Levitra enthält Vardenafil, das eine kürzere Wirkdauer als Tadalafil hat, dafür aber schnell einsetzt. Männer, die diskret bestellen möchten, suchen häufig nach levitra kaufen ohne rezept. Dabei spielt die rechtliche Lage in der Schweiz eine wichtige Rolle.
00_annrheumdistoc.indd

Ofatumumab, a fully human anti-CD20 monoclonal antibody, in biological-naive, rheumatoid arthritis patients with an inadequate response to methotrexate: a randomised, double-blind, placebo-controlled clinical trial
Peter C Taylor, 1 Emilia Quattrocchi, 2 Stephen Mallett, 2 Regina Kurrasch, 3 Jørgen Petersen, 4 David J Chang 3
▶ An additional supplementary
ABSTRACT
human CD20 molecule, distinct from the epitope
table is published online only.
Objectives To evaluate the effi cacy and safety of
recognised by rituximab 1 or by other anti-CD20
To view this fi le please visit
intravenous ofatumumab, a fully human anti-CD20
mAb. 2 3 The membrane proximity of this epitope
the journal online at ( http://ard.
monoclonal antibody, in biological-naive, active
probably accounts for the high effi ciency of B-cell
rheumatoid arthritis (RA) patients despite methotrexate
killing observed with ofatumumab in both in-vitro
1 Kennedy Institute of
and in-vivo preclinical studies. 4 – 7
Rheumatology Division, Imperial College, London, UK
Methods In this double-blind, placebo-controlled, phase
In animal models, ofatumumab induced selec-
2 GlaxoSmithKline Clinical
III study, active RA patients on stable methotrexate
tive and prolonged B-cell depletion primarily
Development, Stockley Park, UK
were randomly assigned to one course of two infusions
mediated by effective complement-dependent
3 GlaxoSmithKline Clinical
of ofatumumab 700 mg (n=130) or placebo (n=130),
cytotoxicity and antibody-dependent cell-mediated
Development, Upper Merion, Pennsylvania, USA
2 weeks apart. The primary endpoint was the ACR20
cytotoxicity. 8 9 Effective complement-dependent
4 Genmab, Copenhagen,
response at week 24. Secondary endpoints included
cytotoxicity may depend on the distance between
ACR50/70, EULAR response, disease activity score based
the plasma membrane and the constant parts of the
on 28 joints using C-reactive protein, adverse events (AE)
sensitising antibody thus enabling the effi cient and
Correspondence to
and immunogenicity.
rapid engagement of complement activation. 10
Professor Peter Taylor, Kennedy Institute of Rheumatology
Results At week 24, a greater proportion of patients
A phase I/II study of ofatumumab, administered
Division, Imperial College,
on ofatumumab compared with placebo achieved an
as two intravenous infusions of 300, 700 or 1000 mg
ACR20 response (50% vs 27%, p<0.001) and a good or
2 weeks apart, in active rheumatoid arthritis (RA)
[email protected]
moderate EULAR response (67% vs 41%, p<0.001). All
patients with an inadequate response to disease-
Accepted 17 July 2011
other key secondary effi cacy endpoints were signifi cantly
modifying antirheumatic drugs (DMARD), dem-
Published Online First
improved on ofatumumab. Effi cacy observed by 8 weeks
onstrated signifi cant clinical benefi t and reasonable
was sustained throughout the study. The most common
tolerability (improved after the implementation of
AE for ofatumumab versus placebo were rash (21% vs
premedication) at all doses investigated when com-
<1%) and urticaria (12% vs <1%), mostly occurring
pared with placebo, with the 700 mg dose consid-
on the fi rst infusion day. Overall, fi rst-dose infusion
ered to be optimal. 11
reactions were 68% for ofatumumab and 6% for placebo,
To characterise further the effi cacy and safety
mostly mild to moderate; second-dose infusion reactions
profi le of ofatumumab we conducted a placebo-
markedly declined (<1% and 0%). Serious AE were
controlled phase III trial in patients with active RA
reported in 5% of ofatumumab versus 3% of placebo
who had an inadequate response to methotrexate
patients. Infection rates were 32% and 26% (serious
therapy and no previous biological treatment expo-
infections <1% and 2%), respectively. One death
sure. This trial was also designed to investigate the
(interstitial lung disease), unrelated to study drug, was
effects of ofatumumab on the extent and duration
reported on ofatumumab. No antidrug antibodies were
of B-cell depletion, biomarkers of clinical response,
detected in ofatumumab patients.
patient-reported outcomes and immunogenicity.
Conclusions Ofatumumab signifi cantly improved
all clinical outcomes in biological-naive, active RA
patients with no detectable immunogenicity at week 24.
Study design and objectives
No unexpected safety fi ndings were identifi ed.
This was a multicentre, randomised, double-
Trial Registry clinical trials.gov registration number
blind, placebo-controlled, parallel group, phase
III trial. Patients were enrolled at 36 sites in west-ern Europe, eastern Europe, South America and Asia Pacifi c. The trial is registered at clinicaltrials.
Ofatumumab (HuMax-CD20) is a human IgG1ĸ gov number NCT00611455. The fi rst patient was lytic monoclonal antibody (mAb) that specifi cally
enrolled in January 2008 and the last visit for the
binds to the human CD20 antigen inducing potent
double-blind phase was in June 2009. The trial
B-cell lysis. The CD20 antigen is expressed only was conducted in accordance with good clinical by B lymphocytes from the pre-B to the plasma-
practice and the Declaration of Helsinki. All par-
cytoid immunoblast stage. Ofatumumab recogn-
ticipating sites received approval from national,
ises a unique membrane-proximal epitope on the regional, or investigational centre ethics committee
Ann Rheum Dis 2011;70:2119–2125. doi:10.1136/ard.2011.151522
10/28/2011 8:01:42 PM
or institutional review boards; each patient provided written
therapy, other autoimmune diseases, signifi cant concurrent,
informed consent.
uncontrolled medical conditions, neutrophils less than 2×10 9 /l,
The trial included a 24-week double-blind, placebo-controlled
platelets less than 100×10 9 /l, IgG less than 6.94 g/l (below lower
period followed by a 120-week open-label extension and a safety
limits of normal), and positive serology for HIV, hepatitis B or
follow-up. This paper summarises results from the completed,
C infection. Patients were screened for JC virus using PCR for JC
placebo-controlled, 24-week double-blind phase only.
viral DNA (Quest Diagnostics, Van Nuys, CA, USA and Heston,
Eligible patients were randomly assigned (1:1) to receive two
Middlesex, UK ) and were excluded if tested positive.
infusions of either ofatumumab 700 mg or placebo 2 weeks apart (one course), added to their stable background methotrexate
Assessments
dose. Randomisation was stratifi ed by rheumatoid factor (RF)
Clinical assessments of disease activity were performed at
seropositivity/negativity and region. GlaxoSmithKline prepared
baseline and every 4 weeks to week 24 and included an evalu-
a computer-generated randomisation schedule and randomisa-
ation of the 68-joint tender joint count and 66-joint swollen
tion was handled centrally through an interactive voice response
joint count conducted by an independent assessor (blinded to
system. An unblinded pharmacist at each site prepared the infu-
patient-rated outcomes), patient's pain assessment (visual ana-
sions; ofatumumab and saline (placebo) infusions were indis-
logue scale (VAS) 0–100 mm), patient's and physician's global
tinguishable. Other study personnel and patients were blinded
assessment of disease activity (VAS 0–100 mm), HAQ-DI and
to treatment allocation until the double-blind period was com-
levels of acute-phase reactants (ESR and CRP). From these data,
plete. Premedication with antihistamine (certirizine 10 mg or
ACR20, ACR50 and ACR70 response rates, mean change in
equivalent), oral paracetamol 1000 mg and intravenous meth-
DAS28–ESR and DAS28–CRP and EULAR response were deter-
ylprednisolone 100 mg was administered 30 min to 2 h before
mined. FACIT-F and SF-36v2 were assessed at baseline, week
each infusion. Patients who did not respond were allowed non-
biological DMARD rescue treatment from week 16; however,
Laboratory investigations included levels of peripheral
the use of rescue treatment precluded subsequent entry into
B lymphocytes measured by fl uorescence-activated cell sorting
the open-label period. Breakthrough pain management such as
analysis by the surrogate marker CD19, peripheral T lympho-
analgesics, non-steroidal anti-infl ammatory drugs and one intra-
cytes measured by CD3, CD4 and CD8 markers, immunoglob-
articular corticosteroid injection in one joint per 6-month period
ulins (IgA, IgM, IgG), RF and immunoglobulins to RF (IgM–RF,
were allowed. The joint receiving an intra-articular injection
IgG–RF and IgA–RF), anticyclic citrullinated peptide antibodies
was scored as both swollen and tender in joint count assess-
(anti-CCP), acute phase serum amyloid A, interleukin 6 (IL-6)
ments during the following 12-week period.
(all performed by Quest Diagnostics) and antibodies to ofatu-
The primary objective was to evaluate the effi cacy of ofa-
mumab measured using a validated electrochemiluminescence
tumumab compared with placebo based on the proportion of
meso-scale discovery immunoassay. Positive samples from the
patients achieving an American College of Rheumatology (ACR)
binding antibody assay were tested in a neutralising antibody
20 12 response at week 24. Secondary endpoints included propor-
assay (Clinical Immunology, Biopharm R&D, GlaxoSmithKline,
tions of patients achieving ACR50, ACR70, European League
King of Prussia, PA, USA).
Against Rheumatism (EULAR) good or moderate responses, 13
Adverse events (AE) and serious adverse events (SAE) were
and mean changes in the disease activity score based on 28
collected throughout the study and coded using the Medical
joints (DAS28) using C-reactive protein (CRP), 14 health assess-
Dictionary for Regulatory Activities (MedDRA) version 12.
ment questionnaire disability index (HAQ–DI),
Infusion-related events occurring during study drug infusion and
health survey (SF-36v2) and functional assessment of chronic ill-
up to 24 h after completion of the infusion (and likely to repre-
ness therapy–fatigue version 4 (FACIT–F) 16 at week 24.
sent clinical signs and symptoms characteristic of ofatumumab infusion reactions in patients with RA) were identifi ed by a safety review team before unblinding. Low CD19 cell counts
Patient population
were not reported as AE and hospitalisation for completion of
Male and non-pregnant, non-lactating female patients 18 years
an infusion was not reported as a SAE. Infections were deter-
and older, diagnosed with active RA according to ACR 1987
mined using the MedDRA system organ class ‘infections and
criteria 17 (RA functional class I, II or III) of 6 months or more
duration were eligible to participate. Active RA was defi ned as eight or more swollen and eight or more tender joint counts, based on 66/68 joint count; either CRP of 1.0 mg/dl or greater or
Sample size estimation
erythrocyte sedimentation rate (ESR) of 22 mm/h or greater; and
A sample size of 124 subjects per group was estimated to pro-
DAS28 based on ESR of 3.2 or greater. Patients were required to
vide at least 90% power to detect differences in the proportions
have an inadequate response to methotrexate and to be receiv-
of patients achieving an ACR20 response at week 24 between
ing methotrexate 7.5–25 mg/week for at least 12 weeks, and at
ofatumumab versus placebo, at a 5% level of signifi cance. This
a stable dose for at least 4 weeks, before baseline. All patients
was based on a χ 2 test comparing two binomial proportions.
underwent a washout period of at least 4 weeks for all DMARD (lefl unomide ≥12 weeks or administration of cholestyramine
Statistical analysis
treatment for washout according to the manufacturer's instruc-
For categorical endpoints, the effi cacy of ofatumumab ver-
tions) but maintained their concomitant stable methotrexate
sus placebo was analysed using the Cochran Mantel Haenszel
therapy, along with folic acid of 5 mg/week or greater. Oral
test, adjusting for baseline stratifi cation factors, RF status and
corticosteroids (≤10 mg/day of prednisolone equivalent), non-
geographical region (ie, eastern Europe, western Europe, South
steroidal anti-infl ammatory drugs and one intra-articular injec-
America, Asia Pacifi c). For continuous endpoints effi cacy was
tion of corticosteroid (80 mg methylprednisolone or equivalent)
analysed using analysis of covariance, adjusting for RF status,
in a single joint were permitted. Key exclusion criteria com-
geographical region and baseline value. For categorical end-
prised previous exposure to any biological and B-cell-depleting
points, patients who took disallowed medication or withdrew
Ann Rheum Dis 2011;70:2119–2125. doi:10.1136/ard.2011.151522
10/28/2011 8:01:43 PM
from the study were imputed as non-responders. For continuous
RF status, the ACR20 response in ofatumumab and placebo
endpoints, data were imputed by carrying forward the last value
groups, respectively, was 50% (54/108) versus 26% (29/111)
recorded before taking disallowed medication or withdrawal
for seropositive patients and 48% (10/21) versus 30% (6/20)
(last observation carried forward). The intent-to-treat (ITT) pop-
for seronegative patients. When examined by anti-CCP status,
ulation comprised all randomly assigned patients who received at least one infusion of the study drug. The safety population was identical to the ITT population except that patients were
Table 1 Demographics and baseline disease characteristics
analysed according to their actual treatment in case this differed
Characteristic
Ofatumumab 700 mg (n=129)
Placebo (n=131)
from their randomised treatment.
Mean (SD) age, years
Mean (SD) disease
Disposition of patients and baseline characteristics
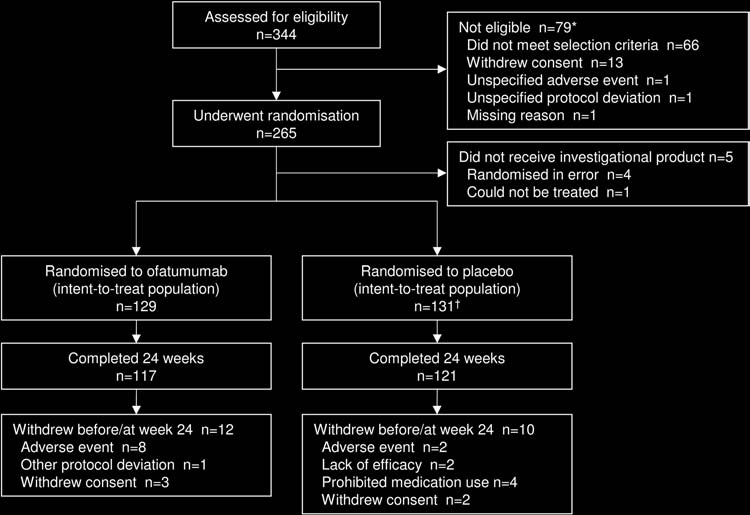
A total of 344 patients was screened and 265 were enrolled and
Median (min, max)
randomly assigned; the reasons for screening failure are shown
methotrexate dose, mg/week
in fi gure 1 . Of the 265 randomly assigned patients, 260 (98%)
Previous DMARD, n (%)
were exposed to investigational product and were included in
the safety and ITT populations ( fi gure 1 ). One patient was ran-
domly assigned to placebo but received ofatumumab and was
Patients receiving oral
included in the placebo group for the ITT population (based on
corticosteroid, n (%)
randomised treatment) and in the ofatumumab group for the
Median (min, max) oral
safety population (based on actual treatment received).
corticosteroid dose, mg/day *
Demographics and baseline RA characteristics were balanced
RF positive, n (%)
between the two groups ( table 1 ). Most patients were women
Median (min, max) CRP, mg/l
Mean (SD) ESR, mm/h
(82%) and RF positive (84%), with a mean age of 53 years. At
Mean (SD) total RF, IU/ml †
baseline, mean RA duration was 8.5 years, mean DAS28–CRP
Mean (SD) SJC (66 joints)
was 5.7 and mean DAS28–ESR was 6.5 ( table 1 ).
Mean (SD) TJC (68 joints)
Mean (SD) DAS28–CRP
Clinical response
Mean (SD) DAS28–ESR
Mean (SD) HAQ–DI
At week 24, a greater proportion of patients administered
Mean (SD) FACIT–F
ofatumumab 700 mg achieved the primary endpoint of ACR20 compared with placebo (50% and 27%, respectively,
* Prednisolone equivalent dose. † At screening.
p<0.001). In addition, signifi cantly greater improvements in
CRP, C-reactive protein; DAS28, disease activity score based on 28 joints; DMARD,
ACR50 and ACR70 were observed with ofatumumab ver-
disease-modifying antirheumatic drug; ESR, erythrocyte sedimentation rate; FACIT–F, functional assessment of chronic illness therapy–fatigue; HAQ–DI, health assessment
sus placebo (ACR50 27% and 11%, p<0.001; ACR70 13%
questionnaire disability index; RF, rheumatoid factor; SJC, swollen joint count;
and 2%, p=0.001) ( table 2 and fi gure 2 ). When examined by
TJC, tender joint count.
Figure 1 Disposition of patients up to week 24. *Patients could have more than one reason for screening failure. †One patient was randomly
assigned to placebo but received ofatumumab. This patient is included in the placebo group for the intent-to-treat population, but in the ofatumumab
group for the safety population.
Ann Rheum Dis 2011;70:2119–2125. doi:10.1136/ard.2011.151522
10/28/2011 8:01:43 PM
Table 2 Summary of disease-activity and quality-of-life clinical endpoints at week 24 for patients receiving ofatumumab or placebo
Ofatumumab 700 mg (n=129)
Placebo (n=131)
OR (95% CI)
2.86 (1.67 to 4.91)
3.29 (1.63 to 6.62)
6.63 (1.87 to 23.51)
EULAR response *
3.07 (1.82 to 5.18)
Clinical remission †
2.09 (0.76 to 5.77)
HAQ–DI response ‡
1.65 (1.01 to 2.70)
Ofatumumab 700 mg (n=129)
Placebo (n=131)
Adjusted mean difference (95% CI)
DAS28–CRP § Baseline, mean (SD)
Week 24, mean (SD)
Adjusted mean (SE) change
−1.00 (−1.29 to −0.72)
DAS28–ESR § Baseline, mean (SD)
Week 24, mean (SD)
Adjusted mean (SE) change
−0.99 (−1.29 to −0.69)
HAQ–DI § Baseline, mean (SD)
Week 24, mean (SD)
Adjusted mean (SE) change
−0.22 (−0.37 to −0.07)
FACIT–F ¶ Baseline, mean (SD)
Week 24, mean (SD)
Adjusted mean (SE) change
3.75 (1.11 to 6.39)
SF-36 physical component summary score * * Baseline, mean (SD)
Week 24, mean (SD)
Adjusted mean (SE) change
2.48 (0.51 to 4.45)
SF-36 mental component summary score * * Baseline, mean (SD)
Week 24, mean (SD)
Adjusted mean (SE) change
3.03 (0.61 to 5.46)
ACR20/50/70, 20%/50%/70% improvement as per American College of Rheumatology (ACR) criteria. * EULAR response of moderate or good, based on DAS28–CRP. † DAS28–CRP score less than 2.6. ‡ Change from baseline HAQ–DI score of 0.22 or greater. § Negative change represents an improvement. Patient numbers assessed: placebo (n=130); ofatumumab (n=126). * * Patient numbers assessed: placebo (n=117); ofatumumab (n=116). ¶ Patient numbers assessed: placebo (n=111); ofatumumab (n=114). Results are reported in accordance with EULAR/ACR collaborative recommendations. 33 CRP, C-reactive protein; DAS28, disease activity score based on 28 joints; EULAR, European League Against Rheumatism; ESR, erythrocyte sedimentation rate; FACIT–F, functional assessment of chronic illness therapy–fatigue; HAQ–DI, health assessment questionnaire disability index; SF-36, short-form health survey.
the ACR20 response in ofatumumab and placebo groups,
group, CD19 cells increased by 3% at week 24. In the ofatu-
respectively, was 50% (56/111) versus 26% (29/113) for sero-
mumab group one patient (1%) had a CD19 B-cell count equal
positive patients and 44% (7/16) versus 31% (5/16) for sero-
to or greater than the lower limit of normal (0.11 GI/l) or the
negative patients.
baseline value at week 24. No trend for a change in periph-
All other secondary effi cacy endpoints, including EULAR
eral CD3, CD4 or CD8 T-cell counts was observed in either
response and mean change from baseline in DAS28–CRP and
DAS28–ESR were signifi cantly improved in the ofatumumab group compared with placebo, except for clinical remission
Biomarkers and assessment of immunogenicity
(DAS28–CRP <2.6) ( table 2 and supplementary table S1, avail-
At week 24, the median change from baseline in absolute bio-
able online only). The mean change from baseline in DAS28–CRP
marker levels for ofatumumab and placebo, respectively, were:
scores over the 24-week period showed sustained improvement
−4.0 versus −0.4 ng/l for IL-6; −53.5 versus −10.9 mg/ml for
compared with placebo from week 8 to week 24 ( fi gure 3 ).
serum amyloid A; −138 versus 0 units for anti-CCP; −6.3 versus
Ofatumumab also provided improvements in patient-reported
0.0 units for RF–IgM; −4.8 versus 0.0 units for RF–IgG and −3.0
outcomes as shown by signifi cantly greater changes from base-
versus 0.0 units for RF–IgA. At week 24, the median change
line in HAQ–DI, FACIT–F and SF36v2 scores compared with
from baseline in immunoglobulin levels for ofatumumab and
placebo ( table 2 ).
placebo, respectively, were: −1.30 versus −0.60 g/l for IgG; −0.30 versus −0.02 g/l for IgM and −0.23 versus −0.09 g/l for
Laboratory fi ndings
IgA. A small number of patients on ofatumumab or placebo had
Peripheral B lymphocytes (CD19) were greatly reduced at each
immunoglobulins equal to or less than the lower limit of nor-
visit relative to baseline in the ofatumumab group: median
mal during the study (IgM 6 vs 4; IgG 3 vs 1; IgA, no patients,
reductions at weeks 2 (before the second infusion), 4, 12 and
respectively). No patients treated with ofatumumab developed
24 were 95%, 96%, 96% and 94%, respectively. In the placebo
detectable anti-ofatumumab antibodies at week 24.
Ann Rheum Dis 2011;70:2119–2125. doi:10.1136/ard.2011.151522
10/28/2011 8:01:44 PM
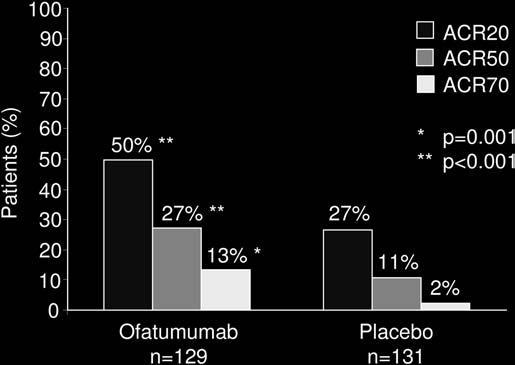
Table 3 Safety of placebo and ofatumumab over 24 weeks (safety
population)
Patients with AE, n (%)
Ofatumumab 700 mg
Placebo (n=130)
Total patient-years of exposure
Any SAE (fatal or non-fatal) *
AE leading to discontinuation of IP or
withdrawal from studyMost common AE (≥5% in either group) Rash
Urinary tract infection
Figure 2 ACR 20/50/70 responses at week 24 in patients receiving
Throat irritation
ofatumumab 700 mg or placebo. ACR20/50/70, 20%/50%/70%
Hypersensitivity
improvement as per American College of Rheumatology (ACR) criteria.
Infusion reactions
Any AE on day of fi rst infusion
Infusion-related
The overall incidence of AE was 89% and 55% in ofatumumab
Any AE on day of second infusion
and placebo groups, respectively ( table 3 ). Within the ofatu-
Infusion-related
mumab group the most commonly reported AE were rash
Patients with an infection
(21%) and urticaria (12%); these events mostly occurred on the
No of infections
Infections/100 patient-years (95% CI)
105.26 (81.86 to
day of fi rst infusion (19% and 12%, respectively). The propor-
(58.07 to 103.82)
tion of patients experiencing an infusion-related AE on the day
Patients with a serious infection
of fi rst infusion was 68% for ofatumumab and 6% for placebo;
No of infections
infusion-related AE on the day of second infusion markedly
Infections/100 patient-years (95% CI)
1.75 (0.42 to 9.77)
declined (<1% and 0%, respectively). Most AE were of mild or
moderate intensity. Severe AE were reported for 8% of patients
Cardiac disorders
on ofatumumab and 2% on placebo, with 5% and less than 1%
occurring on the day of fi rst infusion. AE leading to withdrawal
Vascular disorders
were 9% for ofatumumab and less than 1% for placebo. Four
SAE (bacterial gastroenteritis, pneumonia, myocardial infarc-
tion, ischaemic stroke) were reported for four patients (3%) in
Neoplasms (benign or malignant)
the placebo group and seven SAE (angioedema, interstitial lung
disease (fatal), synovitis, pulmonary embolism, diarrhoea and
pneumonia, pericardial effusion) were reported for six patients
* SAE were bacterial gastroenteritis, pneumonia, myocardial infarction, ischaemic
(5%) in the ofatumumab group. Two of these SAE (angioedema,
stroke in the placebo group and angioedema, interstitial lung disease (fatal, unrelated
pneumonia), both in the ofatumumab group, were considered
to ofatumumab), synovitis, pulmonary embolism, diarrhoea and pneumonia, pericardial effusion in the ofatumumab group.
by the investigator to be related to the investigational product.
† Infusion-related reactions (events likely to represent clinical signs and symptoms
There was one fatal SAE of interstitial lung disease in the ofatu-
characteristic of ofatumumab infusion reactions in patients with RA) were identifi ed by a safety review team before unblinding.
mumab group, which was not considered by the investigator to
AE, adverse event; IP, investigational product; RA, rheumatoid arthritis; SAE, serious
be related to the investigational product but to RA worsening.
AE within the system organ class of infections and infesta-
tions were reported for 26% and 32% of patients on placebo and ofatumumab, respectively. None of the events was of severe
of ofatumumab in a well-defi ned RA patient population with
intensity and none led to discontinuation of the investigational
long-standing disease not controlled by standard methotrexate
product or withdrawal from the study. One SAE of pneumonia
therapy and not previously treated with other available bio-
was reported in each group and one SAE of bacterial gastroen-
logical DMARD therapies. Ofatumumab, at a dose of 700 mg
teritis was reported on placebo ( table 3 ). No serious opportunistic
administered twice, added to a background stable dose of meth-
infections and no cases of progressive multifocal leukoencephal-
otrexate therapy, demonstrated a signifi cantly greater ACR20
opathy were reported. Two neoplasms, both prostatic adenoma
response at week 24 (primary endpoint) compared with placebo.
and both in the placebo group, were reported.
Signifi cantly greater improvements were observed in key sec-ondary endpoints such as ACR50, ACR70, change from baseline in both DAS28–CRP and DAS28–ESR, EULAR response, physi-
DISCUSSION
cal function (HAQ–DI) and fatigue (FACIT–F).
The results from the 24-week, placebo-controlled, double-blind
Effi cacy data from seronegative RA patients, for either RF or
phase of this trial confi rm the previously reported effi cacy of
anti-CCP, as observed in the study, should be interpreted with
one course of intravenous ofatumumab in active RA. 11 In addi-
caution because of the small sample size. Data from a number of
tion, this study provides further information on the effi cacy
clinical trials with rituximab in a range of RA populations seem
Ann Rheum Dis 2011;70:2119–2125. doi:10.1136/ard.2011.151522
10/28/2011 8:01:44 PM
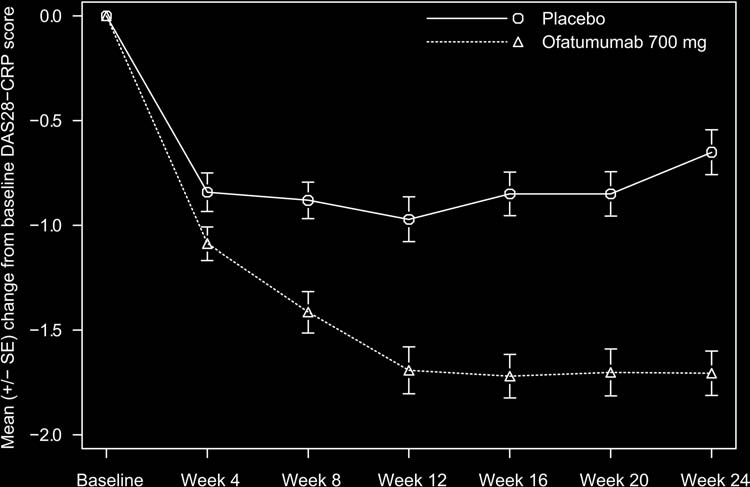
Figure 3 Mean change from baseline for DAS28 using CRP over time. CRP, C-reactive protein; DAS28, disease activity score based on 28 joints.
to suggest that seropositive patients (RF and/or anti-CCP) have
time after receiving rituximab. 26 There was no reported correla-
a higher likelihood of response to B-cell-depleting therapy com-
tion between the development of these human antichimeric anti-
pared with seronegative patients, in particular for improving
bodies and safety or effi cacy; nevertheless, the possibility of loss
signs and symptoms; 18 – 22 however, the statistical signifi cance
or reduction of effi cacy, local reactions, serum sickness/immune
of these fi ndings remains to be determined. Radiographic end-
complex-mediated disease and major allergic reactions (eg, urti-
points were not assessed in this ofatumumab trial and data are
caria, bronchospasm, bronchoconstrictions) is well recognised. 30
not currently available for the persistence of response beyond
In addition to its effi cacy in RA demonstrated in this trial, ofatu-
mumab is approved for the treatment of refractory chronic lym-
The safety information gathered in this study is consistent
phocytic leukaemia. 31 The mechanism of B-cell tumour lysis is
with that observed in short-term studies of rituximab in active
probably through the activation of both complement-dependent
RA. 18 23 24 Although a greater proportion of patients receiving
cytotoxicity and antibody-dependent cell-mediated cytotoxicity.
ofatumumab experienced mild to moderate infusion-related
Compared with rituximab, ofatumumab demonstrates increased
reactions on the day of fi rst infusion, despite steroid premedi-
binding of C1q and more potent complement-dependent cyto-
cation, less than 1% of ofatumumab patients experienced an
toxicity, even in chronic lymphocytic leukaemia cells with low
infusion-related reaction on the day of second infusion. This
CD20 expression levels. 32 It is unknown at this time, however,
fi nding may be explained by the sudden cytokine release that
whether these mechanistic differences can translate to improved
follows the pronounced B-cell lysis occurring after CD20 liga-
safety, tolerability, effi cacy, or potency over rituximab.
tion, as previously reported in non-Hodgkin's lymphoma
In summary, ofatumumab is a fully human mAb binding an
patients treated with rituximab. 25 The rate of serious infections
epitope of CD20 distinct to that recognised by rituximab. A sin-
in patients treated with ofatumumab was low and comparable
gle course of two infusions of 700 mg was effi cacious and safe
to placebo. Although progressive multifocal leukoencephalop-
in biological-naive, active RA patients on background metho-
athy has been reported with rituximab, 26 no such cases were
trexate up to 24 weeks after treatment. As expected for a fully
identifi ed with ofatumumab in the current small study of lim-
human mAb, ofatumumab did not induce immunogenicity.
ited duration, which excluded patients who screened positive for JC virus DNA at baseline (one patient excluded). Similarly,
Acknowledgments The authors would like to thank all participating investiga-
serious opportunistic infections were reported in ocrelizumab
tors and their staff who provided and cared for patients and collected data for the study: Argentina: E Lucero, O Rillo, D Siri, I Strusberg, J Velasco; Australia: S Hall,
trials, but were not observed in this study. 27 28
P Nash, P Youssef; Chile: M Aliste, R Jimenez, P Miranda, F Radrigan, N Stevensen;
Ofatumumab is a fully human mAb, thereby offering a low
Czech Republic: L Bortlik, J Vencovsky, P Vitek; Hungary: B Rojkovich, F Szanyó,
immunogenicity potential. Although all patients in the study
E Vereckei; Peru: E Cabello, O Castañeda; Poland: S Jeka, A Racewicz, Z Ruzga,
were on methotrexate, which may suppress the development of
A Sawicki; Romania: C Zainea; Russian Federation: O Ershova, O Lesnyak, E Nasonov, T Polikarpova, A Rebrov; Spain: RA Blanco, VR Valverde, MI Oyarzabal, FN Sarabia;
antidrug antibodies, 29 and patients only received one course of
UK: A Hammond, T Sheeran.
treatment over 24 weeks, no anti-ofatumumab antibodies were detectable in any of the treated patients. In contrast, 7.9% and
Funding This study was sponsored and funded by Genmab and GlaxoSmithKline
under a collaborative agreement.
5.4% of RA patients who received the chimeric mAb rituximab 500 mg administered twice and 1000 mg administered twice,
Competing interests Genmab and GlaxoSmithKline provided fi nancial support
to the institutions that participated in this study. PCT has received research
respectively, in a study of a similar patient population, developed
grants from Merck, UCB, AstraZeneca, GlaxoSmithKline and Roche and has
human antichimeric antibodies at 24 weeks. 19 Overall, 11% of
been an invited speaker and advisor for Abbott, Bristol-Myers Squibb, Centocor,
patients with RA have tested positive for auto-antibodies at any
Roche, Schering-Plough, Wyeth (acquired by Pfi zer in October 2009), Genmab,
Ann Rheum Dis 2011;70:2119–2125. doi:10.1136/ard.2011.151522
10/28/2011 8:01:45 PM
GlaxoSmithKline and UCB. EQ, SM, RK and DJC are employees of GlaxoSmithKline
longitudinal study of patients with rheumatoid arthritis. Arthritis Rheum
and own stock in GlaxoSmithKline. JP was formerly employed at Genmab and
1995 ; 38 : 44 – 8 .
owned Genmab stocks.
Felson DT, Anderson JJ, Boers M, et al. The American College of Rheumatology
preliminary core set of disease activity measures for rheumatoid arthritis clinical
Patient consent Obtained.
trials. The Committee on Outcome Measures in Rheumatoid Arthritis Clinical Trials.
Contributors Helle Kastberg and Soren Tamer (Genmab) provided advice on
Arthritis Rheum 1993 ; 36 : 729 – 40 .
study design. Peter Critchley, Shilpa Vadher and Janet Perkins (GlaxoSmithKline)
Cella D, Yount S, Sorensen M, et al. Validation of the Functional Assessment of
provided clinical operations, data management and programming support. EQ
Chronic Illness Therapy Fatigue Scale relative to other instrumentation in patients
(GlaxoSmithKline), clinical investigation leader and medical monitor of this trial,
with rheumatoid arthritis. J Rheumatol 2005 ; 32 : 811 – 19 .
authored the manuscript. Editorial support (writing assistance, assembling tables
Arnett FC, Edworthy SM, Bloch DA, et al. The American Rheumatism Association
and fi gures, collating author comments, grammatical editing, and referencing) was
1987 revised criteria for the classifi cation of rheumatoid arthritis. Arthritis Rheum
provided by Julie Taylor of Peak Biomedical Ltd, Macclesfi eld, UK and was funded by
1988 ; 31 : 315 – 24 .
GlaxoSmithKline.
Cohen SB, Emery P, Greenwald MW, et al. Rituximab for rheumatoid arthritis
refractory to anti-tumor necrosis factor therapy: results of a multicenter, randomized,
Ethics approval All participating sites received approval from national, regional,
double-blind, placebo-controlled, phase III trial evaluating primary effi cacy and safety
or investigational centre ethics committee or institutional review boards, as
at twenty-four weeks. Arthritis Rheum 2006 ; 54 : 2793 – 806 .
Emery P, Deodhar A, Rigby WF, et al. Effi cacy and safety of different doses and
Provenance and peer review Not commissioned; externally peer reviewed.
retreatment of rituximab: a randomised, placebo-controlled trial in patients who are biological naive with active rheumatoid arthritis and an inadequate response to methotrexate (Study Evaluating Rituximab's Effi cacy in MTX iNadequate rEsponders
REFERENCES
(SERENE)). Ann Rheum Dis 2010 ; 69 : 1629 – 35 .
Teeling JL, Mackus WJ, Wiegman LJ, et al. The biological activity of human
Rubbert-Roth A, Tak PP, Zerbini C, et al. Effi cacy and safety of various repeat
CD20 monoclonal antibodies is linked to unique epitopes on CD20. J Immunol
treatment dosing regimens of rituximab in patients with active rheumatoid
2006 ; 177 : 362 – 71 .
arthritis: results of a phase III randomized study (MIRROR). Rheumatology (Oxford)
Genovese MC, Kaine JL, Lowenstein MB, et al. Ocrelizumab, a humanized
2010 ; 49 : 1683 – 93 .
anti-CD20 monoclonal antibody, in the treatment of patients with rheumatoid
Tak PP, Rigby WF, Rubbert-Roth A, et al. Inhibition of joint damage and improved
arthritis: a phase I/II randomized, blinded, placebo-controlled, dose-ranging study.
clinical outcomes with rituximab plus methotrexate in early active rheumatoid
Arthritis Rheum 2008 ; 58 : 2652 – 61 .
arthritis: the IMAGE trial. Ann Rheum Dis 2011 ; 70 : 39 – 46 .
Goldenberg DM, Rossi EA, Stein R, et al. Properties and structure–function
Isaacs JD, Olech E, Tak PP, et al. Autoantibody-positive rheumatoid arthritis (RA)
relationships of veltuzumab (hA20), a humanized anti-CD20 monoclonal antibody.
patients (pts) have enhanced clinical response to rituximab (RTX) when compared
Blood 2009 ; 113 : 1062 – 70 .
with seronegative patients [abstract] . Ann Rheum Dis 2009 ; 68 ( Suppl3 ): 442 .
Teeling JL, French RR, Cragg MS, et al. Characterization of new human CD20
Edwards JC, Szczepanski L, Szechinski J, et al. Effi cacy of B-cell-targeted
monoclonal antibodies with potent cytolytic activity against non-Hodgkin lymphomas.
therapy with rituximab in patients with rheumatoid arthritis. N Engl J Med
Blood 2004 ; 104 : 1793 – 800 .
2004 ; 350 : 2572 – 81 .
Cragg MS, Walshe CA, Ivanov AO, et al. The biology of CD20 and its potential as a
Emery P, Fleischmann R, Filipowicz-Sosnowska A, et al. The effi cacy and safety
target for mAb therapy. Curr Dir Autoimmun 2005 ; 8 : 140 – 74 .
of rituximab in patients with active rheumatoid arthritis despite methotrexate
Glennie MJ, French RR, Cragg MS, et al. Mechanisms of killing by anti-CD20
treatment: results of a phase IIB randomized, double-blind, placebo-controlled,
monoclonal antibodies. Mol Immunol 2007 ; 44 : 3823 – 37 .
dose-ranging trial. Arthritis Rheum 2006 ; 54 : 1390 – 400 .
Bleeker WK, Munk ME, Mackus WJ, et al. Estimation of dose requirements for
van der Kolk LE, Grillo-López AJ, Baars JW, et al. Complement activation
sustained in vivo activity of a therapeutic human anti-CD20 antibody. Br J Haematol
plays a key role in the side-effects of rituximab treatment. Br J Haematol
2008 ; 140 : 303 – 12 .
2001 ; 115 : 807 – 11 .
Beum PV, Lindorfer MA, Beurskens F, et al. Complement activation
Genentech . Rituxan (rituximab) Prescribing Information, 2011 . http://www.gene.com/
on B lymphocytes opsonized with rituximab or ofatumumab produces
gene/products/information/pdf/rituxan-prescribing.pdf ( accessed 26 January 2011 ).
substantial changes in membrane structure preceding cell lysis. J Immunol
Emery P, Rigby WFC, Tak PP, et al. Serious infections with ocrelizumab in rheumatoid
2008 ; 181 : 822 – 32 .
arthritis: pooled results from double-blind periods of the ocrelizumab phase III RA
Pawluczkowycz AW, Beurskens FJ, Beum PV, et al. Binding of submaximal C1q
program [abstract] . Arthritis Rheum 2010 ; 62 ( Suppl 10 ): 414 .
promotes complement-dependent cytotoxicity (CDC) of B cells opsonized with
Rigby WFC, Tony HPT, Oelke KR, et al. Effi cacy and safety of ocrelizumab in
anti-CD20 mAbs ofatumumab (OFA) or rituximab (RTX): considerably higher levels of
patients with active rheumatoid arthritis who had an inadequate response to
CDC are induced by OFA than by RTX. J Immunol 2009 ; 183 : 749 – 58 .
methotrexate: results from the phase III STAGE trial [abstract] . Arthritis Rheum
Saphire EO, Stanfi eld RL, Crispin MD, et al. Contrasting IgG structures reveal
2010 ; 62 ( Suppl 10 ): 383 .
extreme asymmetry and fl exibility. J Mol Biol 2002 ; 319 : 9 – 18 .
Maini RN, Breedveld FC, Kalden JR, et al. Therapeutic effi cacy of multiple
Østergaard M, Baslund B, Rigby W, et al. Ofatumumab, a human anti-CD20
intravenous infusions of anti-tumor necrosis factor alpha monoclonal antibody
monoclonal antibody, for treatment of rheumatoid arthritis with an inadequate
combined with low-dose weekly methotrexate in rheumatoid arthritis.
response to one or more disease-modifying antirheumatic drugs: results of a
Arthritis Rheum 1998 ; 41 : 1552 – 63 .
randomized, double-blind, placebo-controlled, phase I/II study. Arthritis Rheum
European Medicines Agency . Draft Guideline on Immunogenicity Assessment
2010 ; 62 : 2227 – 38 .
of Monoclonal Antibodies Intended for In Vivo Clinical Use. EMA/CHMP/
Felson DT, Anderson JJ, Boers M, et al. American College of Rheumatology.
BMWP/86289/2010 , 2010 . http://www.ema.europa.eu/docs/en_GB/document_
Preliminary defi nition of improvement in rheumatoid arthritis. Arthritis Rheum
library/Scientifi c_guideline/2010/11/WC500099362.pdf ( accessed 26 January 2011 ).
1995 ; 38 : 727 – 35 .
GlaxoSmithKline . Arzerra (ofatumumab) Prescribing Information, 2010 . http://us.gsk.
van Gestel AM, Prevoo ML, van ‘t Hof MA, et al. Development and validation
com/products/assets/us_arzerra.pdf ( accessed 26 January 2011 ).
of the European League Against Rheumatism response criteria for rheumatoid
Wierda WG, Kipps TJ, Mayer J, et al. Ofatumumab as single-agent CD20
arthritis. Comparison with the preliminary American College of Rheumatology and
immunotherapy in fl udarabine-refractory chronic lymphocytic leukemia. J Clin Oncol
the World Health Organization/International League Against Rheumatism Criteria.
2010 ; 28 : 1749 – 55 .
Arthritis Rheum 1996 ; 39 : 34 – 40 .
Aletaha D, Landewe R, Karonitsch T, et al. Reporting disease activity in clinical trials
Prevoo ML, van ‘t Hof MA, Kuper HH, et al. Modifi ed disease activity scores that
of patients with rheumatoid arthritis: EULAR/ACR collaborative recommendations.
include twenty-eight-joint counts. Development and validation in a prospective
Ann Rheum Dis 2008 ; 67 : 1360 – 4 .
Ann Rheum Dis 2011;70:2119–2125. doi:10.1136/ard.2011.151522
10/28/2011 8:01:45 PM
Ofatumumab, a fully human anti-CD20
monoclonal antibody, in biological-naive,
rheumatoid arthritis patients with an
inadequate response to methotrexate: a
randomised, double-blind,
placebo-controlled clinical trial
Peter C Taylor, Emilia Quattrocchi, Stephen Mallett, et al.
Ann Rheum Dis 2011 70: 2119-2125 originally published online August22, 2011doi: 10.1136/ard.2011.151522
Updated information and services can be found at:
These include:
"Web Only Data"
This article cites 30 articles, 12 of which can be accessed free at:
Open Access
This paper is freely available online under the BMJ Journals unlockedscheme, see http://ard.bmj.com/info/unlocked.dtl
Receive free email alerts when new articles cite this article. Sign up in
the box at the top right corner of the online article.
To request permissions go to:
To order reprints go to:
To subscribe to BMJ go to:
Source: http://www.protocolosreumatologia.cl/sitio/descargas/OFATUMUMAB.pdf
Dambuza et al. Malar J (2015) 14:505 Open Access Antiplasmodial activity, in vivo pharmacokinetics and anti-malarial efficacy evaluation of hydroxypyridinone hybrids in a mouse modelNtokozo S. Dambuza1*, Peter Smith1, Alicia Evans1, Jennifer Norman1, Dale Taylor1, Andrew Andayi2, Timothy Egan2, Kelly Chibale2 and Lubbe Wiesner1 Abstract Background: During the erythrocytic stage in humans, malaria parasites digest haemoglobin of the host cell, and the toxic haem moiety crystallizes into haemozoin. Chloroquine acts by forming toxic complexes with haem mol-ecules and interfering with their crystallization. In chloroquine-resistant strains, the drug is excluded from the site of action, which causes the parasites to accumulate less chloroquine in their acid food vacuoles than chloroquine-sen-sitive parasites. 3-Hydroxylpyridin-4-ones are known to chelate iron; hydroxypyridone-chloroquine (HPO-CQ) hybrids were synthesized in order to determine whether they can inhibit parasites proliferation in the parasitic digestive vacuole by withholding iron from plasmodial parasite metabolic pathway.Methods: Two HPO-CQ hybrids were tested against Plasmodium falciparum chloroquine-sensitive (D10 and 3D7) and -resistant strains (Dd2 and K1). The pharmacokinetic properties of active compounds were determined using a mouse model and blood samples were collected at different time intervals and analysed using LC–MS/MS. For in vivo efficacy the mice were infected with Plasmodium berghei in a 4-day Peters' test. The parasitaemia was determined from day 3 and the course of the infection was followed by microscopic examination of stained blood films every 2–3 days until a rise in parasitaemia was observed in all test subjects.Results: IC50 values of the two compounds for sensitive and resistant strains were 0.064 and 0.047 µM (compound
University at Albany, State University of New York Thyroid Function and Perfluoroalkyl Acids in Children Living Near a Chemical PlantMaria-Jose Lopez-Espinosa Debapriya MondalUniversity of Salford, [email protected] Ben ArmstrongLondon School of Hygiene & Tropical Science, [email protected] Michael S. BloomUniversity at Albany, State University of New York, [email protected]




