Levitra enthält Vardenafil, das eine kürzere Wirkdauer als Tadalafil hat, dafür aber schnell einsetzt. Männer, die diskret bestellen möchten, suchen häufig nach levitra kaufen ohne rezept. Dabei spielt die rechtliche Lage in der Schweiz eine wichtige Rolle.
38-42. saroj kumar paul.fm
Vol. 6, No. 2, 2015
ISSN 2233-4203/ e-ISSN 2093-8950
www.msletters.org Mass Spectrometry Letters
Identification of Degradation Products in the Phosphodiesterase (PDE-4)Inhibitor Roflumilast Using High Resolution Mass Spectrometry and DensityFunctional Theory Calculations
Saroj Kumar Paul* and Upendra N. Dash
Department of Chemistry, Institute of Technical Education and Research (ITER), Siksha ‘O' Anusandhan University, Bhubaneswar,Odisha, India
Received March 18, 2015; Revised April 12, 2015; Accepted April 20, 2015First published on the web June 30, 2015;o DOI: 10.5478/MSL.2015.6.2.38
Abstract: Roflumilast analogs are a group of drugs which act as selective photodiesterase (PDE-4) inhibitor for the treatment severechronic pulmonary disease associated with chronic brochnonities. Structural identification of degradation products using high resolu-tion mass spectrometry and theoretical investigation by density functional theory have been successfully carried out on roflumilast toidentify four degradation products namely, 3,5-dichloropyridin-4-amine, N-(3,5-dichloropyridin-4-yl)-4-(difluoromethoxy)-3-hydroxybenzamide, N-(3,5-dichloropyridin-4-yl)-3-(cyclopropylmethoxy)-4-(difluoromethoxy) benzamide and 3-(cyclopropylmethoxy)-N-(3,5-dichloro-1-oxidopyridin-4-yl)-4-(difluoro methoxy) benzamide, generated in alkali, acidic and oxidative conditions.
Keywords: Roflumilast, Mass Spectrometry, Degradation products, DFT
The International Conference on Harmonisation of
Phosphodiesterases (PDEs) are a group of enzymes that
Technical Requirements for Registration of Pharmaceuticals
catalyze the breakdown of cyclic adenosine monophosphate
for Human Use (ICH) defines a degradation product as an
and cyclic guanosine monophosphate to their inactive
impurity resulting from a chemical change in the drug
form. PDE4 is the principal selective cyclic adenosine
substance brought about during manufacture and/or storage
monophosphate metabolizing enzyme in inflammatory and
of the new drug product by the effect of, for example, light,
immune cells which is highly expressed in leukocytes and other
temperature, pH, water, or by reaction with an excipient
inflammatory cells involved in the pathogenesis of inflammatory
and/or the immediate container closure system.4 ICH
lung diseases, such as asthma and chronic obstructive pulmonary
guidelines also necessitate the drugs to be subjected to
disease (COPD).1 Roflumilast (RFL) is a second generation
stress decomposition studies followed by identification and
selective phosphodiesterase (PDE-4) inhibitor approved for the
characterization of the degradation products (DP) which
treatment of severe chronic obstructive pulmonary disease
are formed 0.1%.5,6
associated with chronic bronchitis. The IUPAC name for
A thorough literature survey has revealed that only a few
roflumilast is 3-(cyclopropylmethoxy)-N-(3, 5-dichloropyridin-
studies have been reported for the degradation behaviour of
4-yl)-4-(difluoromethoxy) benzamide; CAS 162401-32-3).2
roflumilast. Tarek S. Belal et al. reported significant
Roflumilast has been approved in the EU (as Daxas) and in
degradation of roflumilast under acidic, alkali and
the US (as Daliresp) for treatment of severe COPD
oxidative conditions7 but none have been identified or
associated with chronic bronchitis and a history of
characterized. The drug has been investigated by Barhateet al. to be stable under neutral, thermal and photolyticconditions but unstable to acidic, alkaline and oxidative
*Reprint requests to Saroj Kumar Paul E-mail:
[email protected]
conditions at 80°C. But no attempt has been directed forthe identification of DPs.8
All MS Letters content is Open Access, meaning it is accessible online to
However, there have been reports of process related
everyone, without fee and authors' permission. All MS Letters content ispublished and distributed under the terms of the Creative Commons
Attribution License (http://creativecommons.org /licenses/by/3.0/). Under
this license, authors reserve the copyright for their content; however, they
permit anyone to unrestrictedly use, distribute, and reproduce the content
in any medium as far as the original authors and source are cited. For any
Density Functional Theory (DFT) has been known to
reuse, redistribution, or reproduction of a work, users must clarify the
predict the fragmentation profile of protonated molecular
license terms under which the work was produced.
Identification of Degradation Products in the Phosphodiesterase (PDE-4) Inhibitor Roflumilast
ions with reasonable accuracy.12 P. Wright et al. have
interface at 250oC. Nitrogen was used both as sheath gas
reported a rationalized approach for fragmentation profile
and auxiliary gas. The electro spray and tube lens were set
of maraviroc using DFT.13 Use of DFT for modelling of 15
at 4.5 kv and 90 V respectively. The mass spectrometer
molecules with respect to protonation-induced bond length
was operated in full scan MS with data dependent MS2
changes and subsequent prediction of their fragmentation
mode in positive polarity.
profiles during collision induced dissociation (CID) has
The selected range was from 100 to 1000 m/z and the
also been reported.14
resolution was 70,000 full width half maximum (FWHM)
In this current study the chemical structures of the
with an isolation window applied, followed by a data
different DPs produced under acidic, alkali and oxidative
dependent scan at a resolution of 17,500 FWHM with the
stress conditions are investigated by liquid chromato-
fragmentation energy applied. The target capacity of the C-
graphy-high resolution mass spectrometry. Efforts have also
trap was defined at 1×106 charges and the maximum
been made to rationalize the fragmentation profile of the
injection time was limited to 50 ms.
parent drug molecule by identifying the most favourableposition of protonation and the changes in bond length
Density functional theory
consequent to it, DFT. The DPs were characterized by
Gas-phase basicity, 3D structure and bond length calculations
comparing their collision induced dissociation (CID) mass
were performed using DFT, calculations at the B3LYP level
spectral data with that of the parent drug molecule of RFL.
using the 6-31G* basis set, with Gaussian09. The optimised
This rationalized fragmentation profile can also be
geometry for the neutral molecule was calculated, basic sites
extended to identify any other unknown transfor-mation
were then protonated and the relating minimum energy
products of RFL by comparing their daughter ions
geometry calculated for each possible structure. The energy
obtained under similar experimental condition.
differences between the most favourable cation (highest negativeenergy value in Hartrees) and all others were converted from
Hartrees into kcal/mol using the conversion factor of 627.503.
Materials and reagents
Sample preparation and degradation studies
HPLC grade acetonitrile and methanol were purchased
Standard solution of roflumilast (100 µg/mL) was
from Merck India limited (Mumbai, India). Ultrapure
prepared by dissolving it in acetonitrile. Stress degradation
water (18.2 MΩ) was prepared using a Milli-Q plus water
sample, acidic (1 N HCl, 80oC, 6 h), alkaline (1 N NaOH,
purification system from Millipore (Bedford, MA, USA).
80oC, 6 h) and oxidative (30% H2O2, 80oC, 6 h) were
Formic acid and standard of RFL were obtained from
prepared by dissolving 10 mg of sample in 1 mL of
Sigma-Aldrich Corporation (Bangalore, India). Analytical
acetonitrile, followed by the addition of 2 mL 1 N HCl,
reagent grade ammonium acetate, sodium hydroxide,
3 mL 1 N NaOH and 3 mL 30% H2O2 respectively. All the
hydrochloric acid and hydrogen peroxide were obtained
sample solutions were neutralized and volume made up to
from Qualigens India Limited (Mumbai, India)
10 mL with acetonitrile prior to their injection into themass spectrometer.
Liquid chromatography
All compound solutions were introduced into the ESI
Results and discussion
MS (electrospray ionisation mass spectrometry) source byhigh performance liquid chromatography (HPLC), Dionex
The drug exhibited degradation under acidic, alkali and
ultimate 3000 (Thermo scientific, USA), using a hypersil
oxidative stress conditions. The results of the degradation
BDS C18 column (150×4.6 mm, 5 µm, Thermo scientific,
study have been summarized in Table 1.
USA). A mobile phase consisting of A, 10 mM ammonium
In an attempt to identify the DPs by mass spectral
acetate adjusted to pH 3.2±0.05 with acetic acid and B,
analysis it is highly desirable to have a clear understanding
acetonitrile in gradient mode; T(min)/%B: 0/25, 10/60, 15/85,
of the fragmentation pathway of parent drug RFL. Under
20/85, 25/25, 30/25. Column temperature was maintained
ESI conditions RFL underwent protonation at the nitrogen
at 30 C and the flow rate was 1.0 mL/min. The samples
atom of the pyridine ring and the position of protonation
were injected (10 µL) into the HPLC system in acetonitrile.
has also been supported by DFT calculations. The resultsof DFT calculation for the energies of different protonation
High-resolution mass spectrometry
sites has been depicted in Figure 1. The protonated
The MS and MS/MS studies were performed on
molecular ion of RFL measured accurately to be m/z
Thermofisher Q-exactive mass spectrometer (Thermo
403.0449 Da and produced key fragment ions at m/z
Electron, Bremen, Germany) using electrospray ionization
367.0686 Da, 348.9975 Da, 241.0687 Da, 187.0213 Da
source and orbitrap mass analyzer. Heated electrospray
and 163.9676 Da. The most abundant product ion formed
ionization source was used for ionization. The temperature
at m/z 241.0687 Da due to loss of 3, 5-dichloropyridine
of the heater was kept at 450oC and capillary of the ESI
(C5H3Cl2N, 146.9643) by the cleavage of C-N, which further
Mass Spectrom. Lett. 2015 Vol. 6, No. 2, 38–42
Saroj Kumar Paul and Upendra N. Dash
Table 1. The retention times (RT), measured masses, predicated elemental compositions, theoretical exact masses, mass errorsand major fragment ions of degradation products (DP)
*RFL is not a DP and stands for the parent molecule of Roflumilast.
Figure 1. The energies of roflumilast molecule at different
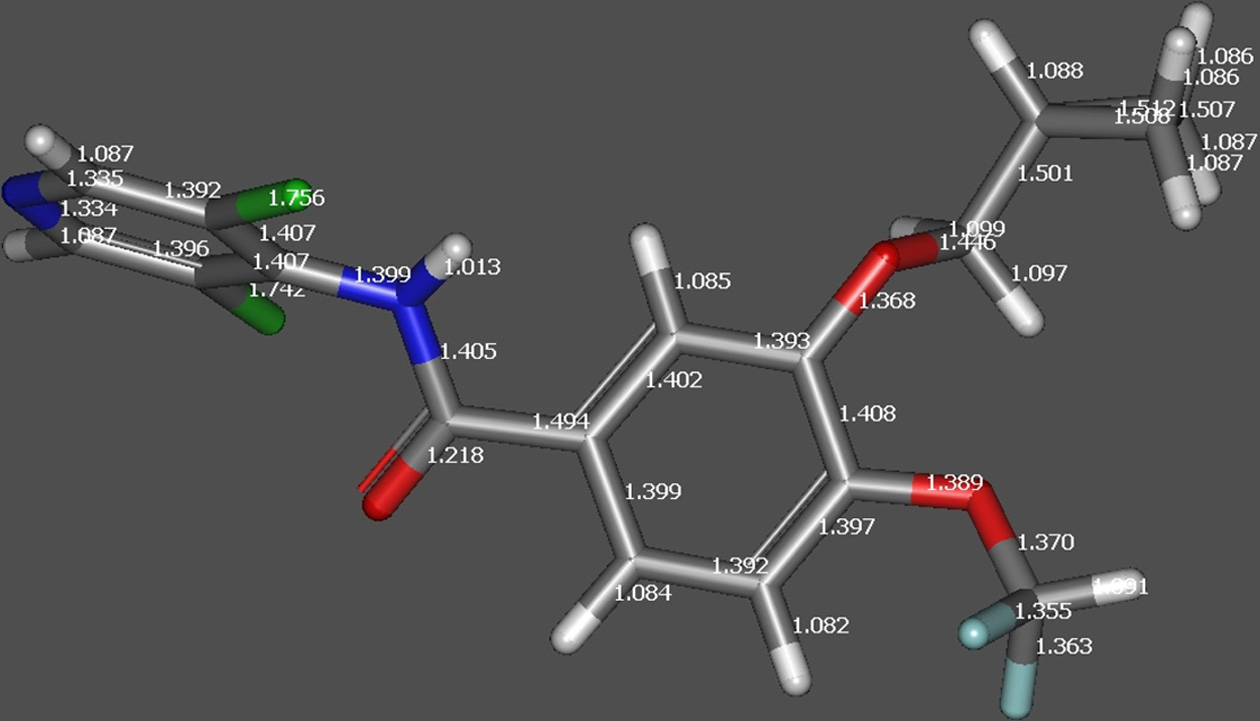
Figure 2a. DFT optimized structure of RFL indicating bond
potential protonation sites in hartee units. (Hartree is the atomic
length before protonation.
unit of energy; Hartrees are converted into kcal mol-1 bymultiplying by 627.503).
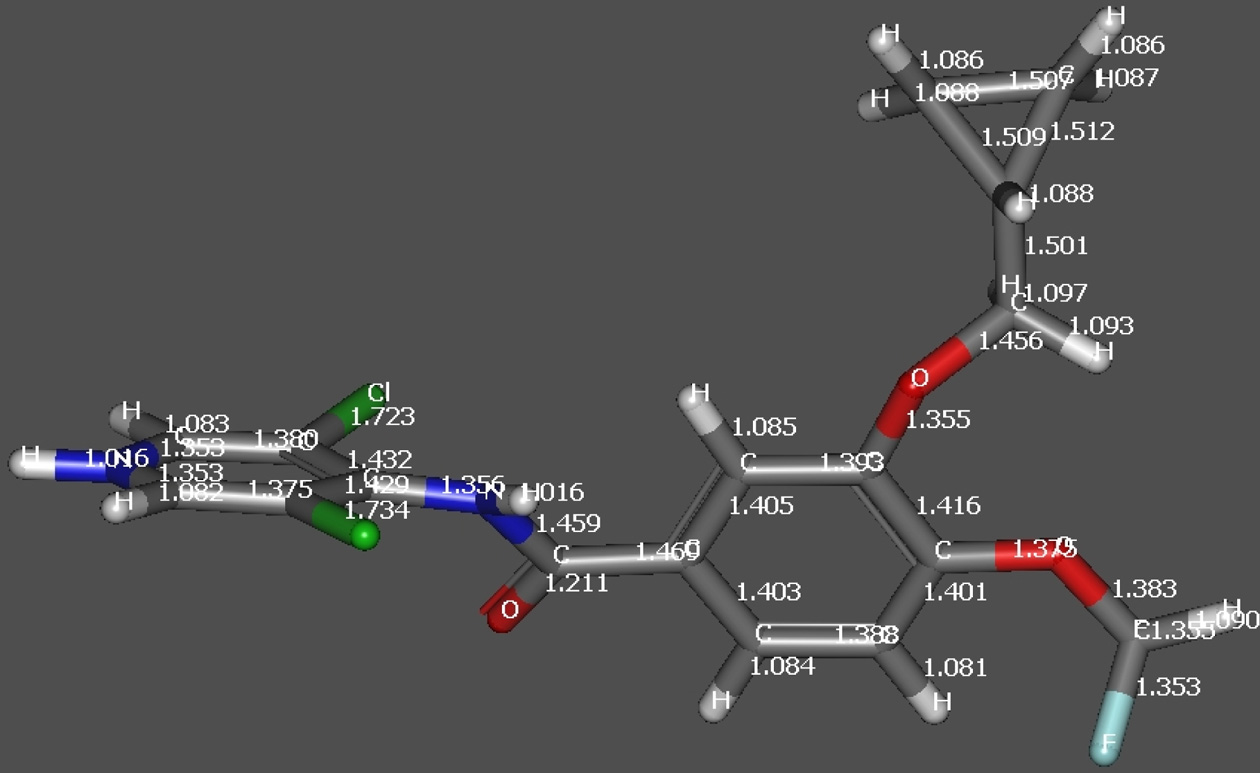
underwent loss of methylene cyclopropane (C4H6, 54.047Da) to generate fragment ion at m/z 187.0213 Da. DFToptimized structure of the protonated molecular ion ofroflumilast also indicated elongation of C-N bond asdepicted in Figure 2. The formation product ion at m/z348.9975 Da is due to loss of methylene cyclopropane(C4H6, 54.047 Da), which in turn under goes rearrangementwith a neutral loss of 185 Da to produce the cation of 3,5-dichloropyridin-4-ol.The schematic presentation of thefragmentation profile of roflumilast is shown in Figure 3a.
DP-1 formed during alkaline degradation showed its
protonated molecular ion at m/z 162.9821 Da and underwent
Figure 2b. DFT optimized structure of RFL indicating change in
fragmentation by losing a molecule of hydrochloric acid to
bond length after protonation.
produce a product ion at m/z 90.9777 Da, as shown inFigure 3b. Based on the mass spectra data DP-1 identifiedas 3, 5-dichloropyridin-4-amine.
evidently supported it to be N-(3,5-dichloropyridin-4-yl)-4-
DP-2 with a measured accurate mass of m/z 348.9946 Da
(difluoro methoxy)-3-hydroxybenzamide, formed by the loss
formed during acidic as well as oxidative stress conditions.
of methyl cyclopropane moiety from roflumilast.
The characteristic isotopic pattern of two chlorine atoms in the
DP-3 was formed under alkaline as well as oxidative stress
mass spectra and product ion spectra containing diagnostic
conditions and showed its protonated molecular ion at m/z
fragment ions of m/z 163.9676 Da, 313.0208 Da (Figure 3c)
353.0453 Da with characteristic isotopic pattern of two
Mass Spectrom. Lett. 2015 Vol. 6, No. 2, 38–42
Identification of Degradation Products in the Phosphodiesterase (PDE-4) Inhibitor Roflumilast
Figure 3a. Plausible fragmentation pathway of roflumilast.
with that of RFL and exhibited similar neutral losses. Basedon this the most possible structure of DP-4 is proposed to bethe N-oxide of roflumilast. The pathway depicting theformation of product ions is shown in Figure 3e.
Figure 3b. Plausible fragmentation pathway of DP-1.
Roflumilast was subjected to stress study and found to be
chlorine atoms. The diagnostic product ions formed at m/z
sensitive in alkaline, acidic and oxidative environment. A
162.9838 Da and 191.0718 Da by the neutral loss of 3-
rationalized approach based on DFT and comparative high
resolution mass spectral analysis with product ion profiling
and 3,5-dichloropyridin-4-amine evidently supported it to be
was used for rapid identification of the degradation
formed by the loss of CHF2 side chain from the molecule of
products. The four degradation products have been identified
roflumilast. Based on this mass spectral analysis as discussed
above and depicted in Figure 3d, the most plausible structure
of DP-3 has been proposed as N-(3,5-dichloropyridin-4-yl)-3-
During oxidative stress study degradant formed with an
accurate mass of m/z 419.0361 Da which is 16 Da higherthan that of RFL and showed isotopic pattern confirming
the presence of two chlorine atoms, is assigned the codename DP-4. The product ions formed in DP-4 are identical
The authors acknowledge the support of Dr. Naresh K.
Figure 3c. Plausible fragmentation pathway of DP-2.
Mass Spectrom. Lett. 2015 Vol. 6, No. 2, 38–42
Saroj Kumar Paul and Upendra N. Dash
Figure 3d. Plausible fragmentation pathway of DP-3.
Figure 3e. Plausible fragmentation pathway of DP-4.
Jena of Stockholm University for DFT calculations, Mr.
6. ICH, Impurities in New Drug Substances Q3A (R2),
Subhrajit Rout of Indian Institute of Technology, Kanpur
International Conference on Harmonization, IFPMA,
and, Thermo fisher application canter, Mumbai for carrying
Geneva. 2006.
out the mass spectral analysis.
7. Tarek, S. B.; Hytham, M. A.; Mohamed, S. M.; Hoda, G.
D.; Mostafa, M. B. Bulletin of Faculty of Pharmacy Cairo
University 2014, 5279.
8. Barhate, V. D.; Deosthalee, P. Indian J. Pharm. Sci. 2010,
1. Beghe, B.; Rabe, K. F.; Fabbri, L. M. American Journal
of Respiratory and Critical Care Medicine 2013, 188,
9. Sun, C.; Lin, Y.; Sun, M.; Wang, D.; Chen, L. Research
on Chemical Intermediates 2013, 1.
2. Giembycz, Mark, A.; Stephen K. F. Drug Design,
10. Zhang, H.; Lin, Y.; Li, Y.; Dong, K.; Chen, L.; Wang, D.
Development and Therapy 2010, 4,147.
Journal of Chemical Research 2014, 38, 507.
3. Boland, S.; Alen, J.; Bourin, A.; Castermans, K.;
11. Lin, Y.; Huang, P.; Qi, H.; Chen, L.; Wang, D. Research
Boumans, N.; Panitti, L.; Vanormelingen, J.; Leysen, D.;
on Chemical Intermediates 2013,39, 3111.
Defert, O. Bioorg. Med. Chem. Lett. 2014, http://
12. Alex, A.; Harvey, S.; Parsons, T.; Pullen, F. S.; Wright, P.;
Riley, J. A. Rapid Commun. Mass Spectrom. 2009, 23,
4. ICH, International Conference on Harmonization
Guidance for Industry, Impurities in New Drug Products
13. Wright, P.; Alex, A.; Nyaruwata1, T.; Parsons, T.; Pullen,
Q3B (R2). 2006.
F. Rapid Commun. Mass Spectrom. 2010, 24, 1025.
5. ICH, Stability Testing of New Drug Substances and
14. Wright, P.; Alex, A.; Pullen, F. S. Rapid Commun. Mass
Products Q1A (R2), International Conference on
Spectrom. 2014, 28, 1127.
Harmonization, IFPMA, Geneva. 2003.
Mass Spectrom. Lett. 2015 Vol. 6, No. 2, 38–42
Source: http://www.kosfa.kr/notReviewEpaperFile.do?method=finalDown&journalSeq=J000011&issueSeq=315&serviceEpaperSeq=1677
PDE5-Inhibitoren 2010 Bewährte, neue und Phosphodiesterase-5 (PDE5)-Inhibitoren waren ursprünglich zur Behandlung kardiovaskulärer Krankheiten konzipiert worden. Ihre eigentliche Popularität verdanken sie aber ihrem Erfolg in der Behandlung von Männern mit erektiler Dysfunktion (ED). In den letzten Jahren haben sich PDE5-Inhibitoren über die Behandlung von ED-Patienten hinaus als vielseitiger anwendbar
TOOLS, TIPS & TECHNIQUES The World of Mesotherapy MESOTHERAPY WORLDWIDE 2009 TERMS & CONDITIONS OF USE DISCLAIMER The information regarding procedures, medications or devices presented in this document has been compiled with all care and presented for educational purposes only, and is not intended to represent the only, nor necessarily the best, methods or


