Slide

Diagnostic criteria for cervical dystonia: Can botulinum
neurotoxin manage, as well as, cure the problem?
Jill L. Ostrem, MD
Professor of Neurology
UCSF Department of Neurology
Movement Disorder and Neuromodulation Center
Bachmann Strauss Dystonia and Parkinson's Disease
Center of Excellence
Disclosures
Educational grant support:
Medtronic Inc., Merz, Inc., Boston Scientific, Allergan
Clinical trial support:
St. Jude Medical Inc., Boston Scientific
Objectives
• Review the diagnostic criteria for cervical
• Understand the role of oral medications in CD • Discuss the use of botulinum neurotoxin
• Outline the methods and procedures for
determining the appropriate muscles for injection
• Understand the possible adverse effects of
neurotoxin injections and the limitations
Dystonia
• A neurologic syndrome dominated by involuntary muscle
contractions that may be sustained, patterned, or repetitive,
frequently causing abnormal postures (twisting, flexion or
extension, abduction or adduction)
Age of Onset: early (<21) or late (>21)
Distribution or body region affected:
focal, segmental, multifocal, generalized
Etiology: idiopathic / primary (sporadic or inherited) or symptomatic /
secondary
• Multiple genes and risk factors known, but still account for
relatively few cases
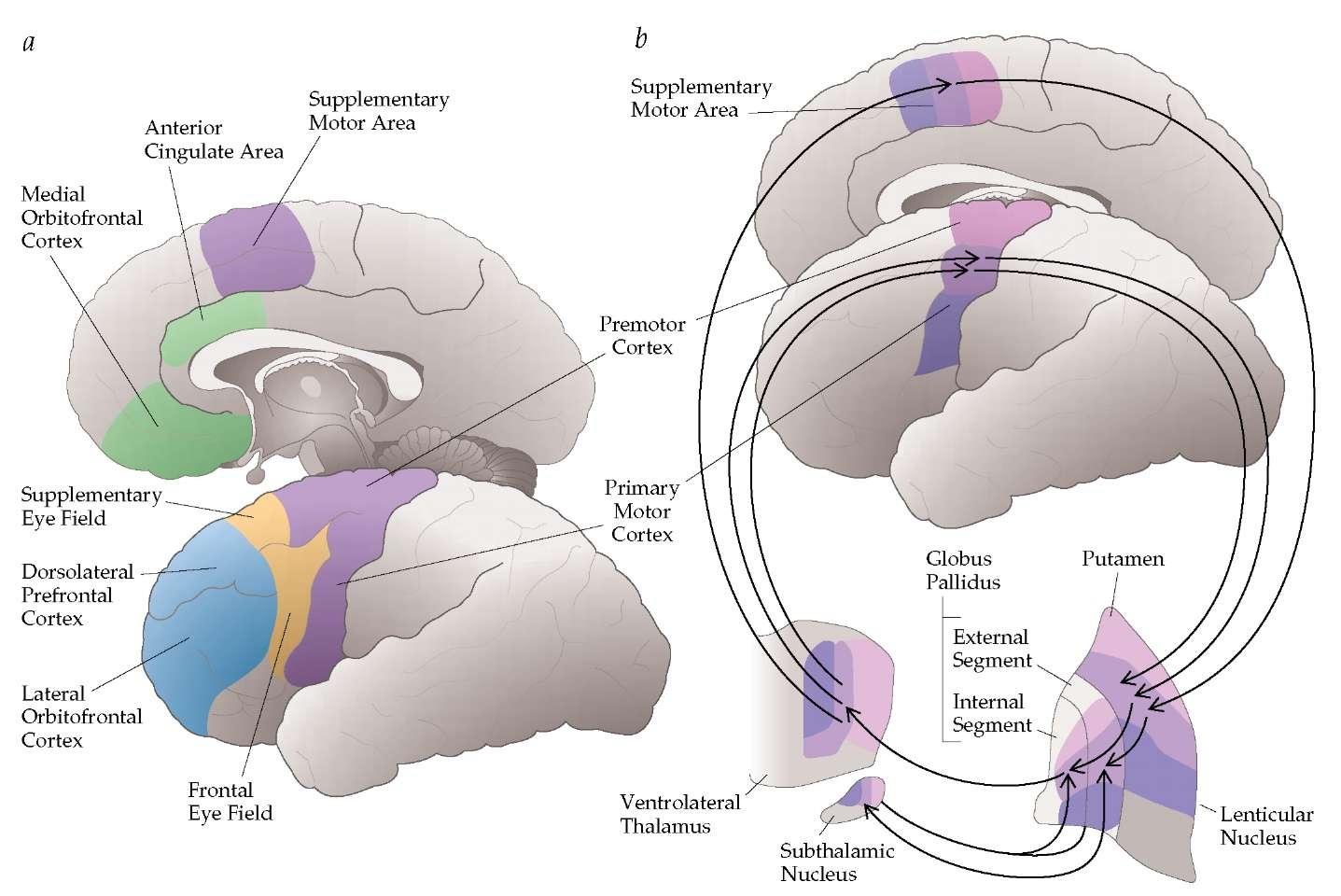
Dystonia is a brain circuit disorder
Cervical Dystonia
• Focal dystonia that produces patterned, repetitive, and
spasmodic or sustained muscle contractions resulting in abnormal movements and postures of the head and neck
• Cervical Dystonia is preferred to the term spasmodic torticollis
as an overarching descriptor, since Cervical Dystonia may or may not be spasmodic and may or may not consist of torticollis (head turning)
• In most cases, the exact cause is unknown • Familial history of dystonia in approximately 12% of cases • Previous neck trauma is common • More common in women, mean onset 41 years
Dystonia Classification - New
• Age of onset (from infancy to late adult onset) • Body distribution (focal forms, segmental, generalized) • Temporal pattern (static or progressive disease course ) • Isolated or combined with another movement disorder
(parkinsonism, myoclonus, or other neurological manifestations)
• Isolated dystonia
– Onset in children = progress to generalized – Onset adulthood = remains focal or segmental
– Inherited (DYT, others) – Acquired (brain injury, tardive syndromes)
Cervical Dystonia
• Cervical Dystonia frequently begins as a pulling or drawing
sensation in the neck or an involuntary twisting or jerking of the neck
• After onset, symptoms typically worsen, although the time course
is highly variable (range: 1 month to 18 years)
• Progression typically plateaus within 5 years of onset • Spontaneous remissions can occur but is rare • No single test exists at this time to confirm diagnosis • Neurologic examination is otherwise usually normal • Sensory tricks—partial, temporary relief
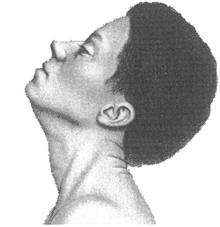
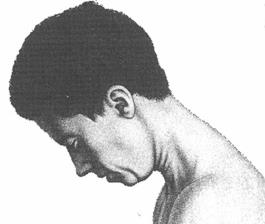
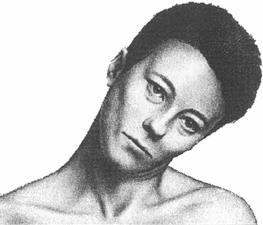
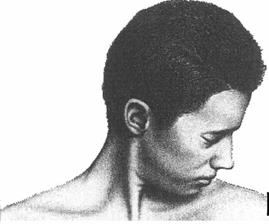
Cervical Dystonia: Characteristics
• Subtypes (Torticollis, Laterocollis, Retrocollis, Anterocollis) • Most patient present with a combination of these movements • Each subtype activates different pattern of muscles resulting in
the abnormal neck/head posture.
• Can also be associated with tremor
Rotational torticollis
Image adapted from Benecke R, et al. Cervical and axial dystonia. In: Moore P, Naumann M, eds. Handbook of Botulinum Toxin Treatment. Malden, MA: Blackwell Science Ltd; 2003:158-194. Reproduced with permission of Blackwell Publishing, Ltd.
Dashtipour K, Lew M. Handbook of Dystonia. 2007: 37-154.
Cervical Dystonia: Treatment Options
• Oral medications have been used off-label
– Trihexyphenidyl (Artane) – Clonazapam (Klonopin) – Baclofen – Other
• Surgery
– Selective denervation – Deep brain stimulation
• Botulinum toxin therapy
Dashtipour K, Lew M. Handbook of Dystonia. 2007: 137-154.
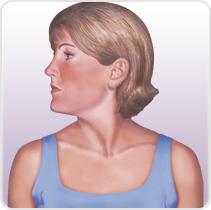
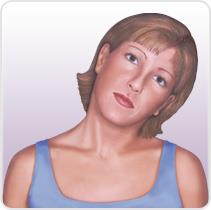
Muscles involved in cervical dystonia
Torticollis
• Contralateral sternocleidomastoid
• Sternocleidomastoid
• Contralateral trapezius
• Ipsilateral splenius capitis
• Ipsilateral splenius capitis
• Ipsilateral scalene complex
• Ipsilateral splenius cervicis
• Ipsilateral semispinalis capitis and
longissimus
Ipsilateral levator scapulae
• Ipsilateral levator scapulae
• Trapezius
Muscles involved in cervical dystonia
Retrocollis
• Bilateral sternocleidomastoid
Bilateral splenius capitis
• Scalene complex
Bilateral levator scapulae
• Posterior vertebral muscles
(semispinalis capitis and
longissimus)
• Upper trapezius
Botulinum Toxin
• Toxin temporarily weakens dystonic muscles, allowing for a
more normal posture and function.
• Benefits depend on location and degree of dystonia of
muscles being injected.
• Can not treat widespread or extremely severe generalized
dystonia, as the toxin dose required would be too high.
• Toxin may be used to target specific dystonic muscles to
improving aspects of care and function or relieve discomfort.
Botulinum Toxin Therapy
• Potent neurotoxin produced by bacterium clostridium
botulinum
• 7 different serotypes – lettered A-G • All serotypes are large proteins that act on cholinergic
neuromuscular junctions to block transmission of synaptic vesicles.
Botulinum Toxins
Serotype
Generic Name
Trade Name
XEOMIN®
WorldMeds/Solstice
• Neurotoxin Products contains highly purified botulinum toxin
protein refined from the bacterium Clostridium botulinum
• Some have A and Some have B Serotypes
• All toxins has a heavy chain and a light chain bound by a di-
Units of neurotoxins and dosing are
not interchangeable
Cervical Dystonia Neurotoxin
Injection Results
• 70% of patients get >60-80% benefit
• Patients with long-duration dystonia respond less well
than those treated earlier
• Side effects occur in 10% of patients (most common,
transient difficulties with swallowing)
• Pain is also often improved
Injection of Botulinum
Toxin Technique
• A small needle is placed into the target muscle
• In large or accessible muscles, confirmation of appropriate
placement may be achieved by feeling the muscle
• In small or deep muscle groups, EMG may be required
• Well tolerated
• Local anesthetic cream or sedation can
Duration of Botulinum Toxin Effects
• The effects of treatment with BTX are usually greatest for a 2-6
week period following injection
• These effects usually fade after about 3 to 6 months.
• Re-injection of the toxin is usually performed every 3 months.
ADVERSE REACTIONS
• Blepharospasm:
WARNING: DISTANT SPREAD OF TOXIN EFFECT Postmarketing reports indicate that the effects of BOTOX® and
– eyelid ptosis (19%)
all botulinum toxin products may spread from the area of injection to produce symptoms consistent with botulinum toxin
– dry mouth (16%)
effects. These may include asthenia, generalized muscle weakness, diplopia, ptosis, dysphagia, dysphonia, dysarthria,
urinary incontinence, and breathing difficulties. These symptoms
visual impairment (12%)
have been reported hours to weeks after injection. Swallowing and breathing difficulties can be life threatening, and there have
– diarrhea (8%)
been reports of death. The risk of symptoms is probably greatest in children treated for spasticity, but symptoms can also occur in
– headache (7%).
adults treated for spasticity and other conditions, particularly in those patients who have an underlying condition that would
predispose them to these symptoms. In unapproved uses,
Cervical Dystonia:
including spasticity in children, and in approved indications, cases of spread of effect have been reported at doses
– dysphagia (13% -18%)
comparable to those used to treat cervical dystonia and at lower doses.
– neck pain (7% -15%) – muscle weakness (7% -11%) – musculoskeletal pain (4% -7%)
• Limb dystonia:
– weakness
Caution for Use
• BTX-should be used with extreme caution in
patients:
– myasthenia gravis – amyotrophic lateral sclerosis (ALS) – Taking anticoagulants – Taking certain antibiotics - aminoglycosides
• Botulinum neurotoxin is a protein that serves as an antigen • The development of an antibody response is dependent on:
– Larger doses of botulinum toxin – Larger cumulative doses – Injections administered at less than 3 months intervals – Controversial if newer Xeomin may have less immunogenicity
• Formation of neutralizing antibodies results in resistance to beneficial
• Antibodies to BTX may be detected using various methodologies • Clinicians often use in vivo tests: frontalis test • If resistance occurs, replacing one serotype with another may be
Final thoughts
• Neurotoxin injections are first line therapy for CD. • They can alleviate symptoms, but not cure the disease. • Injections need to be maintained every three months. • Can allow for greater easy and effectiveness of PT. • Can co-exist with cervical spine degenerative issues.
UCSF and SFVA Movement Disorders Team
Movement Disorder and Neuromodulation Center
Jill L. Ostrem, MD, Medical Director
Philip Starr, MD, PhD, Surgical Director
Neurology
Research Support Staff
Nicholas Galifianakis, MD
Kristen Dodenhoff, BA
Caroline Tanner, MD, PhD
Michael Dodge, BA
Marta San Luciano, MD
Shatara Blackmon
William J. Marks, Jr., MD
Yasmeen Gonzalez
Robert White, MD, PhD
Jeverly Calaunan
James Maas, MD, PHD
Kathleen Comyns, MPH
Chadwick Christine, MD
Samantha Konz, BS
Michael Aminoff, MD
Samantha Betheil, BA
Robert Edwards, MD
Cheryl Meng, MPH
Ken Nakamura, MD, PhD
Alexandra Nelson, MD, PhD Psychiatry
Michael Geschwind, MD
Andrea Seritan, MD
Philip Starr, MD, PhD
Caroline Racine Belkoura, PhD
Social Work
Paul S. Larson, MD
Jennifer Chen, MD
Monica Eisenhardt, LCSW
Edward F. Chang, MD
Cameron Dietiker, MD
Daniel Lim, MD, PhD
Monica Volz, FNP, MS
Nijee Luthra, MD, PhD
Physical Therapy
Krzysztof Bankiewicz, MD, PhD Robin Taylor, FNP, MS
Svjetlana Miocinovic, MD, PhD
Nancy Byl, PT, PhD
Coralie De Hemptinne, PhD
Lorna Beccaria, RN
Heather Bhide, PT
Nicki Swann, PhD
Karen Merchant, MSN
Andrew Miller, BA
Susan Heath, MS, RN
Rigzin Lama, LVN
Doris Wang, MD, PhD
Source: http://www.ccapta.org/resource/collection/C50CF29D-1297-4A8B-9B2D-3A0CE4020C7E/Ostrem_Cervical_Dystonia_29_pages.pdf
Case Study 2: Complex Mixtures of Chemicals in the Aquatic Environment and their Effects John Sumpter,Institute for the Environment,Brunel University The River Thames as a Typical • Catchment covers an area of 12,000 Km2.• The river flows through some densely populated areas.• Population density is 447 people/Km2.• 352 sewage treatment plants discharge into the river,
IN THE SUPREME COURT OF BRITISH COLUMBIA Player v. Janssen-Ortho Inc., Registry: Victoria Estate of Wade Robert Player, Desiree Marine Player, Estate of Daniel Charles Pollock and Elaine Mills Janssen-Ortho Inc., Ranbaxy Pharmaceuticals Canada Inc., Ratiopharm Inc., Sandoz Canada Incorporated, and








