Levitra enthält Vardenafil, das eine kürzere Wirkdauer als Tadalafil hat, dafür aber schnell einsetzt. Männer, die diskret bestellen möchten, suchen häufig nach levitra kaufen ohne rezept. Dabei spielt die rechtliche Lage in der Schweiz eine wichtige Rolle.
Steripen.it

Environmental Consulting Drinking Water Analysis Radon Testing
Testing of SteriPEN™, a Portable Ultraviolet Light Water Purifier, On 1 Liter
Military Canteens to NSF International Protocol P248, Emergency Military
Operations Microbiological Water Purifiers
May 12th, 2008
Research Conducted For:
Miles Maiden
Hydro-Photon, Inc.
262 Ellsworth Road
Blue Hill, Maine 04614
Jonathan T. Dyer
Laboratory Director
Rebecca L. Lebrun
Quality Control Officer
A & L Laboratory Inc. 3100 Hotel Road P.O. Box 1507 Auburn, Maine 04211-1507
Telephone: (207) 784-5354 Fax: (207) 782-5561 Email: [email protected]
NELAP CERT #250103
MAINE CERT #ME021
NH CERT #2501
SteriPEN™ is a portable, handheld device designed to disinfect water by using a short wave germicidal ultraviolet (UV)
light. The device, unlike traditional flow through UV water purifiers, treats batches of water up to 1 liter. Though the method of treatment is slightly different the concept is the same. The SteriPEN™ produces ultraviolet energy that is used to destroy microorganisms, without the use of chemicals. The SteriPEN™ is submerged in the water, where microorganisms are exposed to a dose of ultraviolet light in the 254-nanometer range. Ultraviolet light in this wavelength inactivates a wide range of microorganisms including bacteria, viruses and protozoan cysts. This inactivation occurs as the ultraviolet light disrupts the organism's DNA structure, making reproduction impossible. The intensity of the ultraviolet light and the microorganism's exposure time to the ultraviolet light are factors that influence which microorganisms are inactivated [6].
This study will examine the effects of the SteriPEN™ on 1 Liter US Army Canteens {Figure #1}. The canteens will be
tested as recommended in the NSF International Protocol P248. The Protocol P248 recommends using both a General Test
Water and a Challenge Test Water at normal voltage (nominally 6 - 7 V) and at the lowest possible voltage ( 4.6V).
Test Organism
MS2 Coliphage was chosen as a test subject for this study for several reasons. MS2 offers a high linear response over a
wide range of UV dose levels, UV inactivation results are highly reproducible, it's easily propagated to high titers, and it is non-pathogenic to humans [9].
The MS2 Coliphage
was provided by Thomas Hargy, Clancy Environmental Consultants, P.O. Box 314, St. Albans,
VT, 05478. Clancy Environmental Consultants ran a collimated beam study on samples of this MS2 to determine the virus's UV dose response. Testing concluded that a 2.1 log reduction of MS-2 correlated with a dose of 40mJ/sq.ccm. (see attached collimated beam study).
Test Procedure
The testing procedure was done in accordance with the NSF International Protocol P248 – Emergency Military Operations
Microbiological Water Purifiers. The complete protocol is found in the U.S. Army Center for Health Promotion and
Preventive Medicine publication entitled "WATER SUPPLY MANAGEMENT PROGRAM NO. 31-EC-04TM, NSF
PROTOCOL P248 PURIFIER SPECIFIC TEST PLAN, HYDRO-PHOTON STERIPEN".
Samples of both General Test Water (EPA Test Water # 1) and Challenge Test Water (EPA Test Water #4) were used to
compare the effects of the SteriPEN™ on both visually clear water and water of known contaminant levels. The General
Test Waters and the Challenge Test Waters were created from laboratory reagent water. The required physical and
chemical characteristics of both waters are listed in Table #1. Neither water contained chlorine or any other disinfectant
residuals. pH in both types of water was measured by a Denver Instruments pH-ISE Meter model # 225. The pH was
adjusted using a 1N solution of sodium hydroxide (NaOH) and/or hydrochloric acid (HCL). Total organic carbon (TOC)
analyzed on a Shimadzu TOC-V Combustion Analyzer was adjusted in the challenge water using potassium hydrogen
phthalate. The turbidity in the challenge water was achieved through the addition of A.C. Fine Test Dust
Measurements of turbidity were taken on a Hach 2100A Turbidimeter. Total dissolved solids, measured by a YSI
Conductivity Meter, were increased in both waters to the appropriate concentrations by the use of sea salts. The
alkalinity value was obtained by following Standard Methods Number 4500-CO3. The UV absorption was measured with
a Shimadzu UV-2501PC Spectrophotometer and then the percent transmittance was calculated. Proper water
temperatures were monitored (Sper Scientific Infrared Thermometer 800048) and maintained throughout the entire
experiment. Please refer to Table #2 for the actual readings of each parameter used in the test.
Table #1. Required chemical and physical characteristics of test water per U.S.E.P.A. Guide Standard[7]
Parameter
General Test Water
Challenge Test Water
Chlorine Residual
<0.1 mg/L
<0.1 mg/L
Total Organic Carbon (TOC)
<0.1 mg/L
10.0 – 15.0 mg/L
Turbidity
0.1 NTU - 5 NTU
30 NTU – 50 NTU
Temperature
20ºC +/- 5ºC
4º +/- 1ºC
Total Dissolved Solids (TDS)
50 mg/L - 500 mg/L
1500 mg/L +/- 300 mg/L
Color U.V. Absorption
Color U.V. Transmittance
Alkalinity
80 mg/L – 120 mg/L
80 mg/L – 120 mg/L
Table #2. Actual chemical and physical characteristics of test water.
Parameter
General Test Water
Challenge Test Water
Chlorine Residual
<0.10 mg/L
<0.10 mg/L
Total Organic Carbon (TOC)
<1 mg/L
Turbidity
Temperature
Total Dissolved Solids (TDS)
1430 mg/L
Color U.V. Absorption
0.0075//cm
0.5175/cm
Color U.V. Transmittance
Alkalinity
1. Canteen Test at normal Steripen ™ Voltage (4.9 to 5.0 VDC)
The SteriPEN™ units, General Test Water, and Challenge Test Water were stored at 4°C prior to testing. The General
Test Water was spiked with test organism MS-2 coliphage (Escherichia coli bacteriophage ATCC® 15597-B1™). A control sample was removed from each liter of water. A single UV dose for one liter of water (90 seconds) was applied to each sample. The UV dose was administered according to the manufacturers instructions for treating between 0.5 –1.0 liter of water [5]. The on/off button was pushed once to begin the treatment of a one-liter sample. The green LED flashed indicating the SteriPEN™ was ready for use. The UV lamp end was then submerged into the one liter volume canteen deep enough to create a seal between the canteen and the Steripen™ unit The canteen and the SteriPEN™ were turned up-side–down and swirled to create a "mixing" effect for the duration of the sterilization cycle. After 90 seconds the green indicator light began flashing which indicates the end of the cycle. Upon completion of the treatment, an aliquot of water was removed from each beaker and several dilutions were plated according to the agar layer method described by Adams using E. coli host (Escherichia coli ATCC® 15597™) [1].
The Challenge Test Water was stored at 4°C prior to testing. The Challenge Test Water was spiked with test organism
MS-2 coliphage (Escherichia coli bacteriophage ATCC® 15597-B1™). A control sample was removed from each liter of water. A double UV dose for one liter of water (180 seconds) was applied to each sample. The UV dose was administered according to the manufacturers instructions for treating between 0.5 –1.0 liter of water [5]. The on/off
button was pushed once to begin the treatment of a one-liter sample. The green LED flashed indicating the SteriPEN™ was ready for use. The UV lamp end was then submerged into the one liter volume canteen deep enough to create a seal between the canteen and the Steripen™ unit. The canteen and the SteriPEN™ were turned up-side–down and swirled to create a "mixing" effect for the duration of the sterilization cycle. After 90 seconds the green indicator light began flashing which indicates the end of the cycle. The Steripen™ was removed completely from the canteen, the button was pressed, and when the green light flashed the SteriPEN™ was submerged back into the canteen for the second 90 second dose. Upon completion of the treatment, an aliquot of water was removed from each beaker and several dilutions were plated according to the agar layer method described by Adams using E. coli host (Escherichia coli ATCC® 15597™) [1].
2. Canteen Test at low Steripen ™ Voltage ( 4.6 VDC)
A DC Power Supply made by BK Precision, Model # 1670 was used to reduce the battery voltage. The SteriPEN™ unit is
attached to the DC Power Supply (see Figures #2, #3, and #4) and the voltage was reduced to within a tenth of a volt of shutting the unit off. In this case the unit batteries were operable at 4.6V but shut off at 4.5V. This shut down was indicated by the red LED light appearing on the SteriPEN™ unit.
The SteriPEN™ units, General Test Water, and Challenge Test Water were stored at 4°C prior to testing. The General
Test Water was spiked with test organism MS-2 coliphage (Escherichia coli bacteriophage ATCC® 15597-B1™). A control sample was removed from each liter of water. A single UV dose for one liter of water (90 seconds) was applied to each sample. The UV dose was administered according to the manufacturers instructions for treating between 0.5 –1.0 liter of water [5]. The SteriPEN™ unit was attached to the DC Power Supply and the on/off button was pushed once to begin the treatment of a one-liter sample. The green LED flashed indicating the SteriPEN™ was ready for use. The UV lamp end was then submerged into the one liter volume canteen deep enough to create a seal between the canteen and the SteriPEN™ unit. Once the SteriPEN™ was inserted into the canteen the DC Power Supply was set to the lowest voltage possible without setting off the red LED indicator light (in this case 4.6V) . The canteen and the SteriPEN™ were turned up-side–down and swirled to create a "mixing" effect for the duration of the sterilization cycle. After 90 seconds the green indicator light began flashing which indicates the end of the cycle. Upon completion of the treatment, an aliquot of water was removed from each beaker and several dilutions were plated according to the agar layer method described by Adams using E. coli host (Escherichia coli ATCC® 15597™) [1].
The Challenge Test Water and the SteriPEN™ unit were stored at 4°C prior to testing. The Challenge Test Water was
spiked with test organism MS-2 coliphage (Escherichia coli bacteriophage ATCC® 15597-B1™). A control sample was removed from each liter of water. A double UV dose for one liter of water (180 seconds) was applied to each sample. The UV dose was administered according to the manufacturers instructions for treating between 0.5 –1.0 liter of water [5]. The SteriPEN™ unit was attached to the DC Power Supply and the on/off button was pushed once to begin the treatment of a one-liter sample. The green LED flashed indicating the SteriPEN™ was ready for use. The UV lamp end was then submerged into the one liter volume canteen deep enough to create a seal between the canteen and the SteriPEN™ unit Once the SteriPEN™ was inserted into the canteen the DC Power Supply was set to the lowest voltage possible without setting off the red LED indicator light (in this case 4.6V) . The canteen and the SteriPEN™ were turned up-side–down and swirled to create a "mixing" effect for the duration of the sterilization cycle. After 90 seconds the green indicator light began flashing which indicates the end of the cycle. The SteriPEN™ was removed completely from the canteen, the button was pressed, and when the green light flashed the SteriPEN™ was submerged back into the canteen for the second 90 second dose. Upon completion of the treatment, an aliquot of water was removed from each beaker and several dilutions were plated according to the agar layer method described by Adams using E. coli host (Escherichia coli ATCC® 15597™) [1].
Table #3. Canteen with General Test Water (One 90 Second dose) MS2 Coliphage Titer (PFU/ml)-logarithmic
reductions and percent kill
General Test Water
Log Reduction
Untreated (T=0)
Treated (T=90 Sec)
99.9354%
SteriPEN #1
6.31E+05
4.32E+02
99.9315%
SteriPEN #2
7.10E+05
4.21E+02
99.9407%
SteriPEN #3
6.21E+05
4.10E+02
99.9340%
99.8651%
SteriPEN #1
6.47E+05
8.23E+02
99.8728%
SteriPEN #2
6.09E+05
8.75E+02
99.8563%
SteriPEN #3
6.41E+05
8.58E+02
99.8661%
99.9271%
SteriPEN #1
6.25E+05
5.19E+02
99.9170%
SteriPEN #2
6.76E+05
4.72E+02
99.9302%
SteriPEN #3
6.53E+05
4.29E+02
99.9343%
99.9092%
Table #4. Canteen with Challenge Test Water (Two 90 Second Doses) MS2 Coliphage Titer (PFU/ml)-
logarithmic reductions and percent kill
Challenge Test Water
Log Reduction
Untreated (T=0)
Treated (T=180 Sec)
99.8067%
SteriPEN #1
1.18E+05
2.09E+02
99.8229%
SteriPEN #2
1.08E+05
2.25E+02
99.7917%
SteriPEN #3
1.11E+05
2.16E+02
99.8054%
99.6606%
SteriPEN #1
9.35E+04
3.16E+02
99.6620%
SteriPEN #2
9.02E+04
3.05E+02
99.6619%
SteriPEN #3
9.09E+04
3.11E+02
99.6579%
99.5747%
SteriPEN #1
9.85E+04
4.18E+02
99.5756%
SteriPEN #2
9.44E+04
3.98E+02
99.5784%
SteriPEN #3
9.58E+04
4.12E+02
99.5699%
99.6806%
Table #5. Canteen with General Test Water at Low Voltage [4.6V] (One 90 second dose) MS2 coliphage Titer
(PFU/ml)-logarithmic reductions and percent kill
General Test Water
Log Reduction
Untreated (T=0)
Treated (T=90 Sec)
99.8617%
SteriPEN #1
5.93E+05
7.78E+02
99.8688%
SteriPEN #2
5.57E+05
8.05E+02
99.8555%
SteriPEN #3
5.65E+05
7.86E+02
99.8609%
99.8405%
SteriPEN #1
6.31E+05
9.97E+02
99.8420%
SteriPEN #2
6.12E+05
9.90E+02
99.8382%
SteriPEN #3
6.03E+05
9.57E+02
99.8413%
99.9289%
SteriPEN #1
5.41E+05
4.14E+02
99.9235%
SteriPEN #2
5.69E+05
3.94E+02
99.9308%
SteriPEN #3
5.76E+05
3.89E+02
99.9325%
99.8770%
Table #6. Challenge Test Water at Low Voltage [4.6V] (Two 90 second doses) MS2 coliphage Titer )PFU/ml)-
logarithmic reductions and percent kill
Challenge Test Water
Log Reduction
Untreated (T=0)
Treated (T=180 Sec)
99.7234%
SteriPEN #1
3.68E+05
9.88E+02
99.7315%
SteriPEN #2
3.47E+05
1.01E+03
99.7089%
SteriPEN #3
3.56E+05
9.62E+02
99.7297%
99.4168%
SteriPEN #1
6.08E+05
3.43E+03
99.4359%
SteriPEN #2
5.92E+05
3.65E+03
99.3834%
SteriPEN #3
5.87E+05
3.34E+03
99.4310%
99.5087%
SteriPEN #1
5.12E+05
2.55E+03
99.5020%
SteriPEN #2
4.91E+05
2.41E+03
99.5092%
SteriPEN #3
5.03E+05
2.44E+03
99.5149%
99.5496%
Conclusion
The evaluation of SteriPEN™ on the General Test Water {Table #3} resulted in a 3.07-log reduction (99.91 %) of MS-2
coliphage after a single dose (90 seconds). The use of SteriPEN™ on the Challenge Test Water {Table #4} resulted in a
2.52-log reduction (99.68%) of MS-2 coliphage after a double dose (180 seconds). The increased contaminants in the
challenge test water slightly reduced the effectiveness of SteriPEN™ but not enough to decrease the log reduction to an
inadequate level. Testing of the SteriPEN™ was also conducted at low voltage on the General Test Water and the
Challenge Test Water. The General Test Water {Table #5} resulted in a 2.94-log reduction (99.88%) of MS-2 coliphage
after a single dose (90 seconds). The use of the SteriPen™ on the Challenge Test Water {Table#6} resulted in a 2.37-log
reduction (99.55%) of MS-2 coliphage after a double dose (180 seconds).
The testing conducted on both General Test Waters (3.07- log reduction and 2.94-log reduction respectively) and Challenge Test Waters (2.52- log reduction and 2.37-log reduction respectively) indicates that SteriPEN™ delivered UV doses in excess of the amount of the required 40mJ/sq.cm. and therefore meets the requirements of NSF International P248 Protocol – Emergency Military Operations Microbiological Water Purifiers.
Jonathan T. Dyer / Laboratory Director
Rebecca L. Lebrun / Quality Assurance Officer
Figure #1.
Figure #2

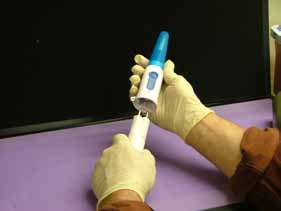


Picture of Canteen with the SteriPen™
Attaching DC Power Supply to SteriPen ™ Unit
Figure #3
Figure #4
Attached DC Power Supply and SteriPen ™ Complete DC Power Supply/SteriPen™ Setup
References
1. Adams, M. H. 1959. Bacteriophages. Interscience Publishers, New York 2. Enriquez, C. and Gerba, c. 2001. Evaluation of the Steri-Pen® Water Treatment System According to the US
Environmental Protection Agency Guide Standard And Protocol For Testing of Microbiological Water Purifiers.
3. Hanson, Anne 2000. Testing of Steri-Pen, a Hand-held Ultraviolet Water Treatment Device using MS2 Coliphage. 4. Hanson, Anne 2001. Testing of Steri-Pen, a Hand-held Ultraviolet Water Treatment Device using MS2 Coliphage on
Visually Turbid Natural Water.
5. Hydro-Photon, Inc., 2005 SteriPEN™ Users Guide, Blue Hill, Maine http://www.hydro-
6. Ultraviolet Light Disinfection Technology In Drinking Water Application - An Overview. United States
Environmental Protection Agency, Office of Water. EPA 811-R-96-002. September, 1996
7. U.S.E.P.A. - Task Force Report, 1987. Guide Standard and Protocol for Testing Microbiological Water Purifiers.
United States Environmental Protection Agency, Registration Division, Office of Pesticide Programs and Criteria and Standards Division, Office of Drinking Water, Washington, DC
8. U.S. Army Center for Health Promotion and Preventive Medicine, 2005. Technical Information Paper; Ultraviolet Light Disinfection in the Use of Individual Water Purification Devices, Aberdeen Proving Ground, MD.
9. Wilson, B.R.P.F. Roessler, E. Van Dellen, M. Abbaszadegan and C.P. Gerba. Coliphage MS2 as a UV Water
Disinfection Efficacy Test Surrogate for Bacterial and Viral Pathogens. University of Arizona, Tuczon, AZ
Appendix

CEC Clancy Environmental Consultants, Inc.
Consulting and Microbiological Laboratory Services
Mr. Miles Maiden Hydro-Photon P.O. Box 675 262 Ellsworth Rd. Blue Hill, ME 04614 Dear Miles, Please find enclosed the results of collimated beam testing performed on the coliphage MS2 lot that was shipped to A&L Laboratory for disinfection system testing. If you have any questions concerning this information, please contact me. Sincerely, CLANCY ENVIRONMENTAL CONSULTANTS, INC.
Thomas M. Hargy Senior Scientist
P.O. Box 314, St. Albans, VT 05478 Telephone (802) 527-2460 Fax (802) 524-3909
CEC Clancy Environmental Consultants, Inc.
Consulting and Microbiological Laboratory Services
MS2 UV Dose Response
For Hydro-Photon
Test date: May 1, 2008
Clancy Environmental Consultants, Inc.
Purpose:
This study was undertaken to determine the sensitivity of a specific lot of coliphage MS2 to UV irradiation.
Methods:
Challenge microorganism: MS-2 phage obtained from the American Type Culture Collection (ATCC #15597-B1) were propagated in
the host bacteria Escherichia coli HS (pFamp)R (ATCC #700891) using a large volume liquid culture method.7 Bacteria were
maintained at Clancy Environmental Consultants, Inc. (CEC) under liquid nitrogen for long-term storage. Working cultures were
prepared regularly from archived sub-samples. All stocks were maintained on trypticase soy agar plates containing ampicillin and
streptomycin. Stock MS2 phage was propagated in batches with titers of approximately 5 X 1011 plaque forming units (pfu) per mL.
Collimated beam dose-response determination: Stock MS2 was diluted to a working volume of 100 mL containing approximately 1 x
106/mL, for dose response determination. The process, known as a collimated beam test (refer to Fig. 1) was carried out at CEC. The
UV source used was a low-pressure mercury vapor lamp (Atlantic Ultraviolet G12T6L). This lamp was housed above a shutter.
When the shutter was opened, light from the lamp passed through a 12 cm collimating tube to irradiate the test organisms suspended
in a 6 cm diameter Petri dish. Prior to irradiations, the lamp was allowed to warm up for a minimum of 30 min. The UV incident to
the surface of the Petri dish was then measured using a radiometer and detector (model X-911, Gigahertz Optik) calibrated at 254 nm.
The incident irradiation across the surface of the Petri dish was measured at 5 mm intervals along an X-Y grid originating at the center
of the dish. Overall irradiance distribution was then determined relative to the center reading, which was confirmed using a second
radiometer (model 1400A, International Light). This value was then used in the calculation of average irradiation incident to the water
surface. Factors influencing average irradiation to the entire volume include reflection from the water surface, divergence of the UV
light, depth of the water, and UV absorption of the inoculated test water. The latter was measured at 254 nm by spectrophotometry
(Spectronic Genesys 10uv™). UV dose was defined as the irradiation multiplied by the exposure time.
P.O. Box 314, St. Albans, VT 05478 Telephone (802) 527-2460 Fax (802) 524-3909
CEC Clancy Environmental Consultants, Inc.
Consulting and Microbiological Laboratory Services
Figure 1 – Collimated Beam Apparatus
Irradiations of sub-samples of the test water were made across a range of exposure times to provide UV doses of 0, 15 30, 45, 60, 75 and 90 mJ/cm2. Ten-milliliter subsamples were transferred to 6 cm diameter Petri dishes containing a 12 mm stir bar. After 1 min of stirring, the UV lamp shutter was opened and the suspension irradiated for a pre-determined length of time. A 0 mJ/cm2 dose was run simultaneously with the irradiation test for the highest mJ/cm2 dose, in the absence of UV. This 0 dose control provides the base count for determination of log inactivation. Each dose was run in duplicate. Results: Concentrations of survivors and resulting log10 inactivations achieved at the above doses are given in Table 1 and Figure 2. The best
fit equation for the average inactivation values is the polynomial equation:
y = 2.831x2 + 13.19 +0.214
Thus if a UV disinfection test achieves 2.1 log inactivation of this MS2, a UV dose of 40.4 mJ/cm2 would have been delivered.
P.O. Box 314, St. Albans, VT 05478 Telephone (802) 527-2460 Fax (802) 524-3909
CEC Clancy Environmental Consultants, Inc.
Consulting and Microbiological Laboratory Services
Table 1. MS2 surviving concentrations and log10 inactivations in collimated dose response test.
Lot #: 120407F
Figure 2. UV Dose response of MS2 Lot 120407F
UV dose response, Lot 120407F
Average Log Inactivation
P.O. Box 314, St. Albans, VT 05478 Telephone (802) 527-2460 Fax (802) 524-3909
Source: http://www.steripen.it/image/Test-Laboratorio-AeLCanteenP248Revised9408.pdf
Foto: Christa Rasch Der Wandel des Leipziger Auenwalds in den letzten 150 Jahren von Dr. Peter Gutte Wie schon 1863 der bekannte Naturforscher und demokratische Schriftsteller Emil Adolf ROßMÄßLER betonte, besitzt Leipzig „einen der schönsten Auwälder Deutschlands". In den letzten Jahrzehnten wurde bereits mehrfach in Fachzeitschriften und in der Presse über
East Timor: Just a political question? The Santa Cruz massacre of 12th November 1991 brought the enduring question of East Timor to the public notice in Australia. Hardly a day goes by without media coverage. It is in this context that Dr Geoffrey Hull's paper is written: the demand for such a paper exists not just in social justice groups, but in the wider Catholic Church and the community at large.






