Levitra enthält Vardenafil, das eine kürzere Wirkdauer als Tadalafil hat, dafür aber schnell einsetzt. Männer, die diskret bestellen möchten, suchen häufig nach levitra kaufen ohne rezept. Dabei spielt die rechtliche Lage in der Schweiz eine wichtige Rolle.
Impactgroupbc.files.wordpress.com
11700 • The Journal of Neuroscience, October 24, 2007 • 27(43):11700 –11711
Neurobiology of Disease
Cannabinoids Elicit Antidepressant-Like Behavior and
Activate Serotonergic Neurons through the Medial
Prefrontal Cortex
Francis Rodriguez Bambico,1
Noam Katz,1,2
Guy Debonnel,1†
and Gabriella Gobbi1,2
1Neurobiological Psychiatry Unit, Department of Psychiatry, McGill University, Montre´al, Quebec, Canada H3A 1A1, and 2Department of Psychiatry, Centre
de Recherche Fernand Seguin, Hoˆpital L.H. Lafontaine, Universite´ de Montre´al, Quebec, Canada H1N 3V2
Preclinical and clinical studies show that cannabis modulates mood and possesses antidepressant-like properties, mediated by the
agonistic activity of cannabinoids on central CB receptors (CB Rs). The action of CB R agonists on the serotonin (5-HT) system, the
major transmitter system involved in mood control and implicated in the mechanism of action of antidepressants, remains however
poorly understood. In this study, we demonstrated that, at low doses, the CB R agonist WIN55,212-2 [R(⫹
)-[2,3-dihydro-5-methyl-3-
[(morpholinyl)]pyrrolo[1,2,3-de]-1,4-benzoxazinyl]-(1-naphthalenyl) methanone mesylate] exerts potent antidepressant-like proper-
ties in the rat forced-swim test (FST). This effect is CB R dependent because it was blocked by the CB R antagonist rimonabant and is 5-HT
mediated because it was abolished by pretreatment with the 5-HT-depleting agent parachlorophenylalanine. Then, using in vivo electro-
physiology, we showed that low doses of WIN55,212-2 dose dependently enhanced dorsal raphe nucleus 5-HT neuronal activity through
a CB R-dependent mechanism. Conversely, high doses of WIN55,212-2 were ineffective in the FST and decreased 5-HT neuronal activity
through a CB R-independent mechanism. The CB R agonist-induced enhancement of 5-HT neuronal activity was abolished by total or
medial prefrontocortical, but not by lateral prefrontocortical, transection. Furthermore, 5-HT neuronal activity was enhanced by the local
microinjection of WIN55,212-2 into the ventromedial prefrontal cortex (mPFCv) but not by the local microinjection of WIN55,212-2 into
the lateral prefrontal cortex. Similarly, the microinjection of WIN55,212-2 into the mPFCv produced a CB R-dependent antidepressant-
like effect in the FST. These results demonstrate that CB R agonists possess antidepressant-like properties and modulate 5-HT neuronal
activity via the mPFCv.
Key words: cannabinoid; CB receptor; serotonin; dorsal raphe nucleus; medial prefrontal cortex; depression; forced swim test
the role of the endocannabinoid system in mood regulation.
Cannabis is the most widely used illicit drug (World Health Or-
Clinical studies have reported benefits of cannabis on mood dis-
ganization, 2006). Its major psychoactive constituent, ⌬9-
orders (Ashton et al., 2005; Ware et al., 2005). Genetic (Haller et
tetrahydrocannabinol, and other cannabinoids exhibit high af-
al., 2002, 2004; Martin et al., 2002) or pharmacological (Navarro
finity to brain CB
et al., 1997; Deroche-Gamonet et al., 2001) CB
1 receptors (CB1Rs). CB1R activation modulates
physiological, affective, cognitive, and psychomotor functions.
murine models yields enhanced expression of depression/
Cannabis intoxication for instance engenders enhanced sociabil-
anxiety-like behaviors. There are conflicting reports on the
ity, mood, and well-being, a condition described as "fatuous eu-
mood-related effects of CB1R agonists and antagonists/inverse
phoria" (Iversen, 2003) whose physiological and subjective com-
agonists with regards to direction and potency of effects. Shear-
ponents are selectively blocked by the CB
man et al. (2003) for example reported instead an antidepressant-
rimonabant (RIM) (Huestis et al., 2001).
like activity of the CB1R inverse agonist AM251 [
N-1-(2,4-
Increased attention has been directed toward understanding
Received April 11, 2007; revised Sept. 11, 2007; accepted Sept. 11, 2007.
been attributed to dose-dependent bidirectional modulation,
This work was supported by the Canadian Psychiatric Research Foundation, Fonds de la Recherche en Sante´ du
sensitivity of cannabimimetic responses to contextual condi-
Que´bec (G.G.), McGill University Health Center Research Institute, and McGill University Faculty of Medicine fellow-
tions, and the kind of animal model and strain used (for review,
ships (F.R.B). We thank Dr. Daniele Piomelli (University of Irvine, Irvine, CA) for his critical feedback on the experi-
see Viveros et al., 2005). Also, the possibility of interaction among
mental design, and Claude Bouchard and Patrick Hattan for their assistance with histology. This work is dedicated inmemory of Guy Debonnel and F. G. David, for their mentorship and friendship.
receptor subtypes including a putative CB3R and vanilloid recep-
†Deceased, November 4, 2006.
tors, which has likewise been demonstrated operating in a pleth-
Correspondence should be addressed to Dr. Gabriella Gobbi, Neurobiological Psychiatry Unit, Department of
ora of cannabimimetic responses, may underlie ambivalent ef-
Psychiatry, Research and Training Building, McGill University, 1033 Pine Avenue West, Montre´al, Quebec, Canada
fects on mood modulation.
H3A 1A1. E-mail:
[email protected].
Despite evidence for the impact of CB
1R activity on mood
Copyright 2007 Society for Neuroscience 0270-6474/07/2711700-12$15.00/0
regulation, information on direct effects of cannabinoids on se-
Bambico et al. • CB Agonism Activates 5-HT Neurons through Prefrontal Cortex
J. Neurosci., October 24, 2007 • 27(43):11700 –11711
• 11701
rotonergic neurotransmission remain meager. Serotonin (5-
0.9% physiological saline. The pH of vehicles and solutions used in the ex-
HT) is the major neurotransmitter implicated in mood patho-
periments was adjusted to 7.2.
physiology and in the mechanism of antidepresssant action(Blier and de Montigny, 1999). Several studies have neverthe-
Experiment 1: forced swim test
less provided indications of functional cannabinoid–5-HT in-
The FST examines the dynamics of transition from an active (swimming
teraction. First, the dorsal raphe (DR), the principal source of
and climbing) to a passive (immobility) mode of coping in an inescap-able cylindrical water pool (20 cm diameter, 50 cm high; 30 cm water
forebrain 5-HT, expresses the endocannabinoid-degrading
depth, 25–27°C water temperature). During the course of the 5 min test
enzyme fatty acid amide hydrolase (FAAH) (Egertova et al.,
swim session, an enhanced transition from activity to immobility result-
1998, 2003), the CB1R in rats (Moldrich and Wenger, 2000),
ing from a 15 min preexposure to the pool (preswim 24 h previously) has
and CB1R mRNA in mice (Ha¨ring et al., 2007). Second, CB1Rs
often been equated to learned behavioral despair (Porsolt et al., 1978).
are abundantly expressed in the prefrontal cortex (PFC) (Mar-
This enhancement of immobility is prevented by antidepressant treat-
sicano and Lutz, 1999; Moldrich and Wenger, 2000), which
ment. Here, rats received 0.05, 0.2, 1.0, or 2.0 mg/kg intraperitoneal
sends excitatory afferents to the DR, coursing from the medial
injections of the potent CB R agonist WIN55,212-2 23, 5, and 0.75 h
PFC (mPFC) (Jankowski and Sesack, 2004). Furthermore, up-
before the test swim, modified after the protocol previously described by
regulation in PFC CB
Page et al. (1999). A second cohort of rats was used to assess the effect of
1R density, likely a compensatory feed-
back, was observed in suicidal depressives (Hungund et al.,
coadministering rimonabant with the dose of WIN55,212-2 that elicitedthe maximal antidepressant-like effect. A third cohort of rats was used to
2004). Imaging studies revealed that cannabis alters PFC regional
verify the role of 5-HT in mediating the effects of WIN55,212-2. Vehicle
cerebral blood flow and metabolic activity. The degree was corre-
or pCPA (350 mg/kg, i.p., once daily), a selective inhibitor of the 5-HT
lated with subjective effects, and the pattern was consistent with
synthesis precursory enzyme tryptophan hydroxylase that therefore de-
CB1R localization (Volkow et al., 1991; Matthew et al., 2002). Third,
pletes endogenous 5-HT, was administered 72 and 48 h before the swim
CB1R agonism alters 5-HT1A and 5HT2A receptor-mediated behav-
test. WIN55,212-2 (0.2 mg/kg, i.p.) or vehicle were administered 23, 5,
ioral responses (Hill et al., 2006) and inhibits 5-HT reuptake
in vitro
and 0.75 h before the swim test (modified after Page et al., 1999). Behav-
(Banerjee et al., 1975; Johnson et al., 1976). In this study, we there-
ioral endpoints were analyzed by an automated tracking system equipped
fore aimed at determining whether the CB
with infrared-sensitive cameras (Videotrack; Viewpoint Life Science,
1R agonist WIN55,212-2
Montreal, Quebec, Canada). The predominant behaviors assessed were
subsumed to one of three categories: immobility, in which the rat wasmaking minimal movements to keep its head above water; swimming, in
exhibits antidepressant-like effects in the forced swim test (FST),
which the rat was engaged in average movements that cause it to move
modulates 5-HT activity through a CB1R-mediated mechanism,
(usually horizontally) within the cylinder; and climbing (burst activity),
and effects dose-dependent bidirectional modulations. Further-
in which forceful thrashing limb movements against the walls of the
more, using local microinfusions, we aimed to demonstrate that
cylinder were observed. It has been shown that antidepressants with
the mPFC is strongly involved in mediating these effects.
5-HT-specific action selectively increase the duration of swimming,whereas those with a predominantly noradrenergic-specific activity in-crease those of climbing (Page et al., 1999). In all sessions, each animal
Materials and Methods
was held on the neck and back, gently immersed in the pool hindlimbs
first. They were removed from the pool using a plastic grid, then dried
Adult male Sprague Dawley rats (Charles River, Saint-Constant, Quebec,
with a towel, and caged near a heat source. The FST is both sensitive and
Canada) weighing 280 –350 g at the time of experiments were housed in
selective for clinically effective antidepressants, has been repeatedly val-
pairs in standard polycarbonate cages. They were kept under standard
idated, and is currently the most popular model for detecting antidepres-
laboratory conditions (12 h light/dark cycle, lights on at 7:30 A.M.; tem-
sant activity attributable to its simplicity, reliability, and high predictive
perature at 20 ⫾ 2°C; 50 – 60% relative humidity). All rats had
ad libitum
validity (Lucki, 1997; Cryan et al., 2005). To control for false positives
access to food and water. One week before the start of experiments, rats
(increased activity in the FST of non-antidepressants), as has been con-
were exposed to the testing environment and allowed to habituate to
sistently observed with psychostimulants (for review, see Cryan et al.,
testing conditions. For rats that have undergone intracerebral cannula-
2005), a test for locomotor activity (5 min) in an open field (80 ⫻ 80 cm),
tion, weights were monitored after surgery, and at least 4 d of postoper-
was also conducted, in which locomotor activity was operationalized as
ative recovery period was observed before additional tests were con-
movement velocity (distance traveled in centimeters per minute).
ducted. Drug administrations and electrophysiological and behavioralexperiments were conducted between 2:00 P.M. and 10:00 P.M. All pro-cedures were approved by the local institutional animal care and use
Experiment 2: electrophysiology
committee and were in accordance to the ethical guidelines set by the
In vivo extracellular single-unit recordings of presumed DR 5-HT neu-
Canadian Institutes of Health Research and the Society for Neuroscience.
rons were performed to determine whether the antidepressant-like prop-erties of WIN55,212-2 in the FST corresponded to a capacity to enhance
5-HT neurotransmission. Recordings were conducted after repeated in-
The CB R agonist WIN55,212-2 (Sigma, Oakville, Ontario, Canada) was
traperitoneal administration following the FST protocol as well as after
emulsified in Tween 80 (polyoxyethylene-sorbitan mono-oleate; Sigma) and
intravenous administration. All stereotaxic coordinates used in the suc-
further dissolved in saline with 5% Tween 80 and 5% poly(ethylene) glycol
ceeding experiments were based on the stereotaxic atlas of Paxinos and
(Sigma). The WIN55,212-2 solution used for intracerebral microinfusions
Watson (1986).
was dissolved in dimethylsulfoxide (DMSO) (Sigma) and vehicle (VEH)
Preparation for electrophysiological experiments. Rats were anesthetized
(1:2) because of the hydrophobic property of WIN55–212-2. The CB R an-
with chloral hydrate (400 mg/kg, i.p.) and mounted in a stereotaxic frame
(David Kopf Instruments, Tujunga, CA) with the skull positioned hori-
(2,4-dichlorophenyl)-4-methyl-pyrazolecarboxamide] (a kind gift from Dr.
zontally (incisor bar at ⫺3.3). To maintain a full anesthetic state charac-
D. Piomelli, University of California, Irvine, CA) and the transient receptor
terized by the absence of a nociceptive reaction to a paw/tail pinch and
potential vanilloid type 1 (TRPV1)/vanilloid receptor antagonist capsaz-
eyeblink response to pressure, chloral hydrate was continuously admin-
epine (CPZ) (Tocris Bioscience, Ballwin, MO) were initially dissolved in
istered intraperitoneally at a dose of 50 –70 mg 䡠 kg ⫺1 䡠 h ⫺1 using an
DMSO and further diluted (1:20) with saline containing 5% Tween 80 and
infusion pump (Braintree Scientific, Braintree, MA). Body temperature
5% poly(ethylene) glycol. Desipramine hydrochloride (DMI), parachloro-
was maintained at 37 ⫾ 0.5°C throughout the experiment using a rectal
phenylalanine (pCPA) (Sigma), and citalopram hydrobromide (CIT)
probe and a heating pad (Seabrook International, Seabrook, NH). Before
(kindly provided by Lundbeck, Copenhagen, Denmark), were dissolved in
electrophysiological recordings, a catheter was inserted into the lateral
11702 • J. Neurosci., October 24, 2007 • 27(43):11700 –11711
Bambico et al. • CB Agonism Activates 5-HT Neurons through Prefrontal Cortex
tail vein to facilitate systemic administration of drugs. Extracellular
Fr3; the ventrolateral and lateral orbital cortices; and some parts of pari-
single-unit recordings were performed using single-barreled glass mi-
etal area 1 (modified after Hajos et al., 1999). 5-HT single-unit record-
cropipettes pulled from 2 mm Stoelting (Wood Dale, IL) capillary glass
ings were conducted 1.5–2 h after transection.
on a Narashige (Tokyo, Japan) PE-21 pipette puller and preloaded withfiberglass strands to promote capillary filling with 2% Pontamine Sky
Experiment 4: combined intracerebral microinfusion
Blue dye in sodium acetate (0.5 M, pH 7.5). The micropipette tips were
broken down to diameters of 1–3 m. Electrode impedances ranged
Experiment 4A: intracerebral microinfusion into the dorsal raphe nucleus.
from 2 to 4 M⍀. At the end of each experiment, the recording site was
Anesthetized rats were implanted with a single guide cannula (22 gauge;
marked by iontophoretic ejection (5–10 A, negative current for 10 min)
of Pontamine Sky Blue for histological verification.
WIN55,212-2. The guide cannula was angled at 25° from the vertical axis
Single-unit extracellular recordings of DR 5-HT neurons. The dose–
and aimed 1 mm above the DR to allow termination of the injector tip
response of putative 5-HT neurons was assessed after single intravenous
within the nucleus (⫹1.2 mm from interaural zero, ⫾1.4 from midline,
(0.05– 0.8 mg/kg) and repeated intraperitoneal (0.05–2 mg/kg) adminis-
and ⫺6.0 mm from the dura mater). The cannula was fixed to the skull
trations of WIN55,212-2. A burr hole was drilled on the midline, 1.2 mm
with the use of skull screws and dental acrylic. A 1.5–2 h postoperative
anterior to interaural zero. Using a hydraulic micropositioner (model
interim period was allowed before the start of electrophysiological re-
650; David Kopf Instruments), the electrode was lowered into the DR.
cordings (as described above in experiment 2). Once a stably firing 5-HT
Putative 5-HT neurons were encountered immediately below the ventral
neuron was found and 1–3 min of baseline activity was established, mi-
border of the Sylvian aqueduct and abound between 5.0 and 6.5 mm
croinfusion of either vehicle (0.5 l) or WIN55,212-2 (5 g/0.5 l) was
ventral to the dura mater. These neurons under normal conditions were
initiated and continuously delivered for 3 min. Substances were infused
identified according to the following criteria: a slow (0.1– 4 Hz) and
into the DR via a 28 gauge stainless steel injector attached by polyethylene
prominently regular firing rate (coefficient of variation ranges from 0.12
tubing to a 1 ml syringe driven by a CMA 400 microdialysis syringe pump
to 0.87) and a broad positive action potential (0.8 –3.5 ms; 1.4 ms first
(CMA Microdialysis, Solma, Sweden). Changes in neuronal discharge
positive and negative deflections) (Baraban and Aghajanian, 1980; Allers
pattern were continually monitored for up to 45 min after the cessation
and Sharp, 2003). An inhibitory response to the GABA agonist baclofen
of microinfusion. The probability that results were confounded by ran-
after intravenous drug administrations also confirmed that neurons were
dom diffusion of solutions to neighboring structures was remote be-
5-HT because GABA receptors are almost exclusively limited to 5-HT
cause, aside from the strictly controlled injection volume, the vehicle
neurons in the DR (Wirtshafter and Sheppard, 2001).
used was relatively viscous and lipophilic compared with conventional
5-HT burst activity. Burst activity pattern was analyzed based on the
solvents. To mark the site of injection at the end of each experiment, a
criteria of Gobbi et al. (2005), such that a train of at least two spikes with
small volume (50 nl/cannula) of Pontamine Sky Blue was infused via the
an onset defined by a maximum initial interspike interval of 20 ms within
same injector that was used for drug injections or was iontophoretically
a regular low-frequency firing pattern was categorized as a burst. The
ejected from the recording pipette (see Fig. 5
C).
longest interspike interval allowed within a burst was 40 ms. In intraperi-
Experiment 4B: intracerebral microinfusion into the prefrontal cortex,
toneal experiments, two to four electrode descents were made to obtain a
intra-mPFCv. Simultaneous bilateral microinfusion of WIN55,212-2 (5
maximal sample of neurons across the rostrocaudal medial extent of the
g/0.5 l) into the ventromedial subregions of the mPFC (mPFCv) (pre-
DR presumed richest in 5-HT neurons (Descarries et al., 1982). Record-
limbic–infralimbic cortices; ⫹2.2 mm from bregma, ⫾0.5 from midline,
ings were also performed after coadministration of rimonabant (1 mg/
⫺3.5 mm from the dura mater) was performed using a bilateral guide
kg) injected intraperitoneally 10 min before the last WIN55,212-2 injec-
cannula (22 gauge, 1 mm center-to-center distance; Plastics One). The
tion. Single-unit activity was recorded as discriminated action potentials
rest of the procedure was as described in the intra-DR microinfusion
amplified by a Tennelec (Oakridge, TN) TB3 MDA3 amplifier, postam-
experiment (see above, experiment 4A).
plified and filtered by a Realistic 10 band frequency equalizer, digitalized
Experiment 4C: intracerebral microinfusion into the prefrontal cortex,
by a CED1401 interface system (Cambridge Electronic Design, Cam-
intra-latPFC. Simultaneous bilateral microinfusion of WIN55,212-2 (5
bridge, UK), processed on-line, and analyzed off-line by Spike2 software
g/0.5 l) into the latPFC (agranular insular cortex; ⫹3.2 mm from
version 5.05 for Windows PC (Microsoft, Seattle, WA). Changes in neu-
bregma, ⫾4.2 from midline, and ⫺4.2 mm from the dura mater) was
ronal firing pattern resulting from drug administrations were continu-
performed with two single guide cannulas (22 gauge; Plastics One). The
ously monitored; the first 30 s immediately after drug injections were not
rest of the procedure was as described in the intra-DR microinfusion
considered to eliminate injection artifacts. Neurons were considered
experiment (see above, experiment 4A).
nonresponding if percentage change from baseline firing activity after
Experiment 4D: intracerebral microinfusion into the prefrontal cortex,
drug administrations was ⬍10%.
intra-mPFCv with PFC transection. To confirm whether the integrity ofthe mPFC–DR pathway is required for the modulatory effect of
Experiment 3: PFC transections
WIN55,212-2 on DR 5-HT neurons, a final negative control procedure
To determine whether cannabinoid-induced modulation of DR 5-HT
was performed by combined microinfusion and electrophysiological
neurons originate in the PFC and are relayed via the descending corti-
techniques on PFC-transected rats. Anesthetized rats first underwent
coraphe fibers, systematic transections of the PFC–DR pathway were
transection of the PFC (1 mm anterior to bregma; see above, experiment
performed on anesthetized rats before electrophysiological recordings.
3) and immediately implanted with a bilateral cannula into the mPFCv,
For a total bilateral PFC transection (tPFC), a fine needle (0.35 mm
as described previously (2.2 mm anterior to bregma; see above, experi-
diameter) was initially positioned at 1 mm anterior to bregma and 0.8
ment 4B) anterior to the transection lesion. Then, following the proce-
mm left of the midline. It was lowered 6.8 mm from the dura mater,
dure used in experiment 4B, WIN55,212-2 was infused while performing
slowly slid leftward 5 mm from the midline (0.5 mm/min), and then
single-unit recording on a DR 5-HT neuron.
slowly retracted vertically using a micropositioner (10 m/min). Toavoid damaging the sinus, the needle was positioned 1.2 mm from the
Experiment 5: intracerebral mPFCv microinfusion and FST
midline on the right hemisphere, lowered 6.8 mm at a 20° angle, and then
Under Equithesin anesthesia (3 ml/kg) (1.96 g of sodium pentobarbital,
moved 5 mm rightward (modified after Diaz-Mataix et al., 2005). Selec-
8.5 g of chloral hydrate, 4.25 g of MgSO hexahydrate, 60 ml of propylene
tive transection of PFC subregions were performed by positioning the
glycol, 20 ml of ethanol, and 58 ml of distilled water, adjusted to 200 ml
needle 2.5 mm anterior to bregma, lowered 6.8 mm from the dura mater,
with distilled water), rats were mounted in a stereotaxic frame as de-
and moved 1.5 mm (on both sides) from the midline for an mPFC
scribed previously. Cannulation procedure was the same as in experi-
transection (areas transected included the dorsal peduncular, infralim-
ment 4B. After surgery, the incisions were stitched and applied with
bic, prelimbic Cg3, and cingulate Cg1 cortices) or from 1.5 to 5 mm for a
antiseptics. The cannulas were occluded with stainless steel wire stylets
transection of the lateral aspect of the prefrontal cortex (latPFC): mainly
(Plastics One) to maintain patency. A postoperative recovery period of
the lateral prefrontal/agranular insular, but also the frontal Fr2, Fr1, and
8 d was allowed. Thereafter, cannulated rats were submitted to the FST

Bambico et al. • CB Agonism Activates 5-HT Neurons through Prefrontal Cortex
J. Neurosci., October 24, 2007 • 27(43):11700 –11711 • 11703
(see above, experiment 1). A 15 min preswim test was conducted. Thenext day, rats were allowed to habituate to the testing room for 2 h beforemicroinfusion. Then, WIN55,212-2 (1 or 5 g in 0.5 l of vehicle) orvehicle (0.5 l) was continuously delivered for 3 min. In some cases,infusion of rimonabant [1 g (Caille´ and Parsons, 2006) in 0.25 l ofvehicle for 1.5 min] was performed 1 min before infusion ofWIN55,212-2 (1 g in 0.25 l of vehicle for 1.5 min). In all groups, totalinfusion volume was 0.5 l. The microinfusion connector assembly wasleft in place 4 more min to allow the drug solution to diffuse into thetarget structure (mPFCv). The rats were then placed in the cylindricalpool and subjected to the FST (5 min test swim recording).
HistologyHistological verification of the extent and selectivity of lesions imprintedby transections and cannula/microinjector trajectories were performedat the end of each experiment. The rats were deeply anesthetized (400mg/kg chloral hydrate, i.p.) and then perfused according to standardprocedures (fixative, 4% paraformaldehyde with 0.1% glutaraldehyde).
The brains were harvested and postfixed in 4% paraformaldehyde over-night and incubated in 30% sucrose for 2 d. Adjacent series of 20 –50 Abrain slices within the vicinity of lesions were cut using a freezing mic-rotome and stained with thionin acetate (Sigma) for light microscopicverification or stored in a cryoprotectant solution at ⫺20% untilinspection.
Statistical analysesSPSS (version 13; SPSS, Chicago, IL) was used to organize and statisti-cally analyze data. All data were expressed as mean ⫾ SEM and weresubmitted to parametric tests (one-way or two-way ANOVA), followed
Figure 1.
Antidepressant-like activity of WIN in the rat FST. A, Behavioral effects of
by Student's t test/Dunnett's test for post hoc comparisons. When as-
intraperitoneally administered vehicle, CIT (5 mg/kg, i.p.), DMI (10 mg/kg, i.p.), and WIN
sumptions of normality and variance homogeneity were not satisfied,
(0.05, 0.1, 0.2, 1.0, and 2.0 mg/kg, i.p.). Single injection of RIM (1 mg/kg, i.p.) 10 min
nonparametric tests (Kruskal–Wallis or Friedman's tests for ANOVA,
before administration of a low dose of WIN (0.2 mg/kg, i.p.) blocked the antidepressant-
Mann–Whitney U test for post hoc comparisons) were performed. Dun-
like effect. Single injection of RIM (1 mg/kg, i.p.) 10 min before administration of a high
nett's post hoc test was used for comparing with baseline drug-induced
dose of WIN (2 mg/kg, i.p.) did not modify the inert effect of WIN. Note that RIM by itself
changes in neuronal activity. Nonlinear curve fitting and the calculation
did not have any significant effect. B, The antidepressant-like effect of WIN (0.2 mg/kg,
were performed using Microcal Software (Northampton, MA)
i.p.) was abrogated by pretreatment with pCPA (150 mg/kg, i.p.) 72 and 48 h before
Origin (version 7). Fisher's exact test was used to assess the differential
pretest. Pretreatment of pCPA by itself did not have any significant effect. All treatments
response to CB R blockade versus TRPV1 blockade after intravenous
were administered 23, 5, and 0.75 h before test swim according to the method of Page et
administrations of rimonabant and capsazepine. Statistical values reach-
al. (1999). n ⫽ 8 –15 per treatment group. Bars represent mean ⫾ SEM total time of
ing p ⱕ 0.05 were considered significant.
behaviors indicated. *p ⬍ 0.05 or **p ⬍ 0.01 versus vehicle.
Results
Effect of subchronic low and high doses of WIN55,212-2 in
swimming behaviors were prevented by the coadministration of
To examine the possible dose-dependent effect of CB
1R antagonist RIM (1 mg/kg, i.p.) (WIN at 0.2 mg/kg plus
RIM at 1 mg/kg, i.p.; immobility, 122.52 ⫾ 26.49, ⫺23.38%, NS
on stress-coping behavior, we tested low and high doses of
vs VEH; swimming, 154.16 ⫾ 25.39, ⫹19.63%, NS vs VEH) (Fig.
WIN55,212-2 in the FST. A low dose of WIN55,212-2 (0.2 mg/kg,i.p.) compared with vehicle elicited a significant decrease in total
1 A). A high dose of WIN55,212-2 (2 mg/kg, i.p.) did not shorten
time spent in immobility (WIN at 0.2 mg/kg, 54.05 ⫾ 11.11;
total immobility duration (174.91 ⫾ 36.57, ⫺0.91%, NS vs VEH)
VEH, 159.9 ⫾ 10.94; ⫺66.2%, p ⬍ 0.01) (Fig. 1A) and a signifi-
and prolonged neither total swim duration (106.42 ⫾ 32.1,
cant increase in total swimming duration (WIN at 0.2 mg/kg,
⫹6.89%, NS vs VEH) nor total climbing duration (17.16 ⫾ 6.5,
220.08 ⫾ 10.39; VEH, 128.86 ⫾ 10.72; ⫹70.79%, p ⬍ 0.01) (Fig.
⫺29.79%, NS vs VEH) (Fig. 1A). This inert activity of the high
1 A) but did not affect total climbing duration [WIN at 0.2 mg/kg,
dose of WIN55,212-2 was not changed during coapplication with
24.73 ⫾ 4.79; VEH, 11.21 ⫾ 15; no significant difference (NS)]
RIM (WIN at 2.0 mg/kg plus RIM at 1 mg/kg, i.p; immobility,
(Fig. 1 A). These effects were comparable with those produced by
122.52 ⫾ 26.49; swimming, 154.16 ⫾ 25.39; or climbing, 23.4 ⫾
the clinically used selective serotonin reuptake inhibitor (SSRI)
2.0 vs VEH, NS) (Fig. 1 A). RIM (1 mg/kg, i.p.) did not induce any
antidepressant citalopram (5 mg/kg, i.p.; immobility, 47.8 ⫾
significant change in behavior (immobility, 128.32 ⫾ 23.26;
15.25, ⫺72.84%, p ⬍ 0.01 vs VEH; swimming, 218.83 ⫾ 25.4,
swimming, 159.34 ⫾ 22.48; or climbing, 12.46 ⫾ 2.0 vs VEH, NS)
⫹119.8%, p ⬍ 0.01 vs VEH; climbing, 33.33 ⫾ 13.78, ⫹36.37%,
(Fig. 1 A). The locomotor activity (movement velocity) of all
NS vs VEH) (Fig. 1 A). Conversely, the selective norepinephrine
drug-treated groups were not significantly different from that of
reuptake inhibitor DMI (10 mg/kg, i.p.) elicited a decrease in
the vehicle-treated group (CIT at 5 mg/kg, 433.24 ⫾ 19.52; DMI
total immobility duration (30 ⫾ 6.57, ⫺82.95%, p ⬍ 0.01 vs
at 10 mg/kg, 423.97 ⫾ 22.44; WIN at 0.05 mg/kg, 423.21 ⫾ 30.12;
VEH) and an increase in total climbing duration (142.01 ⫾ 19.6,
WIN at 0.10 mg/kg, 445.65 ⫾ 29.45; WIN at 0.2 mg/kg, 434.71 ⫾
⫹481.06%, p ⬍ 0.01 vs VEH) but not an increase in total swim-
26.31; WIN at 2.0 mg/kg, 371.26 ⫾ 33.16; WIN at 0.2 mg/kg plus
ming duration (127.99 ⫾ 14.88, ⫹28.56%, NS vs VEH), confirm-
RIM at 1 mg/kg, 476.82 ⫾ 21.46; WIN at 2.0 mg/kg plus RIM at 1
ing its primary action on noradrenergic transmission (Fig. 1 A).
mg/kg, 382.12 ⫾ 37.26; or RIM at 1 mg/kg, 399.92 ⫾ 22.50, NS vs
The effects of the low dose of WIN55,212-2 on immobility and
VEH, 433.23 ⫾ 24.21).

11704 • J. Neurosci., October 24, 2007 • 27(43):11700 –11711
Bambico et al. • CB Agonism Activates 5-HT Neurons through Prefrontal Cortex
Effect of pCPA pretreatment on WIN55,212-2 antidepressant-
like activity in the FST
Because the activity of the low dose of WIN55,212-2 in the FST
was similar to that of the SSRI citalopram, we tested whether this
antidepressant-like activity was via a main action on 5-HT trans-
mission as observed with SSRIs (Page et al., 1999). When injected
with the low dose of WIN55,212-2, rats pretreated with the
5-HT-depleting agent pCPA expressed neither increased swim-
ming behavior (pCPA plus WIN at 0.2 mg/kg, i.p., 166.35 ⫾
15.06 vs VEH plus VEH, 142.54 ⫾ 17.2, NS) (Fig. 1B) nor de-
creased immobility (pCPA plus WIN at 0.2 mg/kg, i.p., 112.54 ⫾
15.35 vs VEH plus VEH, 134.34 ⫾ 18.3, NS) (Fig. 1B). Pretreat-
ment of pCPA alone did not induce any effect significantly dif-
ferent from vehicle pretreatment (pCPA plus VEH; immobility,
118.57 ⫾ 9.72; swimming, 160.6 ⫾ 9.65; or climbing, 20.93 ⫾
2.24 vs VEH plus VEH, NS) (Fig. 1 B).
Effect of subchronic intraperitoneal doses of WIN55,212-2 on
5-HT single-unit activity
To test whether antidepressant-like behavioral effects of
WIN55,212-2 in the FST were paralleled by enhanced 5-HT neu-
ronal firing activity, we performed in vivo extracellular record-
ings of presumed 5-HT neurons following the same treatment
schedule as in the FST. WIN55,212-2 was administered 23, 5, and
0.75 h before electrophysiological recordings. In some animals,
RIM was injected intraperitoneally 10 min before the third ad-
ministration of 0.2 mg/kg WIN55,212-2. Mean spontaneous fir-
ing rate of 5-HT neurons showed a biphasic response profile after
incremental doses of WIN55,212-2. One-way ANOVA revealed a
dose-dependent increase with lower doses of WIN55,212-2
(VEH, 1.14 ⫾ 0.04; WIN at 0.05 mg/kg, 1.35 ⫾ 0.11; WIN at 0.1
mg/kg, 1.88 ⫾ 0.15; WIN at 0.2 mg/kg, 2.58 ⫾ 0.25; F
10.97; p ⬍ 0.01) (Fig. 2A), and the coadministration of RIMprevented this increase. A dose of 0.2 mg/kg WIN55,212-2yielded a maximal 126.32% increase in neuronal activity. Con-versely, a high dose of WIN55,212-2 (2.0 mg/kg) yielded a signif-icant decrease compared with vehicle (WIN at 2.0 mg/kg, 0.41 ⫾0.11, ⫺64%, p ⬍ 0.01 vs VEH) (Fig. 2A). We also calculated the
Figure 2.
Effect of intraperitoneal administration of WIN on DR 5-HT neurons. A, Effect of
WIN on 5-HT neuronal firing activity. WIN (0.05–2.0 mg/kg, i.p.) was administered 23, 5, and
mean number of neurons per electrode descent, which served as
0.75 h before electrophysiological recordings. Coapplication of RIM (1 mg/kg, i.p.) 10 min be-
an indirect measure of spontaneously active neurons (Gobbi et
fore WIN (0.2 mg/kg, i.p.) blocked the increase in spontaneous 5-HT single-unit firing activity.
al., 2007). Compared with vehicle injections (VEH, 3.75 ⫾ 0.16),
B, Effect of WIN on the number of spontaneously active 5-HT neurons. The number of sponta-
there were 28% more spontaneously active 5-HT neurons after
neously active neurons was calculated as the number of recorded 5-HT neurons per electrode
treatment with 0.1 mg/kg WIN55,212-2 (4.8 ⫾ 0.39, p ⬍ 0.05)
descent in each treatment group. Values at the base of each column in A denote the number of
and 33.33% more active neurons with 0.2 mg/kg WIN55,212-2
5-HT neurons recorded. Bars represent mean ⫾ SEM values. **p ⬍ 0.01 versus vehicle.
(5.0 ⫾ 0.46, p ⬍ 0.01), whereas a high dose of WIN55,212-2 hadfewer active neurons than the control (WIN at 2.0 mg/kg, 1.92 ⫾
but was blocked by RIM (1 mg/kg, i.v.) in 100% of neurons tested
0.39, ⫺48.8%, p ⬍ 0.01). The number of spontaneously active
(n ⫽ 4; Fisher's test, p ⫽ 0.01) (Fig. 3A). WIN55,212-2 treatment
neurons in rats treated with a low dose of WIN55,212-2 (0.2
also increased burst activity, a pattern of neural activity that is
mg/kg) coapplied with RIM (1.0 mg/kg) did not significantly
associated with enhanced 5-HT release in postsynaptic regions
differ from those treated with the vehicle (Fig. 2 B).
(Gartside et al., 2000), as well as antidepressant-like activity(Gobbi et al., 2005). The maximal increase in burst frequency
Effects of a single intravenous administration of low doses of
from baseline (percentage recorded spikes contained in bursts)
WIN55,212-2 on 5-HT firing activity
was recorded at 0.2 mg/kg (VEH, 3.71 ⫾ 1.16%; WIN at 0.05
To appraise whether the CB1R agonist WIN55,212-2 rapidly in-
mg/kg, 8.85 ⫾ 3.59%; WIN at 0.1 mg/kg, 9.61 ⫾ 3.15%; WIN at
fluences 5-HT neurotransmission, we measured the spontaneous
0.2 mg/kg, 12.51 ⫾ 4.07; 2
12.56; p ⬍ 0.01, Kruskal–Wallis
single-unit firing activity of DR 5-HT neurons after cumulative
test) (Fig. 3F, top). The maximal increase in the mean number of
intravenous administration of WIN55,212-2. Incremental doses
spikes in a burst was 324% occurring at 0.2 mg/kg (VEH, 0.63 ⫾
of WIN55,212-2 (0.05– 0.2 mg/kg) evoked a dose-dependent in-
0.13; WIN at 0.05 mg/kg, 1.23 ⫾ 0.42; WIN at 0.1 mg/kg, 1.28 ⫾
crease in 5-HT unit firing activity (F
4.64, p ⬍ 0.01, one-
0.32; WIN at 0.2 mg/kg, 2.67 ⫾ 1.3; 2(3)
10.01; p ⬍ 0.01,
way ANOVA; VEH, 1.04 ⫾ 0.10 Hz; WIN at 0.05 mg/kg, 1.17 ⫾
Kruskal–Wallis test) (data not shown). Mean burst length was
0.28 Hz; WIN at 0.1 mg/kg, 1.61 ⫾ 0.37; WIN at 0.2 mg/kg,
82.25, 235.35, and 175.83% greater than baseline (vehicle) after
2.04 ⫾ 0.27) (Fig. 3E), which was half-maximal (ED50) at a dose
incremental doses of 0.05, 0.1, and 0.2 mg/kg WIN55,212-2, re-
of 0.1 mg/kg and was not blocked by capsazepine (20 g/kg, i.v.)
spectively (VEH, 13.24 ⫾ 3.75 ms; WIN at 0.05 mg/kg, 24.13 ⫾

Bambico et al. • CB Agonism Activates 5-HT Neurons through Prefrontal Cortex
J. Neurosci., October 24, 2007 • 27(43):11700 –11711 • 11705
Effects of a single intravenous administration of high doses of
WIN55,212-2 on 5-HT firing activity
Remarkably,
WIN55,212-2 injected intravenously generally produced a de-cline in neuronal excitation significant at both 0.3 and 0.4 mg/kgand achieved a maximal level 45% below baseline (vehicle) after0.4 mg/kg WIN55,212-2 (VEH, 1.04 ⫾ 0.10 Hz; WIN at 0.04mg/kg, 0.59 ⫾ 0.08 Hz; F
6.7; p ⬍ 0.01) (Fig. 3E). A waning
of stimulatory effects was also observed with burst activity: burstfrequency (percentage of total number of recorded spikes; WINat 0.3 mg/kg, 3.9 ⫾ 2.85%; WIN at 0.4 mg/kg, 2.67 ⫾ 1.82; WINat 0.5 mg/kg, 10.51 ⫾ 6.21%) (Fig. 3F, top), mean number ofspikes in a burst (WIN at 0.3 mg/kg, 0.64 ⫾ 0.34; WIN at 0.4mg/kg, 1.83 ⫾ 1.11; WIN at 0.5 mg/kg, 1.5 ⫾ 0.5) (data notshown), mean burst length (WIN at 0.3 mg/kg, 20.73 ⫾ 15.94 ms;WIN at 0.4 mg/kg, 19.54 ⫾ 9.59 ms; WIN at 0.5 mg/kg, 15.53 ⫾7.75 ms) (Fig. 3F, bottom). The CB1R antagonist RIM (1 mg/kg,i.v.) partially reversed this decline only in one of four neurons(Fig. 3B). Three neurons were nonresponsive (Fig. 3C). In two ofthree neurons tested, capsazepine reversed the decrease inducedby high doses of WIN55,212-2 (Fig. 3D). This complex responsepattern suggests that the CB1R may not be involved in the 5-HTeffects induced by high doses of WIN55,212-2 (Fisher's test, p ⫽0.57, NS). Neither RIM (RIM at 1 mg/kg, i.v.) alone nor capsaz-epine (CPZ at 20 g/kg, i.v.) alone had a significant effect on5-HT single-unit firing activity (VEH, 1.3 ⫾ 0.33; CPZ, 1.2 ⫾0.57, n ⫽ 5; RIM, 1.48 ⫾ 0.52, n ⫽ 7).
5-HT single-unit activity after PFC transections
DR 5-HT neurons receive important excitatory inputs from py-
ramidal (glutamatergic) cells of the PFC (Jankowski and Sesack,
2004). To verify whether cortical CB1Rs in the PFC or its subre-
gions are essential in the control of DR 5-HT neurons by canna-
binoids, rats were subjected to a tPFC, latPFC, or mPFC deaffer-
entation by mechanical transection before 5-HT single-unit
recordings.
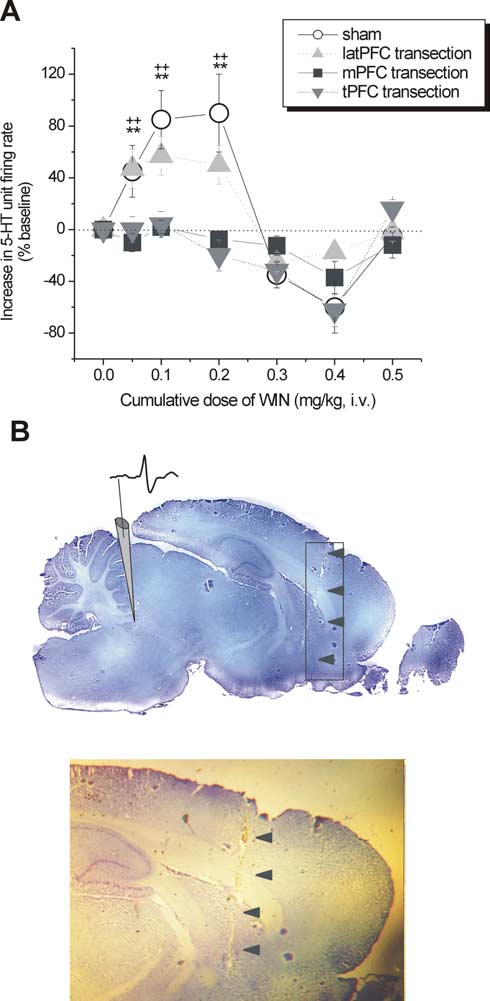
WIN55,212-2 failed to increase 5-HT single-unit firing activity atotherwise stimulatory doses in intact brains (tPFC transection:baseline, 1.46 ⫾ 0.39 Hz, n ⫽ 7; WIN at 0.05 mg/kg, 0.78 ⫾ 0.17Hz, n ⫽ 6; WIN at 0.1 mg/kg, 0.91 ⫾ 0.25 Hz, n ⫽ 5; WIN at 0.2mg/kg, 0.97
Figure 3.
Effect of intravenous administration of cumulative doses of WIN on DR 5-HT neu-
⫾ 0.29 Hz, n ⫽ 5; WIN at 0.3 mg/kg, 1.04 ⫾ 0.18 Hz,
rons. A–D, Integrated firing rate histograms of 5-HT neurons illustrating that low doses of WIN
n ⫽ 4; WIN at 0.4 mg/kg, 0.97 ⫾ 0.17 Hz, n ⫽ 4; WIN at 0.5
(0.1– 0.2 mg/kg, i.v.) rapidly increased single-unit firing activity. A, This effect was reversed by
mg/kg, 1.10 ⫾ 0.15 Hz, n ⫽ 4; WIN at 0.6 mg/kg, 1.31 ⫾ 0.40 Hz,
RIM (1.0 mg/kg, i.v.; n ⫽ 4) but not by CPZ (0.02 mg/kg, i.v.; n ⫽ 4). B–D, High dose of WIN
n ⫽ 3; p ⬍ 0.01) (Fig. 4A). We noted that, at doses higher than 0.6
(0.30 – 0.50 mg/kg, i.v.) rapidly decreased single-unit firing activity. This effect was reversed by
mg/kg intravenously, a moderate increase of firing activity can be
CPZ (0.02 mg/kg, i.v.) in two of three neurons (D) and partially reversed (B) or unreversed (C) by
elicited but was not sensitive to RIM (non-CB1R-selective),
RIM (1 mg/kg, i.v.) in one and three neurons, respectively. 5-HT neuronal firing rate in each
which may indicate unspecific binding (data not shown). To pin-
histogram is plotted as spikes per 10 s. Calibration bar on right side of each histogram, 1 min.
point the specific subregion of the PFC that is critical in mediat-
The vertical lines depicted below each histogram represent the frequency of neuronal burst
ing the modulation of 5-HT single-unit activity, we compared
activity such that each tick corresponds to a burst discharge event. E, WIN (0.05– 0.5 mg/kg,
transection of the mPFC with that of the latPFC. The response of
i.v.) produced a biphasic response profile in 5-HT single-unit activity. F, Line graphs showing
5-HT single units to the latPFC did not significantly differ from
that cumulative doses of WIN modulated 5-HT neuronal burst activity measured as percentageof spikes within bursts (top) and mean burst length (bottom). *p ⬍ 0.05 or **p ⬍ 0.01 vs
the control (baseline, 1.40 ⫾ 0.12 Hz, n ⫽ 4; WIN at 0.05 mg/kg,
baseline (vehicle).
1.97 ⫾ 0.57 Hz, n ⫽ 4; WIN at 0.1 mg/kg, 2.18 ⫾ 0.59 Hz, n ⫽ 4;WIN at 0.2 mg/kg, 0.96 ⫾ 0.02 Hz, n ⫽ 4; NS vs control, between-groups ANOVA) (Fig. 4 A). On the contrary, mPFC transection
10.79 ms; WIN at 0.1 mg/kg, 44.4 ⫾ 18.1 ms; WIN at 0.2 mg/kg,
produced an effect similar to tPFC transection and was signifi-
36.52 ⫾ 14.64 ms; 2
9.03; p ⬍ 0.05, Kruskal–Wallis test)
cantly different from the control (baseline, 0.79 ⫾ 0.22 Hz, n ⫽ 4;
(Fig. 3F, bottom). Among all neurons recorded, 66.67% (n ⫽ 16)
WIN at 0.05 mg/kg, 0.79 ⫾ 0.20 Hz, n ⫽ 4; WIN at 0.1 mg/kg,
of 5-HT neurons responded to increasing dose injections of
0.81 ⫾ 0.23 Hz, n ⫽ 4; WIN at 0.2 mg/kg, 0.69 ⫾ 0.26 Hz, n ⫽ 4;
WIN55,212-2, whereas 33.33% (n ⫽ 8) of neurons were nonre-
WIN at 0.3 mg/kg, ⫺0.62 ⫾ 0.30 Hz, n ⫽ 4; WIN at 0.4 mg/kg,
sponding. All responding and nonresponding neurons showed
0.71 ⫾ 0.21 Hz, n ⫽ 4; WIN at 0.6 mg/kg, 1.03 ⫾ 0.33 Hz, n ⫽ 4;
the same electrophysiological characteristics, were inhibited by
WIN at 0.7 mg/kg, 0.87 ⫾ 0.06, n ⫽ 4; p ⬍ 0.05 vs control,
baclofen, and were localized in the DR.
between-groups ANOVA) (Fig. 4 A), thus indicating that the me-
11706 • J. Neurosci., October 24, 2007 • 27(43):11700 –11711
Bambico et al. • CB Agonism Activates 5-HT Neurons through Prefrontal Cortex
Microinfusion of WIN55,212-2 into the mPFCv and DR:
electrophysiology on 5-HT neurons
To further localize the action of cannabinoids on 5-HT neurons,
local microinfusion experiments were performed. Because the
CB1R (Moldrich and Wenger, 2000) and CB1R mRNA (Ha¨ring et
al., 2007) are present in the DR, it appeared reasonable to begin
our attempt to localize the action of WIN55,212-2 within this
nucleus, with the hypothesis that cannabinoid-induced 5-HT
neuronal modulation occurs through local DR circuits. The mi-
croinfusion of WIN55,212-2 into the DR elicited a rapid increase
(63.64%) and decrease (30%) in single-unit firing activity in one
positive responder and one negative responder, respectively. The
other two of four neurons recorded were nonresponders (Fig.
5 A, B).
The mPFCv is functionally associated with stress and coping
mechanisms through the regulation of the DR (Amat et al., 2005).
Therefore, we further assessed the impact of CB1R activation inthe ventral prelimbic–infralimbic cortex (mPFCv) on 5-HTsingle-unit activity. To strengthen results obtained from the se-lective mPFC transection, we locally microinfused WIN55,212-2into the mPFCv of both cortical hemispheres. Corroborating theresults from the transections (experiment 3), a gradual increasein 5-HT single-unit firing activity was elicited in four of five(80%) neurons recorded. This increase plateaued after 10 –20min and was rapidly nullified by the injection of RIM (1 mg/kg,i.v.) (Fig. 6 A). Furthermore, microinfusion of WIN55,212-2 intothe same site in mPFC-transected brains (n ⫽ 2) (Fig. 6C) as wellas into the latPFC (n ⫽ 2) (Fig. 6D) did not elicit an increase in5-HT single-unit activity in support of the necessity of the mPFCin cannabinoid-induced modulation of DR 5-HT neurons.
Microinfusion of WIN55,212-2 into the mPFCv: behavior in
the FST
Because the results obtained from intracerebral WIN55,212-2
microinfusions with electrophysiology seemed to point to the
mPFCv as a structure that plays an important role in
cannabinoid-induced activation of DR 5-HT neurons, we there-
fore examined whether local bilateral microinfusion of
WIN55,212-2 into the mPFCv is sufficient to alter stress-coping
behaviors in the FST. Both microdoses of WIN55,212-2 used (1
and 5 g in 0.5 l of vehicle), compared with vehicle, produced a
reduction of 47.43 and 36.24%, respectively, in total immobility
time (VEH, 142.92 ⫾ 14.29 s; WIN at 1 g, 75.13 ⫾ 15.74 s; WIN
at 5 g, 90.12 ⫾ 20.93 s; p ⬍ 0.01 vs VEH) (Fig. 7) and an
enhancement of 38.78 and 32.31%, respectively, in total swim-
Figure 4.
Effect of PFC transections on the modulation of DR 5-HT neuronal activity by intravenous
ming time (VEH, 141.09 ⫾ 12.97 s; WIN at 1 g, 195.81 ⫾ 15.49
administration of WIN. A, Line graph showing the modulatory effect of cumulative doses of WIN
s; WIN at 5 g, 186.68 ⫾ 18.84 s; p ⬍ 0.01 vs VEH) (Fig. 7). There
(0 – 0.5 mg/kg) on 5-HT single-unit firing activity after tPFC (shaded inverted triangles), ablation of
were no significant changes observed in climbing behavior (VEH,
mPFC (shaded squares), or latPFC (shaded upright triangles) subregions compared with sham-
16.07 ⫾ 3.23 s; WIN at 1 g, 29.2 ⫾ 5.22 s; WIN at 5 g, 21.50 ⫾
exposed controls (open circles). tPFC and mPFC transections abrogated the excitatory response to low
7.43 s; NS vs VEH) (Fig. 7), implying that enhancement in nor-
doses of WIN (0.05– 0.2 mg/kg, i.v.), whereas latPFC transection did not significantly reduce the
adrenergic transmission may not be as important as enhance-
excitatory response to low doses of WIN (0.05– 0.2 mg/kg, i.v.). n ⫽ 3–7 animals per group. Valuesareexpressedasmean
mPFC transection versus control; ⫹⫹p ⬍ 0.01 tPFC transection versus control. B, Histological verifi-
antidepressant-like effects of WIN55,212-2 in the FST. A micro-
dose of RIM (1 g) that by itself did not induce any significant
all transections, and arrows point to an example of a cortical lesion trace on a midsagittal brain section
effect in the FST (immobility, 05.0 ⫾ 40; swimming, 170.5 ⫾
(⬃0.4 mmlateral tomidline according to Paxinosand Watson,1986).Shown is anillustrativedepic-
20.2; or climbing, 23.5 ⫾ 10 vs VEH, NS) blocked the effect of 1
tion of the electrode placement and a typical action potential waveform of a putative 5-HT neuron
g of WIN55,212-2 when microinfused 1 min before
(top) and a closer inspection of the lesion trace (bottom).
WIN55,212-2. In the open-field test, neither WIN55,212-2, RIM,nor RIM plus WIN55,212-2 induced a change in locomotor ac-
dial, but not lateral, parts are responsible for the 5-HT firing
tivity with the microdoses used, eliminating the possibility of a
activity enhancement by CB1R agonism. The transection proce-
false positive in the FST (VEH, 422.93 ⫾ 41.38; WIN at 1 g,
dure did not significantly modify the basal discharge rate of DR
400.31 ⫾ 50.86; WIN at 5 g, 397.89 ⫾ 64.02; RIM at 1 g plus
5-HT neurons as was also observed by Hajos et al. (1999).
WIN at 1 g, 431.43 ⫾ 42.12; or RIM at 1 g, 455.26 ⫾ 31.9).

Bambico et al. • CB Agonism Activates 5-HT Neurons through Prefrontal Cortex
J. Neurosci., October 24, 2007 • 27(43):11700 –11711 • 11707
direct CB1R activation that modulates 5-HTbecause it was blocked by the CB1R antago-nist rimonabant and the 5-HT-depletingagent pCPA. Highlighting the role of CB1Rin mood regulation, preclinical studies haveindeed shown that its genetic and pharma-cological blockade rendered animals moreemotionally reactive and anxious (Haller etal., 2002, 2004; Martin et al., 2002), suscepti-ble to chronic stress-induced anhedonia(Martin et al., 2002), and liable to impair-ments in hypothalamic–pituitary–adrenalregulation (Barna et al., 2004) reminiscent ofneuroendocrine dysfunction observed in de-pression. Interestingly, CB1R knock-outmice presented impaired extinction of aver-sive memories (Marsicano et al., 2002), in-voking the pathological hallmark of post-traumatic stress disorder, a conditionpossessing overlapping symptomatologyand high rate of comorbidity with majordepression
Antidepressant-like effects in the FSThave also been reported previously withthe
arachidonamide] (Hill and Gorzalka, 2005)and the direct CB1R agonist HU-210[3-(1,1-dimethylheptyl)-(⫺)-11-hydroxy-⌬8-tetrahydro-cannabinol] (Hill and Gorza-lka, 2005; Jiang et al., 2005). ChronicHU-210 treatment was also found to drivehippocampal cell proliferation (Jiang etal., 2005), believed to be a downstream se-quela of antidepressant treatment (Mal-berg et al., 2000). We recently demon-strated that the selective FAAH inhibitorURB597 cyclohexylcarbamic acid 3⬘-carbamoylbiphenyl-3yl
antidepressant-like properties in the FST,tail suspension test (Gobbi et al., 2005),and chronic mild stress paradigm (Borto-
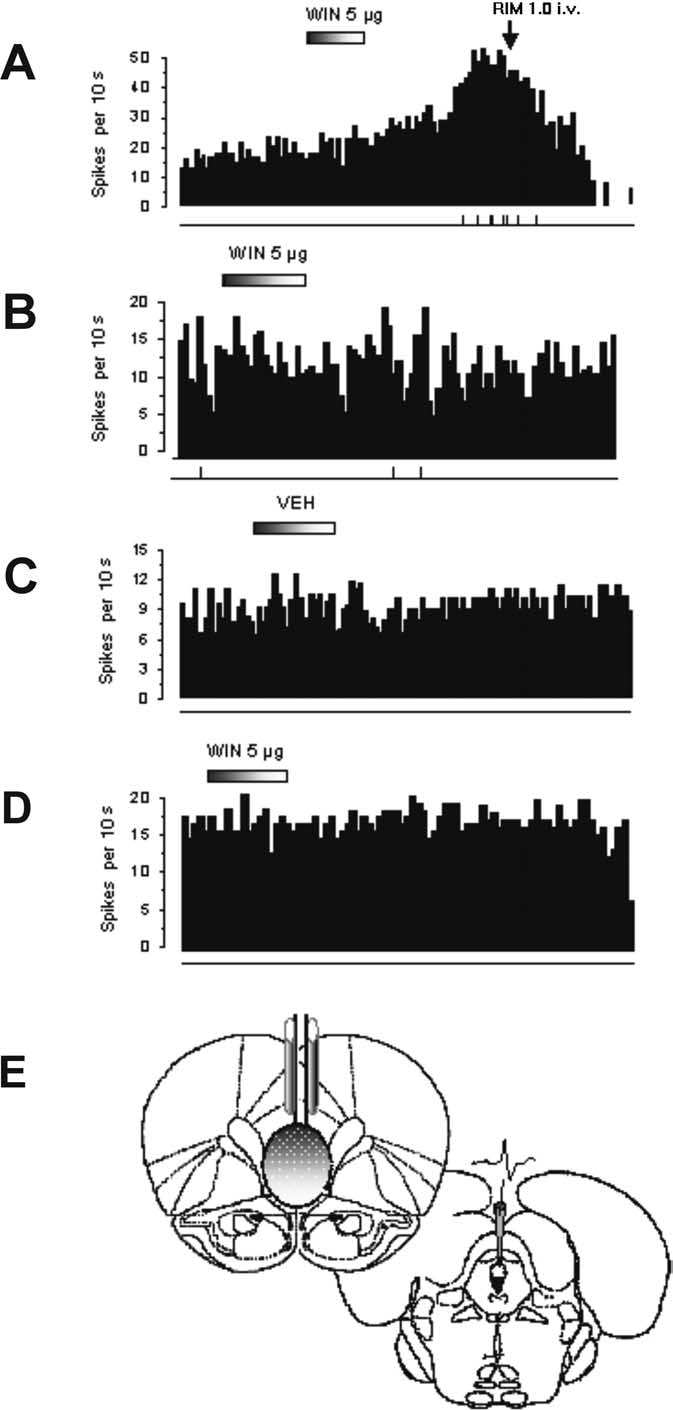
Figure 5.
Effect of WIN microinfused into the DR. A, Integrated firing rate histogram of a 5-HT neuron before and after intra-DR
lato et al., 2007), in support of the concept
microinfusion of vehicle (0.5 l) (n ⫽ 3 neurons). B, Integrated firing rate histogram of a 5-HT neuron before and after intra-DR
that the endocannabinoid system may
microinfusion of WIN (5 g in 0.5 l of vehicle) showing a slight increase in single-unit firing activity immediately after infusion
serve as target for depression therapy with-
observed in one of four neurons. Among the other three neurons, one showed a decrease whereas the other two did not respond
out the unwanted psychotropic effects of
at all. On each histogram, 5-HT neuronal firing rate is plotted as spikes per 10 s. Horizontal bar on top represents the time course
direct CB1R agonists. Moreover, perturba-
of infusion, and vertical lines at the bottom represent the frequency of neuronal burst activity such that each tick corresponds to a
tions in endocannabinoid signaling may
burst event. C, Left, An illustrative depiction of the electrode descent into the DR (shaded gray area) and the trajectory of the
very well be implicated in depression etiol-
microcannula based on the stereotaxic atlas of Paxinos and Watson (1986). Right, Histological verification of lesions imprinted by
ogy, supported by the following. First,
the electrode descent (left arrow) and of the microcannula (right arrow) on a coronal brain section (⬃1.2 anterior to interauralzero) showing the DR (shaded gray area). Bottom, Closer inspection of lesion traces.
chronic unpredictable stress, used tomodel anhedonia, a core depression
symptom, is associated with decreased en-
These results establish that low doses of a CB
docannabinoid 2-arachidonoylglycerol in the rat hippocampus (Hill
1R agonist elicit
potent antidepressant-like behavior and enhance 5-HT neuro-
et al., 2005). Second, in humans, upregulation of PFC CB1R in sui-
transmission, mediated by CB
cidal depressives may indicate an adaptive response to decreased
1R activation in the mPFCv. Con-
versely, high doses nullify antidepressant-like behavior and
endocannabinoids (Hungund et al., 2004). Third, randomized trials
markedly attenuate 5-HT neurotransmission, an effect that ap-
of the CB1R antagonist rimonabant for obesity management in-
pears to be instigated by a non-CB
creased adverse effects of depression and anxiety (Bronander and
1R mechanism.
Similar to antidepressants selectively blocking 5-HT reuptake
Bloch, 2007).
We demonstrated that WIN55,212-2 dose dependently en-
1R agonist WIN55,212-2 potently decreased immo-
bility and increased swim behavior in the FST. This was attributed to
hances the number, firing, and burst activity of spontaneously

11708 • J. Neurosci., October 24, 2007 • 27(43):11700 –11711
Bambico et al. • CB Agonism Activates 5-HT Neurons through Prefrontal Cortex
Figure 7.
Behavioral effects of bilateral microinfusion of WIN and RIM into the mPFCv in the
rat FST. WIN (1 or 5 g in 0.5 l of vehicle infused for 3 min) administered 7–10 min before theFST increased total time spent swimming and decreased total time spent immobile. Bilateralmicroinfusion of RIM (1 g in 0.25 l of vehicle for 1.5 min) 1 min before microinfusion of WIN(1 g in 0.25 l for 1.5 min) abrogated antidepressant-like effect. RIM (1 g in 0.5 l ofvehicle for 3 min) did not have any significant effect. n ⫽ 7–11 animals per treatment group.
**p ⬍ 0.01 versus vehicle.
active DR 5-HT neurons, corroborating microdialysis experi-ments that found increased synaptic 5-HT release in subcorticaltarget regions (Fadda et al., 2006). We reported that elevatingintrinsic anandamide through URB597 similarly elicited in-creased 5-HT activity (Gobbi et al., 2005). Both URB597 andWIN55,212-2 effects were CB1R mediated because these wereblocked by rimonabant. Noteworthy, the effects here observedwith WIN55,212-2 were markedly different from those exertedby URB597 in at least two features. First, WIN55,212-2 elicited arapid response as opposed to the 2 h delay with URB597; thisdifference might be ascribed to the kinetics of FAAH inhibitionpreceding anandamide-induced CB1R activation. Second, higherURB597 doses produced an enduring (plateau effect) excitation(Gobbi et al., 2005), whereas higher doses of WIN55,212-2 re-sulted in a rapid decline in excitation; this difference could beattributable to the fact that direct CB1R agonists activate whole-brain CB1Rs, whereas FAAH inhibitors indirectly activate a sub-population of these receptors colocalized with FAAH.
We identified that a CB1R subpopulation mediating 5-HT
excitatory response to WIN55,212-2 is localized in the mPFC, themain source of cortical afferents to the DR (Hajos et al., 1999;Jankowski and Sesack, 2004). Convergent with results shownhere, limbic 5-HT was enhanced after electrical stimulation of themPFC but not of the latPFC (Juckel et al., 1999). Indeed, transect-ing mPFC efferents to the DR abolished the response of all re-corded DR 5-HT neurons to the excitatory dose of WIN55,212-2.
Figure 6.
Effect of bilateral microinfusion of WIN into the mPFCv and latPFC on DR 5-HT
This was not observed after latPFC transection because of the
neuronal activity. A, Integrated firing rate histogram of a 5-HT neuron showing a robust but
slow-onset increase in single-unit activity after intra-mPFCv infusion of WIN (5
absence of DR afferents from this area (Peyron et al., 1998). We
g in 0.5 l of
vehicle) in four of five neurons. This effect was abrogated by RIM (1.0 mg/kg, i.v.). B, Integrated
surmise that the mPFCv particularly contained this receptor sub-
firing rate histogram of a 5-HT neuron before and after intra-mPFCv infusion of WIN (5 g in 0.5
population because WIN55,212-2 bilaterally infused into this
l of vehicle) showing an abolition of increased single-unit activity resulting from total pre-
subregion markedly decreased FST immobility and increased
frontocortical transection (n ⫽ 2 neurons). The microinfusion site was anterior to the transec-
basal discharge activity of DR 5-HT neurons. These effects were
tion lesion. C, Integrated firing rate histogram of a 5-HT neuron before and after intra-mPFCv
blocked by rimonabant.
infusion of vehicle (0.5 l), showing no apparent effect on neuronal activity (n ⫽ 4 neurons). D,
The action of WIN55,212-2 may be explained by an enhanced
Integrated firing rate histogram showing that intra-latPFC infusion of WIN (5 g in 0.5 l of
feedforward excitatory input along mPFC–DR monosynaptic
vehicle) did not produce an increase in 5-HT single-unit activity (n ⫽ 2 neurons). On each
projections to 5-HT neurons possibly driven by disinhibiting py-
histogram, 5-HT neuronal firing rate is plotted as spikes per 10 s. Horizontal bar represents the
ramidal neurons (Fortin et al., 2004). Disinhibition was likely
time course of infusion, and vertical lines at the bottom represent the frequency of neuronal
burst discharge such that each tick corresponds to a burst event. E, Illustrative depiction of the
engaged by CB1R inhibitory control of cortical interneurons
placement of cannulas directed into the mPFCv (shaded region area, bregma ⫹2.2) and the
(Trettel and Levine, 2002), in agreement with the Gi/o-protein-
electrode descent into the dorsal raphe nucleus (interaural 0 ⫹ 1.2), based on the stereotaxic
linked transduction mechanism known to be associated with it
atlas of Paxinos and Watson (1986).
and the resultant inhibition of voltage-sensitive calcium channels
Bambico et al. • CB Agonism Activates 5-HT Neurons through Prefrontal Cortex
J. Neurosci., October 24, 2007 • 27(43):11700 –11711 • 11709
(Piomelli, 2003). This view is strengthened by the abundant peri-
et al., 2004), periaqueductal gray (Maione et al., 2006), and hip-
somatic expression of CB
pocampus (Hajos and Freund, 2002).
1Rs in neocortical and PFC interneu-
rons (Tsou et al., 1998; Marsicano and Lutz, 1999). Also, canna-
Altogether, these data are highly suggestive of a significant role
binoids have been shown to increase cortical excitatory
of the mPFCv in mood control and in DR 5-HT activity through
transmission and net spiking probability of pyramidal neurons in
CB1R. We cannot, however, completely rule out the contribu-
vivo (Pistis et al., 2001; Fortin et al., 2004), consistent with a
tions of other brain regions and neurotransmitter systems that
concurrent increment of basal glutamate levels and decrement of
can act in concert with the mPFCv. The observed difference be-
basal GABA levels in prefrontocortical microdialysis experiments
tween systemic and intra-mPFCv WIN55,212-2 on the magni-
observed in anesthetized (Pistis et al., 2002) and awake (Ferraro et
tude of FST effects may reflect extra-mPFCv contributions. CB1R
al., 2001) rats, as well as in prefrontocortical in vitro culture (Fer-
agonists also modulate neuronal activity in various subcortical
raro et al., 2001; Tomasini et al., 2002).
structures, e.g., the VTA (Diana et al., 1998), amygdala (Pistis et
The degree of controllability over stressors is processed by the
al., 2004), and locus ceruleus (Muntoni et al., 2006), all known to
mPFCv, which in turn influences brainstem monoaminergic ac-
send afferents to the DR. Additional studies are underway to
tivity, particularly the DR (Amat et al., 2005, 2006; Maier et al.,
evaluate the influences of these areas on the activation of DR by
2006). As such, it can be posited that the integrity of DR function
in normosensitive states in relation to stress-coping and mood-
Finally, this study confirms the emerging concept that the
related behaviors relies on the efficiency of mPFCv pyramidal
CB1R is an important new target in the development of antide-
activity. The therapeutic relevance of increasing pyramidal trans-
pressant drugs. However, the challenge in the discovery of novel
mission becomes explicit on consideration that simply brief ex-
cannabinoid-derived agents lies in the development of agonists
posures to uncontrollable stress already can inflict significant
with selective antidepressant properties, and that minimize the
dendritic retraction of infralimbic pyramidal neurons and impair
unwanted psychotropic effects of cannabis.
stress-coping and fear extinction in murines (Izquierdo et al.,2006). Incidentally, hyperactivating anandamide through FAAH
knock-out, thereby enhancing intrinsic CB1R activity, has been
Allers KA, Sharp T (2003) Neurochemical and anatomical identification of
observed to modulate PFC plasticity, significantly increasing
fast- and slow-firing neurons in the rat dorsal raphe nucleus using juxta-
dendritic spine density (Patel et al., 2007). This neuroplastic
cellular labeling methods in vivo. Neuroscience 122:193–204.
change is as well akin to an antidepressant-like effect on the
Amat J, Baratta MV, Paul E, Bland ST, Watkins LR, Maier SF (2005) Medial
mPFC observed after chronic antidepressant treatment (Sairanen
prefrontal cortex determines how stressor controllability affects behav-iour and dorsal raphe nucleus. Nat Neurosci 8:365–371.
et al., 2007).
Amat J, Paul E, Zarza C, Watkins LR, Maier SF (2006) Previous experience
Finally, we presented evidence that increasing WIN55,212-2 dose
with behavioral control over stress blocks the behavioral and dorsal raphe
produces a bidirectional profile in the modulation of 5-HT neuronal
nucleus activating effects of later uncontrollable stress: role of ventral
firing and burst activity, as well as in FST antidepressant-like behav-
medial prefrontal cortex. J Neurosci 26:13264 –13272.
ior. This effect mirrors classical biphasic/bidirectional biochemical,
American Psychiatric Association (1994) Diagnostic and statistical manual
physiological, and psychopharmacological modulations by cannabi-
of mental disorders (DSM-IV-R), Ed 4. Washington, DC: American Psy-chiatric Association.
noids reported previously (for review, see Chaperon and Thiebot,
Ashton H, Golding J, Marsh VR, Millman JE, Thompson JW (1981) The
1999). Moreover, this bidirectional effect may explain the succession
seed and the soil: effect of dosage on the response to delta-9-
of euphoria and dysphoria during cannabis intoxication (American
tetrahydrocannabinol in man. Br J Clin Pharmacol 12:705–720.
Psychiatric Association, 1994; Iversen, 2003), a phenomenon vali-
Ashton CH, Moore PB, Gallagher P, Young AH (2005) Cannabinoids in
dated by neuropsychological measures (Ashton et al., 1981).
bipolar affective disorder: a review and discussion of their therapeutic
Although we point to mPFCv CB
potential. J Psychopharmacol 19:293–300.
1R as instrumental to
Banerjee SP, Snyder SH, Mechoulam R (1975) Cannabinoids: influence on
WIN55,212-2-induced antidepressant-like effect and 5-HT ac-
neurotransmitter uptake in rat brain synaptosomes. J Pharmacol Exp
tivity enhancement, the 5-HT-decreasing effect appeared to be
Ther 194:74 – 81.
independent of CB1R. The inert effect of high WIN55,212-2
Baraban JM, Aghajanian GK (1980) Suppression of firing activity of 5-HT
doses in the FST and the decline in 5-HT excitation was generally
neurons in the dorsal raphe by alpha-adrenoceptor antagonists. Neuro-
nonsensitive to rimonabant. The TRPV1 antagonist capsazepine
reversed the decline in 5-HT excitation induced by high
Barna I, Zelena D, Arszovszki AC, Ledent C (2004) The role of endogenous
cannabinoids in the hypothalamo-pituitary-adrenal axis regulation: in
WIN55,2212-2 doses. Interestingly, TRPV1 is expressed in both
vivo and in vitro studies in CB receptor knockout mice. Life Sci
DR and PFC (Liapi and Wood, 2005) and is implicated in anxiety,
75:2959 –2970.
conditioned fear, and hippocampal long-term potentiation
Blier P, de Montigny C (1999) Serotonin and drug-induced therapeutic re-
(Marsch et al., 2007) and in schizophrenia (Chahl, 2007), whose
sponses in major depression, obsessive-compulsive and panic disorders.
negative symptoms overlap with depression. Second, we consider
Neuropsychopharmacology 22:346 –356.
the possible role of the putative CB
Bortolato M, Mangieri RA, Fu J, Kim JH, Arguello O, Duranti A, Tontini A,
3R or a non-CB1 cannabinoid
Mor M, Tarzia G, Piomelli D (2007) Antidepressant-like activity of the
receptor possessing a lower affinity to WIN55,212-2 proposed to
fatty acid amide hydrolase inhibitor URB597 in a rat model of chronic
be present in glutamatergic terminals and thus in a position to
mild stress. Biol Psychiatry, in press.
inhibit the release of excitatory amino acids (Hajos and Freund,
Bronander KA, Bloch MJ (2007) Potential role of the endocannabinoid re-
2002). Indeed, a reduction of evoked glutamate-mediated synap-
ceptor antagonist rimonabant in the management of cardiometabolic
tic currents in 5-HT neurons was observed in acute DR slice
risk: a narrative review of available data. Vasc Health Risk Manag
preparations (Haj-Dahmane and Shen, 2005). Third, CB
nists may differentially act on GABAergic and glutamatergic
Caille´ S, Parsons LH (2006) Cannabinoid modulation of opiate reinforce-
ment through the ventral striatopallidal pathway. Neuropsychopharma-
pathways as observed in the ventral tegmental area (VTA) (Melis
cology 31:804 – 813.
et al., 2004; Riegel and Lupica, 2004). Interestingly, a dual recep-
Chahl LA (2007) TRP's: links to schizophrenia. Biochim Biophys Acta
tor mechanism was also reported to occur in the amygdala (Pistis
1772:968 –977.
11710 • J. Neurosci., October 24, 2007 • 27(43):11700 –11711
Bambico et al. • CB Agonism Activates 5-HT Neurons through Prefrontal Cortex
Chaperon F, Thiebot MH (1999) Behavioral effects of cannabinoid agents
Hill MN, Gorzalka BB (2005) Pharmacological enhancement of cannabi-
in animals. Crit Rev Neurobiol 13:243–281.
noid CB receptor activity elicits an antidepressant-like response in the rat
Cryan JF, Valentino RJ, Lucki I (2005) Assessing substrates underlying the
forced swim test. Eur Neuropsychopharmacol 15:593–599.
behavioural effects of antidepressants using the modified rat forced swim-
Hill MN, Patel S, Carrier EJ, Rademacher DJ, Ormerod BK, Hillard CJ, Gor-
ming test. Neurosci Biobehav Rev 29:547–569.
zalka BB (2005) Downregulation of endocannabinoid signaling in the
Deroche-Gamonet V, Le Moal M, Piazza PV, Soubrie P (2001) SR141716A,
hippocampus following chronic unpredictable stress. Neuropsychophar-
a CB receptor antagonist, decreases the sensitivity to the reinforcing
macology 30:508 –515.
effects of electrical brain stimulation in rats. Psychopharmacology (Berl)
Hill MN, Sun JC, Tse MT, Gorzalka BB (2006) Altered responsiveness of
157:254 –259.
serotonin receptor subtypes following long-term cannabinoid treatment.
Descarries L, Watkins KC, Garcia S, Beaudet A (1982) The serotonin neu-
Int J Neuropsychopharmacol 9:277–286.
rons in nucleus raphe dorsalis of adult rat: a light and electron microscope
Huestis MA, Gorelick DA, Heishman SJ, Preston KL, Nelson RA, Moolchan
radioautographic study. J Comp Neurol 207:239 –254.
ET, Frank RA (2001) Blockade of effects of smoked marijuana by the
Diana M, Melis M, Gessa GL (1998) Increase in meso-prefrontal dopami-
CB -selective cannabinoid receptor antagonist SR141716. Arch Gen Psy-
nergic activity after stimulation of CB receptors by cannabinoids. Eur
J Neurosci 10:2825–2830.
Hungund BL, Vinod KY, Kassir SA, Basavarajappa BS, Yalamanchili R, Coo-
Diaz-Mataix L, Scorza MC, Bortolozzi A., Toth M, Celada P, Artigas F (2005)
per TB, Mann JJ, Arango V (2004) Upregulation of CB receptors and
Involvement of 5-HT
receptors in prefrontal cortex in the modulation
agonist-stimulated [35S]GTPgammaS binding in the prefrontal cortex of
of dopaminergic activity: role in atypical antipsychotic action. J Neurosci
depressed suicide victims. Mol Psychiatry 9:184 –190.
Iversen L (2003) Cannabis and the brain. Brain 126:1252–1270.
Egertova M, Giang DK, Cravatt BF, Elphick MR (1998) A new perspective
Izquierdo A, Wellman CL, Holmes A (2006) Brief uncontrollable stress
on cannabinoid signalling: complementary localization of fatty acid
causes dendriticretraction in infralimbic cortex and resistance to fear ex-
amide hydrolase and the CB receptor in rat brain. Proc Biol Sci
tinction in mice. J Neurosci 26:5733–5738.
Jankowski MP, Sesack SR (2004) Prefrontal cortical projections to the rat
Egertova M, Cravatt BF, Elphick MR (2003) Comparative analysis of fatty
dorsal raphe nucleus: ultrastructural features and associations with sero-
acid amide hydrolase and CB cannabinoid receptor expression in the
tonin and gamma-aminobutyric acid neurons. J Comp Neurol
mouse brain: evidence of a widespread role for fatty acid amide hydrolase
468:518 –529.
in regulation of endocannabinoid signaling. Neuroscience 119:481– 496.
Jiang W, Zhang Y, Xiao L, Van Cleemput J, Ji SP, Bai G, Zhang X (2005)
Fadda P, Scherma M, Salis P, Mascia P, Fattore L, Fratta W (2006) Involve-
Cannabinoids promote embryonic and adult hippocampus neurogenesis
serotonergic receptors in the anxiety-like effects in-
and produce anxiolytic- and antidepressant-like effects. J Clin Invest
duced by the CB receptor agonist WIN55,212-2. International Cannabi-
115:3104 –3116.
noid Research Society, 16th Annual Symposium on the Cannabinoids,
Johnson KM, Ho BT, Dewey WL (1976) Effects of delta9-tetrahydrocannabinol
Tihany, Hungary, June 24 –28.
on neurotransmitter accumulation and release mechanisms in rat forebrain
Ferraro L, Tomasini MC, Cassano T, Bebe W, Siniscalchi A, O'Connor WT,
synaptosomes. Life Sci 19:347–356.
Magee P, Tanganelli S, Cuomo V, Antonelli T (2001) Cannabinoid re-
Juckel G, Mendlin A, Jacobs BL (1999) Electrical stimulation of rat medial
ceptor agonist WIN55,212-2 inhibits rat cortical dialysate gamma-
prefrontal cortex enhances forebrain serotonin output: implications for
aminobutyric acid levels. J Neurosci Res 66:298 –302.
electroconvulsive therapy and transcranial magnetic stimulation in de-
Fortin DA, Trettel J, Levine ES (2004) Brief trains of action potentials en-
hance pyramidal neuron excitability vie endocannabinoid-mediated sup-
Liapi A, Wood JN (2005) Extensive co-localization and heteromultimer for-
pression of inhibition. J Neurophysiol 92:2105–2112.
mation of the vanilloid receptor-like protein TRPV and the capsaicin
Gartside SE, Hajos-Korcsok E, Bagdy E, Harsing Jr LG, Sharp T, Hajos M
in the adult rat cerebral cortex. Eur J Neurosci
(2000) Neurochemical and electrophysiological studies on the func-
22:825– 834.
tional significance of burst firing in serotonergic neurons. Neuroscience
Lucki I (1997) The forced swimming test as a model for core and compo-
nent behavioural effects of antidepressant drugs. Behav Pharmacol
Gobbi G, Bambico FR, Mangieri R, Bortolato M, Campolongo P, Solinas M,
Cassano T, Morgese MG, Debonnel G, Duranti A, Tontini A, Tarzia G,
Maier SF, Amat J, Baratta MV, Paul E, Watkins LR (2006) Behavioural con-
Mor M, Trezza V, Goldberg SR, Cuomo V, Piomelli D (2005)
trol, the medial prefrontal cortex, and resilience. Dialogues Clin Neurosci
Antidepressant-like activity and modulation of brain monoaminergic
8:397– 406.
transmission by blockade of anandamide hydrolysis. Proc Natl Acad Sci
Maione S, Bisogno T, de Novellis V, Palazzo E, Cristino L, Valenti M,
USA 102:18620 –18625.
Petrosino S, Guglielmotti V, Rossi F, Di Marzo V (2006) Elevation of
Gobbi G, Cassano T, Radja F, Morgese MG, Cuomo V, Santarelli L, Hen R,
endocannabinoid levels in the ventrolateral periaqueductal grey through
Blier P (2007) Neurokinin 1 receptor antagonism requires norepineph-
inhibition of fatty acid amide hydrolase affects descending nociceptive
rine to increase serotonin function. Eur Neuropsychopharmacol
pathways via both cannabinoid receptor type 1 and transient receptor
17:328 –338.
potential vanilloid type-1 receptors. J Pharmacol Exp Ther 316:969 –982.
Haj-Dahmane S, Shen RY (2005) The wake-promoting peptide orexin-B
Malberg JE, Eisch AJ, Nestler EJ, Duman RS (2000) Chronic antidepressant
inhibits glutamatergic transmission to dorsal raphe nucleus serotonin
treatment increases neurogenesis in adult rat hippocampus. J Neurosci
neurons through retrograde endocannabinoid signaling. J Neurosci
20:9104 –9110.
25:896 –905.
Marsch R, Foeller E, Rammes G, Bunck M, Kossl M, Holsboer F, Zieglgan-
Hajos N, Freund TF (2002) Pharmacological separation of cannabinoid
sberger W, Landgraf R, Lutz B, Wotjak CT (2007) Reduced anxiety, con-
sensitive receptors on hippocampal excitatory and inhibitory fibers. Neu-
ditioned fear, and hippocampal long-term potentiation in transient re-
ceptor potential vanilloid type 1 receptor-deficient mice. J Neurosci
Hajos M, Hajos-Korcsok E, Sharp T (1999) Role of the medial prefrontal
27:832– 839.
cortex in 5-HT1A receptor-induced inhibition of 5-HT neuronal activity
Marsicano G, Lutz B (1999) Expression of the cannabinoid receptor CB in
in the rat. Br J Pharmacol 126:1741–1750.
distinct neuronal subpopulations in the adult mouse forebrain. Eur
Haller J, Bakos N, Szirmay M, Ledent C, Freund TF (2002) The effects of
J Neurosci 11:4213– 4225.
genetic and pharmacological blockade of CB cannabinoid receptor on
Marsicano G, Wotjak CT, Azad SC, Bisogno T, Rammes G, Cascio MG,
anxiety. Eur J Neurosci 16:1395–1398.
Hermann H, Tang J, Hofmann C, Zieglgansberger W, Di Marzo V, Lutz B
Haller J, Varga B, Ledent C, Barna I, Freund TF (2004) Context-dependent
(2002) The endogenous cannabinoid system controls extinction of aver-
effects of CB cannabinoid gene disruption on anxiety-like and social
sive memories. Nature 418:530 –534.
behaviour in mice. Eur J Neurosci 19:1906 –1912.
Martin M, Ledent C, Parmentier M, Maldonado R, Valverde O (2002) In-
Ha¨ring M, Marsicano G, Lutz B, Monory K (2007) Identification of the
volvement of CB cannabinoid receptors in emotional behaviour. Psy-
cannabinoid receptor type 1 in serotonergic cells of raphe nuclei in mice.
chopharmacology (Berl) 159:379 –387.
Matthew RJ, Wilson WH, Turkington TG, Hawk TC, Coleman RE, DeGrado
Bambico et al. • CB Agonism Activates 5-HT Neurons through Prefrontal Cortex
J. Neurosci., October 24, 2007 • 27(43):11700 –11711 • 11711
TR, Provenzale J (2002) Time course of tetrahydrocannabinol-induced
mice: strain differences and the effects of imipramine. Eur J Pharmacol
changes in regional cerebral blood flow measured with positron emission
tomography. Psychiatry Res Neuroimaging 116:173–185.
Riegel AC, Lupica CR (2004) Independent presynaptic and postsynaptic
Melis M, Pistis M, Pera S, Muntoni AL, Pillola G, Gessa GL (2004) Endo-
mechanisms regulate endocannabinoid signaling at multiple synapses in
cannabinoids mediate presynaptic inhibition of glutamatergic transmis-
the ventral tegmental area. J Neurosci 24:11070 –11078.
sion in rat ventral tegmental area dopamine neurons through activation
Sairanen M, O'Leary OF, Knuuttila JE, Castre´n (2007) Chronic antidepres-
of CB receptors. J Neurosci 24:53– 62.
sant treatment selectively increases expression of plasticity-related pro-
Moldrich G, Wenger T (2000) Localization of the CB cannabinoid receptor
teins in the hippocampus and medial prefrontal cortex of the rat. Neuro-
in the rat brain: an immunohistochemical study. Peptides 21:1735–1742.
science 144:368 –374.
Muntoni AL, Pillolla G, Melis M, Perra S, Gessa GL, Pistis M (2006) Canna-
Shearman LP, Rosko KM, Fleischer R, Wang J, Xu S, Tong XS, Rocha BA
binoids modulate spontaneous neuronal activity and evoked inhibition of
(2003) Antidepressant-like and anorectic effects of the cannabinoid re-
locus coeruleus noradrenergic neurons. Eur J Neurosci 23:2385–2394.
ceptor inverse agonist AM251 in mice. Behav Pharmacol 14:573–582.
Navarro M, Hernandez E, Mun
˜oz RM, del Arco I, Villanua MA, Carrera MR,
Tomasini MC, Ferraro L, Bebe BW, Tanganelli S, Cassano T, Cuomo V,
Rodriguez de Fonseca F (1997) Acute administration of the CB canna-
Antonelli T (2002) Delta(9)-tetrahydrocannabinol increases endoge-
binoid receptor antagonist SR141716A induces anxiety-like responses in
nous extracellular glutamate levels in primary cultures of rat cerebral
the rat. NeuroReport 8:491– 496.
cortex neurons: involvement of CB receptors. J Neurosci 68:449 – 453.
Page ME, Detke MJ, Dalvi A, Kirby LG, Lucki I (1999) Serotonergic media-
Trettel J, Levine ES (2002) Cannabinoids depress inhibitory synaptic inputs
tion of the effects of fluoxetine, but not desipramine, in the rat forced
received by layer 2/3 pyramidal neurons of the neocortex. J Neurophysiol
swimming test. Psychopharmacology (Berl) 147:162–167.
88:534 –539.
Patel S, Wang HD, Hillard CJ, Deutch AY (2007) Endocannabinoid signal-
Tsou K, Brown S, Sanudo-Pena MC, Mackie K, Walker JM (1998) Immu-
ing modulates dendritic spine density in prefrontal cortical pyramidal
nohistochemical distribution of cannabinoid CB receptors in the rat
cells. International Cannabinoid Research Society, 17th Annual Sympo-
central nervous system. Neuroscience 83:393– 411.
sium on the Cannabinoids, Saint-Sauveur, Quebec, Canada, June 26 –30.
Vieweg WV, Julius DA, Fernandez A, Beatty-Brooks M, Hettema JM, Pan-
Paxinos G, Watson C (1986) The rat brain in stereotaxic coordinates. San
durangi AK (2006) Posttraumatic stress disorder: clinical features,
Diego: Academic.
pathophysiology, and treatment. Am J Med 119:383–390.
Peyron C, Petit JM, Rampon C, Jouvet M, Luppi PH (1998) Forebrain af-
Viveros MP, Marco EM, File SE (2005) Endocannabinoid system and stress
ferents to the rat dorsal raphe nucleus demonstrated by retrograde and
and anxiety responses. Pharmacol Biochem Behav 81:331–342.
anterograde tracing methods. Neuroscience 82:443– 468.
Volkow ND, Gillespie H, Mullani N, Tancredi L, Grant C, Ivanovic M, Hol-
Piomelli D (2003) The molecular logic of endocannabinoid signaling. Nat
lister L (1991) Cerebellar metabolic activation by delta-9-tetrahydro-
Rev Neurosci 4:873– 884.
cannabinol in human brain: a study with positron emission tomography
Pistis M, Porcu G, Melis M, Diana M, Gessa GL (2001) Effects of cannabi-
and 18F-2-fluoro-2-deoxyglucose. Psychiatry Res 40:69 –78.
noids on prefrontal neuronal responses to ventral tegmental area stimu-
Ware MA, Adams H, Guy GW (2005) The medicinal use of cannabis in the
lation. Eur J Neurosci 14:96 –102.
UK: results of a nationwide survey. Int J Clin Pract 59:291–295.
Pistis M, Ferraro L, Pira L, Flore G, Tanganelli S, Gessa GL, Devoto P (2002)
Wirtshafter D, Sheppard AC (2001) Localization of GABA(B) receptors in
Delta(9)-tetrahydrocannabinol decreases extracellular GABA and in-
midbrain monoamine containing neurons in the rat. Brain Res Bull 56:
creases extracellular glutamate and dopamine levels in the rat prefrontal
cortex: an in vivo microdialysis study. Brain Res 948:155–158.
World Health Organization (2006) Annex 5.a: prevalence of use, adverse
Pistis M, Perra S, Pillola G, Melis M, Gessa GL, Muntoni AL (2004) Canna-
health effects of and interventions for— cannabis, cocaine, amphet-
binoids modulate neuronal firing in the rat basolateral amygdala: evi-
amines and—MDMA use and dependence. In: Disease control priorities
dence for CB- and non-CB -mediated actions. Neuropharmacology
related to mental, neurological, developmental and substance abuse dis-
orders, p 87. Geneva: Department of Mental Health and Substance Abuse,
Porsolt RD, Bertin A, Jalfre M (1978) "Behavioural despair" in rats and
World Health Organization.
Source: http://impactgroupbc.files.wordpress.com/2009/01/asantidepressant.pdf
⏐ PUBLIC HEALTH MATTERS ⏐ What Has a Decade of Daubert Wrought? Margaret A. Berger, JD Demands for tort reform may also have There have been changes within the judicial system that may be attributable to played a part in the Court's willingness to ac- opinions on the admissibility of expert testimony that began with the Supreme cept the Daubert case for review. There had
Manuale CITES pergli orti botanici Manuale CITES per gli orti botanici Manuale CITES per gli orti botanici Manuale CITES per gli Orti Botanici Sara Oldfield e Noel McGough Citazione del manuale: Oldfield S. e McGough N. (Comp.), 2007. A CITES manual for botanic gardens. Second edition. Botanic Gardens Conservation International,







