Levitra enthält Vardenafil, das eine kürzere Wirkdauer als Tadalafil hat, dafür aber schnell einsetzt. Männer, die diskret bestellen möchten, suchen häufig nach levitra kaufen ohne rezept. Dabei spielt die rechtliche Lage in der Schweiz eine wichtige Rolle.
Maternal risk factors for gastroschisis in canada
Maternal Risk Factors for Gastroschisis in Canada
Erik D. Skarsgard*1, Christopher Meaney2, Kate Bassil3, Mary Brindle4, Laura Arbour5,Rahim Moineddin2, and the Canadian Pediatric Surgery Network (CAPSNet)
Background: Gastroschisis is a congenital abdominal wall defect that occurs
confidence interval, 0.83–0.87; p < 0.0001), smoking (odds ratio, 2.86; 95%
in one per 2200 pregnancies. Birth defect surveillance in Canada has shown
confidence interval, 2.22–3.66; p < 0.0001), a history of pregestational or
that the prevalence of gastroschisis has increased threefold over the past 10
gestational diabetes (odds ratio, 2.81; 95% confidence interval, 1.42–5.5;
years. The purpose of this study was to compare maternal exposures data
p 5 0.0031), and use of medication to treat depression (odds ratio, 4.4; 95%
from a national gastroschisis registry with pregnancy exposures from vital
confidence interval, 1.38–11.8; p 5 0.011) emerged as significant
statistics to understand maternal risk factor associations with the occurrence
associations with gastroschisis pregnancies. Conclusion: Gastroschisis in
of gastroschisis. Methods: Using common definitions, pregnancy cohorts were
Canada is associated with maternal risk factors, some of which are
developed from two databases. The Canadian Pediatric Surgery Network
modifiable. Further studies into sociodemographic birth defect risk are
database, a population-based dataset was used to record maternal exposures
necessary to allow targeted improvements in perinatal health service delivery
for women who experienced a gastroschisis pregnancy, while a
and health policy.
contemporaneous, geographically cross-sectional "control" cohort of pregnant
women and their exposures was developed from Canadian Community Health
Birth Defects Research (Part A) 103:111–118, 2015.
Survey data. Groups comparison of maternal risk factors was performed using
C 2015 Wiley Periodicals, Inc.
univariate and multivariate logistic generalized estimating equation techniques.
Results: A total of 692 gastroschisis pregnancies (from Canadian Pediatric
Key words: gastroschisis; population-based registry; maternal risk factors;
Surgery Network) and 4708 pregnancies from Canadian Community Health
maternal age; teratogenesis
Survey were compared. Younger maternal age (odds ratio, 0.85; 95%
The Public Health Agency of Canada has documented a
Gastroschisis (GS) is a congenital abdominal wall defect
threefold rise in prevalence of GS over the last 10 years,
which results in the extrusion of the developing fetal intes-
to approximately 1 per 2200 births (Moore et al., 2013). A
tines into the amniotic space. It is usually detected prena-
similarly observed increase in GS prevalence has been
tally by maternal serum screening and ultrasound, and
made in many other countries, including the United States
tends to occur as an isolated congenital anomaly. When a
and several European nations (Laughon et al., 2003; Inter-national Clearinghouse for Birth Defects Surveillance and
prenatal diagnosis of GS is made, arrangements are made
Research, 2009; Langlois et al., 2011), prompting referen-
for delivery at an obstetrical center that is functionally
ces to a "Gastroschisis Epidemic" (Kilby, 2006; Mastroia-
linked to a specialty pediatric hospital with the capability
covo et al., 2006; Keys et al., 2008). Epidemiologic studies
of providing surgical treatment after birth as well as essen-
of causation of GS have emerged from single state/region/
tial neonatal intensive care. Survival following birth of an
country birth defect registries, to pooled data from a net-
infant with GS exceeds 90%, however, survivors may
work of population-based congenital anomaly reporting
require prolonged hospitalization in high intensity nurs-
registries, all with an intent to better understand modifi-
eries, which makes them among the most expensive of con-
able risk factors for GS.
genital anomalies to treat (Sydorak et al., 2002; Skarsgard
Since 2006, the Canadian Pediatric Surgery Network
et al., 2008). The specific cause of GS remains unknown,
(CAPSNet) has collected standardized pre and postnatal
although the available evidence suggests interactions of
data on all cases of GS admitted to each of the 17 hospi-
multiple maternal risk factors lead to occurrence.
tals in Canada that provide specialty pediatric care forbirth defects. The collected data include maternal demo-graphic (age, home postal code) and exposures (e.g., smok-
1Department of Surgery, University of British Columbia, Vancouver, Canada
ing, alcohol, recreational drug use) data for all GS
2Department of Family and Community Medicine, University of Toronto,
pregnancies. The purpose of this study was to explore the
association between maternal factors and GS in Canada by
Dalla Lana School of Public Health, University of Toronto, Toronto, Canada
4Department of Surgery, University of Calgary, Calgary, Canada
comparing maternal exposures data for GS cases identified
5Department of Medical Genetics, University of British Columbia, Vancouver,
in CAPSNet with household exposures data for a geograph-
ically "cross-sectional" group of pregnant women from aCanadian Vital Statistics database.
This work was Supported by the Canadian Institutes of Health Research(CIHR) Funding Reference # Sec 117139.
*Correspondence to: Erik D. Skarsgard, K0–110 ACB, 4480 Oak Street, Van-
Materials and Methods
couver, BC, V6H 3V4 Canada. E-mail:
[email protected]
The two primary data sources for this study consisted ofthe CAPSNet registry, and the Canadian Community Health
Published online 12 February 2015 in Wiley Online Library (wileyonlinelibrary.
com). Doi: 10.1002/bdra.23349
Survey (CCHS, which is administered by Statistics Canada),
C 2015 Wiley Periodicals, Inc.
RISK FACTORS FOR GASTROSCHISIS IN CANADA
a cross-sectional survey which collects information related
survey was conducted in alternate years). The CCHS sur-
to health status and health determinants of Canadians at
vey of 65,000 Canadians per year, targets persons aged 12
the household level, across all geographic regions (dissem-
years or older who are living in private dwellings in the
ination areas) in Canada (Canadian Community Health Sur-
ten provinces and the three territories. Persons living on
vey [CCHS], 2014). Through integration of data from these
Indian Reserves or Crown lands, clientele of institutions,
two sources, we were able to develop health and maternal
full-time members of the Canadian Armed Forces, and res-
exposure risk profiles of mothers who gave birth to infants
idents of certain remote regions are excluded from this
with GS (from CAPSNet), to pregnant women sampled
survey. The sampling method ensures that all provinces'
from the CCHS. The variables that were consistently col-
health regions (provincially designated health service
lected across the two data sources include: history of alco-
areas) are sampled in proportion to the size of their
hol, tobacco, marijuana, cocaine, methamphetamine and
heroin use during pregnancy, history of diabetes (type 1,
The CCHS covers approximately 98% of the Canadian
type 2, or gestational), use of depression medication dur-
population aged 12 or older, and collects information
ing pregnancy, and use of folic acid during pregnancy.
related to health status, health care usage, and healthdeterminants. These data can then be used to estimate, on
a health regional basis, potential relationships between
The CAPSNet consists of the 17 perinatal/surgical centers
health outcomes and economic, demographic, occupational,
that provide population-based pre- and postnatal care for
and environmental factors. Ultimately, the data are meant
GS in Canada (The Canadian Pediatric Surgery Network
to provide a better understanding of the health of Cana-
[CAPSNet], 2014). The CAPSNet registry was designed spe-
dians and inform public policy, through health surveillance
cifically for outcomes research and contains rigorously
and the facilitation of population health research.
defined fields that allow discrimination of risk variablesand treatment, as well as relevant clinical outcomes fromthe birth hospitalization to death or discharge. Data are
collected from maternal and infant charts by trained
This study used a cross-sectional design. In this design,
abstractors using a customized data entry program with
the GS (case) sample consisted of all mothers identified
built-in error checking and a standard manual of opera-
from the CAPSNet database between 2006 and 2012, who
tions and definitions. Abstracted prenatal information
had a GS pregnancy resulting in a live birth, stillbirth or
details maternal risk variables including demographics
termination of pregnancy, while the non-GS (control) sam-
(postal code of residence), prenatal exposures including
ple consisted of all pregnant mothers responding to the
smoking, alcohol, and a variety of nonprescription drugs;
CCHS surveys (Cycle 1.1 [2001], Cycle 2.1 [2003], Cycle
medical comorbidities, quantitative ultrasound and other
3.1 [2005], CCHS 2007, and CCHS 2010). Although CCHS
prenatal diagnostic data, and information on all pregnancy
data do not differentiate women whose pregnancy was or
All data collection
was not complicated by GS (or any other birth defect), it
"observational" and is not used to influence the care of
is assumed that the CCHS cohort represents a reasonable
any individual patient.
control group because the sample is large and the overall
Data from each CAPSNet center are de-identified and
birth defect rate is known to be rare, and because controls
transmitted electronically to a centralized repository for
misclassification causes bias toward the null for the risk
cleaning, quality assurance and storage. Thereafter, the
aggregate dataset is overseen by a research coordinator
The comparison variables of interest between cases
and controls are summarized in Table 1. Definitions of
steering committee comprised of pediatric surgeons, neo-
"exposure" in both databases included any use, on at least
natologists, maternal-fetal medicine specialists, and an epi-
one occasion of the listed substances, during a time when
demiologist. Aggregate data use for research purposes is
the woman was pregnant, whether known or unknown
enabled by inter-institutional data sharing agreements,
(Canadian Pediatric Surgery Network, 2013). A coincident
and requires that each CAPSNet center maintain institu-
history of diabetes, could mean that the woman had pre-
tional review board approval for data collection. Aggregate
existing diabetes (type 1 or 2) diagnosed by a health pro-
data release requires project-specific institutional review
fessional or had gestational diabetes requiring medication
board approval from the principal investigator's institu-
or dietary modification. A history of depression requiring
tion, and complies with Health Information Portability and
medication meant that the woman had a mood disorder
Accountability Act (HIPPA) requirements.
diagnosed by a health professional and received anti-depressive medication at any time in CAPSNet, (or within
past month for CCHS), for any duration during her preg-
CCHS is a cross-sectional household survey administered
nancy. Folic acid use meant that the woman used a multi-
by Statistics Canada on an annual basis (before 2007 the
vitamin containing folic acid before or upon realization of
BIRTH DEFECTS RESEARCH (PART A) 103:111–118 (2015)
TABLE 1. CCHS and CAPSNet Variables Definitions
CAPSNet data definitionsa
Did you drink any alcohol during your last
Record if any alcohol use during pregnancy
Cigarette smoking
Did you smoke during your last pregnancy?b
Record if cigarettes were smoked during this preg-
nancy. If unknown or if no cigarettes were
During your last pregnancy, did you smoke daily,
smoked during pregnancy, leave the box
occasionally or not at all?c
1 Daily 2 Occasionally 3 Not at all
Have you used marijuana in the past 12 months?
Record whether or not the mother used any of the
following during this pregnancy.
Have you used cocaine or crack in the past 12
Methamphetamine or crystal meth
Have you used speed (amphetamines) in the past 12
Heroin: includes methadone
Have you used heroin in the past 12 months?
Do you have diabetes?
Record the mother's status as a diabetic during this
Pre-existing DM diagnosed prior to conception
Gestational diabetes
In the past month, did you take anti-depressants
Record use of antidepressants during this preg-
such as Prozac, Paxil or Effexor?:
nancy: includes selective-serotonin reuptake
inhibitors (SSRI) (i.e. Zoloft, Paxil or Prozac)
Did you take a vitamin supplement containing folic
Record whether the mother has been taking regular
acid before your pregnancy, that is, before you found
prenatal vitamins during this pregnancy.
out that you were pregnant?
None: no folic acid nor prenatal vitamins taken
before the start of the second trimester.
Folic acid: initiated prior to pregnancy or within the
first trimester.
Vitamins: initiated prior to pregnancy or within the
aFrom CAPSNet Abstractors' Manual vol 5.1.0, April 2013.
bQuestion from CCHS 1.1.
cQuestion from CCHS 2.1, 3.1, 2007, 2010.
her pregnancy. Age was estimated from maternal date of
residence was assigned using dissemination area (DA), a
birth, as reported through both datasets, and was analyzed
small, relatively stable geographic unit composed of one or
as a continuous variable. Geographic location of home
more adjacent dissemination blocks. It is the smallest
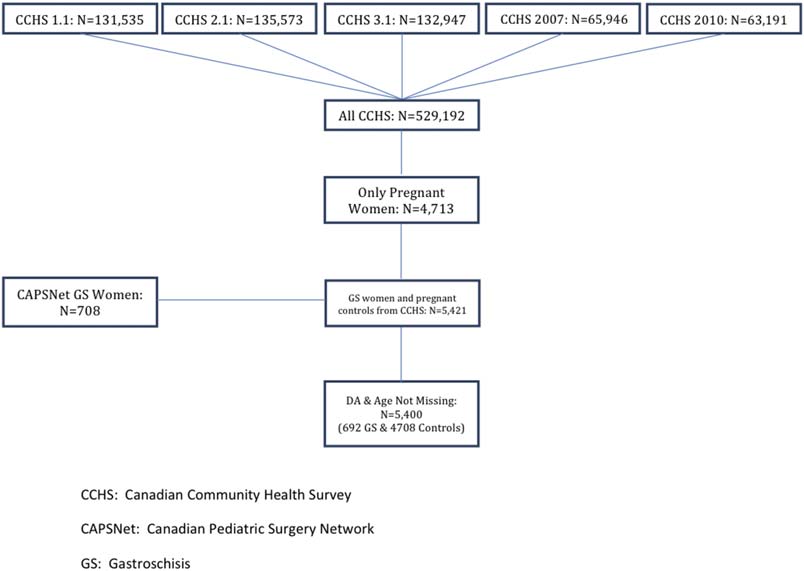
RISK FACTORS FOR GASTROSCHISIS IN CANADA
FIGURE 1. Inclusion/exclusion flow diagram of patients
analyzed in this study.Patients were collected from
five cycles of the CCHS (1.1, 2.1, 3.1, 2007 and
2010). Patients who were pregnant during the time of
the survey were included. The CAPSNet registry was
used to identify women who had gastroschisis preg-
nancies between 2006 and 2012, and those with
complete data were included.
standard geographic area for which all census data are
cies (from CAPSNet) and 4708 "control" pregnancies from
disseminated within Canada, and can be assigned using
the Postal Code Conversion File software (Wilkins and
The 692 GS mothers came from a total of 465 DAs and
Khan, 2010).
the 4708 control mothers came from a total of 1285 DAs.
Initially, we intended to perform a 1:1 matched case
The mean age of the GS mothers was significantly lower
control study where mothers were matched on age (a
than that of the controls (23.64 years; SD 5 4.79 years vs.
known GS risk factor) and DA, which would have allowed
28.84 years; SD 5 6.11 years; p < 0.0001). When maternal
us to control for some predisposing environmental factors.
age was interrogated as a predictive variable using bivari-
When analysis was completed under the proposed design
ate and multivariate generalized estimating equation mod-
many associations between categorical risk factors and
independently predictive
outcome of a GS pregnancy had low counts in some cells
occurrence of a GS pregnancy (odds ratio [OR], 0.85; 95%
of the contingency tables. As a result, these data could not
confidence interval [CI], 0.83–0.87; p < 0.0001).
be released from Statistics Canada due to anonymity con-
The relationships between maternal risk factors during
cerns and an alternative design was necessary.
pregnancy and the occurrence of GS are summarized inTables 2 and 3. Table 2 displays 2x2 tables, estimated
STATISTICAL ANALYSIS
odds ratios, 95% confidence intervals and p-values associ-
To avoid issues related to low cell counts, we chose not to
ated with a variety of maternal substance exposures dur-
match based on any demographic variables a priori. Rather
ing pregnancy, folic acid use, depression medication use or
we treated GS mothers and CCHS controls as being
a history of diabetes. Due to low cell counts, exposures
"clustered" within DAs and used logistic generalized esti-
data for cocaine, heroin, and methamphetamine were sup-
mating equation methods to account for this design fea-
pressed, and so an aggregate variable (any illicit drug use
ture. Moreover, we treated maternal age, (a known risk
inclusive of marijuana) was created. Table 2 suggests that
factor for GS occurrence) as a covariate in each model and
exposure to alcohol, tobacco, marijuana, illicit drugs or
estimated the adjusted odds of a GS birth, as a function of
medication for depression during pregnancy, as well as a
other hypothesized maternal risk factors after controlling
history of diabetes increases the likelihood of a GS preg-
for age. Where sample size allows we have summarized
nancy. Conversely, the use of folic acid appears to be pro-
the association between maternal factors (all categorical
tective against a GS pregnancy.
variables) and GS occurrence using contingency tables.
Given the awareness of young maternal age as a risk
factor for GS, age adjustment was performed in the logisticgeneralized estimating equation risk modeling, as summar-
ized in Table 3. After adjustment for maternal age, the
The process of deriving the case (GS pregnancies from
association between maternal exposures to cocaine and
CAPSNet) and control (non-GS pregnancies from CCHS)
marijuana individually, (and illicit drugs collectively) and
maternal cohorts is illustrated in Figure 1. After exclusions
the occurrence of GS persists. In the multivariate model,
for incomplete data there were a total of 692 GS pregnan-
BIRTH DEFECTS RESEARCH (PART A) 103:111–118 (2015)
(OR, 3.54; 95% CI, 2.22–5.63; p < 0.0001), and use of med-
TABLE 2. 2 x 2 Contingency Tables with Odds Ratios, 95% Confidence Inter-
ication to treat depression (OR, 4.4; 95% CI, 1.38–11.8;
vals, and p-Values Describing the Relationship between the Association
between Maternal Risk Factors and the Occurrence of a Gastroschisis
DiscussionGastroschisis is among the most common structural birth
defects, and its cause remains unknown. The phenomenon
of increased prevalence has been observed in several juris-
583 (92.98) 4454 (95.64)
dictions, and continues to be a stimulus for epidemiologic
evaluation of risk factors, both maternal (physiological,teratogens, socioeconomic) and environmental (Torfs et al.,
231 (33.38) 610 (13.06) 3.34 2.79, 3.99 <0.0001
1994; Reefhuis and Honein, 2004; Rittler et al., 2007; Cas-
461 (66.62) 4061 (86.94)
tilla et al., 2008; Salemi et al., 2009; Waller et al., 2010;
Agopian et al., 2013).
8.03 5.63, 11.46 <0.0001
The most widely observed association in GS pregnancy
614 (88.73) 3477 (98.44)
occurrence is its inverse relationship with maternal age.
The risk seems to be highest in the teenage cohort. Aggre-
gate data from EUROCAT (a consortium of birth defect
registries which combines registry data from 23 countries)
report a relative risk of 7.0 in the under 20 age cohort,
and a RR of 2.4 in the 20 to 24 age cohort, compared withthe age 25 to 29 reference group (Reefhuis and Honein,
2004). While a strong association with maternal age is
certain, what is less clear is whether the increased preva-
lence of GS is due exclusively to an increased prevalence
within the teenage mother population, or to a GS preva-
lence increase across all maternal age strata (Kazauraet al., 2004; Loane et al., 2007). Regardless of the exact
nature of this association, the relationship between mater-
9.35 6.64, 13.15 <0.0001
nal age and GS should be factored in to all studies of GS
600 (86.71) 3475 (98.39)
epidemiology, with analyses of all putative risk factors
being subject to age-adjustment.
Several epidemiologic studies of causation suggest a
moderate risk of GS associated with smoking during preg-
672 (97.25) 4651 (98.81)
nancy (Haddow et al., 1993; Draper et al., 2008; Feldkamp
et al., 2008), and data from Canada identify a higher rate
4.65 2.61, 8.30 <0.0001
of smoking during pregnancy in mothers with GS (Wein-
670 (96.82) 3544 (99.30)
sheimer et al., 2008). Not only is there a maternal smokingassociation with GS prevalence, there is also an association
with clinical outcome, with GS infants of smoking mothers
134 (19.36) 1289 (27.83) 0.62 0.51, 0.76 <0.0001
having more severe bowel injury at birth (Weinsheimer
558 (80.64) 3342 (72.17)
et al., 2008; Brindle et al., 2012). In addition to smoking,
Empty cells, suppressed due to low counts.
illicit drug use is another purported risk factor for GS,with cocaine, marijuana, and methamphetamine observed
significant predictor of GS occurrence. Similarly, although
to have a significant age-adjusted association with GS
alcohol exposure remained predictive after age adjustment,
occurrence (Draper et al., 2008; Weinsheimer et al., 2008;
its predictive association with GS occurrence disappeared
Brindle et al., 2012).
with multivariate analysis. The other significant variable
The current study provides further insight into GS epi-
change associated with age-adjustment was the loss of the
demiology through integration of a contemporary, Cana-
apparent protection associated with folic acid use.
dian population-based GS dataset with Vital Statistics data
Younger maternal age, smoking (OR, 2.86; 95% CI,
from a cross-sectional, representative cohort of pregnant
2.22–3.66; p < 0.0001), a history of diabetes (OR, 2.81;
mothers from the Canadian Community Health Survey.
95% CI, 1.42–5.5; p 5 0.0031), history of illicit drug use
Critical to the accuracy and reliability of this dataset
RISK FACTORS FOR GASTROSCHISIS IN CANADA
TABLE 3. Bivariate, Age-Adjusted, and Multivariate Logistic Regression Models Evaluating Risk Factor Prediction of a Gastroschisis Pregnancy
Bivariate logistic gee model
Age-adjusted logistic GEE model
Multivariate logistic GEE model
—, not used in multivariate model, rather combined in composite "illicit drug" variable.
GEE, general estimating equation.
integration, is the accuracy of case and control ascertain-
for maternal age and race (Polen et al., 2013). Conversely,
ment, and equivalence of risk factor definitions. One of the
two other studies looking at associations between selective
limitations of birth defect registry ascertainment (for
serotonin-reuptake inhibitors in pregnancy did not demon-
example EUROCAT) is the accuracy of discharge diagnosis
strate a significantly increased rate of common structural
abstraction from hospital charts. The diagnostic code for
birth defects among exposed infants (Alwan et al., 2007;
GS (International Classification of Disease, ICD-9 756.7) is
Louik et al., 2007). It is likely that an association between
shared with another congenital defect of the abdominal
depression and/or its treatment and the occurrence of GS
wall (omphalocele), which differs dramatically from GS
has many confounders, and, therefore, caution should be
and is frequently associated with genetic patterns of inher-
exercised in inferring a direct relationship in the absence
itance. The British Pediatric Association modification of
of other supportive studies.
ICD-9 is used by some registries, and allows differentiation
Our data identify a maternal history of pregestational
of gastrochisis from omphalocele, however it is not used
type 1 or type 2 or gestational diabetes mellitus as being
uniformly. The Canadian Pediatric Surgery Network (CAP-
independently predictive of a GS pregnancy. While a rela-
SNet) database, on the other hand, is a research database,
tionship between pregestational/gestational diabetes and
for which cases are ascertained by clinicians who are
increased rates of several birth defects (specifically, cardio-
directly involved in either prenatal diagnosis or postnatal
vascular defects), is well established, no specific associa-
treatment of GS, which improves diagnostic accuracy
tion with human GS has been reported previously.
Although the concept of abdominal wall malformations
Our study reinforces the inverse relationship between
associated with a hyperglycemic state is plausible and sup-
maternal age and GS occurrence, as well as associations
ported by observations of GS in pregnant rats who were
with maternal smoking and illicit drug use, which were
made diabetic by intraperitoneal streptozotocin (Padma-
both independently predictive of occurrence on multivari-
nabhan and al-Zuhair, 1987–1988), this relationship is
ate logistic regression modeling. Although it was observed
intuitively at odds with the observed protective effect of
to be protective on univariate analysis, use of folic acid
overweight or obese prepregnancy BMI on the risk of hav-
lost its predictive effect following adustment for maternal
ing a GS pregnancy (Lam et al., 1999; Waller et al., 2007;
age. We also observed that treatment of depression with
Stothard et al., 2009). A potential explanation for our find-
any anti-depressive medication was associated with an
ings is an underreporting of gestational diabetes in the
increased risk of GS (OR, 4.04; 95% CI, 1.38 5 11.8). A
CCHS cohort. A population-based study of rates of bio-
recent report from the National Birth Defects Prevention
chemically validated gestational diabetes in the province
Study using a case control methodology, looked at the
of Ontario, showed a doubling of age-adjusted rate from
effect of periconceptual use of the anti-depressant venla-
2.7 to 5.6% between 1996 and 2010 (Feig et al., 2014),
faxine on the occurrence of birth defects, and observed a
which is substantially higher than the rate of 1.2%
statistically significant association with GS, after adjusting
observed in our CCHS cohort, and suggests the possibility
BIRTH DEFECTS RESEARCH (PART A) 103:111–118 (2015)
that a diagnosis of gestational diabetes was unknown to
many pregnant women at the time they were surveyed.
The authors thank Alison Butler, CAPSNet coordinator, for
We are reluctant to ascribe much significance to this
her administrative efforts in support of this work
observation, other than to note its statistical significance,and suggest that future studies of GS epidemiology should
evaluate this potential association further.
Agopian AJ, Langlois PH, Cai Y, et al. 2013. Maternal residential
While this study has some unique strengths, it also has
atrazine exposure and gastroschisis by maternal age. Matern
limitations. Combining unrelated sources of data (despite
Child Health J 17:1768–1775.
common definitions) for cases and controls raises concernover the comparability of maternal exposures between
Alwan S, Reefhuis J, Rasmussen SA, et al. 2007. Use of selective
groups. Both data sources reflect self-reporting and are,
serotonin-reuptake inhibitors in pregnancy and the risk of birth
therefore, subject to recall bias, and potentially, a reluc-
defects. N Engl J Med 356:2684–2692.
tance to admit to risky behavior during pregnancy. Neither
Brindle ME, Flageole H, Wales PW, et al. 2012. Influence of
source specifically identifies pre or peri-conceptual expo-
maternal factors on health outcomes in gastroschisis: a Canadian
sure from exposure during pregnancy. The inquiry around
population-based study. Neonatology 102:45–52.
timing of exposure in the CCHS database is variable, rang-ing from "past month" (antidepressant use), "during preg-
Canadian Community Health Survey (CCHS). 2014. Available at:
nancy" (alcohol, smoking), or "past 12 months" (illicit
5Accessed August 29, 2014.
drugs, which could capture exposures before pregnancy).
As previously acknowledged, the occurrence of birth
Canadian Pediatric Surgery Network. 2013. CAPSNet Data
defects, including GS within the "control" CCHS cohort can-
Abstractors Manual. V 5.1.0. Available at:
not be excluded, yet we contend that it represents a rea-
Accessed August 29, 2014.
sonable control sample based on its size and the low birthdefect rate.
Castilla EE, Mastroiacovo P, Orioli IM. 2008. Gastroschisis: inter-
Finally, the time periods reflected by the two databases
national epidemiology and public health perspectives. Am J MedGenet C Semin Med Genet 148C:162–179.
are different: the CCHS controls represent an aggregatecohort from surveys done in 2001, 2003, 2005, 2007, and
Draper ES, Rankin J, Tonks AM, et al. 2008. Recreational drug
2010, while the CAPSNet cases were accrued between
use: a major risk factor for gastroschisis? Am J Epidemiol 167:
2006 and 2012). Another limitation is the inability to con-
trol for geographic factors (location of maternal resi-dence). Although our initial intent was to undertake case/
Feig DS, Hwee J, Shah BR, et al. 2014. Trends in incidence of dia-
control matching by maternal age and dissemination area,
betes in pregnancy and serious perinatal outcomes: a large,population-based study in Ontario, Canada, 1996–2010. Diabetes
the low counts in many of the DA cells meant that these
data could not be released. We have, therefore, had toassume, for the purpose of this study, that GS occurs with
Feldkamp ML, Alder SC, Carey JC. 2008. A case control
the same prevalence across Canada, which we know is not
population-based study investigating smoking as a risk factor for
the case, based on spatial mapping analysis of GS cases
gastroschisis in Utah, 1997–2005. Birth Defects Res A Clin Mol
across Canada (K. Bassil, personal communication, 2014).
We are also unable to make any observations of potential
Haddow JE, Palomaki GE, Holman MS. 1993. Young maternal age
associations between maternal race and GS. In another
and smoking during pregnancy as risk factors for gastroschisis.
CAPSNet study of GS epidemiology, we have observed a
higher than expected prevalence of GS pregnancies in Abo-riginal women, although maternal ethnicity does not
International Clearinghouse for Birth Defects Surveillance and
emerge as independently predictive of occurrence (Brindle
Research. 2009. Annual Report 2009 with data for 2007. Rome, Italy:
et al., 2012). The fact that the CCHS excludes Canadians
International Center on Birth Defects. Available at: Accessed
living on Indian reserves means that Aboriginals may be
Month day, year.
underrepresented in our control group.
Future studies on causation of GS and other high
Kazaura MR, Lie RT, Irgens LM, et al. 2004. Increasing risk of
impact structural birth defects will be enabled by our abil-
gastroschisis in Norway: an age-period-cohort analysis. Am J Epi-
ity to integrate data from different sources, and speak to
the necessity of having access to and linkages for a varietyof clinical and administrative datasets. Improvements in
Keys C, Drewett M, Burge DM. 2008. Gastroschisis: the cost of anepidemic. J Pediatr Surg 43:654–657.
perinatal health service delivery and health policy repre-sent some of our greatest opportunities for outcome
Kilby MD. 2006. The incidence of gastroschisis. BMJ 332:250–
improvement for birth defects like GS.
RISK FACTORS FOR GASTROSCHISIS IN CANADA
Lam PK, Torfs CP, Brand RJ. 1999. A low pregnancy body mass
Rittler M, Castilla EE, Chambers C, et al. 2007. Risk for gastroschi-
index is a risk factor for an offspring with gastroschisis. Epidemi-
sis in primigravidity, length of sexual cohabitation, and change in
ology 10:717–721.
paternity. Birth Defects Res A Clin Mol Teratol 79:483–487.
Langlois PH, Marengo LK, Canfield MA. 2011. Time trends in the
Salemi JL, Pierre M, Tanner JP, et al. 2009. Maternal nativity as a
prevalence of birth defects in Texas 1999–2007: real or artifac-
risk factor for gastroschisis: a population-based study. Birth
tual? Birth Defects Res A Clin Mol Teratol 91:902–917.
Defects Res A Clin Mol Teratol 85:890–896.
Laughon M, Meyer R, Bose C, et al. 2003. Rising birth prevalence
Skarsgard ED, Claydon J, Bouchard S, et al. 2008. Canadian Pedi-
of gastroschisis. J Perinatol 23:291–293.
atric Surgical Network: a population-based pediatric surgery net-work and database for analyzing surgical birth defects. The first
Loane M, Dolk H, Bradbury I; EUROCAT Working Group. 2007.
100 cases of gastroschisis. J Pediatr Surg 43:30–34.
Increasing prevalence of gastroschisis in Europe 1980–2002: aphenomenon restricted to younger mothers? Paediatr Perinat
Stothard KJ, Tennant PW, Bell R, et al. 2009. Maternal overweight
and obesity and the risk of congenital anomalies: a systematicreview and meta-analysis. JAMA 301:636–650.
Louik C, Lin AE, Werler MM, et al. 2007. First-trimester use ofselective serotonin-reuptake inhibitors and the risk of birth
Sydorak RM, Nijagal A, Sbragia L, et al. 2002. Gastroschisis: small
defects. N Engl J Med 356:2675–2683.
hole, big cost. J Pediatr Surg 37:669–672.
Mastroiacovo P, Lisi A, Castilla EE. 2006. The incidence of gastro-
The Canadian Pediatric Surgery Network (CAPSNet). 2014. Avail-
schisis: research urgently needs resources. BMJ 332:423–424.
able at: Accessed August 29, 2014.
Moore A, Rouleau J, Skarsgard ED. 2013. Chapter 7: Gastroschi-
Torfs CP, Velie EM, Oechsli FW, et al. 1994. A population-based
sis. In: Public Health Agency of Canada. Congenital anomalies in
study of gastroschisis: demographic, pregnancy, and lifestyle risk
Canada 2013: a perinatal health surveillance report. Ottawa, Can-
factors. Teratology 50:44–53.
ada: Public Health Agency of Canada. pp. 57–64. Available at:
Waller DK, Shaw GM, Rasmussen SA, et al. 2007. Prepregnancy
Accessed Month day, year.
obesity as a risk factor for structural birth defects. Arch PediatrAdolesc Med 161:745–750.
Padmanabhan R, al-Zuhair AG. 1987–1988. Congenital malfor-mations and intrauterine growth retardation in streptozotocin
Waller SA, Paul K, Peterson SE, et al. 2010. Agricultural-related
induced diabetes during gestation in the rat. Reprod Toxicol 1:
chemical exposures, season of conception, and risk of gastroschi-
sis in Washington State. Am J Obstet Gynecol 202:241 e1–e6.
Polen KN, Rasmussen SA, Riehle-Colarusso T, et al. 2013. Associ-
Weinsheimer RL, Yanchar NL, Canadian Pediatric Surgical Net-
ation between reported venlafaxine use in early pregnancy and
work. 2008. Impact of maternal substance abuse and smoking
birth defects, national birth defects prevention study, 1997–
on children with gastroschisis. J Pediatr Surg 43:879–883.
2007. Birth Defects Res A Clin Mol Teratol 97:28–35.
Wilkins R, Khan S. Automated geographic coding based on the
statistics Canada postal code conversion files. Including postal
chromosomal birth defects, Atlanta — 1968–2000: teenager or
codes through October 2010. Available at:
thirty-something, who is at risk? Birth Defects Res A Clin Mol
Accessed Month day, year.
Source: http://canadanews.ga/polopoly_fs/1.3010179!/httpFile/file.pdf
Facultad de Ciencias Veterinarias -UNCPBA- Actualización sobre las bases terapéuticas para la Peritonitis Infecciosa Felina (PIF) y presentación de tres casos clínicos de PIF tratados con Talidomida. Alarcón, Gabriela Verónica; Paludi, Alejandro Esteban; Nejamkin, Pablo. Mayo, 2016
Carbamate and Pyrethroid Resistance in the Leafminer J. A. ROSENHEIM,' AND B. E. T ABASHNIK Department of Entomology, University of Hawaii at Manoa, Honolulu, Hawaii 96822 J. Econ.Entomol.83(6): 2153-2158 (1990) ABSTRACT Populations of D1glyphus begini (Ashmead), a parasitoid of Lirlomyza leafminers, showed resistance to oxamyl, methomyl, fenvalerate, and permethrin in labo-ratory bioassays. Relative to a susceptible strain from California, maximum resistance ratiosfor these pesticides were 20, 21, 17, and 13, respectively. Three populations that had beentreated frequently with insecticides were significantly more resistant to all four insecticidescompared with an untreated Hawaii population and a California population with an unknownspray history. Parasitoids from a heavily sprayed tomato greenhouse on the island of Hawaiihad LC",'s for permethrin and fenvalerate that were 10 and 29 times higher than the fieldrate, respectively. Populations resistant to oxamyl and methomyl had LC",'s two- and sixfoldbelow the field rate, respectively. D. begini is one of the few parasitoids resistant to pyre-throids, with LC",'s exceeding field application rates. Resistant D. begini may be useful forcontrolling leafminers in management programs that integrate biological and chemical con-trols.

