Levitra enthält Vardenafil, das eine kürzere Wirkdauer als Tadalafil hat, dafür aber schnell einsetzt. Männer, die diskret bestellen möchten, suchen häufig nach levitra kaufen ohne rezept. Dabei spielt die rechtliche Lage in der Schweiz eine wichtige Rolle.
Cmrf.research.uiowa.edu
The Journal of Neuroscience, May 1, 1999,
19(9):3423–3429
Regulation of Calcitonin Gene-Related Peptide Secretion by a
Serotonergic Antimigraine Drug
Paul L. Durham and Andrew F. Russo
Department of Physiology and Biophysics, University of Iowa, Iowa City, Iowa 52242
We have investigated the regulation of calcitonin gene-related
tion rate. Unexpectedly, sumatriptan did not lower cAMP levels,
peptide (CGRP) release from trigeminal neurons by the seroto-
in contrast to the classical role ascribed to the 5-HT receptors.
nergic antimigraine drug sumatriptan. Serum levels of the neu-
Instead, activation of 5-HT receptors caused a slow and re-
ropeptide CGRP are elevated during migraine. Treatment with
markably prolonged increase in intracellular calcium. The inhi-
the drug sumatriptan returns CGRP levels to normal coincident
bition of CGRP secretion is attenuated by the phosphatase
with the alleviation of headache. However, despite this clinical
inhibitor okadaic acid, suggesting that sumatriptan action is
efficacy, the cellular target and mechanism of sumatriptan ac-
mediated by calcium-recruited phosphatases. These results
tion are not well understood beyond the pharmacology of its
suggest that 5-HT agonists may block a deleterious feedback
recognition of the 5-HT class of serotonin receptors. We have
loop in migraine at the trigeminal neurons and provide a general
mechanism by which this class of drugs can attenuate stimu-
sumatriptan can directly repress CGRP secretion from sensory
lated neuropeptide release.
neurons. The stimulated secretion in response to depolarization
Key words: CGRP; serotonin receptors; trigeminal neurons;
or inflammatory agents was inhibited, but not the basal secre-
calcium; phosphatase; migraine; neuropeptide
Calcitonin gene-related peptide (CGRP) is a 37 amino acid
of the previous studies have used
in vivo model systems and 5-HT1
regulatory neuropeptide derived from alternative splicing of the
receptors are expressed by both cerebral blood vessels and tri-
calcitonin/CGRP gene (Rosenfeld et al., 1983). During migraine,
geminal nerves (Bouchelet et al., 1996), the site of sumatriptan's
a painful neurological disorder afflicting 16% of the general
action, let alone the cellular mechanism, has remained unclear.
population (Stewart et al., 1994), activation of trigeminal neurons
In this study, we have demonstrated that sumatriptan and other
leads to increased secretion of CGRP (Moskowitz, 1993). To-
5-HT1 receptor agonists can directly repress the stimulated, but
gether with substance P and neurokinin A, CGRP helps mediate
not basal, release of CGRP from cultured trigeminal neurons.
neurogenic inflammation, a condition characterized by vasodila-
Somewhat surprisingly, we found that sumatriptan did not medi-
tion, plasma protein extravasation, and mast cell degranulation
ate a decrease in intracellular cAMP levels, a function typically
(Buzzi et al., 1995). CGRP is the most potent vasodilatory neu-
associated with activation of the 5-HT1 receptors (Boess and
ropeptide known (McCulloch et al., 1986) and recently has been
Martin, 1994). Rather, sumatriptan treatment resulted in a slow
shown to cause dural mast cell degranulation (Ottosson and
and remarkably prolonged increase in intracellular calcium in
Edvinsson, 1997). CGRP is also believed to convey nociceptive
trigeminal neurons. We also demonstrated that a phosphatase
information from the vasculature to the CNS (Van Rossum et al.,
inhibitor effectively blocked the inhibitory effect of sumatriptan
1997). On the basis of these data, CGRP is believed to play a key
on stimulated CGRP release. These data are suggestive that
role in the painful phase of migraine.
sumatriptan mediates an increase in phosphatase activity via a
This belief has been strongly supported by the clinical efficacy
calcium-dependent pathway. On the basis of our results, we have
of the selective 5-HT1 receptor drug sumatriptan (Ferrari, 1998).
elucidated a novel mechanism by which the antimigraine drug
Sumatriptan has been shown to decrease the elevated CGRP
sumatriptan may block a deleterious feedback loop in migraine
levels in migraine patients, coincident with relief of headache
and restore CGRP to normal levels.
pain (Goadsby and Edvinsson, 1993). Trigeminal nerves play an
important role in the regulation of cerebral blood flow during
normal and disease states and are the major source of sensory and
MATERIALS AND METHODS
CGRP innervation to the cerebral vasculature (McCulloch et al.,
Cell culture. Trigeminal ganglia primary cultures were established as
1986; O'Conner and Van Der Kooy, 1988). However, because all
described previously (Durham et al., 1997). Briefly, ganglia from ,1-
week-old Sprague Dawley rats were dissociated with Dispase II. The cells
from three to four ganglia were plated on glass coverslips coated with
Received Nov. 23, 1998; revised Feb. 1, 1999; accepted Feb. 12, 1999.
mouse Engelbreth-Holm-Swarm laminin or plastic tissue culture dishes
This work was supported by grants from National Institutes of Health (HD25969,
coated with poly-D-lysine and laminin. Cells were incubated in L15
NS37386, HL14388) and the American Heart Association (96013860) to A.R., with
medium, 10% fetal bovine serum (FBS), and 10 ng/ml mouse 2.5 S nerve
tissue culture support provided by the Diabetes and Endocrinology Center
growth factor at 37°C in 5% CO2. Penicillin and streptomycin were
(DK25295) and an Iowa Cardiovascular Interdisciplinary Research Fellowship
added to all media. Cultures of trigeminal ganglia used for CGRP
(HL07121) to P.D. We thank members of the lab and K. Campbell for comments on
secretion and calcium studies were subcultured for 24 hr in serum-free
this manuscript and discussions and M. Hamblin for generously providing reagents.
Correspondence should be addressed to Dr. Andrew F. Russo, Department of
medium (Durham et al., 1997). HeLa cells stably expressing the 5-HT1B
Physiology and Biophysics, University of Iowa College of Medicine, Iowa City, IA
receptor (HeLa1B) were kindly provided by Dr. Mark Hamblin (Seattle
Veterans Affairs Medical Center, Seattle, WA) (Hamblin et al., 1992)
Copyright 1999 Society for Neuroscience 0270-6474/99/193423-07$05.00/0
and were maintained in F-12 medium supplemented with 10% FBS. CGS
3424 J. Neurosci., May 1, 1999, 19(9):3423–3429
Durham and Russo • Serotonergic Repression of CGRP Secretion
12066A monomaleate (CGS) and L-694,294 were purchased from RBI
(Natick, MA). Sumatriptan succinate was obtained from the University
of Iowa Pharmacy, methiothepin was from RBI, and okadaic acid was
from Sigma (St. Louis, MO).
Immunohistochemistry. Trigeminal ganglion cells at various times in
culture were fixed and stained as described previously for neurofilament
protein using anti-rat NF-M monoclonal antibodies (Boehringer Mann-
heim Biochemicals, Indianapolis, IN) and FITC-conjugated secondary
antibodies (Sigma). Expression of CGRP in trigeminal cultures was
detected using CGRP-specific polyclonal antibodies (RBI) and Cy-3-
conjugated secondary antibodies (Sigma).
Calcium measurements. Intracellular calcium levels in cultured trigem-
inal neurons were measured essentially as described previously (Durham
et al., 1997). Briefly, dissociated trigeminal ganglia grown on laminin-
coated 25 mm glass coverslips were maintained in phenol- and serum-free
medium 24 hr before the start of the calcium imaging procedure. Cells
were incubated in DMEM (high glucose) containing 0.2% BSA and 1 mM
fura-2 AM for 25–30 min at 37°C in 5% CO2. After the cells were washed
twice with DMEM/BSA, they were incubated in the same buffer for 30
min before analysis. Basal calcium levels were measured for a minimum
of 180 sec before addition of 5-HT1 receptor agonists or other agents.
Statistical analyses were performed using the Student's t test (two-tailed,
unpaired samples).
CGRP and cAMP assays. For the CGRP secretion studies, cells were
incubated in HBS (22.5 mM HEPES, 135 mM NaCl, 3.5 mM KCl, 1 mM
MgCl, 2.5 mM CaCl, 3.3 mM glucose, and 0.1% BSA, pH 7.4) (Vasko et
al., 1994), and the amount of CGRP released from trigeminal neurons
into the culture media was determined using a specific CGRP radioim-
munoassay (Peninsula Labs, Belmont, CA). As a control, the basal
(unstimulated) rate of CGRP secreted into the media in 1 hr was
determined, and these values were used to normalize for differences
between dishes. Cells were pretreated with the indicated concentrations
of 5-HT1 receptor agonists, with or without the 5-HT1 antagonist me-
thiothepin, or appropriate vehicle for 30 min before addition of either
HBS (control), KCl, or inflammatory cocktail. The inflammatory cocktail
(Strassman et al., 1996) contained 10 mM each of bradykinin, prostaglan-
din, and serotonin, and 50 mM histamine in HBS at pH 5.5. This
combination and concentration of agents was based on previous studies
that elicited physiological responses (Steen et al., 1992; Strassman et al.,
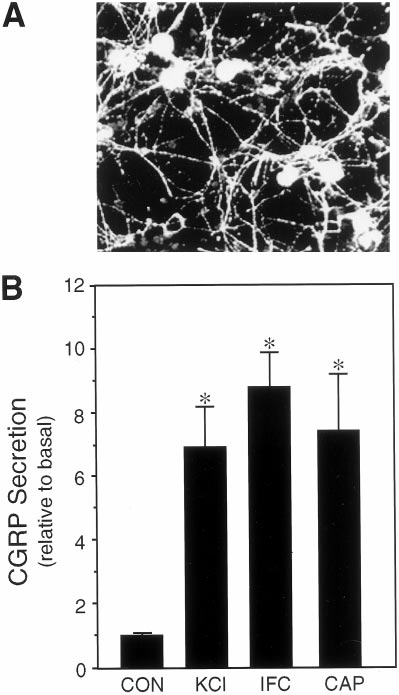
Figure 1. Expression and regulated release of CGRP from cultured
1996). Although it is difficult to know what the local concentrations of
trigeminal ganglia neurons. A, Fluorescent micrograph of CGRP-
these agents would be during neurogenic inflammation, high hydrogen
immunoreactive trigeminal neurons 7 d after plating on poly-
ion concentrations have been found in inflammation, pH 5.4, and the
laminin. B, The relative amount of CGRP secreted in 1 hr from untreated
relatively high, perhaps unphysiological, concentrations of the chemical
control cells (CON ) or cells treated with 60 m
agents have been reported to be necessary for in vitro responses (Steen et
M potassium chloride (KCl ),
a cocktail of inflammatory agents (IFC), or 10 m
M capsaicin (CA P) is
shown. The mean basal rate of CGRP release was 148 6 5 pg/hr per dish
For the cAMP measurements, trigeminal cultures were incubated with
(SE, n 5 36). The secretion rate for each condition was normalized to the
either 2 mM forskolin (Sigma) in the presence or absence of sumatriptan
basal rate for each dish. The means and the SE from at least four
(20 mM) or CGS (10 mM) for 30 min at 37°C. The HeLa1B cell line was
independent experiments are shown. *p , 0.001 when compared with
incubated with 100 mM forskolin and 0.1 mM sumatriptan or CGS under
control levels.
the same conditions. These concentrations were chosen on the basis of
previous studies with this cell line (Hamblin et al., 1992). Intracellular
cAMP was determined using a cAMP-specific radioimmunoassay (Pen-
insula Labs). Each experimental condition was repeated in at least three
occurred at a steady-state rate of 148 pg/hr per dish of cells
independent experiments, and statistical analyses were performed using
(approximately two ganglia). Treatment with potassium chloride
the Student's t test (two-tailed, unpaired samples).
(KCl) to mimic neuronal depolarization caused approximately a
sevenfold increase in the rate of CGRP release (Fig. 1B). Treat-
ment with capsaicin, which selectively activates sensory C-fibers
Regulated release of CGRP from cultured trigeminal
via a vanilloid receptor (Caterina et al., 1997), resulted in a
similar sevenfold increase (Fig. 1B). The rate of CGRP release
To determine whether sumatriptan could directly repress CGRP
was also markedly stimulated by a mixture of agents known to
secretion from trigeminal neurons, we took a reductionist ap-
mediate physiological responses of neurogenic inflammation and
proach by establishing primary cultures of rat trigeminal ganglia
sensitization of nociceptive afferents (Strassman et al., 1996) (Fig.
enriched for neuronal cells (estimated to be .80%). Expression
1B). Because the release of CGRP during migraine is thought to
of a neuron-specific protein, 160 kDa neurofilament subunit was
result in the production and/or release of agents that escalate and
detected in all cells exhibiting a neuronal morphology. A unique
sustain the inflammatory response, our results indicate that these
feature of our culture conditions is that almost all of the neuronal
agents can also act to maintain CGRP secretion after the initial
cells are CGRP positive (Fig. 1A), although it is estimated that
nerve activation.
only 23% of the neurons in the trigeminal ganglia express CGRP
in vivo (O'Conner and Van Der Kooy, 1988). A likely reason for
Sumatriptan represses stimulated CGRP release
this bias is that only nerve growth factor, and not BDNF or NT-3,
Having established that cultured trigeminal neurons exhibit reg-
which are required for survival of other neurons, was included in
ulated CGRP secretion, we then asked whether sumatriptan could
the culture media (Buchman and Davies, 1993). CGRP secretion
directly inhibit this release. We found that sumatriptan inhibited
Durham and Russo • Serotonergic Repression of CGRP Secretion
J. Neurosci., May 1, 1999, 19(9):3423–3429 3425
Figure 3. Effect of 5-HT1 agonists on stimulated CGRP release. 5-HT1
repression of CGRP release after stimulation by inflammatory agents.
The amount of CGRP secreted per hour was normalized to the basal rate
determined for 1 hr before addition of 60 mM KCl or 5-HT1 agonists. The
relative rates after addition of buffer (CON ), inflammatory cocktail (IFC),
and IFC plus 10 mM sumatriptan (Suma), 10 mM CGS, or 10 mM L-694,247
(L694 ) are shown. The mean basal rate was 137 6 2 pg/hr per dish. The
means and the SE from at least three independent experiments are
shown. *p , 0.001 when compared with control values. # p , 0.05 when
compared with IFC values.
normal, nonmigranuer individuals (Goadsby and Edvinsson,
1993). The specificity of sumatriptan action via the 5-HT1 recep-
tors was confirmed by addition of a 5-HT1 receptor antagonist,
methiothepin. Methiothepin completely blocked the action of
sumatriptan on CGRP secretion from the cultured neurons (Fig.
2B). Methiothepin treatment alone had little or no effect on
secretion. We have shown previously that this antagonist is able
to block the elevation of intracellular calcium by the 5-HT1
agonist CGS (Durham et al., 1997), and it can also block the
increase in intracellular calcium after the sumatriptan treatment
described below (data not shown). These results demonstrate that
sumatriptan activation of trigeminal 5-HT1 receptors is sufficient
Figure 2. Effect of 5-HT1 receptor agonists on CGRP release. A, CGRP
to directly inhibit CGRP release.
secretion as a function of sumatriptan concentration and treatment time.
We also investigated whether sumatriptan could inhibit the
The effect of sumatriptan was determined on unstimulated and KCl-
release of the vasoactive neuropeptide substance P, because it has
stimulated trigeminal neurons (cultured for 4–7 d). The amount secreted
been reported to colocalize with CGRP in sensory neurons (Ed-
per hour was normalized to the basal rate determined before addition of
buffer, 60 mM KCl, or sumatriptan (Suma) for 1 hr. Where indicated, 10
vinsson and Goadsby, 1994) and is involved in mediating neuro-
mM sumatriptan was added for 2 or 4 hr, and the amount per hour was
genic inflammation (Buzzi et al., 1995). In preliminary studies, we
normalized to basal. The mean basal CGRP secretion rate was 131 6 4
determined that sumatriptan could also inhibit the KCl-
pg/hr per dish. The means and the SE from at least three independent
stimulated release of substance P (data not shown). Thus, the
experiments are shown. *p , 0.001 when compared with control values.
effectiveness of sumatriptan in reducing or abolishing the pain
, 0.05 when compared with KCl-only values. B, The 5-HT1 receptor
antagonist methiothepin blocks inhibitory effect of sumatriptan on KCl-
associated with migraine is likely caused by its ability to coordi-
stimulated CGRP release. The relative rates after addition of buffer
nately inhibit the release of vasoactive neuropeptides from tri-
(CON ), KCl, KCl plus 10 mM sumatriptan (SUMA) and/or 20 mM me-
geminal ganglion nerves.
thiothepin (SUMA1MET) are shown. The mean basal rate was 122 6 5
Because we had found that a mixture of inflammatory agents
pg/hr per dish. The means and the SE from the two independent exper-
iments are shown for each study. *p , 0.01 when compared with control
caused a marked increase in the rate of CGRP secretion (Fig. 1),
values or sumatriptan values. # p , 0.05 when compared with KCl values.
we wanted to determine whether sumatriptan could also block
this type of stimulated CGRP release. The increase in CGRP
potassium-stimulated CGRP secretion in a dose-dependent man-
release caused by the inflammatory cocktail was inhibited more
ner (Fig. 2A). The secretion rate remained relatively stable
than twofold by pretreatment with sumatriptan (Fig. 3). In addi-
throughout 4 hr of potassium stimulation, and a single dose of
tion, we showed that pretreatment with two other 5-HT1 receptor
sumatriptan was able to maintain a steady inhibition throughout
agonists, CGS and L-694,294, caused a similar inhibition of
this period (Fig. 2A). In contrast, sumatriptan had no significant
CGRP secretion (Fig. 3). These results demonstrate that multiple
effect on the basal secretion of CGRP from unstimulated trigem-
5-HT1 agonists can repress CGRP secretion by at least two
inal neurons (Fig. 2A). This finding is consistent with clinical
different stimuli, suggesting that these agents target a common
reports that sumatriptan does not lower serum CGRP levels in
downstream step.
3426 J. Neurosci., May 1, 1999, 19(9):3423–3429
Durham and Russo • Serotonergic Repression of CGRP Secretion
Table 1. cAMP levels in cultured trigeminal neurons
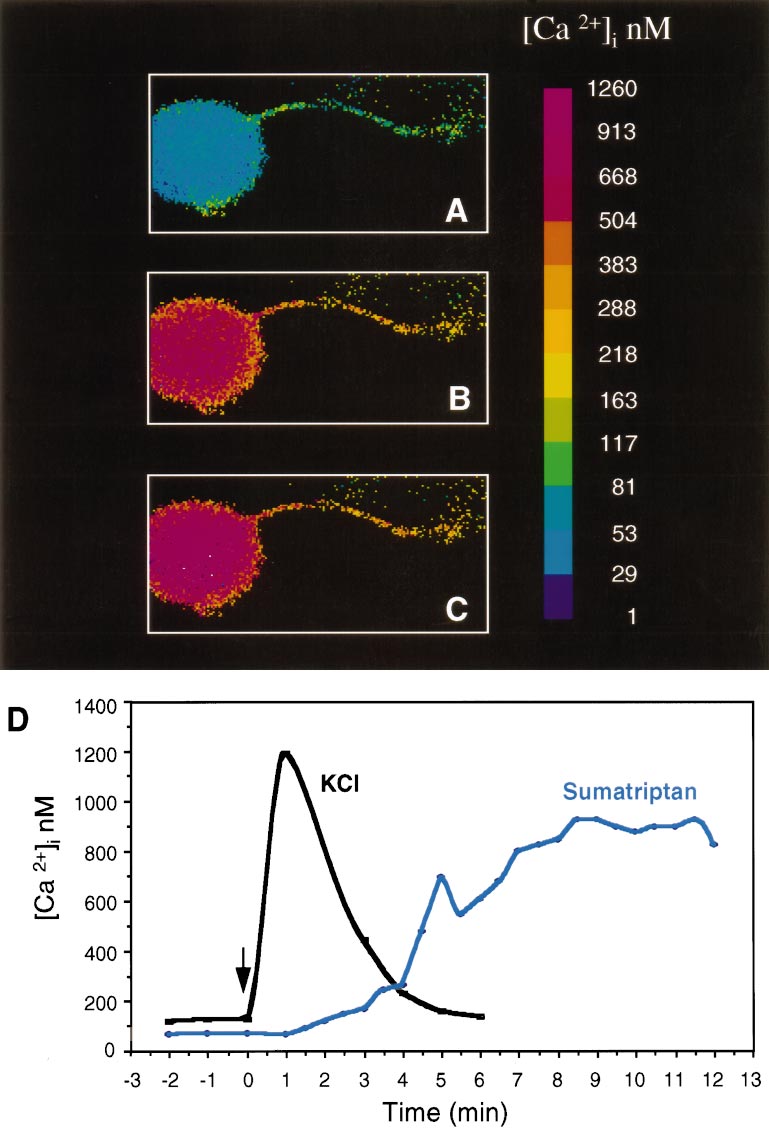
tested whether a similar pathway was activated by sumatriptan in
cultured trigeminal neurons. We found that sumatriptan caused a
slow, but markedly prolonged increase in intracellular calcium in
the neurons. There was approximately a fivefold increase in
intracellular calcium when compared with basal calcium levels
(Fig. 4, Table 3). The increased calcium levels did not reach the
FSK 1 sumatriptan
maximal levels until ;8 min after treatment but were maintained
for at least 30 min (longest time sampled). The calcium reached
a maximum concentration of ;600 nM on average and as high as
IFC 1 sumatriptan
1 mM in some cells. We estimate that ;40% of the neuronal cells
cAMP levels were measured from untreated control cultures or cells treated for 30
did not respond to sumatriptan treatment. The viability of these
min with forskolin (FSK) or with a cocktail of inflammatory agents (IFC). The
neuronal cells was confirmed after the sumatriptan treatment by
cultures were cotreated with vehicle, sumatriptan, or CGS. The means and SE from
five independent experiments with duplicate samples, and the fold increases in
the elevation of calcium levels in response to high concentrations
cAMP levels relative to control cells are shown.
of KCl. The reason for this heterogeneity is not known but may
*p , 0.001 when compared with control levels.
indicate that not all of the neurons are expressing sufficient levels
of 5-HT1 receptors. In contrast to the delayed increase in calcium
Table 2. cAMP levels in 5-HT1B-expressing HeLa cells
after sumatriptan treatment, addition of depolarizing levels of
KCl caused a very rapid and transient increase in calcium (Fig. 4).
These data demonstrate that activation of endogenous trigeminal
neuron 5-HT1 receptors is coupled to a calcium-signaling path-
way and not to a Gi/o-coupled decrease in cAMP.
FSK 1 sumatriptan
Okadaic acid blocks inhibitory effect of sumatriptan
How does a prolonged calcium elevation inhibit secretion? One
cAMP levels were measured from untreated control cultures or cells treated for 15
possibility is that protein phosphorylation states are changed. It is
min with forskolin (FSK). The cultures were cotreated with vehicle, sumatriptan, or
CGS. The means and SE from two independent experiments with duplicate samples,
generally accepted that changes in calcium can alter protein
and the fold increases in cAMP levels relative to control cells are shown.
phosphorylation and that phosphorylation plays an important role
*p , 0.02 when compared with control levels.
in regulating neuropeptide release from sensory neurons (Green-
gard et al., 1993). We have used okadaic acid, a potent inhibitor
of serine threonine protein phosphatases, especially PP1 and
Sumatriptan does not cause a decrease in intracellular
PP2A (Denhardt, 1996), to test the possibility that 5-HT1 agonists
cAMP levels
may be activating a phosphatase to attenuate stimulated secre-
We then characterized the signaling pathway(s) used by
tion. Okadaic acid treatment blocked the inhibitory effect of
sumatriptan in primary trigeminal ganglion cultures. Pharmaco-
sumatriptan on stimulated CGRP release (Fig. 5). Okadaic acid
logical studies have demonstrated that sumatriptan has high se-
treatment alone increased CGRP release that was similar in
lectivity and potency at the 5-HT1B, 1D, and 1F receptors, all of
magnitude to that caused by depolarization (Fig. 5), which is in
which are expressed by trigeminal neurons (Martin, 1997). The
agreement with previous studies by Vasko and colleagues (Hingt-
classical 5-HT1 signaling pathway based on studies using brain
gen and Vasko, 1994) using cultured sensory neurons from dorsal
slices and non-neuronal cell lines overexpressing 5-HT1 receptors
root ganglia. Cotreatment with okadaic acid and KCl did not
has been that these receptors inhibit adenylate cyclase and de-
result in a greater increase in CGRP release than observed with
crease cAMP levels via pertussis toxin-sensitive Gi/o proteins
each agent alone. These results indicate that sumatriptan acts by
(Boess and Martin, 1994). However, in contrast to these reports,
stimulating a serine threonine phosphatase. Although the identity
neither sumatriptan nor CGS inhibited forskolin-stimulated
of this phosphatase is not known, it is intriguing that okadaic acid
cAMP accumulation (Table 1). In addition, treatment with the
has recently been reported to inhibit a MAP kinase phosphatase
mixture of inflammatory agents that stimulated CGRP release
activity (Runden et al., 1998), and we have shown previously that
did not elevate cAMP levels, nor did the cAMP levels change
5-HT1 agonists cause a long-term increase in MAP kinase phos-
after cotreatment with sumatriptan (Table 1). As a positive con-
phatase activity in the CA77 cells (Durham and Russo, 1998).
trol, we confirmed that we would be able to detect inhibition of
cAMP production in cells known to couple 5-HT1B receptors to
Gi/Go. Sumatriptan or CGS treatment essentially blocked
forskolin-induced elevation of cAMP levels in HeLa cells stably
Our results support a model in which the trigeminal ganglion
expressing the 5-HT
nerves are activated during migraine and release CGRP to cause
1B receptor (Table 2). The degree of inhibi-
tion is similar to previously published results with this cell line
vasodilation and mast cell degranulation leading to the release of
(Hamblin et al., 1992). Thus, sumatriptan could lower cAMP
inflammatory agents (Fig. 6). On the basis of our data using CA77
levels in HeLa1B cells but did not cause a decrease in either the
cells (Durham and Russo, 1998), these agents may stimulate
stimulated or unstimulated cAMP levels in trigeminal neurons.
MAP kinase pathways leading to an increase in CGRP synthesis
and secretion that could potentially maintain elevated CGRP
Sumatriptan mediates an increase in
levels for the long duration (up to 72 hr) of a migraine. Activation
of this pathway ultimately leads to sensitization of the trigeminal
We had shown previously that activation of 5-HT1 receptors by
neurons and nociceptive transmission to the CNS contributing to
sumatriptan and other 5-HT1 receptor agonists caused a sus-
the pain, nausea, and photophobia associated with migraine
tained increase in calcium in the neuronal-like CA77 thyroid
(Buzzi et al., 1995). It is likely that sumatriptan is able to block
C-cell line (Durham et al., 1997). With this in mind, we then
this pathway via activation of the 5-HT1 receptors leading to a
Durham and Russo • Serotonergic Repression of CGRP Secretion
J. Neurosci., May 1, 1999, 19(9):3423–3429 3427
Figure 4. Sumatriptan increases the
concentration of intracellular calcium in
trigeminal neurons. A, Intracellular cal-
cium concentrations, [Ca 21]i, from day 4
cultures were measured using fura-2 and
a microscopic digital imaging system.
The pseudo-color scale indicates the
[Ca 21]i. Basal levels are in a single neu-
ron with a neurite. B, The same cell 6
min after addition of 10 mM sumatriptan.
C, The same cell after 12 min. D, A
graphic representation of the change in
[Ca 21]i as a function of time after
sumatriptan treatment of a representa-
tive cell (same cell as above). For com-
parison, a trace of a different cell treated
with only KCl (60 mM) is superimposed.
prolonged elevation in calcium that mediates the recruitment of
In the process of demonstrating this point, we have uncovered
phosphatases. The concentration of sumatriptan required for
several unexpected findings. First, activation of endogenous tri-
inhibition in vitro is higher than the estimated plasma concentra-
geminal ganglion neuron 5-HT1 receptors did not decrease
tion in patients (;0.2 mM) (Fowler et al., 1991). Possible expla-
cAMP levels, which contradicts the commonly held belief (Boess
nations are that the effective receptor number may be low because
and Martin, 1994). These observations are consistent with our
of the culture conditions and/or lack of colocalization of recep-
findings that the 5-HT1 receptor agonist CGS also did not de-
tors and secretory machinery at nerve terminals. Alternatively,
crease cAMP levels in a model neuronal cell line and that CGS
higher concentrations may be required to counteract chronic
actions were pertussis toxin independent (Durham et al., 1997).
stimulation of the cultures. In either case, the ability to block
The simplest explanation is that the cellular context is critical for
stimulated CGRP secretion in the absence of vascular contribu-
assigning second messenger pathways to receptors. In support of
tions strongly supports the neurogenic model of migraine.
this conclusion, others have reported that terminal 5-HT1 auto-
3428 J. Neurosci., May 1, 1999, 19(9):3423–3429
Durham and Russo • Serotonergic Repression of CGRP Secretion
Table 3. Effect of sumatriptan on calcium levels in cultured
1.1 6 0.8a
Intracellular calcium levels were measured from untreated control neurons or
neurons treated with 10 mM sumatriptan. The means and SE are given.
*p , 0.001 when compared with control levels.
aThere was no significant change in calcium in control cells, so for comparison with
the sumatriptan-treated cells, calcium levels at 488 sec were used to calculate peak
and fold increase values for the control cells.
Figure 6. Model of 5-HT1 receptor-mediated inhibition of CGRP release
from trigeminal neurons. A depolarizing stimulus causes the initial re-
lease of CGRP from trigeminal nerves, leading to neurogenic inflamma-
tion, which then further stimulates the release of CGRP. Activation of
5-HT1 receptors blocks this cycle by inhibiting CGRP release via an
increase in phosphatase activity that is likely mediated by a sustained
elevated level of intracellular calcium.
the amplitude, duration, and localization of increased calcium can
differentially activate transcription factors (Dolmetsch et al.,
Our working model is that there is a balance between kinase
and phosphatase activity that controls CGRP secretion (Fig. 6).
Support for this type of mechanism is provided by our data
showing that the protein phosphatase inhibitor okadaic acid
blocks the inhibitory effect of sumatriptan on stimulated CGRP
release. Under basal conditions the phosphorylation state is at an
intermediate level. Depolarization, inflammatory agents, and
okadaic acid change the balance, leading to increased secretion.
Figure 5. Okadaic acid treatment blocks sumatriptan-mediated inhibi-
tion of potassium-stimulated CGRP release. The relative amount of
In agreement with this model, okadaic acid treatment alone
CGRP secreted from trigeminal neurons stimulated with 60 mM KCl, 600
stimulated neuropeptide release from cultured trigeminal (this
nM (unless indicated as 300 nM) okadaic acid (OA), or the combination of
study) and dorsal root ganglia neurons (Vasko et al., 1994).
KCl and OA, with or without cotreatment with 10 mM sumatriptan
Sumatriptan is able to blunt the increased secretion in response to
(Suma) is shown. The mean basal rate of CGRP secretion was 99 6 4
pg/hr per dish. The means and SE from at least three independent
depolarization and inflammatory agents, but not okadaic acid,
experiments are shown. *p , 0.001 when compared with control values.
suggesting that specific phosphatases are recruited by 5-HT1
# p , 0.05 when compared with KCl values. 1 p , 0.05 when compared
receptor activation. The possibility of coordinated regulation by
with KCl plus sumatriptan values.
phosphatases is suggested by our previous studies showing that
CGRP promoter activity was repressed in CA77 cells by a
receptors in hippocampal neurons may not be coupled to Gi/o
calcium-dependent increase in MAP kinase phosphatase-1 activ-
proteins (Blier, 1991).
ity via 5-HT1 receptor activation (Durham and Russo, 1998). A
The second and perhaps most intriguing finding is that the
remaining question is how sumatriptan selectively inhibits stim-
inhibition of neuropeptide secretion by 5-HT1 receptor activation
ulated but not basal release of CGRP. To our knowledge, this is
is paradoxically coupled to an unusually prolonged intracellular
the first report of a drug that selectively targets only one of these
calcium signal. At face value, this observation is paradoxical
events. One possible explanation would be if sumatriptan causes
because increased calcium is well known to be a signal to increase
dephosphorylation of proteins responsible for the assembly, fu-
secretion (Matthews, 1996). Indeed, this dogma held true for the
sion, and/or recycling of vesicles in response to depolarization or
potassium treatment, which caused a more typical transient in-
crease in calcium, with increased CGRP release from cultured
In conclusion, our results have demonstrated that activation of
trigeminal ganglia neurons. There is precedence in parathyroid
the 5-HT1 receptor class of antimigraine drugs is able to directly
endocrine cells for coupling of elevated intracellular calcium with
block CGRP release from trigeminal nerves. The inhibitory effect
inhibition of peptide secretion (Shoback et al., 1984). Our data
of sumatriptan occurs via a paradoxical elevation in calcium and
demonstrate that activation of endogenous trigeminal neuron
activation of an okadaic acid-sensitive phosphatase. During mi-
5-HT1 receptors is coupled to a calcium-dependent signaling
graine, CGRP helps mediate neurogenic inflammation that may
pathway that differs from depolarization-induced changes in cal-
result in the release of inflammatory agents. These agents could
cium. This raises the possibility that the amplitude and duration
in turn feed back to sensitize the trigeminal ganglia neurons to
of increased calcium can differentially regulate neuropeptide se-
sustain an elevated rate of CGRP release (Fig. 6). On the basis of
cretion from sensory neurons, analogous to recent evidence that
our data, the effectiveness of sumatriptan is attributable in part to
Durham and Russo • Serotonergic Repression of CGRP Secretion
J. Neurosci., May 1, 1999, 19(9):3423–3429 3429
its ability to break this deleterious feedback loop at trigeminal
Hingtgen CM, Vasko MR (1994) The phosphatase inhibitor, okadaic
ganglia nerve terminals by inhibiting CGRP secretion.
acid, increases peptide release from rat sensory neurons in culture.
Neurosci Lett 178:135–138.
Martin GR (1997) Serotonin receptor involvement in the pathogenesis
Blier P (1991) Terminal serotonin autoreceptor function in the rat hip-
and treatment of migraine. In: Headache (Goadsby PJ, Silberstein SD,
pocampus is not modified by pertussis and cholera toxins. Naunyn
eds), pp 25–39. Boston: Butterworth-Heinemann.
Schmiedebergs Arch Pharmacol 344:160–166.
Matthews G (1996) Neurotransmitter release. In: Annual review of neu-
Boess FG, Martin IL (1994) Molecular biology of 5-HT receptors. Neu-
roscience (Cowan WM, ed), pp 219–233. Palo Alto, CA: Annual
Reviews, Inc.
Bouchelet I, Cohen Z, Case B, Seguela P, Hamel E (1996) Differential
McCulloch J, Uddman R, Kingman TA, Edvinsson L (1986) Calcitonin
expression of sumatriptan-sensitive 5-hydroxytryptamine receptors in
gene-related peptide: functional role in cerebrovascular regulation.
human trigeminal ganglia and cerebral blood vessels. Mol Pharmacol
Proc Natl Acad Sci USA 83:5731–5735.
Moskowitz MA (1993) Neurogenic inflammation in the pathophysiology
Buchman VL, Davies AM (1993) Different neurotrophins are expressed
and treatment of migraine. Neurology 43:S16–20.
and act in a developmental sequence to promote the survival of em-
O'Conner TP, Van Der Kooy D (1988) Enrichment of vasoactive neu-
bryonic sensory neurons. Development 118:989–1001 .
ropeptide calcitonin gene-related peptide in the trigeminal sensory
Buzzi MG, Bonamini M, Moskowitz MA (1995) Neurogenic model of
projections to the intracranial arteries. J Neurosci 8:2468–2476.
migraine. Cephalagia 15:277–280.
Ottosson A, Edvinsson L (1997) Release of histamine from dural mast
Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Julius D (1997)
cells by substance P and calcitonin gene-related peptide. Cephalagia
The capsaicin receptor: a heat-activated ion channel in the pain path-
way. Nature 389:816–824.
Rosenfeld MG, Mermod J-J, Amara SG, Swanson LW, Sawchenko PE,
Denhardt DT (1996) Signal-transducing protein phosphorylation cas-
cades mediated by Ras/Rho proteins in the mammalian cell: the po-
Rivier J, Vale WW, Evans RM (1983) Production of a novel neu-
tential for multiplex signaling. Biochem J 318:729–747.
ropeptide encoded by the calcitonin gene via tissue-specific RNA pro-
Dolmetsch RE, Lewis RS, Goddnow CC, Healy JI (1997) Differential
cessing. Nature 304:129–135.
activation of transcription factors induced by Ca 21 response amplitude
Runden E, Seglen PO, Haug F, Ottersen OP, Wieloch T, Shamloo M,
and duration. Nature 386:855–858.
Laake JH (1998) Regional selective neuronal degeneration after pro-
Durham PL, Russo AF (1998) Serotonergic repression of mitogen-
tein phosphatase inhibition in hippocampal slice cultures: evidence for
activated protein kinase control of the calcitonin gene-related peptide
a MAP kinase-dependent mechanism. J Neurosci 18:7296–7305.
enhancer. Mol Endocrinol 12:1000–1008.
Shoback DM, Thatcher J, Leombruno R, Brown EM (1984) Relation-
Durham PL, Sharma R, Russo AF (1997) Repression of the calcitonin
ship between parathyroid hormone secretion and cytosolic calcium
gene-related peptide promoter by 5-HT1 receptor activation. J Neurosci
concentration in dispersed bovine parathyroid cells. Proc Natl Acad Sci
USA 81:3113–3117.
Edvinsson L, Goadsby PJ (1994) Neuropeptides in migraine and cluster
Steen KH, Reeh PW, Anton F, Handwerker HO (1992) Protons selec-
headache. Cephalagia 14:320–327.
tively induce lasting excitation and sensitization to mechanical stimu-
Ferrari MD (1998) Migraine. Lancet 351:1043–1051.
lation of nociceptors in rat skin, in vitro. J Neurosci 12:86–95.
Fowler PA, Lacey LF, Thomas M, Keene ON, Tanner RJ, Baber NS
Stewart WF, Schechter A, Rasmussen BK (1994) Migraine heterogene-
(1991) The clinical pharmacology, pharmacokinetics and metabolism
ity: disability, pain intensity, and attack frequency and duration. Neu-
of sumatriptan. Eur Neurol 31:291–294.
rology 44:S24–39.
Goadsby PJ, Edvinsson L (1993) The trigeminovascular system and mi-
Strassman AM, Raymond SA, Burstein R (1996) Sensitization of men-
graine: studies characterizing cerebrovascular and neuropeptide
changes seen in humans and cats. Ann Neurol 33:48–56.
ingeal sensory neurons and the origin of headaches. Nature 384:
Greengard P, Valtorta F, Czernik AJ, Benfenati F (1993) Synaptic ves-
icle phosphoproteins and regulation of synaptic function. Science
Van Rossum D, Hanisch U-K, Quirion R (1997) Neuroanatomical lo-
calization, pharmacological characterization and functions of CGRP,
Hamblin MW, Metcalf MA, McGuffin RW, Karpells S (1992) Molecular
related peptides and their receptors. Neurosci Biobehav Rev
cloning and functional characterization of a human 5-HT
receptor: a homologue of the rat 5-HT
Vasko MR, Campbell WB, Waite KJ (1994) Prostaglandin E
1B receptor with 5-HT1D-like
pharmacological specificity. Biochem Biophys Res Commun
bradykinin-stimulated release of neuropeptides from rat sensory neu-
rons in culture. J Neurosci 14:4987–4997.
Source: http://cmrf.research.uiowa.edu/files/cmrf.research.uiowa.edu/files/Regulation%20of%20Calcitonin%20Gene-Related%20Peptide%20Secretion.pdf
Limitations of New LORNA WEIR The term "new social movements" (NSMs) entered the lexicon of social theory during the 1980s. At the most obvious level, "new social movements" de- signates the broad range of contemporary social movements,including environmental, peace, feminist, ethnic, anti-racistand national minority organizing. These movements arethought to be defined by an orientation to identity and cul-tural politics rather than to state and class politics. NSMs
Radiation Measurements 43 (2008) 315 – 318 Characteristics of LiF:Mg,Cu,P thermoluminescence at ultra-high dose range P. Bilskia,∗, B. Obryka, P. Olkoa, E. Mandowskab, A. Mandowskib, J.L. Kimc a Institute of Nuclear Physics (IFJ), Krakow, Poland bInstitute of Physcis, Jan Dlugosz University (AJD), Czestochowa, Poland cKorean Atomic Energy Research Institute (KAERI), Dejoan, Republic of Korea


