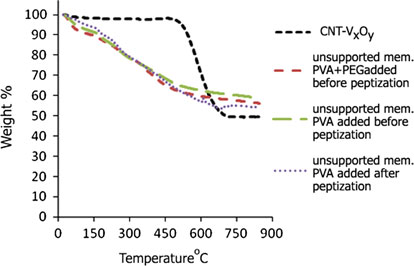
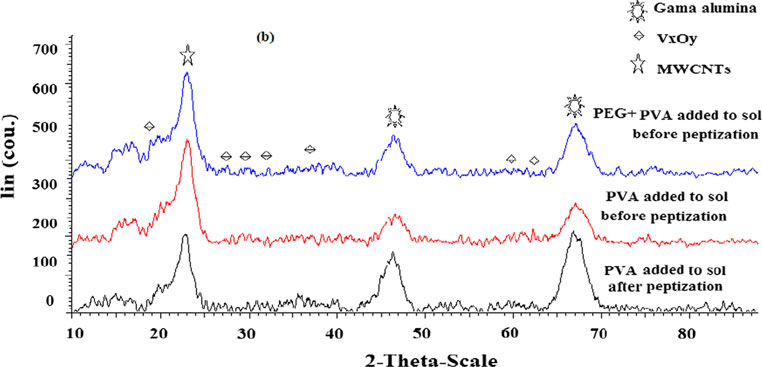
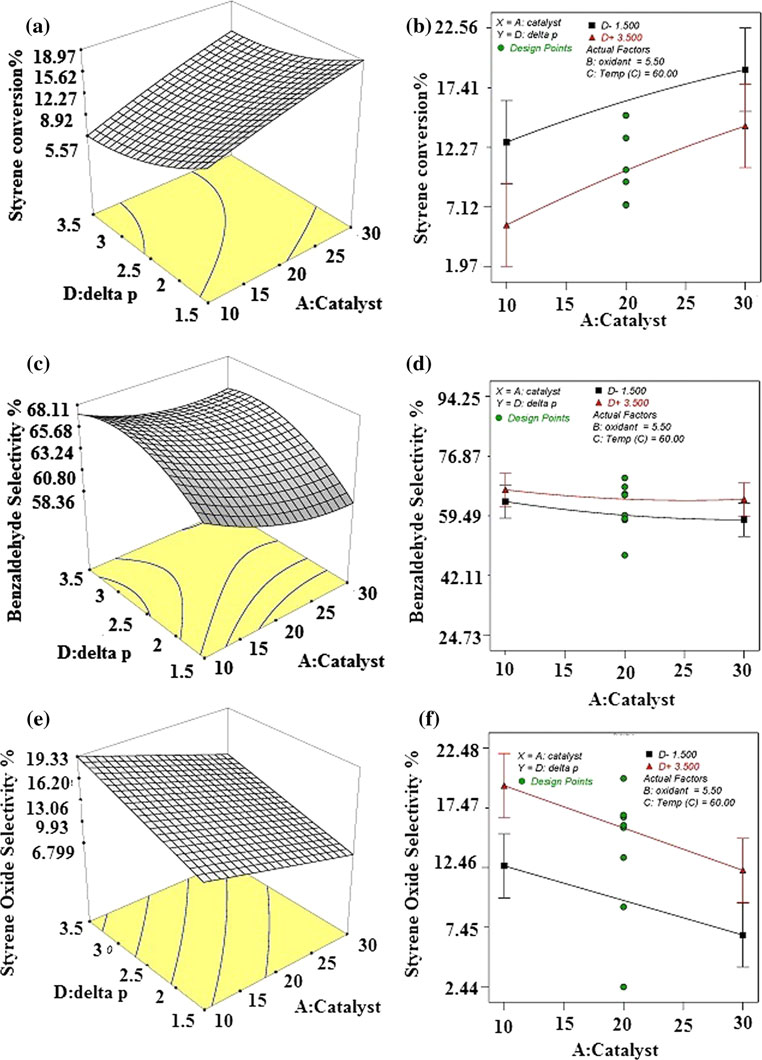
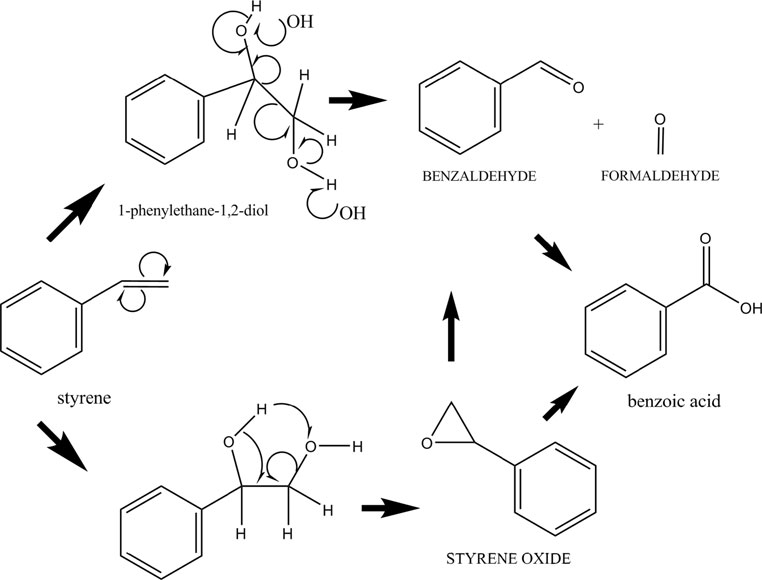
Guideline vulvovaginal candidosis (2010) of the german society for gynecology and obstetrics, the working group for infections and infectimmunology in gynecology and obstetrics, the german society of dermatology, the board of german dermatologists and the german speaking mycological society
Diagnosis,Therapy and Prophylaxis of Fungal Diseases Guideline vulvovaginal candidosis (2010) of the german society forgynecology and obstetrics, the working group for infections andinfectimmunology in gynecology and obstetrics, the german societyof dermatology, the board of german dermatologists and the germanspeaking mycological society W. Mendling and J. Brasch Prof. Dr. med. Werner Mendling, Vivantes – Klinikum im Friedrichshain and Am Urban, Clinics for Obstetrics and Gynecology (2011 retired), 10249 Berlin,Landsberger Allee 49Prof. Dr. med. Jochen Brasch, University Hospitals of Schleswig – Holstein, Campus Kiel, Department of Dermatology, Venerology and Allergology,Schittenhelmstrasse 7, 24105 Kiel, Germany