Levitra enthält Vardenafil, das eine kürzere Wirkdauer als Tadalafil hat, dafür aber schnell einsetzt. Männer, die diskret bestellen möchten, suchen häufig nach levitra kaufen ohne rezept. Dabei spielt die rechtliche Lage in der Schweiz eine wichtige Rolle.
Asthma-edu.org.tw
POCKET GUIDE FOR
ASTHMA MANAGEMENT
AND PREVENTION REPRODUCE
(for Adults and Children Older than 5 Years)
A Pocket Guide for Physicians and Nurses
BASED ON THE GLOBAL STRATEGY FOR ASTHMA
MANAGEMENT AND PREVENTION
Global Initiative for Asthma

GLOBAL INITIATIVE
FOR ASTHMA
POCKET GUIDE FOR HEALTH PROFESSIONALS
Updated 2015
GINA Board of Directors
Chair: J Mark FitzGerald, MD
GINA Science Committee
Chair: Helen Reddel, MBBS PhD
GINA Dissemination and Implementation Committee
Chair: Louis-Philippe Boulet, MD
GINA Assembly
The GINA Assembly includes members from 45 countries, listed on the GINA website
GINA Program
Scientific Director: Suzanne Hurd, PhD
Names of members of the GINA Committees are listed on p
TABLE OF CONTENTS
TABLE OF FIGURES
Abbreviations used in this Pocket Guide are found on pa
Asthma affects an estimated 300 million individuals worldwide. It is a serious global health problem affecting all age groups, with increasing prevalence in many developing countries, rising treatment costs, and a rising burden for patients and the community. Asthma still imposes an unacceptable burden on health care systems, and on society through loss of productivity in the workplace and, especially for pediatric asthma, disruption to the family.
Health care providers managing asthma face different issues around the
world, depending on the local context, the health system, and access to resources.
The Global Initiative for Asthma (GINA) was established to increase
awareness about asthma among health professionals, public health
authorities and the community, and to improve prevention and management
through a coordinated worldwide effort. GINA prepares scientific reports on
asthma, encourages dissemination and implementation of the
recommendations, and promotes international collaboration on asthma
The Global Strategy for Asthma Management and Prevention was
extensively revised in 2014 to provide a comprehensive and integrated
approach to asthma management that can be adapted for local conditions and for individual patients. It focuses not only on the existing strong evidence base, but also on clarity of language and on providing tools for feasible implementation in clinical practice. The report was updated in 2015.
This Pocket Guide is a brief summary of the GINA 2015 report for primary
health care providers. It does NOT contain all of the information required for
managing asthma, for example, about safety of treatments, and should be
used in conjunction with the full GINA 2015 report. GINA cannot be held liable
or responsible for healthcare administered with the use of this document,
including any use which is not in accordance with applicable local or national
regulations or guidelines.
The GINA 2015 report and other GINA publications (listed on page 28) can be obtained from
WHAT IS KNOWN ABOUT ASTHMA?
Asthma is a common and potentially serious chronic disease that
imposes a substantial burden on patients, their families and the community. It
causes respiratory symptoms, limitation of activity, and flare-ups (attacks) that
sometimes require urgent health care and may be fatal.
Fortunately…asthma can be effectively treated, and most patients can
achieve good control of their asthma. When asthma is under good control,
patients can:
Avoid troublesome symptoms during day and night Need little or no reliever medication Have productive, physically active lives
Have normal or near normal lung function Avoid serious asthma flare-ups (exacerbations, or attacks)
What is asthma? Asthma causes symptoms such as wheezing, shortness of
breath, chest tightness and cough that vary over time in their occurrence,
frequency and intensity.
These symptoms are associated with variable expiratory airflow, i.e. difficulty breathing air out of the lungs due to bronchoconstriction (airway narrowing), airway wall thickening, and increased mucus. Some variation in airflow can
also occur in people without asthma, but it is greater in asthma.
Factors that may trigger or worsen asthma symptoms include viral
infections, domestic or occupational allergens (e.g. house dust mite, pollens,
cockroach), tobacco smoke, exercise and stress. These responses are more likely when asthma is uncontrolled. Some drugs can induce or trigger asthma, e.g. beta-blockers, and (in some patients), aspirin or other NSAIDs.
Asthma flare-ups (also called exacerbations or attacks) may occur, even in
people taking asthma treatment. When asthma is uncontrolled, or in some
high-risk patients, these episodes are more frequent and more severe, and
may be fatal.
A stepwise approach to treatment, customized to the individual patient,
takes into account the effectiveness of available medications, their safety, and
their cost to the payer or patient.
Regular controller treatment, particularly with inhaled corticosteroid (ICS)-
containing medications, markedly reduces the frequency and severity of
asthma symptoms and the risk of having a flare-up.
Asthma is a common condition, affecting all levels of society. Olympic
athletes, famous leaders and celebrities, and ordinary people live successful
and active lives with asthma.

MAKING THE DIAGNOSIS OF ASTHMA
Asthma is a disease with many variations (heterogeneous), usually characterized by chronic airway inflammation. Asthma has two key defining features:
• a history of respiratory symptoms such as wheeze, shortness of breath,
chest tightness and cough that vary over time and in intensity, AND
• variable expiratory airflow limitation.
A flow-chart for making the diagnosis in clinical practice is shown in Box 1,
with the specific criteria for diagnosing asthma in Box 2.
Box 1. Diagnostic flow-chart for asthma in clinical practice
The diagnosis of asthma should be confirmed and, for future reference, the
evidence documented in the patient's notes. Depending on clinical urgency
and access to resources, this should preferably be done before starting
controller treatment. Confirming the diagnosis of asthma is more difficult after
treatment has been started (s
CRITERIA FOR MAKING THE DIAGNOSIS OF ASTHMA
Box 2. Features used in making the diagnosis of asthma
1. A history of variable respiratory symptoms
Typical symptoms are wheeze, shortness of breath, chest tightness, cough • People with asthma generally have more than one of these symptoms • The symptoms occur variably over time and vary in intensity • The symptoms often occur or are worse at night or on waking • Symptoms are often triggered by exercise, laughter, allergens or cold air
• Symptoms often occur with or worsen with viral infections
2. Evidence of variable expiratory airflow limitation
• At least once during the diagnostic process when FEV1 is low,
document that the FEV1/FVC ratio is reduced. The FEV1/FVC ratio is normally more than 0.75–0.80 in adults, and more than 0.90 in children.
• Document that variation in lung function is greater than in healthy
people. For example:
o FEV1 increases by more than 12% and 200mL (in children, >12%
of the predicted value) after inhaling a bronchodilator. This is called ‘bronchodilator reversibility'.
o Average daily diurnal PEF variability* is >10% (in children, >13%)
o FEV1 increases by more than 12% and 200mL from baseline (in
children, by >12% of the predicted value) after 4 weeks of anti-inflammatory treatment (outside respiratory infections)
• The greater the variation, or the more times excess variation is seen,
the more confident you can be of the diagnosis
• Testing may need to be repeated during symptoms, in the early
morning, or after withholding bronchodilator medications.
• Bronchodilator reversibility may be absent during severe exacerbations
or viral infections. If bronchodilator reversibility is not present when it is first tested, the next step depends on the clinical urgency and availability of other tests.
• For other tests to assist in diagnosis, including bronchial challenge
tests, see Chapter 1 of the GINA 2015 report.
*Calculated from twice daily readings (best of 3 each time), as ([the day's highest PEF minus the day's lowest PEF]) divided by the mean of the day's highest and lowest PEF, and averaged over 1-2 weeks. If using PEF at home or in the office, use the same PEF meter each time.
Physical examination in people with asthma is often normal, but the most
frequent finding is wheezing on auscultation, especially on forced expiration.
DIAGNOSING ASTHMA IN SPECIAL POPULATIONS
Patients with cough as the only respiratory symptom
This may be due to chronic upper airway cough syndrome (‘post-nasal drip'), chronic sinusitis, gastroesophageal reflux (GERD), vocal cord dysfunction, or eosinophilic bronchitis, or cough variant asthma. Cough variant asthma is characterized by cough and airway hyperresponsiveness, and documenting variability in lung function is essential to make this diagnosis. However, lack of variability at the time of testing does not exclude asthma. For other diagnostic
tests, see Box 2, and Chapter 1 of the GINA 2015 report, or refer the patient for specialist opinion.
Occupational asthma and work-aggravated asthma
Every patient with adult-onset asthma should be asked about occupational exposures, and whether their asthma is better when they are away from work. It is important to confirm the diagnosis objectively (which often needs specialist referral) and to eliminate exposure as soon as possible.
Pregnant women
Ask all pregnant women and those planning pregnancy about asthma, and advise them about the importance of asthma treatment for the health of both mother and baby.
The elderly
Asthma may be under-diagnosed in the elderly, due to poor perception, an assumption that dyspnea is normal in old age, lack of fitness, or reduced
activity. Asthma may also be over-diagnosed in the elderly through confusion with shortness of breath due to left ventricular failure or ischemic heart disease. If there is a history of smoking or biomass fuel exposure, COPD or asthma-COPD overlap syndrome (ACOS) should be considered (see Chapter 5 of the GINA 2015 report).
Smokers and ex-smokers
Asthma and COPD may co-exist or overlap (asthma-COPD overlap
syndrome, ACOS), particularly in smokers and the elderly. The history and pattern of symptoms and past records can help to distinguish asthma with fixed airflow limitation from COPD. Uncertainty in diagnosis should prompt early referral, as ACOS has worse outcomes than asthma or COPD alone.
Confirming an asthma diagnosis in patients taking controller treatment:
For many patients (25–35%) with a diagnosis of asthma in primary care, the diagnosis cannot be confirmed. If the basis of the diagnosis has not already been documented, confirmation with objective testing should be sought.
If standard criteria for asthma (Box 2) are not met, consider other investigations. For example, if lung function is normal, repeat reversibility testing after withholding medications for 12 hours. If the patient has frequent symptoms, consider a trial of step-up in controller treatment and repeat lung function testing after 3 months. If the patient has few symptoms, consider stepping down controller treatment, but ensure the patient has a written asthma action plan, monitor them carefully, and repeat lung function testing.
ASSESSING A PATIENT WITH ASTHMA
Take every opportunity to assess patients with a diagnosis of asthma, particularly when they are symptomatic or after a recent exacerbation, but also when they ask for a prescription refill. In addition, schedule a routine
review at least once a year.
Box 3. How to assess a patient with asthma
1. Asthma control – assess both symptom control and risk factors
Assess symptom control over the last 4 weeks (Box 4, p9)
• Identify any other risk factors for poor outcomes (Box 4) • Measure lung function before starting treatment, 3–6 months later, and
then periodically, e.g. yearly
2. Treatment issues
• Record the patient's treatment (Box 7, p14), and ask about side-effects •
Watch the patient using their inhaler, to check their technique (p18)
• Have an open empathic discussion about adherence (p18) • Check that the patient has a written asthma action plan (p22) • Ask the patient about their attitudes and goals for their asthma
3. Are there any comorbidities?
• These include rhinitis, rhinosinusitis, gastroesophageal reflux (GERD),
obesity, obstructive sleep apnea, depression and anxiety.
• Comorbidities should be identified as they may contribute to respiratory
symptoms and poor quality of life. Their treatment may complicate asthma management.
HOW TO ASSESS ASTHMA CONTROL
Asthma control means the extent to which the effects of asthma can be seen
in the patient, or have been reduced or removed by treatment. Asthma control
has two domains: symptom control (previously called ‘current clinical control')
and risk factors for future poor outcomes.
Poor symptom control is a burden to patients and a risk factor for flare-ups.
Risk factors are factors that increase the patient's future risk of having
exacerbations (flare-ups), loss of lung function, or medication side-effects.
Box 4. Assessment of symptom control and future risk
A. Level of asthma symptom control
In the past 4 weeks, has the patient had:
controlled controlled
Daytime symptoms more than twice/week? Yes No Any night waking due to asthma?
Yes No None
Reliever needed* more than twice/week? Yes No of these
Any activity limitation due to asthma?
B. Risk factors for poor asthma outcomes
Assess risk factors at diagnosis and periodical y, particularly for patients experiencing
exacerbations. Measure FEV1 at start of treatment, after 3–6 months of controller treatment to record
personal best lung function, then periodically for ongoing risk assessment. Potential y modifiable independent risk factors for exacerbations include:
• Uncontrolled asthma symptoms (as above)
• ICS not prescribed; poor ICS adherence; incorrect inhaler technique
• High SABA use (with increased mortality if >1x200-dose canister/month)
Having one or more
• Low FEV1, especially if <60% predicted
of these risk factors
Major psychological or socioeconomic problems
increases the risk of
Exposures: smoking; allergen exposure if sensitized
exacerbations even
Comorbidities: obesity; rhinosinusitis; confirmed food allergy
• Sputum or blood eosinophilia
if symptoms are well
Other major independent risk factors for flare-ups (exacerbations) include:
• Ever being intubated or in intensive care for asthma
• Having 1 or more severe exacerbations in the last 12 months.
Risk factors for developing fixed airflow limitation include lack of ICS treatment; exposure to tobacco
smoke, noxious chemicals or occupational exposures; low FEV1; chronic mucus hypersecretion; and
sputum or blood eosinophilia Risk factors for medication side-effects include:
• Systemic: frequent OCS; long-term, high dose and/or potent ICS; also taking P450 inhibitors
• Local: high-dose or potent ICS; poor inhaler technique

What is the role of lung function in monitoring asthma?
Once asthma has been diagnosed, lung function is most useful as an indicator of future risk. It should be recorded at diagnosis, 3–6 months after starting treatment, and periodically thereafter. Patients who have either few or many symptoms relative to their lung function need more investigation.
How is asthma severity assessed?
Asthma severity can be assessed retrospectively from the level of treatment (p14) required to control symptoms and exacerbations. Mild asthma is asthma
that can be controlled with Step 1 or 2 treatment. Severe asthma is asthma that requires Step 4 or 5 treatment, to maintain symptom control. It may appear similar to asthma that is uncontrolled due to lack of treatment.
HOW TO INVESTIGATE UNCONTROLLED ASTHMA
Most patients can achieve good asthma control with regular controller treatment, but some patients do not, and further investigation is needed.
Box 5. How to investigate uncontrolled asthma in primary care
This flow-chart shows the most common problems first, but the steps can be carried out in a different order, depending on resources and clinical context.
MANAGEMENT OF ASTHMA
GENERAL PRINCIPLES
The long-term goals of asthma management are symptom control and
risk reduction. The aim is to reduce the burden to the patient and their risk of
exacerbations, airway damage, and medication side-effects. The patient's
own goals regarding their asthma and its treatment should also be identified.
Population-level recommendations about ‘preferred' asthma treatments
represent the best treatment for most patients in a population.
Patient-level treatment decisions should take into account any individual
characteristics or phenotype that predict the patient's likely response to
treatment, together with the patient's preferences and practical issues such as inhaler technique, adherence, and cost.
A partnership between the patient and their health care providers is
important for effective asthma management. Training health care providers in
communication skills may lead to increased patient satisfaction, better
health outcomes, and reduced use of health care resources.
Health literacy – that is, the patient's ability to obtain, process and
understand basic health information to make appropriate health decisions –
should be taken into account in asthma management and education.
TREATING TO CONTROL SYMPTOMS AND MINIMIZE RISK
Treatment of asthma for symptom control and risk reduction includes:
• Medications. Every patient with asthma should have a reliever
medication, and most adults and adolescents with asthma should have a controller medication
• Treating modifiable risk factors • Non-pharmacological therapies and strategies
Importantly, every patient should also be trained in essential skills and guided asthma self-management, including:
• Asthma information • Inhaler skills (p18) • Adherence (p18) • Written asthma action plan (p22) • Self-monitoring • Regular medical review (p8)

CONTROL-BASED ASTHMA MANAGEMENT
Asthma treatment is adjusted in a continuous cycle to assess, adjust
treatment and review response. The main components of this cycle are
shown in Box 6.
Box 6. The control-based asthma management cycle
INITIAL CONTROLLER TREATMENT
For the best outcomes, regular daily controller treatment should be initiated as soon as possible after the diagnosis of asthma is made, because:
• Early treatment with low dose ICS leads to better lung function than if
symptoms have been present for more than 2–4 years
• Patients not taking ICS who experience a severe exacerbation have
lower long-term lung function than those who have started ICS
• In occupational asthma, early removal from exposure and early treatment
increase the probability of recovery
Regular low dose ICS is recommended for patients with any of the
following:
• Asthma symptoms more than twice a month • Waking due to asthma more than once a month • Any asthma symptoms plus any risk factor(s) for exacerbations
(e.g. needing OCS for asthma within the last 12 months; low FEV1; ever in intensive care unit for asthma)
Consider starting at a higher step (e.g. medium/high dose ICS, or ICS/LABA) if the patient has troublesome asthma symptoms on most days; or is waking from asthma once or more a week, especially if there are any risk factors for exacerbations.
If the initial asthma presentation is with severely uncontrolled asthma, or with an acute exacerbation, give a short course of OCS and start regular controller treatment (e.g. high dose ICS, or medium dose ICS/LABA).
Low, medium and high dose categories for different ICS medications are shown in Box 8 (
Before starting initial controller treatment
• Record evidence for the diagnosis of asthma, if possible • Document symptom control and risk factors • Assess lung function, when possible • Train the patient to use the inhaler correctly, and check their technique •
Schedule a follow-up visit
After starting initial controller treatment
• Review response after 2–3 months, or according to clinical urgency • See Box 7 for ongoing treatment and other key management issues • Consider step down when asthma has been well-controlled for 3 months
Box 7. Stepwise approach to asthma treatment
*For children 6–11 years, theophylline is not recommended, and the preferred Step 3 treatment is medium dose ICS. **Low dose ICS/formoterol is the reliever medication for patients prescribed low dose budesonide/formoterol or low dose beclometasone/formoterol ## Tiotropium by soft-mist inhaler is an add-on treatment for patients with a history of exacerbations; it is not indicated in children <18 years.
For medication Glossary, se For details about treatment recommendations, supporting evidence, and clinical advice about implementation in different populations see the full GINA 2015 report.
Box 8. Low, medium and high daily doses of inhaled corticosteroids (mcg)
Inhaled corticosteroid
Adults and adolescents
Children 6–11 years
Beclometasone dipropionate (CFC)*
Beclometasone dipropionate (HFA)
Budesonide (DPI)
Budesonide (nebules)
Ciclesonide (HFA)
Fluticasone propionate( DPI)
Fluticasone propionate (HFA)
Mometasone furoate
≥220–<440
Triamcinolone acetonide
CFC: chlorofluorocarbon propellant; DPI: dry powder inhaler; HFA: hydrofluoroalkane propellant. *Included for comparison with older literature.
STEPWISE APPROACH FOR ADJUSTING TREATMENT
Once asthma treatment has been started, ongoing decisions are based on a cycle to assess, adjust treatment and review response. The preferred treatments at each step are summarized below and in Box 7 (p14); for details, see full GINA 2015 report. See Box 8 ( for ICS dose categories.
STEP 1: As-needed SABA with no controller (this is indicated only if
symptoms are rare, there is no night waking due to asthma, no exacerbations in the last year, and normal FEV
Other options: regular low dose ICS for patients with exacerbation risks.
STEP 2: Regular low dose ICS plus as-needed SABA
Other options: LTRA are less effective than ICS; ICS/LABA leads to faster improvement in symptoms and FEV1 than ICS alone but are more expensive and the exacerbation rate is similar. For purely seasonal allergic asthma, start ICS immediately and cease 4 weeks after end of exposure.
STEP 3: Low dose ICS/LABA either as maintenance treatment plus as-
needed SABA, or as ICS/formoterol maintenance and reliever therapy
For patients with ≥1 exacerbation in the last year, low dose BDP/formoterol
or BUD/formoterol maintenance and reliever strategy is more effective than
maintenance ICS/LABA with as-needed SABA.
Other options: Medium dose ICS Children (6–11 years): Medium dose ICS. Other options: low dose ICS/LABA
STEP 4: Low dose ICS/formoterol maintenance and reliever therapy, or
medium dose ICS/LABA as maintenance plus as-needed SABA
Other options: Add-on tiotropium by soft-mist inhaler for adults (≥18 years)
with a history of exacerbations; high dose ICS/LABA, but more side-effects
and little extra benefit; extra controller, e.g. LTRA or slow-release
theophylline (adults)
Children (6–11 years): Refer for expert assessment and advice.
STEP 5: Refer for expert investigation and add-on treatment
Add-on treatments include anti-IgE (omalizumab) for severe allergic asthma. Sputum-guided treatment, if available, improves outcomes. Other options: Add-on tiotropium by soft-mist inhaler for adults (≥18 years) with a history of exacerbations. Some patients may benefit from low dose OCS but long-term systemic side-effects occur.
REVIEWING RESPONSE AND ADJUSTING TREATMENT
How often should patients with asthma be reviewed?
Patients should preferably be seen 1–3 months after starting treatment and every 3–12 months after that, except in pregnancy when they should be reviewed every 4–6 weeks. After an exacerbation, a review visit within 1 week should be scheduled. The frequency of review depends on the patient's initial level of control, their response to previous treatment, and their ability and willingness to engage in self-management with an action plan.
Stepping up asthma treatment
Asthma is a variable condition, and periodic adjustment of controller treatment
by the clinician and/or patient may be needed.
• Sustained step-up (for at least 2–3 months): if symptoms and/or
exacerbations persist despite 2–3 months of controller treatment, assess the following common issues before considering a step-up
o Incorrect inhaler technique
o Poor adherence
o Modifiable risk factors, e.g. smoking
o Are symptoms due to comorbid conditions, e.g. allergic rhinitis
• Short-term step-up (for 1–2 weeks) by clinician or by patient with written
asthma action plan (p22), e.g. during viral infection or allergen exposure
• Day-to-day adjustment by patient for patients prescribed low dose
beclometasone/formoterol or budesonide/formoterol as maintenance and reliever therapy.
Stepping down treatment when asthma is well-controlled
Consider stepping down treatment once good asthma control has been achieved and maintained for 3 months, to find the lowest treatment that controls both symptoms and exacerbations, and minimizes side-effects.
• Choose an appropriate time for step-down (no respiratory infection,
patient not travelling, not pregnant)
• Document baseline status (symptom control and lung function), provide a
written asthma action plan, monitor closely, and book a follow-up visit
• Step down through available formulations to reduce the ICS dose by
25–50% at 2–3 month intervals (see full GINA report for details)
• Do not completely withdraw ICS (in adults or adolescents) unless it is
needed temporarily to confirm the diagnosis of asthma
INHALER SKILLS AND ADHERENCE
Provide skills training for effective use of inhaler devices
Most patients (up to 80%) cannot use their inhaler correctly. This contributes to poor symptom control and exacerbations. To ensure effective inhaler use:
• Choose the most appropriate device for the patient before prescribing:
consider medication, physical problems e.g. arthritis, patient skills, and cost; for ICS by pressurized metered dose inhaler, prescribe a spacer.
• Check inhaler technique at every opportunity. Ask the patient to show
you how they use the inhaler. Check their technique against a device-specific checklist.
• Correct using a physical demonstration, paying attention to incorrect
steps. Check technique again, up to 2–3 times if necessary.
• Confirm that you have checklists for each of the inhalers you prescribe,
and can demonstrate correct technique on them.
Information about inhaler devices and techniques for their use can be found
on the GINA websit and the ADMIT website
Check and improve adherence with asthma medications
Around 50% of adults and children do not take controller medications as prescribed. Poor adherence contributes to poor symptom control and exacerbations. It may be unintentional (e.g. forgetfulness, cost, misunderstandings) and/or non-intentional (e.g. not perceiving the need for
treatment, fear of side-effects, cultural issues, cost).
To identify patients with adherence problems:
• Ask an empathic question, e.g. "Most patients don't take their inhaler
exactly as prescribed. In the last 4 weeks, how many days a week have you been taking it? 0 days a week, or 1, or 2 days [etc]?", or "Do you find it easier to remember your inhaler in the morning or night?"
• Check medication usage, from prescription date, inhaler date/dose
counter, dispensing records
• Ask about attitudes and beliefs about asthma and medications
Only a few adherence interventions have been studied closely in asthma.
• Shared decision-making for medication and dose choice • Inhaler reminders for missed doses • Reduced complexity of the regimen (once- vs twice-daily) • Comprehensive asthma education with home visits by asthma nurses • Clinicians reviewing feedback on their patients' dispensing records
TREATING MODIFIABLE RISK FACTORS
Exacerbation risk can be minimized by optimizing asthma medications, and by identifying and treating modifiable risk factors. Some examples of risk modifiers with consistent high quality evidence are:
• Guided self-management: self-monitoring of symptoms and/or PEF, a
written asthma action plan (p22), and regular medical review
• Use of a regimen that minimizes exacerbations: prescribe an ICS-
containing controller. For patients with 1 or more exacerbations in the last
year, consider a low dose ICS/formoterol maintenance and reliever regimen
• Avoidance of exposure to tobacco smoke
• Confirmed food allergy: appropriate food avoidance; ensure availability
of injectable epinephrine for anaphylaxis
• For patients with severe asthma: refer to a specialist center, if
available, for consideration of add-on medications and/or sputum-guided treatment.
NON-PHARMACOLOGICAL STRATEGIES AND INTERVENTIONS
In addition to medications, other therapies and strategies may be considered where relevant, to assist in symptom control and risk reduction. Some
examples with consistent high quality evidence are:
• Smoking cessation advice: at every visit, strongly encourage smokers to
quit. Provide access to counselling and resources. Advise parents and
carers to exclude smoking in rooms/cars used by children with asthma
• Physical activity: encourage people with asthma to engage in regular
physical activity because of its general health benefits. Provide advice about management of exercise-induced bronchoconstriction.
• Occupational asthma: ask all patients with adult-onset asthma about their
work history. Identify and remove occupational sensitizers as soon as possible. Refer patients for expert advice, if available.
• NSAIDs including aspirin: always ask about asthma before prescribing.
Although allergens may contribute to asthma symptoms in sensitized patients, allergen avoidance is not recommended as a general strategy for asthma. These strategies are often complex and expensive, and there are no validated methods for identifying those who are likely to benefit.
Some common triggers for asthma symptoms (e.g. exercise, laughter) should
not be avoided, and others (e.g. viral respiratory infections, stress) are
difficult to avoid and should be managed when they occur.
TREATMENT IN SPECIAL POPULATIONS OR CONTEXTS
Pregnancy: asthma control often changes during pregnancy. For baby and
mother, the advantages of actively treating asthma markedly outweigh any
potential risks of usual controller and reliever medications. Down-titration has
a low priority in pregnancy. Exacerbations should be treated aggressively.
Rhinitis and sinusitis often coexist with asthma. Chronic rhinosinusitis is
associated with more severe asthma. For some patients, treatment with
intranasal corticosteroids improves asthma control.
Obesity: to avoid over- or under-treatment, it is important to document the
diagnosis of asthma in the obese. Asthma is more difficult to control in
obesity. Weight reduction should be included in the treatment plan for obese patients with asthma; even 5–10% weight loss can improve asthma control.
The elderly: comorbidities and their treatment should be considered and may
complicate asthma management. Factors such as arthritis, eyesight,
inspiratory flow, and complexity of treatment regimens should be considered
when choosing medications and inhaler devices.
Gastroesophageal reflux (GERD) is commonly seen in asthma.
Symptomatic reflux should be treated for its general health benefits, but there
is no benefit from treating asymptomatic reflux in asthma.
Anxiety and depression: these are commonly seen in people with asthma,
and are associated with worse symptoms and quality of life. Patients should
be assisted to distinguish between symptoms of anxiety and of asthma.
Aspirin-exacerbated respiratory disease (AERD): a history of exacerbation
following ingestion of aspirin or other NSAIDs is highly suggestive. Patients
often have severe asthma and nasal polyposis. Confirmation of the diagnosis
of AERD requires challenge in a specialized center with cardiopulmonary
resuscitation facilities, but avoidance of NSAIDs may be recommended on the
basis of a clear history. ICS are the mainstay of treatment, but OCS may be
required. Desensitization under specialist care is sometimes effective.
Food allergy and anaphylaxis: food allergy is rarely a trigger for asthma
symptoms. It must be assessed with specialist testing. Confirmed food allergy
is a risk factor for asthma-related death. Good asthma control is essential;
patients should also have an anaphylaxis plan and be trained in appropriate
avoidance strategies and use of injectable epinephrine.
Surgery: whenever possible, good asthma control should be achieved pre-
operatively. Ensure that controller therapy is maintained throughout the peri-
operative period. Patients on long-term high dose ICS, or having more than 2
weeks' OCS in the past 6 months, should receive intra-operative
hydrocortisone to reduce the risk of adrenal crisis.
ASTHMA FLARE-UPS (EXACERBATIONS)
A flare-up or exacerbation is an acute or sub-acute worsening in symptoms and lung function from the patient's usual status; occasionally it may be the initial presentation of asthma.
For discussion with patients, the word ‘flare-up' is preferred. ‘Episodes', ‘attacks' and ‘acute severe asthma' are often used, but they have variable meanings, particularly for patients.
The management of worsening asthma and exacerbations should be
considered as a continuum, from self-management by the patient with a written asthma action plan, through to management of more severe
symptoms in primary care, the emergency department and in hospital.
Identifying patients at risk of asthma-related death
These patients should be identified, and flagged for more frequent review.
• A history of near-fatal asthma requiring intubation and ventilation •
Hospitalization or emergency care for asthma in last 12 months
• Not currently using ICS, or poor adherence with ICS • Currently using or recently stopped using OCS (this indicates the severity
of recent events)
• Over-use of SABAs, especially more than 1 canister/month • Lack of a written asthma action plan • History of psychiatric disease or psychosocial problems •
Confirmed food allergy in a patient with asthma
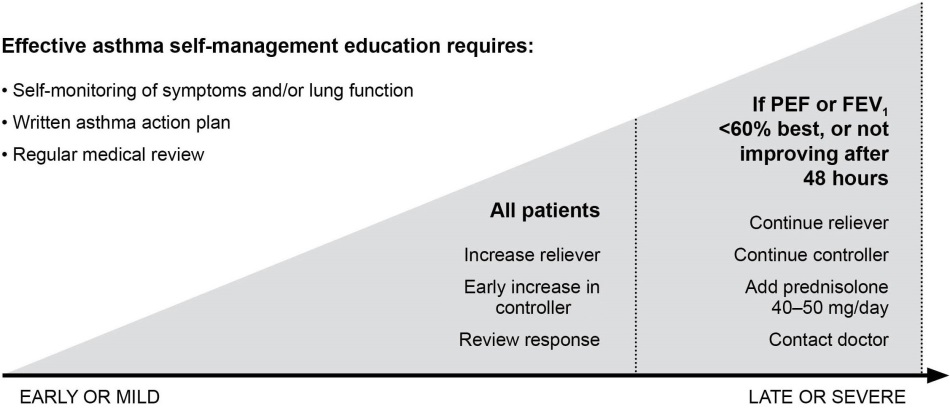
WRITTEN ASTHMA ACTION PLANS
All patients should be provided with a written asthma action plan appropriate for their level of asthma control and health literacy, so they know how to recognize and respond to worsening asthma.
Box 9. Self-management with a written action plan
The written asthma action plan should include:
• The patient's usual asthma medications • When and how to increase medications, and start OCS
• How to access medical care if symptoms fail to respond
The action plan can be based on symptoms and/or (in adults) PEF. Patients who deteriorate quickly should be advised to go to an acute care facility or
see their doctor immediately.
Medication changes for written asthma action plans
Increase frequency of inhaled reliever (SABA, or low dose ICS/formoterol if
using maintenance and reliever regimen); add spacer for pMDI.
Increase controller: Rapid increase in ICS component up to max. 2000mcg
BDP equivalent. Options depend on usual controller medication, as follows:
• ICS: At least double dose, consider increasing to high dose.
• Maintenance ICS/formoterol: Quadruple maintenance ICS/formoterol
dose (to maximum formoterol dose of 72 mcg/day).
• Maintenance ICS/salmeterol: Step up at least to higher dose formulation;
consider adding separate ICS inhaler to achieve high ICS dose.
• Maintenance and reliever ICS/formoterol: Continue maintenance dose;
increase as-needed ICS/formoterol (maximum formoterol 72 mcg/day).
Oral corticosteroids (preferably morning dosing):
• Adults - prednisolone 1mg/kg/day up to 50mg, usually for 5–7 days. • For children, 1–2 mg/kg/day up to 40mg, usually for 3–5 days. • Tapering not needed if treatment has been given for less than 2 weeks.
MANAGING EXACERBATIONS IN PRIMARY OR ACUTE CARE
Assess exacerbation severity while starting SABA and oxygen. Assess
dyspnea (e.g. is the patient able to speak sentences, or only words),
respiratory rate, pulse rate, oxygen saturation and lung function (e.g. PEF).
Check for anaphylaxis.
Consider alternative causes of acute breathlessness (e.g. heart failure,
upper airway dysfunction, inhaled foreign body or pulmonary embolism).
Arrange immediate transfer to an acute care facility if there are signs of
severe exacerbation, or to intensive care if the patient is drowsy, confused, or has a silent chest. For these patients, immediately give inhaled SABA, inhaled
ipratropium bromide, oxygen and systemic corticosteroids.
Start treatment with repeated doses of SABA (usually by pMDI and spacer),
early oral corticosteroids, and controlled flow oxygen if available. Check
response of symptoms and saturation frequently, and measure lung function
after 1 hour. Titrate oxygen to maintain saturation of 93–95% in adults and
adolescents (94–98% in children 6–12 years).
For severe exacerbations, add ipratropium bromide, and consider giving
SABA by nebulizer. In acute care facilities, intravenous magnesium sulfate
may be considered if the patient is not responding to intensive initial
Do not routinely perform chest X-ray or blood gases, or prescribe antibiotics, for asthma exacerbations.
REVIEWING RESPONSE
Monitor patients closely and frequently during treatment, and titrate
treatment according to response. Transfer the patient to higher level care if
worsening or failing to respond.
Decide about need for hospitalization based on clinical status,
symptomatic and lung function, response to treatment, recent and past history
of exacerbations, and ability to manage at home.
Before discharge, arrange ongoing treatment. For most patients, prescribe
regular controller therapy (or increase current dose) to reduce the risk of
further exacerbations. Continue increased controller doses for 2–4 weeks,
and reduce reliever to as-needed. Check inhaler technique and adherence.
Provide an interim written asthma action plan.
Arrange early follow-up after any exacerbation, preferably within 1 week.
Consider referral for specialist advice for patients with an asthma hospitalization, or repeated emergency department presentations.
Box 10. Management of asthma exacerbations in primary care
O2: oxygen; PEF: peak expiratory flow; SABA: short-acting beta2-agonist (doses are for salbutamol)
FOLLOW-UP AFTER AN EXACERBATION
Exacerbations often represent failures in chronic asthma care, and they provide opportunities to review the patient's asthma management. All patients must be followed up regularly by a health care provider until symptoms and lung function return to normal.
Take the opportunity to review:
• The patient's understanding of the cause of the exacerbation • Modifiable risk factors for exacerbations, e.g. smoking
• Understanding of purposes of medications, and inhaler technique skills • Review and revise written asthma action plan
Discuss medication use, as adherence with ICS and OCS may fall to 50% within a week after discharge.
Comprehensive post-discharge programs that include optimal controller management, inhaler technique, self-monitoring, written asthma action plan and regular review are cost-effective and are associated with significant
improvement in asthma outcomes.
GLOSSARY OF ASTHMA MEDICATION CLASSES
For more details, see full GINA 2015 report and Appendix and
Product Information from manufacturers.
Medications
Action and use
Adverse effects
CONTROLLER MEDICATIONS
Inhaled corticosteroids
The most effective anti-inflammatory
Most patients using ICS do
(ICS) (pMDIs or DPIs) e.g. medications for persistent asthma. ICS
not experience side-effects.
reduce symptoms, increase lung function, Local side-effects include
budesonide, ciclesonide,
improve quality of life, and reduce the risk oropharyngeal candidiasis and
fluticasone propionate,
of exacerbations and asthma-related
dysphonia. Use of spacer with
fluticasone furoate,
hospitalizations or death. ICS dif er in
pMDI, and rinsing with water
mometasone, triamcinolone their potency and bioavailability, but most and spit ing out after
of the benefit is seen at low doses (see
inhalation, reduce local side
Box 8 (for low, medium and high
effects. High doses increase
doses of dif erent ICS).
the risk of systemic side-
ICS and long-acting beta2 When a medium dose of ICS alone fails to The LABA component may be
agonist bronchodilator
achieve good control of asthma, the
associated with tachycardia,
combinations (ICS/LABA) addition of LABA to ICS improves
headache or cramps. Current
(pMDIs or DPIs) e.g.
symptoms, lung function and reduces
recommendations are that
beclometasone/ formoterol, exacerbations in more patients, more
LABA and ICS are safe for
budesonide/formoterol,
rapidly, than doubling the dose of ICS.
asthma when used in
fluticasone furoate/
Two regimens are available: maintenance combination. Use of LABA
vilanterol, fluticasone
ICS/LABA with SABA as reliever, and low- without ICS in asthma is
propionate/formoterol,
dose combination beclometasone or
associated with increased risk
fluticasone propionate/
budesonide with formoterol for
of adverse outcomes.
maintenance and reli
mometasone/formoterol.
Leukotriene modifiers
Target one part of the inflammatory
Few side-effects except
(tablets) e.g. montelukast, pathway in asthma. Used as an option for elevated liver function tests
pranlukast, zafirlukast,
controller therapy, particularly in children. with zileuton and zafirlukast.
Used alone: less effective than low dose
ICS; added to ICS: less effective than
Chromones (pMDIs or
Very limited role in long-term treatment of Side effects are uncommon
DPIs) e.g. sodium
asthma. Weak anti-inflammatory effect,
but include cough upon
cromoglycate and
less effective than low-dose ICS. Require inhalation and pharyngeal
nedocromil sodium
meticulous inhaler maintenance.
Anti-IgE (omalizumab)
A treatment option for patients with severe Reactions at the site of
persistent allergic asthma uncontrolled on injection are common but
Step 4 treatment (high dose ICS/LABA). minor. Anaphylaxis is rare.
Long-acting
An add-on option at Step 4 or 5 bny soft- Side-effects are uncommon
anticholinergic, tiotropium mist inhaler for adults (≥18 years) whose but include dry mouth.
asthma is uncontrolled by ICS ± LABA
Medications
Action and use
Adverse effects
Systemic corticosteroids Short-term treatment (usually 5–7 days in Short-term use: some adverse
(tablets,suspension or
adults) is important early in the treatment effects e.g. hyperglycaemia,
intramuscular (IM) or
of severe acute exacerbations, with main gastro-intestinal side-effects,
intravenous (IV) injection) effects seen after 4–6 hours. Oral
e.g. prednisone,
corticosteroid (OCS) therapy is preferred Long-term use: limited by the
and is as effective as IM or IV therapy in risk of significant systemic
methylprednisolone,
preventing relapse. Tapering is required if adverse effects e.g. cataract,
treatment given for more than 2 weeks.
glaucoma, osteoporosis,
Long-term treatment with OCS may be
adrenal suppression. Patients
required for some patients with severe
should be assessed for
osteoporosis risk and treated
RELIEVER MEDICATIONS
Short-acting inhaled
Inhaled SABAs are medications of choice Tremor and tachycardia are
for quick relief of asthma symptoms and commonly reported with initial
bronchodilators (SABA) bronchoconstriction including in acute
use of SABA, but tolerance to
(pMDIs, DPIs and, rarely, exacerbations, and for pre-treatment of
these effects usually develops
solution for nebulization or exercise-induced bronchoconstriction.
rapidly. Excess use, or poor
injection) e.g. salbutamol SABAs should be used only as-needed at response indicate poor
(albuterol), terbutaline.
the lowest dose and frequency required. asthma control.
Long-term use: ipratropium is a less
Dryness of the mouth or a
anticholinergics (pMDIs effective reliever medication than SABAs. bitter taste.
or DPIs) e.g. ipratropium
Short-term use in acute asthma: inhaled
ipratropium added to SABA reduces the
oxitropium bromide
risk of hospital admission
Abbreviations used in this pocket guide
Beclometasone dipropionate
Dry powder inhaler
Forced expiratory volume in 1 second
Forced vital capacity
Inhaled corticosteroids
LABA Long-acting beta2-agonists
Oral corticosteroids
Peak expiratory flow
pMDI Pressurized metered dose inhaler
SABA Short-acting beta2-agonists
ACKNOWLEDGEMENTS
The activities of the Global Initiative of Asthma are supported by the work of members of the GINA Board of Directors and Committees (listed below). The members of the GINA committees are solely responsible for the statements and recommendations presented in this and other GINA publications.
GINA Board of Directors (2014)
J Mark FitzGerald, Canada, Chair; Eric Bateman, South Africa; Louis-Philippe Boulet*, Canada; Alvaro Cruz*, Brazil; Tari Haahtela*, Finland; Mark Levy*, United Kingdom; Paul O'Byrne, Canada; Pierluigi Paggiaro*, Italy; Soren Pedersen, Denmark; Manuel
Soto-Quiroz*, Costa Rica; Helen Reddel, Australia; Gary Wong*, Hong Kong ROC.
GINA Scientific Director: Suzanne Hurd, USA
GINA Science Committee (2014)
Helen Reddel, Australia, Chair; Eric Bateman, South Africa.; Allan Becker, Canada ; Johan de Jongste, The Netherlands; Jeffrey M. Drazen, USA ; J. Mark FitzGerald, Canada; Hiromasa Inoue, Japan; Robert Lemanske, Jr., USA; Paul O'Byrne, Canada; Soren Pedersen, Denmark; Emilio Pizzichini, Brazil; Stanley J. Szefler, USA.
GINA Dissemination and Implementation Committee (2014)
Louis-Philippe Boulet, Canada, Chair; other members indicated by asterisks (*) above.
GINA Assembly
The GINA Assembly includes members from 45 countries. Their names are listed on
GINA PUBLICATIONS
• Global Strategy for Asthma Management and Prevention (updated 2015). This
report, provides an integrated approach to asthma that can be adapted for a wide range of health systems. The report was extensively revised in 2014, and has been updated in 2015. The report has a user-friendly format with practical summary tables and flow-charts for use in clinical practice.
• GINA Online Appendix (updated 2015). Detailed background information to support
the main GINA report.
• Pocket Guide for asthma management and prevention for adults and children
older than 5 years (updated 2015). Summary for primary health care providers, to
be used in conjunction with the main GINA report.
• Pocket guide for asthma management and prevention in children 5 years and
younger (updated 2015). A summary of patient care information about pre-schoolers
with asthma or wheeze, to be used in conjunction with the main GINA 2015 report.
• Diagnosis of asthma-COPD overlap syndrome (ACOS) (updated 2015). This is a
stand-alone copy of the corresponding chapter in the main GINA report. It is co-published by GINA and GOLD (the Global Initiative for Chronic Obstructive Lung Diseas
• Clinical practice aids and implementation tools will be available on the GINA
GINA publications and other resources are available from
Visit the GINA website at www.ginasthma.org
2015 Global Initiative for Asthma
Source: http://www.asthma-edu.org.tw/asthma/photo/GINA_Pocket_2015.pdf
Jae-Min Jung and Ho-Yeon Kim: Third-person Effects in the Stock Market:Perception of Experts & Non-experts and Impacts on Attitude Third-person Effects in the Stock Market: Perception of Experts & Non-experts and Impacts on Attitude Jae-Min Jung* and Ho-Yeon Kim** Abstract: The third-person effect was tested by examining whether people perceive a greater influence of unidentified information recommending stocks in the Internet on others than on themselves. Findings confirm the third-person effect but also show subjects with stock market experience perceive a greater influence on others than did subjects with no stock market experience. Additionally, subjects demonstrated a larger third-person effect when "others" are specified as novice investors who have little knowledge or experience in stock trading than when "others" are identified as experienced traders. After controlling for SES and interest and experience in stock trading, the third-person perception remained. Keywords: Third-person perception, Behavioral effect, Stock market, Internet information, Expert
11700 • The Journal of Neuroscience, October 24, 2007 • 27(43):11700 –11711 Neurobiology of Disease Cannabinoids Elicit Antidepressant-Like Behavior andActivate Serotonergic Neurons through the MedialPrefrontal Cortex Francis Rodriguez Bambico,1 Noam Katz,1,2 Guy Debonnel,1† and Gabriella Gobbi1,21Neurobiological Psychiatry Unit, Department of Psychiatry, McGill University, Montre´al, Quebec, Canada H3A 1A1, and 2Department of Psychiatry, Centrede Recherche Fernand Seguin, Hoˆpital L.H. Lafontaine, Universite´ de Montre´al, Quebec, Canada H1N 3V2





