for the laser to release
irradiated wells with serum had significantly lower glycerol
triglycerides from the fat cell and that the action is not
levels than the non-irradiated group (0.14 ± 0.01 vs.
0.17 ± 0.03 mmol/L, p = 0.01). The glycerol levels in thelaser-irradiated wells containing heat-inactivated serumwere not different from the non-irradiated wells with heat-inactivated serum (0.14 ± 0.01 vs. 0.15 ± 0.02 mmol/L,
p = 0.3). Before and after laser irradiation in the presence ofserum in which triglycerides were released, the cells
Low-level laser therapy is a light source treatment that
continued to appear intact without evidence of lysis
generates light of a single wavelength. Low-level laser
(Fig. Laser treatment did not release glycerol into the
therapy emits no heat, sound, or vibration. Instead of
Fig. 7 Woman who lost 2.7 kgbefore and after 4 weeks andeight treatments with the place-bo LAPEX 2000 LipoLaser
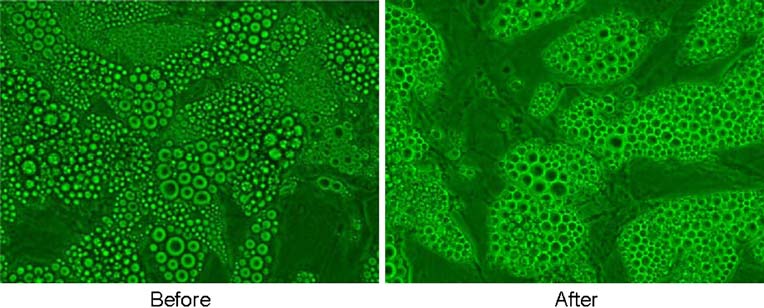
Fig. 8 The number of live anddead cells measured by propi-dium iodide and the cellularmetabolism measured by calceinwith and without laser treatment
producing a thermal effect, low-level laser therapy acts via
fluctuation was to accommodate the effect of menstruation-
nonthermal or photochemical reactions in the cells, also
related fluid shifts in women while representing a more
referred to as photobiological or biostimulatory ].
conservative value in one man with a weight fluctuation of
A single LAPEX 2000 LipoLaser treatment yielded girth
loss, and repeated treatments remained effective giving
Girth loss over the course of the study was greater than
approximately a 0.4 to 0.5 cm girth loss per treatment. This
2 cm and statistically significant. The subjects in this study
difference was statistically significant at treatment 3,
were not obese and an approximate 1 inch (2.54 cm)
demonstrating that the effect of the LAPEX 2000 Lip-
reduction in waist girth over the course of 8 treatments and
oLaser does not appear to diminish with repeated treat-
4 weeks was clinically significant and cosmetically rele-
ments through time. The 1.74 cm girth loss at treatment 3
vant. The blinded ratings of the baseline pictures compared
suggests that the LAPEX 2000 LipoLaser treatments twice
to treatment 8 (week 4) pictures taken in a standardized
a week are cumulative in their effect on girth loss.
way demonstrated an improvement in appearance that was
It is likely that weight change over the course of
highly statistically significant. As expected, the improve-
treatment would change waist circumference and confound
ment was greater when limiting the comparison to only
the results. The subjects selected for the study were asked
those subjects that remained within 1.5 kg of their baseline
not to lose or gain weight over the course of the study.
Since some subjects did gain or lose a significant amount of
The mechanism by which the laser reduces fat from fat
weight over the 4-week study, the cumulative fat loss was
cells observed by Neira et al. [] was unclear. Fat cell lysis,
analyzed only on those subjects whose weight was within
lipolysis, followed by glycerol and fatty acid release, or
1.5 kg of their baseline weight. The 1.5-kg weight
leakage of fat from fat cells are some possible explanations.
Fig. 9 Human adipocytes inculture before and after LAPEX2000 LipoLaser irradiation for10 min in the presence of serumin which triglycerides were re-leased—cells remain intactwithout evidence of lysis
First, we determined if the laser-induced cell lysis by a
mobilize subcutaneous fat for body contouring without
complement-mediated process. Gay-Crosier et al. found
weight loss, future investigations should involve larger
that a pulsed dye laser activated complement in normal skin
samples and explore the application of this technique to
and confirmed this phenomenon by measuring a rise in
other body parts for cosmetic contouring.
membrane attack complex of complement [Our experi-ments revealed that plasma with complement lysed cells
This study was supported by a grant from
with or without the laser and that heat-inactivated serum
Meridian Medical, Inc. Mary Katherine Caruso-Davis received
without complement did not. Confirming the findings of
support for her assistantship through the Bissoon Mesotherapy
Niera [], we found that the cells were not killed by laser
Foundation. The mechanistic in vitro studies were partially supportedby a CNRU Center Grant # 1P30 DK072476 entitled "Nutritional
treatment, but had increased clearance of the dye, consistent
Programming: Environmental and Molecular Interactions", sponsored
with pores being present in the membranes of the cells.
by NIDDK with the assistance of Jeffrey Gimble, M.D., Ph.D. and
Interestingly, laser treatment of human fat cells without
Ying Yu, MS. The authors wish to thank Eleanor Meador for
serum present did not result in the release of triglycerides,
coordinating the study and performing the LAPEX 2000 LipoLasertreatments, Lindsay Southard and Canaan Heard, undergraduates
but in the presence of normal serum or heat-inactivated
working on the study, and Mary Beth Burnett who assisted in
serum, triglyceride in the media was increased by laser
irradiation. Presence of serum along with fat cells simulatesin vivo environment to release triglycerides in the presenceof laser irradiation and further confirms the ability of the
laser to influence fat loss. There was no increase of glycerolin the media, confirming that the laser did not stimulate
1. King PR. Low level laser therapy: a review. Lasers Med Sci.
Fat that is mobilized by the laser presumably enters the
2. Neira R, Arroyave J, Ramirez H, et al. Fat liquefaction: effect of
low-level laser energy on adipose tissue. Plast Reconstr Surg.
blood stream via the lymphatics in fat tissue much like fat
2002;110:912–22. discussion 923–5.
in food enters the body from the intestinal lymphatics into
3. Benedicenti A, Verrando M, Cherlone F, et al. Effect of a 904 nm
the blood stream. The amount of fat mobilized with a
laser on microcirculation and arteriovenous circulation as evalu-
single lipolaser treatment, based on the average circum-
ated using telethermographic imaging. Parodontol Stomatol(Nuova). 1984;23:167–78.
ference changes, is a mean of about 52 grams. This
4. Dortbudak O, Haas R, Mallath-Pokorny G. Biostimulation of
amount of fat can be consumed in a large meal and is less
bone marrow cells with a diode soft laser. Clin Oral Implants Res.
than one third the amount of fat that is administered
intravenously when people cannot use their intestinal tract.
5. Garavello-Freitas I, Baranauskas V, Joazeiro PP, et al. Low-power
laser irradiation improves histomorphometrical parameters and
If weight is stable, the mobilized fat from the lipolaser
bone matrix organization during tibia wound healing in rats. J
treatment will either be burned for food in the body or be
Photochem Photobiol B. 2003;70:81–9.
distributed into fat depots typical of that person's fat
6. Hall G, Anneroth G, Schennings T, et al. Effect of low level
distribution. Redistribution of fat using the laser does not
energy laser irradiation on wound healing. An experimental studyin rats. Swed Dent J. 1994;18:29–34.
change the body's lipolytic thresholds. Thus, without
7. Mester E, Mester AF, Mester A. The biomedical effects of laser
periodic treatments, the body will redistribute fat in its
application. Lasers Surg Med. 1985;5:31–9.
normal pattern. The laser, therefore, should add no more
8. Campana V, Moya M, Gavotto A, et al. Effects of diclofenac
risk of developing atherosclerosis than the routine eating
sodium and He:Ne laser irradiation on plasmatic fibrinogen levelsin inflammatory processes. J Clin Laser Med Surg. 1998;16:317–
Thus, the LAPEX 2000 LipoLaser gives a significant waist
9. Caruso MK, Pekarovic S, Raum WJ, et al. Topical fat reduction
girth loss that is sustained over repeated treatments and is
from the waist. Diabetes Obes Metab. 2007;9:300–3.
cumulative over 4 weeks of eight treatments. This waist girth
10. Bunnell BA, Estes BT, Guilak F, et al. Differentiation of adipose
stem cells. Methods Mol Biol. 2008;456:155–71.
loss was almost 1 inch (2.54 cm) in magnitude.
11. Rogers PM, Fusinski KA, Rathod MA, et al. Human adenovirus
Therefore, the LAPEX 2000 LipoLaser gave a clinically
Ad-36 induces adipogenesis via its E4 orf-1 gene. Int J Obes
meaningful, a cosmetically detectable, and a statistically
significant improvement in appearance. The fat loss was
12. Karu T. Photobiological fundamentals of low power laser therapy.
IEEE J Quantum Electron. 1987;23:1703–18.
probably a consequence of the laser creating temporary
13. Robinson MF, Watson PE. Day-to-day variations in body-weight
pores in the fat cells through which triglycerides were
of young women. Br J Nutr. 1965;19:225–35.
leaked, a process that requires serum, but is not
14. Gay-Crosier F, Polla LL, Tschopp J, et al. Complement activation
by pulsed tunable dye laser in normal skin and hemangioma. JInvest Dermatol. 1990;94:426–31.
Current options for cosmetic body contouring include
15. Dhami LD, Agarwal M. Safe total corporal contouring with large-
surgery or cream application [, Although low-level
volume liposuction for the obese patient. Aesthetic Plast Surg.
laser therapy appears to offer a non-surgical option to
Source: http://www.ilipo.cl/docs/Meridian.pdf
January—April 2013 AfCiC News Action for Children in Conflict (AfCiC) "Working towards a world of equal and sustainable opportunities for every Child From street freedom to a life of Hope Who is AfCiC? The Interim Care Centre for street chil- sional authority. They are incorporated in streets, things were not different. We
La Gazette de l' N° 22 Mensuel - Avril 2009 Bonjour à tous et à toutes !!! Voici le traditionnel mot du CEI qui accompagne chaque numéro de votre gazette préférée. Tout d'abord bravo à tous nos mi-thésards pour leur exposé de mi-thèse. En espérant que la dernière moitié de la thèse soit fructueuse. Ça y est, c'est le printemps ! Le soleil n'est pas encore vraiment au rendez-vous, mais ça ne saurait tarder. En avril








