Levitra enthält Vardenafil, das eine kürzere Wirkdauer als Tadalafil hat, dafür aber schnell einsetzt. Männer, die diskret bestellen möchten, suchen häufig nach levitra kaufen ohne rezept. Dabei spielt die rechtliche Lage in der Schweiz eine wichtige Rolle.
Stress.cl
Neuroscience 169 (2010) 98 –108
CHRONIC FLUOXETINE TREATMENT INDUCES STRUCTURAL
PLASTICITY AND SELECTIVE CHANGES IN GLUTAMATE RECEPTOR
SUBUNITS IN THE RAT CEREBRAL CORTEX
E. AMPUERO,a F. J. RUBIO,a R. FALCON,a
The selective serotonin reuptake inhibitor fluoxetine (flx) is
M. SANDOVAL,a G. DIAZ-VELIZ,b R. E. GONZALEZ,a
one of the most commonly prescribed antidepressant drugs.
N. EARLE,a A. DAGNINO-SUBIABRE,c F. ABOITIZ,d
The mechanisms underlying its antidepressant action, how-
F. ORREGOa AND U. WYNEKENa*
ever, are still unclear. Although serotonin levels rise rapidly
aLaboratorio de Neurociencias, Universidad de los Andes, Santiago,
after acute administration, several weeks are required before
therapeutic benefits are achieved. The delayed onset of an-
bFacultad de Medicina, Universidad de Chile, Santiago, Chile
tidepressant action suggests that plastic changes over
cLaboratorio de Neurobiologia y Conducta, Facultad de Medicina,
protracted periods of time might be causally related to the
Universidad Catolica del Norte, Santiago, Chile
therapeutic effect. Repetitive antidepressant administration
dDepartamento de Psiquiatria, Pontificia Universidad Catolica de
promotes plastic changes such as neurogenesis, synapto-
Chile, Santiago, Chile
genesis, neurotrophin signalling, and changes in chromatinstructure and gene expression
Abstract—It has been postulated that chronic administration
Increasing evidence implicates glutamatergic circuit-
of antidepressant drugs induces delayed structural and mo-
ries in these plastic changes
lecular adaptations at glutamatergic forebrain synapses that
might underlie mood improvement. To gain further insight
into these changes in the cerebral cortex, rats were treated
We found that 2 weeks of flx treat-
with fluoxetine (flx) for 4 weeks. These animals showed de-
ment induced brain-derived neurotrophic factor (BDNF)
creased anxiety and learned helplessness. N-methyl-D-aspar-
signalling at excitatory forebrain synapses
Glutamatergic synapses are situated on dendritic
propionate (AMPA) receptor subunit levels (NR1, NR2A,
spines containing postsynaptic densities (PSDs), which
NR2B, GluR1 and GluR2) were analysed in the forebrain by
allow glutamate receptors to anchor through interactions
both western blot of homogenates and immunohistochemis-
with scaffolding proteins. Spines and PSDs are plastic
try. Both methods demonstrated an upregulation of NR2A,
structures, and large spines are associated with more
GluR1 and GluR2 that was especially significant in the retro-
efficient synapses The devel-
splenial granular b cortex (RSGb). However, when analysing
subunit content in postsynaptic densities and synaptic mem-
opment of dendritic spines begins with the formation of
branes, we found increases of NR2A and GluR2 but not
filopodia. After establishing contact with pre-synaptic ter-
GluR1. Instead, GluR1 was augmented in a microsomal frac-
minals, they mature into spines acquiring thin, then stubby,
tion containing intracellular membranes. NR1 and GluR2
and finally mushroom morphologies. The latter represent
were co-immunoprecipitated from postsynaptic densities
the most mature and stable spines.
and synaptic membranes. In the immunoprecipitates, NR2A
Ionotropic glutamate receptors include the alpha-amino-
was increased while GluR1 was decreased supporting a
3-hydroxy-5-methyl-4-isoxazole propionic acid (AMPA-R)
change in receptor stoichiometry. The changes of subunit
and N-methyl-D-aspartate (NMDA-R) types. NMDA-Rs are
levels were associated with an upregulation of dendritic
heterotetramers composed of NR1 and NR2 subunits that
spine density and of large, mushroom-type spines. These
coassemble to form a functional channel. A single NR1 sub-
molecular and structural adaptations might be involved in
neuronal
unit exists in eight splice isoforms, and there are four distinct
treatment. 2010 IBRO. Published by Elsevier Ltd. All rights
NR2 subunits (NR2A–D). AMPA-Rs are homo- or heterotet-
ramers composed mainly of GluR1 and 2/3 subunits in theadult forebrain. NMDA-R and AMPA-R subunit composition
Key words: antidepressants, glutamate, ionotropic receptors,
is a major determinant of biophysical properties, association
dendritic spines, cerebral cortex.
to protein complexes, downstream signalling, receptor traf-ficking and synaptic targeting Glutamate receptor availability de-
*Corresponding author. Tel: ⫹56-2-4129353; fax: ⫹56-2-2141752.
termines spine structure, and NMDA-Rs, especially those
E-mail address: (U. Wyneken).
Abbreviations: BDNF, brain-derived neurotrophic factor; EPM, ele-
containing NR2A subunits, are present in large and stable
vated plus maze; flx, fluoxetine; FST, forced swim test; IHC, immuno-
histochemistry; NMDA, N-methyl-D-aspartate; NSF, novelty-sup-
larly, GluR2 subunits promote the formation and growth of
pressed feeding; PrL, prelimbic cortex; PSDs, postsynaptic densities;
RSGb, retrosplenial granular b cortex; TST, tail suspension test; WB,western blots.
0306-4522/10 $ - see front matter 2010 IBRO. Published by Elsevier Ltd. All rights reserved.
doi:10.1016/j.neuroscience.2010.04.035
E. Ampuero et al. / Neuroscience 169 (2010) 98 –108
The present study examined glutamate receptor levels
with 2 cm of wooden bedding. Twenty-four hours before behav-
following long-term flx treatment in the prelimbic (PrL), retro-
ioural testing (immediately following the last drug injection), ani-
splenial granular b (RSGb) and secondary motor (M2) corti-
mals were deprived of food in the home cage. At the time oftesting, one food pellet was placed on a piece of round filter paper
ces, as well as in subcellular fractions obtained from the
(10 cm in diameter) positioned in the centre of the box. The animal
forebrain. Spine morphology was analysed in the same re-
was placed in a corner of the cage. The latency to begin feeding
gions. Our results strongly suggest that flx induces plastic
was recorded (maximum time, 15 min). Afterwards, the amount of
changes in extensive forebrain networks that are consistent
food consumed in 5 min in the home cage was measured.
with a functional stabilization of synaptic connections.
Spontaneous motor activity.
Rats were individually placed
in a plexiglass cage (30⫻30⫻35 cm3) located inside a soundproof
chamber. The spontaneous motor activity and rearing was moni-tored during a period of 30 min and evaluated as described
We used adult male Sprague–Dawley rats (250 – 400 g) in all of
The apparatus consisted of a central
the experiments. Procedures involving animals and their care
platform (10⫻10 cm2), two opposed open arms (50⫻10 cm2) and
were performed in accordance with the Universidad de los, Andes
two opposed closed arms of the same size with 40-cm-high
Bioethical Committee and the Guide for the Care and Use of
opaque walls. The maze was elevated 83 cm above the ground.
Laboratory Animals from the National Institutes of Health. All
Each animal was placed at the centre of the maze facing one of
efforts were made to minimize animal suffering. Flx at doses of 0.7
the open arms. During a 5-min interval, the number of open and
or 3.5 mg/kg (Ely-Lilly Co., Indianapolis, USA) or 0.9% NaCl (sal)
closed arms entries, plus the time spent in the open and closed
were administered daily by i.p. injection between 9:00 and 10:00
arms were measured in dim light.
AM for 28 days. Body weight was controlled daily and the percent-age of weight gain was calculated. In total, 224 rats were sacri-
Tail suspension test.
Rats were individually suspended by
ficed 24 h following the last flx or sal injection to perform immu-
the tail to a horizontal ring stand bar (distance from the table, 40
nohistochemistry (
n⫽6 per group), Golgi staining (
n⫽6 per group)
cm) at 4 –5 cm from the beginning of the tail. A 6-min test session
or subcellular fractionation (
n⫽10 per group, in total 10 indepen-
was recorded. The behavioural parameter measured was the
dent preparations were performed). For immunohistochemistry or
number of seconds spent in a completely immobile posture.
for Golgi staining, rats were sacrificed under ketamine (50 mg/kg)and xylazine (5 mg/kg) anaesthesia and then perfused intracardi-
Forced swim test.
The forced swim test was performed
ally with sal followed by 300 ml of 4% paraformaldehyde in PBS.
according to a modification, suggested by Lucki of
After perfusion, brains were removed immediately and processed
the traditional method described by Porsolt
for immunohistochemistry or for Golgi staining.
The following behavioural responses were recorded: escape orclimbing behaviour, which was defined as upward-directed move-
ment of the forepaws along the side of the swim chamber; swim-ming behaviour, which was defined as movement throughout the
All chemical reagents were purchased from Sigma (St Louis, MO,
swim chamber; and immobility, which was recorded when the rat
USA), unless otherwise stated. Protein G Sepharose was from
made no further attempts to escape except the movement nec-
Amersham Biosciences (Freiburg, Germany).
essary to keep its head above the water.
The primary antibodies used for immunohistochemistry (IHC),
synaptic membrane staining and western blots (WB) were as
Tissue preparation and subcellular fractionation
follows: guinea pig anti-ProSAP2/Shank3 (synaptic membranes,1:2000) (kindly donated by Eckart D. Gundelfinger at the Leibniz
Rats were sacrificed by rapid decapitation and the cortices and
Institute for Neurobiology, Magdeburg, Germany); anti-GluR1
hippocampi were immediately separated on ice and placed in
(synaptic membranes, 1:10; IHC, 1:50; WB, 1:1000), anti-GluR2
homogenization buffer (0.32 M sucrose, 0.5 mM EGTA, 5 mM
(synaptic membranes, 1:50; IHC, 1:400) and anti-NR2A (IHC,
Hepes, pH 7.4) supplemented with a mixture of protease inhibitors
1:100; Chemicon International, Temecula, CA, USA); anti-GluR2
(Boehringer, Mannheim). Subcellular fractionation was performed
(WB, 1:1000; BD Biosciences, Pharmingen, San Jose, CA, USA);
following the method of Wyneken et al.
anti-NR2A (synaptic membranes, 1:2000; WB, 1:1000; Millipore
Synaptosomes were collected from the first sucrose gradient at
Corporation, MA, USA); anti-NR1 (IHC, 1:250; Pharmingen, San
the 1/1.2 M interphase and submitted to a hypo-osmotic shock to
Diego, CA, USA); and anti-NR2B (IHC, 1:100; WB, 1:1000), anti-
release intracellular organelles. Synaptic membranes were col-
SAP102 (WB, 1:1000) and anti-PSD-95 (WB, 1:250; BD Trans-
lected from the second sucrose gradient at the 1/1.2 M interphase
duction Laboratories, San Jose, CA, USA).
and delipidated in 0.5% Triton to yield PSDs. The microsomalfraction (P3) resulted from the centrifugation of the supernatant
(S2) at 100,000 g for 1 h. Protein concentrations were determined
Rats were housed in groups of three in a 12-h (8:00 –20:00)
using the BCA assay (Pierce, Rockford, IL, USA).
light/dark cycle at 22⫾1 °C with standard rodent pellet food andwater available
ad libitum. Each animal behaviour was evaluated
only once, 24 h after the final injection using the following se-
Protein (250 g) was solubilized for 2 h in 1 ml of solubilization
quence: novelty-suppressed feeding (NSF), spontaneous motor
buffer (50 mM Tris–Cl, 1% deoxycholate plus protease inhibitors,
activity, elevated plus maze (EPM), tail suspension test (TST) and
pH 9.0). The corresponding primary antibody or control IgG (2 g)
the pretest session of the forced swim test (FST). The test session
was added to the supernatant and incubated overnight. Protein G
of the FST was applied on the second day (i.e., 48 h after the last
Sepharose (20 l pre-washed with solubilization buffer and
drug injection). Scores were generated from live observations byan experimenter blinded to the treatment condition, and video
blocked with 0.2% BSA) was added and incubated for 1 h. The
sequences were used for later reanalysis when necessary.
samples were centrifuged for 5 min at 1000 g and the superna-tants were discarded. The immunoprecipitates were washed three
Novelty-suppressed feeding test.
The testing apparatus
times with solubilization buffer and were resuspended in 60 l of
consisted of a plastic cage (80⫻70⫻40 cm3) with its floor covered
electrophoresis loading buffer.
E. Ampuero et al. / Neuroscience 169 (2010) 98 –108
Over 1200 spines from 18 to 23 neurons (four to five neurons
per animal) were analysed per condition. In addition, images from
Western blots.
Equal amounts of protein (20 g) were sep-
each analysed dendritic segment were captured as visual support
arated by 4 –20% SDS gradient gel electrophoresis, transferred to
at 1 m-spaced focal plane in the region of interest using a BX61
nitrocellulose membranes and immunoblotted with the indicated
Olympus microscope (100⫻ objective, numeric aperture 1.3) at-
primary and corresponding secondary antibodies.
tached to a Diagnostic Instruments 25.4/2 MP camera (final mag-nification of 4700⫻; see movie in supplementary material).
Synaptic membranes were immuno-
stained following the procedure of Ciruela
Synaptic membranes were plated on polylysine-coated slides,fixed in 4% paraformaldehyde in PBS containing 4% sucrose for
Data were analysed using Graph Pad Prism 4.0 software and
10 min and washed with PBS containing 25 mM glycine. Mem-
presented as mean⫾SEM. The statistical test used for behav-
branes were permeabilized, blocked and incubated with the indi-
ioural data was one-way analysis of variance (ANOVA) followed
cated primary antibody. After incubation with the primary antibody,
by Newman–Keuls post hoc test for multiple comparisons. For
the membranes were washed and stained with the corresponding
morphological and immunochemical staining, the Mann–Whitney
secondary antibody (1:600; Alexa Fluor® 647 conjugated goat
U-test was applied. In WBs, the relative optic densities of bands
anti-Guinea-pig IgG, Alexa Fluor® 488 conjugated goat anti-rabbit
were compared by two-tailed
t-tests. A probability level of
P⬍0.05
IgG or Alexa Fluor® 555 conjugated donkey anti-mouse IgG;
(*) or
P⬍0.01 (**) was accepted as significant.
Invitrogen, Eugene, OR, USA). Images of serial sections werecaptured with a Nikon Eclipse TE2000-U inverted epifluorescence
microscope with a Plan Fluor 60⫻/1.25 numeric aperture oil-immersion objective attached to a cooled monochrome camera
Twenty-eight days of 0.7 mg/kg fluoxetine reduced
DS-2MBWc (final magnification 3500⫻).
anxiety and learned helplessness in rats
After perfusion, brains were cryopre-
To evaluate meaningful changes elicited by flx, we first
served and cut serially in 30-m frozen coronal sections. Staining
examined behaviour using two different flx doses, 0.7
was performed according to Ampuero and collaborators
mg/kg (0.7 flx) or 3.5 mg/kg (3.5 flx), to select a dose with
Immunoreactivity for the RSGb was quantified in coronalsections restricted to interaural 5.86 mm/bregma ⫺3.14 mm and
optimal effects following 4 weeks of treatment. Although
interaural 4.84 mm/ bregma ⫺4.16 mm. Quantification of immuno-
selective serotonin reuptake inhibitors are known to induce
reactivity in the PrL and M2 regions was restricted to interaural 13.20
anorectic effects in rats, there was no significant difference
mm/bregma 4.20 mm and interaural 11.20/bregma 2.20 mm
in body weight gain among the experimental groups ex-
Layer V pyramidal neurons were examined
amined in this study (sal group, 140.8⫾6.2%; 0.7 flx group,
in the RSGb and PrL area. In M2, layer II/III pyramidal neurons were
126⫾4%; 3.5 flx group, 131.4⫾4.1%; compared to day 1 of
analysed. Brain slices were visualized under a light microscope(AxiosKop, Zeiss, Germany; 10⫻ magnification; numeric aperture
treatment). This may be due to the low doses of flx used in
0.3), and images of serial sections were captured with a digital
the present study. Interestingly, treatment with 0.7 mg/kg
camera (Nikon, CoolPIX 995) with a final magnification of 880⫻.
flx has already been shown to lead to plasma levels con-
Digitized images were analysed with ImageJ program. The number
sidered therapeutically effective in humans, and this dose
of positive cells was counted in equal sample areas of 0.0289 mm2.
stimulates BDNF/TrkB signalling at excitatory synapses
At least five sections per animal were analysed in control and treated
animals (
n⫽5 per group).
We first assessed anxiety-like behaviour in the NSF
test left panel). The NSF test is a behavioural
paradigm that is sensitive to chronic antidepressant treat-
Brains were processed using the FDRapid GolgiStainTM kit (FD
ments and acute treatments with anxiolytics (such as ben-
Neuro Technologies, Baltimore, USA) and
zodiazepines) but not subchronic antidepressant treat-
analysed by an individual blind to the experimental conditions.
ments. In the NSF test, the latency to feed decreased from
Eighteen to twenty-three randomly selected layer V pyramidal
396.9⫾39.8 s to 257.3⫾34.2 s in the 0.7 flx group and to
neurons were examined per experimental condition in the RSGb
272.3⫾37.6 s in the 3.5 flx group (
F
and PrL area. In M2, layer II/III pyramidal neurons were analysed.
The selected neurons were required to have no breaks in staining
groups
P⬍0.05 compared to sal). After the test session,
along the dendrites. Starting from the origin of the soma (for
animals were returned to the home cage and allowed to
primary dendrites) or the branch point (for secondary dendrites)
eat. Next, we measured spontaneous motor activity be-
and continuing away from the cell soma, spines were counted in
cause a change in this activity might indirectly influence
8 m segments along an 80 m stretch of the dendrite. Dendritic
behavioural tests. Although the flx-treated rats revealed a
spines were classified under the microscope at different focal
trend towards decreased motor activity, this was not sig-
planes, by a single rater, blinded to the experimental groups, intothree shape categories filopodia/thin, stubby
nificant when counted over a 30 min period (sal group,
and mushroom/branched spines. Thin spines had a greater length
1482⫾119.7, 0.7 flx group, 1388⫾113.3, 3.5 flx group,
than neck diameter and similar head and neck diameters. Stubby
1303⫾140.3; (
F
⫽0.4963)). Because rearings are pos-
spines had neck diameters that were similar to the total spine
itively correlated with anxiety we also ex-
length. For mushroom-shaped spines, the diameter of the head
amined the number of rearings as an index of vertical
was much greater than the diameter of the neck. Branched spines
exploratory behaviour. We found significantly increased
had more than one head emerging from a single neck originating
rearing in the 3.5 flx group compared to the 0.7 flx group
from the dendrite. If there was ever any doubt in a classification(less than 10%), spine and neck diameters were measured from
(
P⬍0.05) (sal group, 30⫾2.2, 0.7 flx group, 21.1⫾2.2, 3.5
the picture taken at the focal plane in which it appeared at its
flx group, 37.6⫾7.3;
F
⫽3.872;
P⬍0.05). Although a
maximal size.
trend towards decreased rearing with 0.7 flx treatment was

E. Ampuero et al. / Neuroscience 169 (2010) 98 –108
ses of structural and molecular effects at glutamatergic fore-brain synapses.
Long-term flx induces subunit-specific changes in
glutamate receptor subunit levels
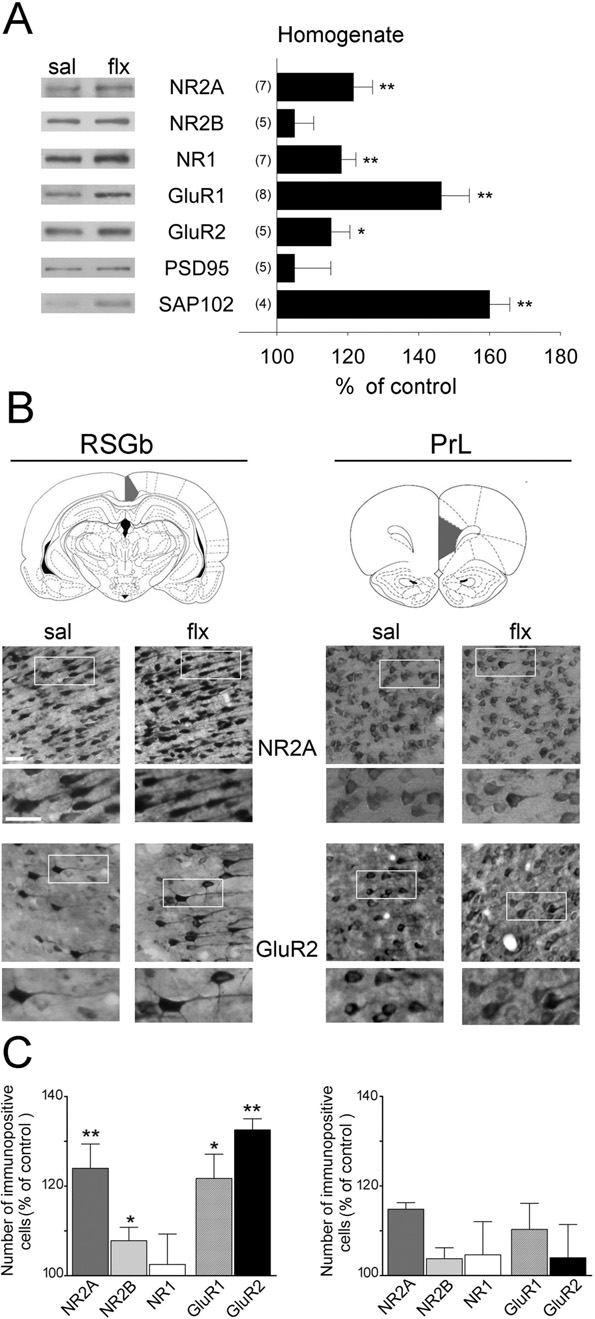
Initially, we measured the levels of glutamate receptor sub-units in forebrain homogenates by WB We detecteda large increase in GluR1 (P⬍0.01), GluR2 (P⬍0.05), NR2Aand NR1 (P⬍0.01%), in the homogenates, but no changewas found for NR2B. PSD-95 and SAP102 are examples ofglutamate receptor scaffolding proteins, which have beenimplicated in the trafficking and anchoring of glutamate re-ceptor subunits. In the present study, we did not detect anychanges in PSD-95, but SAP102 increased by 60⫾6%. Tocheck these results in cerebrocortical subregions, we per-
Fig. 1. Fluoxetine decreases depressive-like behaviours in rats. Rats
formed IHC in coronal sections of the forebrain. In
were treated for 28 days with sal (white bars), 0.7 (black bars) or 3.5
(upper panel), two of the selected regions are shown: the PrL
(grey bars) mg/kg of flx. (A) Anxiety was analysed using the NSF test andthe EPM test. Performances were video-recorded and analysed blindly. In
cortex, a subregion of the prefrontal cortex thought to mod-
the EPM, the time spent in the open arms divided by the number of entries
ulate depressive symptoms
into the open arms is shown. (B) Learned helplessness was assessed by
and the RSGb, a limbic cortex on the posterior
the TST and the FST. In both cases, immobility was scored (in sec-
cingulate gyrus that has recently been implicated in the con-
onds). In addition, swimming and climbing behaviours were measured
trol of emotions
separately in the FST. Results are presented as mean⫾SEM.
* P⬍0.05; ** P⬍0.01: compared to sal; # P⬍0.05: 3.5 flx compared
(lower panel) shows representative stainings of NR2A and
to 0.7 flx; ## P⬍0.01: 3.5 flx compared to 0.7 flx (one-way ANOVA
GluR2 at two different magnifications. The quantification of
followed by post hoc test).
layer V-immunopositive cells revealed an increase of NR2A,
evident, the difference between the sal and 0.7 or 3.5 flx
NR2B, GluR1 and GluR2 in the RSGb area, whereas no
groups did not reach significance. Anxiety-like behaviour
change was found in the PrL area
was also evaluated in the EPM test, which has been widely
To determine whether the upregulated receptors were
validated to measure anxiety in rodents
transported to the synapse, glutamate receptor subunits
In the EPM test right
were detected by WB in isolated PSDs We found
panel), the time in the open arm per entry increased in the
no change in NR2B even though NR1 and NR2A subunits
rats treated with 0.7 flx (F
⫽5.227; P⬍0.05). The New-
increased. In addition, the AMPA-R subunit GluR2 did not
man–Keuls post hoc multiple comparisons revealed that
change, but GluR1 was decreased by 12⫾5% and the
the scores of the 3.5 flx group were not different from
glutamate receptor scaffolding proteins PSD-95 and
controls. In addition, the 0.7 flx group scores differed from
SAP102 were increased by 23⫾7% and 30⫾4%, respec-
those of both the sal and the 3.5 flx groups (P⬍0.05).
tively (not shown). Thus, treatment with flx appeared to
These results confirm that the 0.7 mg/kg dose is more
favour synapses with higher ratios of NR2A to NR2B and
efficient in decreasing anxiety.
GluR2 to GluR1. A possible explanation of decreased
Learned helplessness was assessed by the TST and the
AMPA-R detection in PSDs is that GluR1 and GluR2 sub-
FST In both tests, immobility decreased following
units, although increased in homogenates, might be pref-
0.7 or 3.5 flx treatment. In the TST (F
erentially localized intracellularly. To test this hypothesis,
the post hoc Newman-Keuls test revealed a significant de-
we used WB to determine that glutamate receptor subunits
crease for both flx doses compared with sal (P⬍0.01). In the
in the microsomal P3 fraction This fraction is a
⫽4.655, P⬍0.05), the post hoc test revealed a
heterogeneous membrane compartment known to be en-
significant decrease for both 0.7 and 3.5 flx (P⬍0.01 and
riched in endoplasmic reticulum, Golgi network, endo-
P⬍0.05, respectively). However, only the 0.7 flx dose caused
somes, trafficking vesicles and synaptic vesicles, but not in
a significant increase in swimming behaviour when com-
PSDs (see Suppl. Fig. 1). Consistent with our previous
pared to the sal and 3.5 flx doses (F
data, we found increases of NR2A (P⬍0.05) whereas
The post hoc test revealed a significant effect (P⬍0.01) for
NR2B decreased (P⬍0.01). NR1 content was not modi-
0.7 flx compared to sal and 3.5 flx. In this same test, the
fied. Significantly elevated GluR1 and GluR2 were found
climbing behaviour was increased by 3.5 flx but not by 0.7 flx
(P⬍0.05 in both cases) suggesting that these subunits are
⫽3.934, P⬍0.05, and P⬍0.05 in the Newman-Keuls
present in intracellular membranes, which contain a variety
test comparing sal with 3.5 flx). Although both flx doses
of cellular components including trafficking compartments.
decreased immobility, the lower dose enhanced swimming
Another possibility was that AMPA-Rs were lost during the
behaviour, and the higher dose increased climbing behav-
PSD purification procedure because they are loosely at-
iour. Overall, these results established that 0.7 flx for 28 days
tached to PSDs and a proportion of them is localized on
consistently induced antidepressant-like behavioural effects
their periphery. Therefore, we used immunodetection to
(reducing both anxiety and learned helplessness). Therefore,
determine AMPA-R subunit localization in synaptic mem-
the 0.7 mg/kg dose of flx was chosen for subsequent analy-
branes, which are the cellular fractions from which PSDs
E. Ampuero et al. / Neuroscience 169 (2010) 98 –108
are obtained by delipidation. Synaptic membranes corre-sponded to synaptosomes that had been subjected to anosmotic shock to release intracellular membranes (i.e.,their microsomal compartment). For increased sensitivityin this analysis compared to WBs, we stained synapticmembranes fixed on slides and quantified the levels ofreceptor subunits present exclusively in membrane com-partments containing the scaffolding protein ProSAP2/Shank3, a reliable marker of excitatory synapses As a positive control, we quantified the co-localization ofNR2A and ProSAP2. Consistent with the WBs and immu-nohistochemical analysis, we found a significant increaseof NR2A localization at excitatory synapses after flx treat-ment left panel, P⬍0.01). Interestingly, the size ofthe stained membranous structures, corresponding tospines that frequently retain their presynaptic terminal, wasaugmented right panel, P⬍0.05). This observa-tion was consistent with the idea that NR2A subunits arepresent on larger spines. A similar analysis for GluR1 andGluR2 reflected no change in GluR1/ProSAP2 co-localiza-tion but a significant increase in GluR2/ProSAP2 colocal-ization (P⬍0.05). In both cases, the synaptic membranearea increased (P⬍0.05). These results suggested thatAMPA-Rs present in the spine membrane preferentiallycontain GluR2 subunits and confirm that NMDA-Rs pref-erentially contain NR2A over NR2B. To test whether thesubunit composition of N-methyl-D-aspartate (NMDA) andAMPA-Rs changed following flx, we co-immunoprecipi-tated relevant subunits from PSDs, synaptic membranesand synaptosomes as starting material. showsrepresentative immunoprecipitations of NR1 and GluR2subunits from PSDs. Following flx, NR2A increased by4.50⫾0.88 times over control in NR1 immunoprecipitates(n⫽3, P⬍0.05) and no change was observed for NR2B.
GluR1 decreased in GluR2 immunoprecipitates to 0.62⫾0.11when compared to sal (n⫽4, P⬍0.05). This was confirmedby immunoprecipitations from synaptic membranes inwhich the decrease of GluR1 was found to be 0.59⫾0.13(n⫽3, P⬍0.05). However, when synaptosomes (i.e. syn-aptic membranes containing intracellular membranes)were taken as starting material for immunoprecipitation(n⫽4), no significant differences in AMPA-R stoichiometrywere found (1.07⫾0.14). These results indicate that at thelevel of the spine membrane, flx induced a switch towardsAMPA-Rs preferentially enriched in GluR2 subunits and of
Fig. 2. Fluoxetine induces region-specific changes in glutamate receptor
levels. (A) WBs of glutamate receptor subunits (NR2A, NR2B, NR1,
NMDAR-Rs enriched in NR2A subunits. In contrast, GluR1
GluR1 and GluR2) and scaffolding proteins (PSD-95 and SAP102) in
was increased but it might accumulate in intracellular lo-
cortical homogenates are shown. The left panel shows representative
cations. Taken together, these results confirm that GluR1
western blots for each protein, and the right panel shows the mean
might not reach dendritic spine membranes or PSDs even
change of control ⫾ SEM obtained by densitometric quantification of
though immunohistochemistry and WBs demonstrated that
WBs. The number of independent experiments is indicated. (B) Gluta-mate receptor subunits were immunodetected in coronal sections through
it was increased.
the rat cortex in the indicated brain areas (after Paxinos and Watson,2008) (RSGb: retrosplenial granular cortex b; PrL: prelimbic cortex). Be-
Changes in dendritic spine density and morphology
low, representative photomicrographs of NR2A and GluR2-stained sec-
after 28 days of flx
tions are shown (scale bar: 25 m). (C) Quantification of immunopositivecells expressed as change over control. Significant increases were found
To determine whether changes in glutamate receptor sub-
in the number of NR2A, NR2B, GluR1 and GluR2 positive cells in the
unit composition were associated with changes in spine
RSGb but not in the PrL. Results are presented as mean⫾SEM and were
morphology, we performed Golgi stainings in the described
determined from analysing 4 – 6 sections of five rats per group. Data werestatistically evaluated with Mann–Whitney U-test, * P⬍0.05; ** P⬍0.01.
cortical subregions and movie in SupplementaryMaterial). shows representative inverted images,

E. Ampuero et al. / Neuroscience 169 (2010) 98 –108
Fig. 3. Fluoxetine-induced changes on AMPA and NMDA-R subunit levels. (A) Western blots of glutamate receptor subunits in isolated postsynaptic
densities (PSDs) are shown. Left panel shows representative western blots for each protein, and the right panel shows the mean change of control ⫾ SEM
obtained by densitometric quantification of western blots. Note that GluR1 decreases in PSDs. The number of independent determinations is indicated. (B)
Quantification of glutamate receptor subunits detected by western blots in the microsomal fraction (P3), which is enriched in intracellular membranes but not
PSDs. (C) Immunocytochemical identification of NR2A (green) in the glutamatergic population of synaptic membranes (identified as ProSAP2/Shank3
immunoreactive; red). In the superimposed picture of the double immunocytochemical labelling (merge) of these representative fields, co-localization was
quantified (scale bar: 5 m). (D) The left panel shows the quantification of co-localization of five different fields per slide from four independent experiments
using different membrane preparations and confirmed the augmented co-localization of NR2A and GluR2 but not of GluR1 with isolated glutamatergic
synapse compartments. In the right panel, the size of the double-positive membrane compartments was calculated. Note that the synaptic membranes
correspond to synaptosomes that were subjected to a hypo-osmotic shock in order to release intracellular membranes. Results are presented as
mean⫾SEM. Data were statistically evaluated with a paired t-test, * P⬍0.05; ** P⬍0.01. (E) Equal amounts of NR1 and GluR2 were immunoprecipitated from
PSDs to evaluate changes in receptor stoichiometry. WBs of the corresponding subunits are shown. In NR1 immunoprecipitates, a large increase in NR2A
(4.5⫾0.88; P⬍0.05) was found whereas a decrease of GluR1 (0.62⫾0.11; P⬍0.05) was found in GluR2-containing AMPA-Rs (n⫽3).
taken at three different focal planes, of a primary dendrite
the RSGb, which was the same region that revealed sig-
present in the RSGb of sal and flx treated animals and
nificant increases in glutamate receptor subunits excluding
indicates the main morphological types (filopodia/thin,
NR2B. To evaluate whether this is a more general phe-
stubby and mushroom). Spine density was measured from
nomenon, secondary dendrites in these areas were exam-
its emergence from the soma along an 80 m stretch of the
ined (not shown), and the analysis was extended to the
primary apical dendrite that was subdivided into 10 seg-
secondary motor cortex (M2, supplementary material),
ments The number of spines per 8 m segment
which is not thought to be directly involved in the modula-
increased beginning at the fifth segment in the RSGb and
tion of depressive behaviours. For secondary dendrites,
at the seventh segment in the PrL. This was accompanied
shorter segments (from 32 to 80 m) were measured with
by a robust increase in the percentage of mushroom-type
the premise that the selected segment had to be continu-
spines and a decrease of thin spines in the RSGb
ous with the primary dendrite and the cell soma. In the
right panel, P⬍0.01). In the PrL, thin spines also de-
case of the RSGb, total spine density (measured as the
creased and stubby spines increased (P⬍0.05), but there
number of spines per m) increased from 0.70⫾0.04 to
was no change in mushroom-type spines. Therefore, the
0.84⫾0.04 (P⬍0.05). Mushroom-type spines increased
change in spine morphology was especially significant in
from 32.9% to 43.7% (P⬍0.05) with a concomitant de-

E. Ampuero et al. / Neuroscience 169 (2010) 98 –108
Fig. 4. The effect of flx on spine density and morphology. Cortical pyramidal neurons were visualized by the rapid Golgi impregnation method. (A)
Representative images taken at three different focal planes in sal and flx-treated rats were inverted for better visualization. The original image, used
for quantification at 12 different focal planes per dendritic segment, is presented in the supplementary material. The three shape categories of spines
were shown as follows: filopodia/thin (T), stubby (S) and mushroom/branched (M). The numbers in parentheses indicate superimposed spines that
were resolved by observation at different focal planes: (2) represents a thin and a mushroom-type spine in both cases and (3) represents a thin, a
stubby and a mushroom-type spine. See experimental procedures and the movie in supplementary material for details. The scale bar was 1 m. (B)
The left panels show spine number along primary dendrites in consecutive dendritic segments of 8 m away from the soma in the RSGb and the PrL.
The right panels show the classification of dendritic spines, which revealed a significant increase of mushroom-type spines in the RSGb, but no
differences in the PrL, between sal and flx treated animals. All results are presented as mean⫾SEM determined from analysing 18 –23 cells/area
obtained from five rats per group, * P⬍0.05; ** P⬍0.01.
crease of thin and stubby-like spines (P⬍0.05). In the PrL,
Taken together, the results indicated that flx induced
no change in secondary spine density was found, and this
morphological and molecular changes affecting large fore-
was associated with no change in spine morphology. In-
brain areas. Increased mushroom-type spines in selected
terestingly, the changes in both spine morphology and
regions were accompanied by global increases of NR2A-
glutamate receptor subunit levels extended to layer II/III of
containing NMDA-Rs and of GluR2-containing AMPA-Rs.
M2 (Suppl. Fig. 2).
GluR1 subunits, although upregulated, might be retained
E. Ampuero et al. / Neuroscience 169 (2010) 98 –108
in the cell soma, or they might be located near, or even
mance). In the future, behavioural studies of flx dosing
within, spines. Surprisingly, such changes were highly sig-
should be undertaken in animals in which depressive-like
nificant in the RSGb but less prominent in the PrL cortex.
behaviour is induced (e.g., by exposure to chronic stress).
Glutamate receptors and antidepressants
A strong relationship between mood disorders and gluta-
Our results were the first to demonstrate that long-term
mate neurotransmission has already been postulated
behaviourally relevant doses of flx induced an enrichment
of specific glutamate receptor subunits in the rat forebrain.
like activity of NMDA-R antagonists and participation of
Whereas GluR2 and NR2A increased in spine membranes
AMPA-Rs, especially its GluR2 subunit
and PSDs, GluR1, although increased in homogenates
In agreement with our results, imipra-
and in immunohistochemical analysis, did not reach these
mine upregulated synaptic GluR2 and
synaptic compartments. Changes in subunits levels were
increased GluR1 in synaptosomes In-
associated with an increase of mushroom-type dendritic
creased GluR1 levels were found in a hippocampal syn-
spines in a region-specific manner and were especially
aptic fraction obtained from ovariectomized, but not con-
significant in the RSGb. The molecular changes observed
trol, rats treated with 5 mg/kg flx for 21 days
here may underlie the restoration of plasticity previously ob-
The difference in our results may be due to the flx
served in the visual cortex following flx
dose or the time course of the molecular adaptations. Flx
These changes are compatible with a "maturation-
might counteract the decrease of NR2A, but not NR2B,
like" process leading to stabilization of synaptic connections
observed in major depression
that may be related to a functional recovery of glutamatergic
or the decreases of NR1, NR2A and SAP102
forebrain networks
observed in bipolar disorder
Our behavioural data showed that the flx dose used in
the long-term studies is an important variable to be con-
NMDA and AMPA-Rs may be particularly important targets
sidered. Consistent with our results, a similar low-dose of
for the treatment of mood disorders, we postulated that
flx (1 mg/kg) induced antidepressant-like effects and effec-
subunit-specific strategies should be considered.
tively modulated neuronal firing rates
Role of glutamate receptor subunits in structural and
Conflicting data re-
garding mechanisms involved in antidepressant actionmight be due to the use of potentially harmful doses
The importance of NMDA-Rs in synaptic plasticity and
Increased fear has been reported
memory are well described
following 10 mg/kg flx treatment in the EPM
However, the potential opposing contributions of NR2A
Our results indicated that 3.5 mg/kg flx was not
and NR2B subunits to LTP versus LTD have been highly
effective in reducing anxiety-like behaviour in the EPM or
controversial LTP itself might
rearing but was effective in the NSF. Although both the
induce an immediate switch favouring NR2A over NR2B-
NSF and EPM are conflict tests to assess anxiety, they
containing receptors
recruit different neuronal circuits. NSF depends crucially
The switch of NR2B to NR2A containing
on appetite drive whereas the EPM depends on explor-
NMDA-Rs during development is accompanied by synap-
atory behaviour Consistent with our
tic maturation, stabilization and growth
findings, contrasting results using both tests have previ-
In contrast to NR2B subunits, NR2A subunits are
ously been reported, and it has been proposed that the
predominantly present on large spines
EPM may show a decreased sensitivity to chronic antide-
In accordance with this, the
pressant treatment as well as high drug doses
synaptic enrichment of NR2A induced by flx was accom-
A differential dose-dependent effect was confirmed
panied by a higher proportion of mushroom-type spines,
in the FST. Climbing, a behaviour considered to be depen-
suggesting that flx might induce a maturation-like state.
dent on noradrenergic neurotransmission, was favoured
NMDA-Rs regulate synaptic strength by controlling the
over swimming after 3.5 flx but not 0.7 flx
trafficking of AMPA-Rs in and out of postsynaptic sites
In addition, both flx doses
Synaptic strengthening in-
caused opposing trends on rearing behaviour that were not
volves activity-dependent addition of GluR subunits con-
significant when compared to sal: treatment with 0.7 flx
taining a long intracellular C-terminal tail (e.g. GluR1-con-
induced a decrease in the amount of rearing, and 3.5 flx
taining) to synapses, whereas short-tailed subunits (GluR2
treatment induced an increase resulting in a significant
and GluR3) constitutively replace existing receptors.
difference between the 0.7 and 3.5 flx doses (P⬍0.05).
Therefore, inserted GluR1 subunits are later replaced by
Increased rearing has been shown to correlate positively
GluR2, although the requirement of this transient process
with anxiety-like behaviour
is currently debated A temporal
Our results suggested that the 3.5 dose, in
characterization of synaptic changes induced by flx would
addition to modulating noradrenergic neurotransmission,
be necessary to determine whether the switch towards
might negatively affect anxiety-like behaviour (such as
GluR2-containing receptors required a preceding GluR1
rearing) or have no effect (for instance, on EPM perfor-
insertion. The fact that we did not find changes in GluR1 or
E. Ampuero et al. / Neuroscience 169 (2010) 98 –108
NR1 content in PSDs 2 weeks after flx treatment indicates
ies it was found that the antagonists acted in an excitatory
that these adaptations, measured after 4 weeks, are slowly
manner because the blocked NMDA-Rs were localized on
GABAergic interneurons
A highly positive correlation also exists between GluR2
levels and synaptic size, spine density and mEPSC fre-
It is possible that the thera-
quency, a relationship that has not been established for
peutic effect of NMDA receptor antagonists, at the sub-
anaesthetic doses currently under investigation for the
In addition, GluR2 is more stably tethered to the
treatment of depression, is in part due to their pro-excita-
synapse and its incorporation is necessary for the long-term
tory effect in the retrosplenial cortex
expression of synaptic plasticity
In line with these re-
The increased levels of the scaffolding proteins
sults, the gray matter in bipolar disorder was reduced in the
PSD-95 and SAP102 in homogenates and PSDs are consis-
posterior cingulate/retrosplenial cortex of unmedicated
tent with their role in anchoring glutamate receptors and
subjects relative to medicated patients
delivering critical elements to growing spines.
The interesting possibility that the plastic changes
It is likely that the flx-induced effects on spine morphol-
induced by flx in this cerebrocortical region might be re-
ogy and glutamate receptor content were mediated by
lated with its positive effects on emotion and cognition
neurotrophic factors Several growth
should be investigated in the near future. In general, these
factors, including BDNF and vascular endothelial growth
findings underscore the importance of discriminating be-
factor (VEGF) and their signalling pathways are necessary
tween cortical subregions affected by antidepressants.
for a response to antidepressant drug treatment
Several questions should be addressed in the future. For
example, what are the cellular mechanisms that induce a
specific contribution to the observed changes needs to be
coordinated switch of both NMDA and AMPA-R subunits?
addressed in the future.
Are plastic changes in the glutamatergic system causallyrelated to mood recovery? Can we identify brain circuits that
Morphological changes in the forebrain
are specifically related to a subset of depressive behaviours,and are the RSGb and M2 part of such circuits? In the future,
Animal models currently used to elicit depressive-like
relevant brain circuitries might be preferentially targeted by
symptoms lead to structural changes in neuronal networks,
antidepressant treatments (e.g., by transcranial magnetic
including dendritic length and complexity and spine mor-
stimulation) in a personalized manner depending on individ-
ual symptomatology Our findings might
These changes can be reversed by antidepressant
contribute to the search of new and faster-acting antidepres-
sant interventions that target specific glutamate receptor sub-
gesting that the flx-induced spine remodelling that we ob-
units in concert as well as forebrain circuits critically involved
served is involved in antidepressant action. Increases in
in depressive symptoms.
spine density and mushroom-type spines in the RSGb and
The delayed molecular adaptations in extensive corti-
M2 might reflect enhanced basal neurotransmission. We
cal networks reported here may underlie the therapeutic
observed region-specific changes that correlated with
action of antidepressants. Our findings that a widely pre-
changes in glutamate receptor subunits. However, these
scribed antidepressant in humans induces structural and
effects probably extend to wide forebrain areas because
molecular plasticity in the adult forebrain suggests a po-
changes in glutamate receptor subunits could be detected
tential clinical application for antidepressants in neurolog-
in subcellular fractions obtained from whole forebrain ho-
ical disorders in which synaptic function is compromised.
mogenates. Consistent with this idea, flx induced structuralplasticity in the rat somatosensory cortex Cognitive-emotional behaviours rely on complex
Acknowledgments—This work was supported by Proyecto AnilloACT09-06 (U Wyneken). We are grateful to Soledad Sandoval for
interactions of networks in several brain areas
The contribution of specific areas to depressive-likebehaviours and antidepressant treatment is not completely
understood. Besides mood disturbance, depression is ac-companied by sensorimotor disturbances, and manipulat-
Adesnik H, Nicoll RA (2007) Conservation of glutamate receptor
ing the motor system (e.g., by physical exercise) improves
2-containing AMPA receptors during long-term potentiation. J Neu-rosci 27:4598 – 4602.
mood It is therefore conceivable that the
Al-Hallaq RA, Conrads TP, Veenstra TD, Wenthold RJ (2007) NMDA
structural modulation of M2 has some therapeutic signifi-
di-heteromeric receptor populations and associated proteins in rat
cance. In turn, the RSGb is connected with cortices known
hippocampus. J Neurosci 27:8334 – 8343.
to be involved in emotional control, such as the hippocam-
Alvarez VA, Ridenour DA, Sabatini BL (2007) Distinct structural and
pal formation and the midline limbic cortices
ionotropic roles of NMDA receptors in controlling spine and syn-
Activation of the RSGb by emotionally
apse stability. J Neurosci 27:7365–7376.
Ampuero E, Dagnino-Subiabre A, Sandoval R, Zepeda-Carreno R,
salient stimuli has already been shown
Sandoval S, Viedma A, Aboitiz F, Orrego F, Wyneken U (2007)
Interestingly, the retrosplenial cortex has been implicated
Status epilepticus induces region-specific changes in dendritic
in an experimental model of schizophrenia induced by
spines, dendritic length and TrkB protein content of rat brain cortex.
NMDA receptor antagonists. Unexpectedly, in these stud-
Brain Res 1150:225–238.
E. Ampuero et al. / Neuroscience 169 (2010) 98 –108
Antal M, Fukazawa Y, Eordogh M, Muszil D, Molnar E, Itakura M,
Gray NA, Du J, Falke CS, Yuan P, Manji HK (2003) Lithium regulates
Takahashi M, Shigemoto R (2008) Numbers, densities, and colo-
total and synaptic expression of the AMPA glutamate receptor
calization of AMPA- and NMDA-type glutamate receptors at indi-
GluR2 in vitro and in vivo. Ann N Y Acad Sci 1003:402– 404.
vidual synapses in the superficial spinal dorsal horn of rats. J Neu-
Grosshans DR, Clayton DA, Coultrap SJ, Browning MD (2002) LTP
leads to rapid surface expression of NMDA but not AMPA recep-
Bellone C, Nicoll RA (2007) Rapid bidirectional switching of synaptic
tors in adult rat CA1. Nat Neurosci 5:27–33.
NMDA receptors. Neuron 55:779 –785.
Guirado R, Varea E, Castillo-Gomez E, Gomez-Climent MA, Rovira-
Beneyto M, Meador-Woodruff JH (2008) Lamina-specific abnormali-
Esteban L, Blasco-Ibanez JM, Crespo C, Martinez-Guijarro FJ,
ties of NMDA receptor-associated postsynaptic protein transcripts
Nacher J (2009) Effects of chronic fluoxetine treatment on the rat
in the prefrontal cortex in schizophrenia and bipolar disorder. Neu-
somatosensory cortex: activation and induction of neuronal struc-
tural plasticity. Neurosci Lett 457:12–15.
Bessa JM, Ferreira D, Melo I, Marques F, Cerqueira JJ, Palha JA,
Hajszan T, Szigeti-Buck K, Sallam NL, Bober J, Parducz A, Maclusky
Almeida OF, Sousa N (2009) The mood-improving actions of an-
NJ, Leranth C, Duman RS (2010) Effects of estradiol on learned
tidepressants do not depend on neurogenesis but are associated
helplessness and associated remodeling of hippocampal spine
with neuronal remodeling. Mol Psychiatry 14:764 –773, 739.
synapses in female rats. Biol Psychiatry 67:168 –174.
Bleakman D, Alt A, Witkin JM (2007) AMPA receptors in the therapeu-
Harris KM, Jensen FE, Tsao B (1992) Three-dimensional structure of
tic management of depression. CNS Neurol Disord Drug Targets
dendritic spines and synapses in rat hippocampus (CA1) at postnatal
day 15 and adult ages: implications for the maturation of synaptic
Borta A, Schwarting RK (2005) Inhibitory avoidance, pain reactivity,
physiology and long-term potentiation. J Neurosci 12:2685–2705.
and plus-maze behavior in Wistar rats with high versus low rearing
Holmes A, Kinney JW, Wrenn CC, Li Q, Yang RJ, Ma L, Vishwanath
activity. Physiol Behav 84:387–396.
J, Saavedra MC, Innerfield CE, Jacoby AS, Shine J, Iismaa TP,
Canbeyli R (2010) Sensorimotor modulation of mood and depression:
Crawley JN (2003) Galanin GAL-R1 receptor null mutant mice
an integrative review. Behav Brain Res 207:249 –264.
display increased anxiety-like behavior specific to the elevated
Castren E (2005) Is mood chemistry? Nat Rev Neurosci 6:241–246.
Castren E, Voikar V, Rantamaki T (2007) Role of neurotrophic factors
Holsboer F (2008) How can we realize the promise of personalized
in depression. Curr Opin Pharmacol 7:18 –21.
antidepressant medicines? Nat Rev Neurosci 9:638 – 646.
Ciruela F, Casado V, Rodrigues RJ, Lujan R, Burgueno J, Canals M,
Isaac JT, Ashby M, McBain CJ (2007) The role of the GluR2 subunit in
Borycz J, Rebola N, Goldberg SR, Mallol J, Cortes A, Canela EI,
AMPA receptor function and synaptic plasticity. Neuron 54:859 – 871.
Lopez-Gimenez JF, Milligan G, Lluis C, Cunha RA, Ferre S, Franco R
Kessels HW, Malinow R (2009) Synaptic AMPA receptor plasticity and
(2006) Presynaptic control of striatal glutamatergic neurotransmission
behavior. Neuron 61:340 –350.
by adenosine A1-A2A receptor heteromers. J Neurosci 26:2080 –
Kobayashi C, Aoki C, Kojima N, Yamazaki H, Shirao T (2007) Drebrin
a content correlates with spine head size in the adult mouse
Contreras CM, Rodriguez-Landa JF, Gutierrez-Garcia AG, Bernal-
cerebral cortex. J Comp Neurol 503:618 – 626.
Morales B (2001) The lowest effective dose of fluoxetine in the
Kopec C, Malinow R (2006) Neuroscience. Matters of size. Science
forced swim test significantly affects the firing rate of lateral septal
314:1554 –1555.
nucleus neurones in the rat. J Psychopharmacol 15:231–236.
Krishnan V, Nestler EJ (2008) The molecular neurobiology of depres-
Cryan JF, Markou A, Lucki I (2002) Assessing antidepressant activity
sion. Nature 455:894 –902.
in rodents: recent developments and future needs. Trends Phar-
Kumar P, Waiter G, Ahearn T, Milders M, Reid I, Steele JD (2008)
macol Sci 23:238 –245.
Abnormal temporal difference reward-learning signals in major
Cryan JF, O'Leary OF, Jin SH, Friedland JC, Ouyang M, Hirsch BR,
depression. Brain 131:2084 –2093.
Page ME, Dalvi A, Thomas SA, Lucki I (2004) Norepinephrine-
Lira A, Zhou M, Castanon N, Ansorge MS, Gordon JA, Francis JH,
deficient mice lack responses to antidepressant drugs, including
Bradley-Moore M, Lira J, Underwood MD, Arango V, Kung HF,
selective serotonin reuptake inhibitors. Proc Natl Acad Sci U S A
Hofer MA, Hen R, Gingrich JA (2003) Altered depression-related
101:8186 – 8191.
behaviors and functional changes in the dorsal raphe nucleus of
Chen W, Prithviraj R, Mahnke AH, McGloin KE, Tan JW, Gooch AK,
Inglis FM (2009) AMPA glutamate receptor subunits 1 and 2 reg-
serotonin transporter-deficient mice. Biol Psychiatry 54:960 –971.
ulate dendrite complexity and spine motility in neurons of the
Liston C, Miller MM, Goldwater DS, Radley JJ, Rocher AB, Hof PR,
developing neocortex. Neuroscience 159:172–182.
Morrison JH, McEwen BS (2006) Stress-induced alterations in pre-
Diaz-Veliz G, Mora S, Gomez P, Dossi MT, Montiel J, Arriagada C,
frontal cortical dendritic morphology predict selective impairments in
Aboitiz F, Segura-Aguilar J (2004) Behavioral effects of manganese
perceptual attentional set-shifting. J Neurosci 26:7870 –7874.
injected in the rat substantia nigra are potentiated by dicumarol, a
Lucki I (1997) The forced swimming test as a model for core and
DT-diaphorase inhibitor. Pharmacol Biochem Behav 77:245–251.
component behavioral effects of antidepressant drugs. Behav
Dickerson J, Sharp FR (2006) Atypical antipsychotics and a Src kinase
inhibitor (PP1) prevent cortical injury produced by the psychomi-
Maddock RJ (1999) The retrosplenial cortex and emotion: new insights
metic, noncompetitive NMDA receptor antagonist MK-801. Neuro-
from functional neuroimaging of the human brain. Trends Neurosci
psychopharmacology 31:1420 –1430.
22:310 –316.
Du J, Creson TK, Wu LJ, Ren M, Gray NA, Falke C, Wei Y, Wang Y,
Malenka RC, Bear MF (2004) LTP and LTD: an embarrassment of
Blumenthal R, Machado-Vieira R, Yuan P, Chen G, Zhuo M, Manji
riches. Neuron 44:5–21.
HK (2008) The role of hippocampal GluR1 and GluR2 receptors in
Maya Vetencourt JF, Sale A, Viegi A, Baroncelli L, De Pasquale R,
manic-like behavior. J Neurosci 28:68 –79.
O'Leary OF, Castren E, Maffei L (2008) The antidepressant fluox-
Du J, Gray NA, Falke CA, Chen W, Yuan P, Szabo ST, Einat H, Manji
etine restores plasticity in the adult visual cortex. Science
HK (2004) Modulation of synaptic plasticity by antimanic agents:
the role of AMPA glutamate receptor subunit 1 synaptic expres-
McClung CA, Nestler EJ (2008) Neuroplasticity mediated by altered
sion. J Neurosci 24:6578 – 6589.
gene expression. Neuropsychopharmacology 33:3–17.
Gould TD, O'Donnell KC, Dow ER, Du J, Chen G, Manji HK (2008)
McCullumsmith RE, Kristiansen LV, Beneyto M, Scarr E, Dean B,
Involvement of AMPA receptors in the antidepressant-like effects
Meador-Woodruff JH (2007) Decreased NR1, NR2A, and SAP102
of lithium in the mouse tail suspension test and forced swim test.
transcript expression in the hippocampus in bipolar disorder. Brain
Res 1127:108 –118.
E. Ampuero et al. / Neuroscience 169 (2010) 98 –108
McEwen BS (2007) Physiology and neurobiology of stress and adap-
Passafaro M (2007) Extracellular interactions between GluR2 and
tation: central role of the brain. Physiol Rev 87:873–904.
N-cadherin in spine regulation. Neuron 54:461– 477.
Medvedev NI, Rodriguez-Arellano JJ, Popov VI, Davies HA, Tigaret
Sairanen M, O'Leary OF, Knuuttila JE, Castren E (2007) Chronic
CM, Schoepfer R, Stewart MG (2008) The glutamate receptor 2
antidepressant treatment selectively increases expression of plas-
subunit controls post-synaptic density complexity and spine shape
ticity-related proteins in the hippocampus and medial prefrontal
in the dentate gyrus. Eur J Neurosci 27:315–325.
cortex of the rat. Neuroscience 144:368 –374.
Monyer H, Burnashev N, Laurie DJ, Sakmann B, Seeburg PH (1994)
Sanacora G, Zarate CA, Krystal JH, Manji HK (2008) Targeting the
Developmental and regional expression in the rat brain and func-
glutamatergic system to develop novel, improved therapeutics for
tional properties of four NMDA receptors. Neuron 12:529 –540.
mood disorders. Nat Rev Drug Discov 7:426 – 437.
Morimoto T, Hashimoto K, Yasumatsu H, Tanaka H, Fujimura M,
Schulz D, Buddenberg T, Huston JP (2007) Extinction-induced "despair"
Kuriyama M, Kimura K, Takehara S, Yamagami K (2002) Neuro-
in the water maze, exploratory behavior and fear: effects of chronic
pharmacological profile of a novel potential atypical antipsychotic
antidepressant treatment. Neurobiol Learn Mem 87:624 – 634.
drug Y-931 (8-fluoro-12-(4-methylpiperazin-1-yl)-6H-[1] benzothi-
Shepherd JD, Huganir RL (2007) The cell biology of synaptic plasticity:
AMPA receptor trafficking. Annu Rev Cell Dev Biol 23:613– 643.
eno[2, 3-b] [1,5] benzodiazepine maleate). Neuropsychopharma-
Shinohara Y, Hirase H, Watanabe M, Itakura M, Takahashi M, Shige-
cology 26:456 – 467.
moto R (2008) Left-right asymmetry of the hippocampal synapses
Muigg P, Hetzenauer A, Hauer G, Hauschild M, Gaburro S, Frank E,
with differential subunit allocation of glutamate receptors. Proc Natl
Landgraf R, Singewald N (2008) Impaired extinction of learned fear
Acad Sci U S A 105:19498 –19503.
in rats selectively bred for high anxiety— evidence of altered neu-
Sousa N, Almeida OF, Wotjak CT (2006) A hitchhiker's guide to
ronal processing in prefrontal-amygdala pathways. Eur J Neurosci
behavioral analysis in laboratory rodents. Genes Brain Behav 5
28:2299 –2309.
(Suppl 2):5–24.
Nugent AC, Milham MP, Bain EE, Mah L, Cannon DM, Marrett S,
Tanaka J, Horiike Y, Matsuzaki M, Miyazaki T, Ellis-Davies GC, Kasai
Zarate CA, Pine DS, Price JL, Drevets WC (2006) Cortical abnor-
H (2008) Protein synthesis and neurotrophin-dependent structural
malities in bipolar disorder investigated with MRI and voxel-based
plasticity of single dendritic spines. Science 319:1683–1687.
morphometry. Neuroimage 30:485– 497.
Vaisanen J, Linden AM, Lakso M, Wong G, Heinemann U, Castren E
O'Leary OF, Wu X, Castren E (2009) Chronic fluoxetine treatment
(1999) Excitatory actions of NMDA receptor antagonists in rat
increases expression of synaptic proteins in the hippocampus of
entorhinal cortex and cultured entorhinal cortical neurons. Neuro-
the ovariectomized rat: role of BDNF signalling. Psychoneuroen-
Van Groen T, Wyss JM (2003) Connections of the retrosplenial gran-
Olney JW, Farber NB (1995) NMDA antagonists as neurotherapeutic
ular b cortex in the rat. J Comp Neurol 463:249 –263.
drugs, psychotogens, neurotoxins, and research tools for studying
Velisek L (2006) Prenatal exposure to betamethasone decreases anx-
iety in developing rats: hippocampal neuropeptide y as a target
Olney JW, Labruyere J, Wang G, Wozniak DF, Price MT, Sesma MA
molecule. Neuropsychopharmacology 31:2140 –2149.
(1991) NMDA antagonist neurotoxicity: mechanism and preven-
Vizi ES, Zsilla G, Caron MG, Kiss JP (2004) Uptake and release of
tion. Science 254:1515–1518.
norepinephrine by serotonergic terminals in norepinephrine trans-
Passafaro M, Nakagawa T, Sala C, Sheng M (2003) Induction of
porter knock-out mice: implications for the action of selective se-
dendritic spines by an extracellular domain of AMPA receptor
rotonin reuptake inhibitors. J Neurosci 24:7888 –7894.
subunit GluR2. Nature 424:677– 681.
Warner-Schmidt JL, Duman RS (2008) VEGF as a potential target for
Paxinos G, Watson C (1998) The Rat brain in stereotaxic coordinates.
therapeutic intervention in depression. Curr Opin Pharmacol
San Diego: Academic Press.
Pellow S, Chopin P, File SE, Briley M (1985) Validation of open: closed
Wyneken U, Sandoval M, Sandoval S, Jorquera F, Gonzalez I,
arm entries in an elevated plus-maze as a measure of anxiety in
Vargas F, Falcon R, Monari M, Orrego F (2006) Clinically rele-
the rat. J Neurosci Methods 14:149 –167.
vant doses of fluoxetine and reboxetine induce changes in the
Pessoa L (2008) On the relationship between emotion and cognition.
TrkB content of central excitatory synapses. Neuropsychophar-
Nat Rev Neurosci 9:148 –158.
Pittenger C, Duman RS (2008) Stress, depression, and neuroplastic-
Wyneken U, Smalla KH, Marengo JJ, Soto D, de la Cerda A, Tischmeyer
ity: a convergence of mechanisms. Neuropsychopharmacology
W, Grimm R, Boeckers TM, Wolf G, Orrego F, Gundelfinger ED
33:88 –109.
(2001) Kainate-induced seizures alter protein composition and N-
Popoli M, Gennarelli M, Racagni G (2002) Modulation of synaptic plas-
methyl-D-aspartate receptor function of rat forebrain postsynaptic
ticity by stress and antidepressants. Bipolar Disord 4:166 –182.
densities. Neuroscience 102:65–74.
Porsolt RD, Anton G, Blavet N, Jalfre M (1978) Behavioural despair in
Yao Y, Kelly MT, Sajikumar S, Serrano P, Tian D, Bergold PJ, Frey JU,
rats: a new model sensitive to antidepressant treatments. Eur
Sacktor TC (2008) PKM zeta maintains late long-term potentiation
J Pharmacol 47:379 –391.
by N-ethylmaleimide-sensitive factor/GluR2-dependent trafficking
Radley JJ, Rocher AB, Miller M, Janssen WG, Liston C, Hof PR,
of postsynaptic AMPA receptors. J Neurosci 28:7820 –7827.
McEwen BS, Morrison JH (2006) Repeated stress induces den-
Yashiro K, Philpot BD (2008) Regulation of NMDA receptor subunit
expression and its implications for LTD, LTP, and metaplasticity.
dritic spine loss in the rat medial prefrontal cortex. Cereb Cortex
Rodriguez-Landa JF, Contreras CM, Gutierrez-Garcia AG, Bernal-Mo-
rales B (2003) Chronic, but not acute, clomipramine or fluoxetinetreatment reduces the spontaneous firing rate in the mesoaccumbens
neurons of the rat. Neuropsychobiology 48:116 –123.
Saglietti L, Dequidt C, Kamieniarz K, Rousset MC, Valnegri P,
Supplementary data associated with this article can be found, in
Thoumine O, Beretta F, Fagni L, Choquet D, Sala C, Sheng M,
the online version, at doi:
(Accepted 16 April 2010)
(Available online 22 April 2010)
Source: http://www.stress.cl/pdf/17.pdf
R ESEARCH ARTICLE The effect of mirtazapine on methotrexate-inducedtoxicity in rat liver Bunyami Ozogula, Abdullah Kisaoglua, Mehmet Ibrahim Turanb,∗, Durdu Altunerc, Ebru Senerd,Nihal Cetine, Cengiz Ozturke,f a Department of Surgery, Faculty of Medicine, Ataturk University, 25240, Erzurum, Turkeyb Department of Paediatrics, Faculty of Medicine, Ataturk University, 25240, Erzurum, Turkeyc Department of Pharmacology, Faculty of Medicine, Recep Tayyip Erdogan University, 53100, Rize, Turkeyd Department of Pathology, Erzurum Region Education and Research Hospital, Erzurum, Turkeye Department of Pharmacology-Toxicology, Faculty of Veterinary Medicine, Ataturk University, 25240,
The Collapse of Conventional Medicine Copyright © 2002 Bill Sardi, Knowledge of Health, Inc All rights reserved. No part of this book may be produced or transmitted in any form or by any means, electronic or mechanical, without written permission from the publisher, except for the inclusion of brief quotations in a review.




