Levitra enthält Vardenafil, das eine kürzere Wirkdauer als Tadalafil hat, dafür aber schnell einsetzt. Männer, die diskret bestellen möchten, suchen häufig nach levitra kaufen ohne rezept. Dabei spielt die rechtliche Lage in der Schweiz eine wichtige Rolle.
Cnpdia.embrapa.br
PLANT CELL M EM BRANES AND SALINITY: STRUCTURAL,
BIOCHEM ICAL AND BIOPHYSICAL CHANGES
Institut für Botanik, Fachbereich Biologie, Technische Hochschule
Darmstadt, Schnittspahnstrasse 3-5, D-64287 Darmstadt
ABSTRACT- The fundamental basis of the adaptation of plants
e Ca2+ , trocadores neutros H+ /Na+ e canais iônicos.
to salinity stress is the control of transport of salt across
Conclui-se que evidência s concretas para alterações
membranes. Two major membranes of plant cells, the
adaptativas genuinas de componentes moleculares
plasmalemma and the tonoplast, are particularly involved in
particulares da membrana em resposta a salinidade existe
this process of compartmentation. The opulence of literature
apenas com respeito ao atiporter H+ /Na+ da mebrana
on plant reactions to salinity notwithstanding, there is still rather
plasmática e tonoplasto e ao transportador H+ -ATPase do
little understanding of responses of the molecular constituents
tonoplasto, pelo menos em certos materiais de plantas
of the membranes. The literature is reviewed with respect to
superiores. Progressos recentes sobre bioquímica de
current knowledge regarding lipids, proteins, interactions
membranas e biologia molecular oferece perspectivas para
between lipids and proteins and specific transport enzymes,
maiores avanços da pesquisa nesta área no futuro.
such as H+ - transporting ATPases at the plasmalemma andtonoplast and the H+ - transporting pyrophosphatase at the
Termos adicionais para indexação: Ca2+ -ATPase, canais
tonoplast, other membrane ATPases serving primary active
iônicos, Cl--ATPase, H+ -ATPase, H+ /Na+ -antiport, lipidios,
transport of Cl- and Ca2+ , H+ /Na+ - exchange carriers and
plasmalema, proteinas, tonoplasto.
ion channels. It is concluded that concrete evidence for genuine
adaptive changes of particular molecular membranecomponents in response to salinity only exists with respect to
Salinity is one of the most intensely studied stressors (i.e.
the H+ /Na+ -antiporters of the plasmalemma and tonoplast
environmental stress factors) in the ecophysiology of plants.
and the H+ -transporting ATPase of the tonoplast at least in
The major impact of stressful NaCl-loads is on functions of
certain materials of higher plants. Recent progress in
proteins and membranes as stabilized by water structures in
membrane biochemistry and molecular biology offers
the cell. One option in adaptation is salt exclusion. However,
perspectives for better advances of research in this area in the
this is of limited utility mainly for osmotic reasons and only
effective under conditions of moderate salt stress. The
Additional index terms: Ca2+ -ATPase, Cl--ATPase, H+ -ATPase,
alternative option, viz. salt inclusion, for the reasons mentioned
H+ /Na+ -antiport, ion channels, lipids, plasmalemma, proteins,
above requires compartmentation of the NaCl taken up. The
metabolically functional enzymes and membranes in thecytoplasm must be protected from the NaCl, potentially
M EM BRANAS CELULARES DE PLANTAS
destroying their tertiary and quaternary structures, by
E SALINIDADE: ALTERAÇÕES
sequestration of the salt in the cel sap of the central vacuole.
ESTRUTURAIS, BIOQUÍM ICAS E
A recent review on physiological processes limiting plantgrowth in saline soils concludes with the statement "advances
in salt tolerance at the molecular level will be in manipulating
RESUMO- A base fundamental da adaptação das plantas ao
the expression and structure of proteins that control the
estresse salino é o controle do transporte através de
transport of salt across membranes" (Munns 1993).
membranas. Duas membranas principais das células, o
Two major membranes of plant cells, the plas-
plasmalema e o tonoplasto, são particularmente envolvidas
malemma and the tonoplast, are particularly involved in
neste processo de compartimentalização. Apesar da opulência
this process of compartmentation. There is an ever-
da literatura sobre reações das plantas à salinidade, ainda há
growing opulent wealth of literature regarding mem-
muito pouco entendimento sobre as respostas moleculares
brane fluxes, ion distribution by cell compartmentation
dos constituintes das membranas. A literatura foi revista com
and whole-plant partitioning and the associated water
respeito ao conhecimento corrente sobre lipídeos, proteínas e
relations under salt stress. By contrast to these pheno-
enzimas transportadoras específicas como as H+ -ATPases do
menological consequences of molecular membranefunctions, very much less is known about the responses
plasmalema e do tonoplasto e o transportador
of the membranes themselves. The plasmalemma and
H+ -pirofosfatase do tonoplasto, outras ATPases de
the tonoplast of plant cells contain proteins catalyzing
membrana, que servem para o transporte ativo primário de Cl-
primary- and secondary-active processes of ion trans-port, which are essential in the salt compartmentation
1Palestra apresentada ao
IV Congresso Brasileiro de Fisiologia
involved in adaptation to salinity. Particularly the pri-
R. Bras. Fisiol. Veg., 5(2):217-224,1993.
mary-active transport of protons by membrane ATPases
(1990a) found no clear differences between the polypep-
which also provides the energy for secondary-active ion
tide composition of the tonoplast of the halophyte S.
fluxes and the secondary-active H+ /Na+ -exchange car-
maritima and data for tonoplasts of glycophytes. On the
rier proteins are important entities of these membranes.
other hand, Ben-Hayyim et al. (1989) report on salt-in-
Recent progress of membrane biochemistry and mo-
duced increases and decreases respectively, in the level
lecular biology appears to offer perspectives for a more
of a large number of proteins under salt shock and long-
detailed understanding of the interactions between the
term adaptation in cultured cells of different plant spe-
membranes and the stressor NaCl, and biophysical and
cies. Rather regularly in their various plant materials
electron-microscopical approaches also allow to assess
(barley, tobacco, tomato, Citrus) authors found salt in-
responses of the molecular structure of membranes. The
duced peptides with a molecular mass of 26 - 27 kDa
question is, if just preformed membrane structures are
(Singh et al., 1985; Hurkman et al., 1988; Ben-Hayyim et
involved in functional regulation networks, or if salt has
al., 1989). While in many cases cellular localization of
direct impact on the membranes themselves. It is the
these peptides is not clear, let alone their function, in
aim of this article to review briefly some more recent
some studies it was in fact shown, that they may be
advances in this area and to delineate trends which ap-
membrane proteins (Hurkman et al., 1988), although a
pear to emerge.
clear function, e.g. as an H+ /Na+ -exchange carrier,could not be associated with them. Potentially, at least
2) Lipids and Proteins
based on their molecular mass, these peptides could
A debate, revived at times, regards the question
well belong to the group of "membrane intrinsic pro-
whether lipids or proteins are the major membrane com-
teins", especially the tonoplast intrinsic proteins, which
ponents responding to stress. Clearly, the proteins of
are capable of forming membrane channels and which
membrane enzymes are the decisive functional ele-
could be involved in the transport of ions (Ludevid et al.,
ments, whereas lipids determine passive membrane
1992) and also may be regulated by membrane bound
properties but may also be important in influencing the
Ca2+ dependent protein-kinases (Johnson & Chris-
proteins by affecting their molecular environment.
peels, 1992).
c) Lipids and Proteins, Membrane Fluidity and Perme-
The more recent literature leaves the question open if
in fact qualitative differences in lipid composition of
Using electron-spin resonance methods it has been
membranes are important in salinity adaptation. In roots
noted that in halophytes and under salinity stress mem-
of the halotolerant species Cochlearia anglica lipid con-
branes become more rigid. In principle this could be due
tent increased considerably under exposure to NaCl but
to changes in lipids or proteins, or both. As mentioned
the relative proportions of phospholipids, galactolipids
above, Leach et al. (1990b) argue that in Suadea mari-
and neutral lipids or the fatty acids remained unchanged
tima this is due to the sterols. In Mesembryanthemum
(Prud'homme et al., 1990). Haschke et al. (1990) also
crystallinum higher rigidity of the leaf-cell tonoplast un-
observed that the qualitative lipid composition of purified
der NaCl-stress as compared to the tonoplast of non-
tonoplast-membrane fractions from leaves of Mesem-
stressed plants (Kliemchen et al., 1993) cannot be
bryanthemum crystallinum did not change in response
attributed to the lipids per se because the qualitative
to NaCl stress. In genotypes of Citrus differing in NaCl-
lipid-composition does not change (Haschke et al.,
tolerance by chloride exclusion, variations of galac-
1990). It was shown that in the facultative annual halo-
tolipids and phospholipids also appear not to be in-
phyte M.crystallinum during the change from growth in
volved (Douglas 1985; Douglas and Sykes 1985), in
an NaCl-free medium to a medium with up to 400 mM
contrast to older observations of P.J.C. Kuiper and col-
NaCl many tonoplast peptides change their staining in-
leagues, which indicate a role of galactolipids in grape-
tensity in sodium-dodecyl sulphate polyacrylamide gel
vines (Kuiper, 1968), Plantago species (Kuiper & Kuiper,
electrophoresis (SDS-PAGE) and hence their abun-
1978) and sugar beet (Stuiver et al., 1984). However, in
dance (Bremberger and Lüttge, 1992a; Richter, 1993),
Citrus free sterols may be important in the regulation of
and in particular the amount of the H+ -transporting AT-
Cl- compartmentation and the degree of Cl- exclusion.
Pase is increased (Klink & Lüttge, 1992; Richter, 1993;
Planar sterols integrate more readily into the liquid lipid
see also below section 3b). It is likely that it is the higher
phase of the membranes than less planar sterols, and
protein/lipid ratio in these membranes, which brings
the latter thus allow higher Cl--permeability (Douglas
about a larger rigidity, where 3.6oC higher temperatures
1985; Douglas and Sykes 1985). Leach et al. (1990a, b)
are needed for the tonoplast isolated from leaves of NaCl
conclude from studies with purified isolated vacuoles of
treated plants to reach the same fluidity as the tonoplast
the halophyte Suaeda maritima that the lipid charac-
of non-treated plants (Kliemchen et al., 1993). It is gen-
teristics of the tonoplast, viz. various types of membrane
erally assumed that higher membrane rigidity implies
lipids and the degree of saturation of the fatty acids, are
lower permeability. Thus, more rigid tonoplasts would
essential in NaCl compartmentation and much more im-
provide a stronger resistance for remobilization of salt
portant than the polypeptide composition.
once it is sequestered in the vacuoles by secondary ac-
tive tansport (see section 4b). In M. crystallinum the is-
Besides studies of specific transport proteins of mem-
sue is complicated though by the fact that salt stress
branes (see sections 3 and 4), membrane polypeptides
also elicits a change from C3-photosynthesis to crasssu-
in general have been analyzed in relation to salinity
lacean-acid-metabolism (CAM), and it needs to be evalu-
stress. As already noted above (section 2a), Leach et al.
R. Bras. Fisiol. Veg., 5(2):217-224,1993.
ated which changes are due to salinity per se and to
adapted cells, reacted with an increase in m-RNA abun-
induction of CAM respectively (Richter, 1993).
dance within 24 h after exposure to NaCl. In tomato-
3) H
+ Transporting ATPases
roots Vmax of the plasmalemma ATPase was reduced
In a nice study Sanchez-Aguayo et al. (1991) localized
by salt stress, from 69 to 39 mol Pi mg protein-1 h-1
ATPase activity cytochemically in different root zones of
(Gronwald et al., 1990). More subtle kinetic responses
tomato. NaCl-dependent changes were observed in a
have been documented by Ben-Hayyim & Ran (1990) for
medial root region 200-500µm upwards from the root tip,
the H+ -ATPase of the plasmalemma of cultured Citrus
i.e. a decrease of ATPase activity in the plasmalemma
cells. They suggest that the native form of the ATPase
and an increase in the tonoplast.
has more than one substrate-binding site, where bothsites interact in the presence of salt and act inde-
Several attempts have been made to compare H+ -AT-
pendently in its absence.
Pase activities in membrane fractions of halophytes,moderately salt-resistant glycophytes and salt-sensitive
Comparisons also include marine and halophilic algae,
glycophytes. in the halophyte Atriplex nummularia the
but again spectacular differences are not found
membrane ATPases are only inhibited by ten-times
(Gimmler et al., 1989; Smahel et al., 1990, Balnokin et al.,
higher concentrations of NaCl and KCl than in the salt
1993). Although there are differences in some parame-
sensitive glycophyte Pisum sativum (Lerner et al., 1983).
ters, e.g. requirement of an excess of Mg2+ over ATP,
Salinity during growth somewhat changes the properties
which may reflect the adaptations of the ATPase and its
of the ATPases in A. nummularia, e.g. the pH-depend-
lipid environment to the high saline habitat, generally the
ence profile becomes sharpper and the system is defec-
plasmalemma ATPase is similar to that of glycophytes.
tive in non-salt-grown plants. Thus A. nummularia
b) H
+ -ATPase of the Tonoplast
appears to be not merely salt-tolerant but salt-requiring
Specific tonoplast-ATPase activities of salt-sensitive
(Braun et al., 1986). In moderately salt-resistant cotton
Plantago media and salt-tolerant Plantago maritima
there are no such effects of NaCl (Hassidim et al., 1986).
were comparable and did not alter after application of
The ATPases of A. nummularia were not affected by 140
NaCl stress (Staal et al., 1991). The properties of the
mM NaCl, while in cotton 50 mM NaCl was already in-
tonoplast H+ -ATPase of the halophyte Suaeda maritima
hibitory (Braun et al., 1988). However, in all of these stud-
were found to be similar to those of tonoplast H+ -AT-
ies, crude membrane fractions have been used, and,
Pases of glycophytes (Leach et al., 1990a, Maathuis et
hence, it remains unclear to which cellular membranes
al., 1992). Tonoplasts of higher plants also possess H+ -
the observed effects are to be referred. Koyro et al.
transporting pyrophosphatases, and the above conclu-
(1993) used inhibitors differentiating between various
sion also refers to this enzyme (Leach et al., 1990a).
ATPases in crude extracts of isolated protoplasts of
By contrast, studies with tonoplast fractions obtained
roots of drought-resistant Sorghum and drought-sensi-
from cultured cells of tobacco clearly reveal specific re-
tive, salt-tolerant Spartina townsendii plants grown with
sponses of the tonophast H+ -ATPase to salinity stress.
and without 40 mM NaCl and found increases in the
The enzyme changed kinetics from hyperbolic in cells
activity of both the plasmalemma ATPase (vanadate sen-
grown without NaCl to sigmoidal in cells grown with 428
sitive) and the tonoplast ATPase (NO3 --sensitive, azide
mM NaCl (Reuveni, 1992). The specific H+ - transport
resistant) under the salt treatment. Purified membrane
activity increased four-fold. Salt adaptation of the cells in
fractions allow a separate study of plasmalemma and
fact resulted in a reduction in the amount of the H+ -
tonoplast H+ -ATPases.
ATPase in the tonoplast but this was compensated by an
a) H
+ -ATPase of the Plasmalemma
increase in the capacity of the enzyme for H+ -transport
In a series of studies comparing salt-tolerant and salt-
and ATP- hydrolysis. Thus, adaptation involves quantita-
resistant species of Plantago Brüggemann and Janiesch
tive and qualitative alterations in the enzyme (Reuveni et
(1987, 1988, 1989) found no changes of the activities
and properties of the plasmalemma H+ -ATPase during
The tonoplast ATPase of higher plants is composed of
the physiological adaptation of the plants to saline envi-
several peptide subunits and has a head and stalk struc-
ronments, and there were high similarities between gly-
cophytic and halophytic Plantago species regarding
OF1-ATPases or coupling factors of mito-
chondria and chloroplasts (Sze 1985, Pederson &
substrate specificity, divalent cation requirement, kinetic
Carafoli, 1987; Forgac, 1989; Nelson & Taiz, 1989). The
data and stimulation by monovalent cations. Comparing
head is composed of three copies each of an
A and
B
the plasmalemma H+ -ATPase of the halophyte Atriplex
subunit, with molecular masses of 60 to 70 kDa. The
A
nummularia and the glycophyte Avena sativa Mills and
subunit bears the catalytic ATP-hydrolyzing site, and the
Hodges (1988) arrive at a similar conclusion. Their re-
B subunit has a regulatory function (Mandala & Taiz,
sults suggest that the enzyme is essentially the same in
1986; Rea et al.,1987; Forgac, 1989). Narasimhan et al.
both species and that the differences in Na+ transport in
(1991) have shown that in cultured tobacco cells the
the roots are unrelated to the plasmalemma ATPase.
transcription of the gene of the catalytic 70 kDa subunit
However, more recently Niu et al. (1993) found that in A.
or the stability of its m-RNA was induced by short-term
nummularia the gene expression for the plasmalemma
NaCl treatment in NaCl adapted cells or by abscisic-acid
H+ -ATPase may be regulated by NaCl in an apparently
treatment in both adapted and unadapted cells. There
rather complex way. Levels of m-RNA were similar in
were up to four genes encoding for this subunit.
cells unadapted, adapted and deadapted, i.e. previouslyadapted, to 342 mM NaCl. Deadapted cells, but not un-
R. Bras. Fisiol. Veg., 5(2):217-224,1993.
Quantitative and qualitative changes of the tonoplast
stress also elicits the induction of CAM. Correlations with
H+ -ATPase were also observed in leaf cells of Mesem-
the time-courses of NaCl accumulation and CAM induc-
bryanthemum crystallinum during ageing of the plants
tion respectively, in leaves of M. crystallinum sofar sug-
and during adaptation to salinity of 400 mM NaCl in the
gest that the increased amount of tonoplast H+ -ATPase
root medium (review see Lüttge, 1993).
is related to the NaCl-load and is a response to the re-
Quantitative changes, i.e. an increased ATPase activity
quirements of vacuolar salt sequestration, while the new
associated with an increased amount of the enzyme pro-
subunits are related to CAM and the requirements of
tein as determined by radial immune-diffusion using an
diurnal fluctuation of vacuolar acid levels (Richter, 1993).
antibody against the purified ATPase-holoenzyme, were
c) Other Membrane-ATPases
correlated in time with the stress given by the salt load
a) Cl--ATPases? Staal et al. (1987) have suggested that
imposed on the M. crystallinum plants. There was a
in the plasmalemma of the root-cells of Plantago major
much smaller increase with ageing of the plants during
ssp. pleiosperma in addion to the well known (Mg2+ +
growth. The salt-elicited effect was reversible to the ex-
K+ )-dependent H+ -ATPase a Cl- -dependent ATPase is
tent that was not reached by ageing per se (Richter,
occurring. A Cl---ATPase has also been proposed to be
1993). The activity of the H+ -pyrophosphatase of the
active in the salt glands of Limonium vulgare. In addition
tonoplast rapidly decreased with ageing and was unre-
to intracellular NaCl compartmentation excretion of
lated to the salt treatment.
NaCl via salt glands is another means of salt includersfor managing high salt loads. Early studies of Hill & Hill
The observation of an increase of the amount of the
(1973) have suggested that a Cl
ATPase in the tonoplast membrane due to NaCl stress
--ATPase may be in-
volved in salt excretion by the salt glands of the leaves
was corroborated by the entirely independent approach
of the halophyte L. vulgare. For another species regu-
of using quantitative electron microscopy of replicas of
larly growing in haline media, the marine chlorosiphon-
freeze-fractured tonoplast vesices. Protoplasmic freeze-
alous alga Acetabularia was studied in some detail. Early
fracture faces of tonoplast vesicles show intramembra-
work of Goldfarb & Gradmann (1983) and Goldfarb et al.
neous particles (Klink et al., 1990), which can be
(1984) in fact had suggeted that these algae possess an
demonstrated to belong to the H+ -ATPase, e.g. by puri-
ATPase serving primary active membrane-transport of
fication and reconstitution into arteficial liposomes. Soy-
Cl-, i.e. moving Cl- directly on account of the energy
bean-phospholipid proteoliposomes with the
available from ATP hydrolysis. More recently, Ikeda et al.
reconstituted ATPase show H+ -transport activity, SDS-
(1990) and Ikeda & Oesterhelt (1990) indeed appeared
PAGE of the proteoliposomes reveals that almost exclu-
to have purified and characterized such a novel type of
sively the subunits of the ATPase have been
ATPase and reconstituted it into liposomes. However,
incorporated, and electron microscopy of freeze-frac-
Smahel et al. (1992) critically looked at the plas-
ture replicas shows that similar intramembraneous parti-
malemma-ATPase of Acetabularia and found very differ-
cles are present as in native vesicles (Behre et al., 1992).
ent properties as those described for the Cl--ATPase by
A quantitative and statistical evaluation of particle densi-
lkeda and colleagues. They concluded that this Cl--AT-
ties and particle sizes in the native membranes revealed
Pase is not localized in the plasmalemma and possibly
that both increased during salt treatment, and this
might be mitochondrial. With another halophilic alga, the
clearly implies that the amount of the ATPase must have
volvocalous unicellular Dunaliella bioculata, Smahel et
also increased (Klink and Lüttge, 1992). Koyro et al.
al. (1990) also found strong similarities of the plas-
(1993) also observed increased densities of intramem-
malemma ATPase with the enzyme of other species and
braneous particles in the tonoplast and also in the plas-
argue that these results further undermine the hypothe-
malemma of rhizodermal cells of Sorghum and Spartina
sis of a wider distribution of a high-salt-load Cl--type
townsendii plants when grown at 40 mM NaCl.
plasmalemma ATPase.
In M. crystallinum certain qualitative changes were
b) Ca2+ -ATPases. It is well known that Ca2+ interacts
seen in the staining intensities of tonoplast peptides in-
with salinity stress in that external Ca2+ alleviates ad-
cluding subunits of the H+ -ATPase in SDS-PAGE of
verse effects of external NaCl. It is also well known that
solubilisates of purified tonoplast membranes. A clearer
the plasmalemma of higher plant cells possesses a
picture of the ATPase subunits emerged after immuno-
Ca2+ -ATPase exporting Ca2+ from the cytoplasm to the
blotting using antibodies against the ATPase-holoen-
outside via primary active transport of Ca2+ . To the
zyme. The most conspicuous result was that two new
knowledge of the author nothing appears to be known
subunits with molecular masses of 27 and 31 kDa ap-
sofar, however, about a possible relation of the two ob-
peared during the salt treatment (Richter, 1993) as al-
servations. The major function of the Ca2+ - ATPase is
ready noted by Bremberger et al. (1988) and
thought to be in maintenance of intracellular Ca2+ - ho-
Bremberger & Lüttge (1992a,b). Subunits of this molecu-
lar mass belong to the stalk-region of the ATPase (For-gac, 1989). They are probably important in stabilizing the
4) H
+ /Na+ -Exchange Carriers
attachment of the head to the membrane integral H+ -
H+ /Na+ -exchange carriers (or antiporters) have been
channel via the stalk (Puopolo et al., 1992; Ward et al.
identified both in the plasmalemma and in the tonoplast,
1992) and thus may regulate activity under stress.
e.g. in the halophyte Atriplex nummularia and the glyco-phyte cotton (Hassidim et al., 1990). The antiport is a
As already mentioned above (section 2c), in M. crys-
secondary-active transport using the electro-chemical
tallinum complications arise from the fact that salinity
H+ -gradients established by the H+ -ATPases of the
R. Bras. Fisiol. Veg., 5(2):217-224,1993.
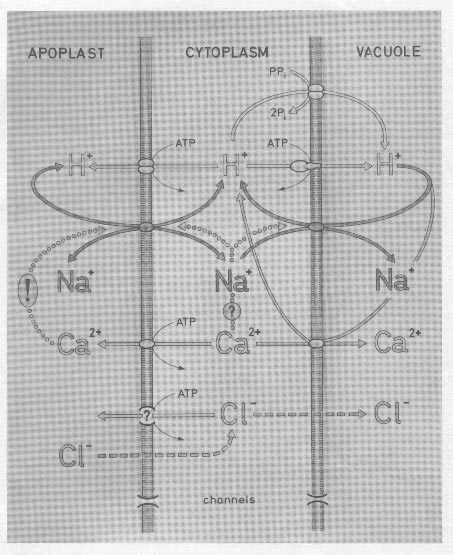
FIGURE 1- Scheme of a plant cel withthe membranes of plasmalemma andtonoplast separating the cytoplasm fromthe apoplast and the vacuolerespectively. Molecular components ortranspot-systems which reportedly orhypothetically ar involved in salinityresponses of cells are depicted asdiscussed in this review. (Drawing byDoris Schäfer, Darmstadt.)
membranes to move Na+ . As the ATPases extrude pro-
existing protein rather than to de novo protein synthesis
tons from the cytoplasm to the outside or into the vacu-
(Garbarino & DuPont, 1989). Exposure of salt-tolerant
oles respectively, the antiporters take protons back into
Plantago maritima and salt-sensitive Plantago media to
the cytoplasm in exchange for Na+ and thus keep cyto-
50 mM NaCl for 6 days resulted in the expression of
plasmic Na+ -concentrations at a low level. H+ /Na+ -an-
H+ /Na+ -antiport activity in tonoplast-vesicles of P. mari-
tiporters are not ubiquitous though. A screening of 16
tima but not of P. media, and in the controls not treated
crop species showed that 4 species had the antiporter,
with NaCl there was no activity in either of the two spe-
which, however, was absent in 5 species, and the situ-
cies (Staal et al., 1991).
ation was unclear in 7 species (Mennen et al., 1990).
The H+ /Na+ -antiporter of the tonoplast of sugar-beet
a) H+ /Na+ -Exchange Carriers of the Plasmalemma
cells was characterized in great detail by Blumwald and
Specifically for the plasmalemma, H+ /Na+ -antiporters
colleagues. An early observation showed that Na+ in the
were described for red beet (Jacoby and Teomy, 1988),
growth medium of cultured cells did not change the ap-
Atriplex nummularia, where the antiporter saturated at
parent Km for Na+ but increased Vmax to about twice
Na+ - concentrations above 100 mM (Braun et al., 1988)
the control value, suggesting a specific induction of an-
and the halophilic alga Dunaliella salina, where adapta-
tipoter synthesis by NaCl, which increases the number
tion to high salinity induced overproduction of the an-
of antiporter molecules present in the membrane (Blum-
tiporter (Katz et al., 1992).
wald & Poole, 1987). The inhibitor of vacuolar H+ /Na+ -
b) H+ /Na+ -Exchange Carriers of the Tonoplast
The H+ /Na+ -antiporter of tonoplasts is characterized
assisted biochemical investigations (Barkla et al., 1990).
by its pronounced inducibility. In barley specific
Thus, the group succeeded in identification of a 170 kDa-
H+ /Na+ -exchange was detected in tonoplast mem-
protein associated with vacuolar H+ /Na+ antiport.
branes from roots of plants grown in 100 mM NaCl, and
Growth of the cells in the presence of NaCl did not affect
it was absent in control roots (Garbarino & DuPont,
the affinity of the antiporter for Na+ (Km) but markedly
1988). Induction of H+ /Na+ -exchange by NaCl was very
increased the maximal velocity of H+ /Na+ -exchange,
rapid, and it appears that it is due to activation of an
corroborating the earlier conclusion that the increase inactivity was due to more protein of the exchange carrier.
R. Bras. Fisiol. Veg., 5(2):217-224,1993.
This was correlated with increased synthesis of the 170
which are not compatible with cytoplasmic membrane
kDa polypeptide (Barkla & Blumwald, 1991).
and protein structures.
5) Ion Channels in the Plasmalemma and Tonoplast
These results were worked out by use of modern ap-
The patch-clamp technique allows the identification
proaches on the structural level (electron microscopy),
and characterization of gated and rectified ion channels
on the biochemical and molecular levels and on the bio-
in membranes, which might also be involved in cellular
physical level (e.g. electron spin resonance). Much more
ion compartmentation under salinity stress. The
such work is definitely needed to establish progress in
K+ /Na+ -selectivity of a cation channel in the plas-
the future, and, as the new techniques now become
malemma of root cells of wheat did not differ in salt-tol-
more readily available it also becomes possible. Hence,
erant and salt-sensitive species (Schachtman et al.,
looking back the conclusions are meagre, but looking
1991). In salt-tolerant Plantago maritima and salt-sensi-
forward, the prospects appear more promising.
tive Plantago media there was a great similarity intonoplast channel properties, notwithstanding the large
differences in ion uptake and distribution. NaCl-supply
BALNOKIN, Y.V.; POPOVA, L. & MYASOEDOV, N.A. Plasma
left the channel selectivity and single-channel conduc-
membrane ATPase of marine unicellular alga Platymonas
tance unchanged but reduced the open-probability
viridis. Plant Physiology and Biochemistry, 31(2):159-
(Maathuis & Prins, 1990).
Measurements on tonoplasts of isolated vacuoles ob-
BARKLA, B.J. & BLUMWALD, E. Identification of a 170-kDa
tained from Suaeda maritima grown in the presence and
protein associated with the vacuolar Na+ /H+ antiport of
absence respectively, of 200 mM NaCl did not indicate
Beta vulgaris. Proceedings of the National Academy
any special adaptation of the tonoplast ion channas in
of Sciences, 88(24):11177-11181, 1991.
the halophyte. A low open-probability must be assumed
BARKLA, B.J.; CHARUK, J.H.M.; CRAGOE, E.J. & BLUM-
in vivo, otherwise NaCl efflux from the vacuoles would
WALD, E. Photolabeling of tonoplast from sugar beet cell
kill the cells (Maathuis et al., 1992).
suspensions by [ 3H] 5-(N-methyl-N-isobutyl)-amiloride,
6) Conclusions and Outlook
an inhibitor of the vacuolar Na+ /H+ antiport. Plant
Physiology, 93(3):924-930, 1990.
In drastic contrast to the immense literature published
on effects of salinity on plants, work trying to come to
BEHRE, B.; RATAJCZAK, R. & LÜTTGE, U. Selective recon-
stitution of the tonoplast H+ -ATPase of the crassulacean
grips more directly with the major membranes involved,
acid metabolism plant Kalanchoe daigremontiana. Bo-
i.e. the plasmalemma and tonoplast themselves, is very
tanica Acta, 105(4):260-265, 1992.
limited sofar. The major molecular components of these
BEN-HAYYIM, G. & RAN, U. Salt-induced cooperativity in
two membranes and their functions as discussed in the
ATPase activity of plasma membrane-enriched fractions
present report are depicted in Figure 1. From the reports
from cultured Citrus cells: kinetic evidence. Physiologia
available it follows that the role of membrane lipids is
Plantarum, 80(2):210-216, 1990.
unclear. Proteins in principle appear to be important, but
BEN-HAYYIM, G.; VAADIA, Y. & WILLIAMS, B.G. Proteins
details are not clear with the exception of three specific
associated with salt adaptation in citrus and tomato cells:
transport proteins, viz. the H+ -ATPase of the tonoplast
involvement of 26 kDa polypeptides. Physiologia Plan-
and the H+ /Na+ antiporters of the tonoplast and the
tarum, 77(3):332-340, 1989.
plasmalemma. The Ca2+ -ATPase remains dubious. The
BLUMWALD, E. & POOLE, R.J. Salt tolerance in suspension
role of the Ca2+ -ATPase has barely been studied and
cultures of sugar beet. Induction of Na+ /H+ antiport
the roles of the plasmalemma ATPase and cation-chan-
activity at the tonoplast by growth in salt. Plant Physiol-
nels remain unclear. Perhaps specific changes, adapta-
ogy, 83(4):884-887, 1987.
tions and expressions of membrane-lipid compositions,
BRAUN, Y.; HASSIDIM, M.; LERNER, H.R. & REINHOLD, L.
plasmalemma- H+ -ATPases and ion channels are really
Studies on H+ -translocating ATPases in plants of varying
not involved in reactions to NaCl-stress and these enti-
resistance to salinity. 1. Salinity during growth modulates
ties just function via their normal role in cellular regula-
the proton pump in the halophyte Atriplex nummularia.
tion networks. Perhaps the somewhat uncertain situation
Plant Physiology, 81(4):1050-1056, 1986.
however, also arises from the fact that frequently apples
BRAUN, Y.; HASSIDIM, M.; LERNER, H.R. & REINHOLD, L.
and pears are compared and a clear overall conclusion
Evidence for a Na+ /H+ antiporter in membrane vesicles
cannot emerge from phenomenological comparisons of
isolated from roots of the halophyte Atriplex nummularia.
more or less salt-tolerant or salt-sensitive glycophytes
Plant Physiology, 87(1):104-108, 1988.
and halophytes grown with and without salt.
BREMBERGER, C.; HASCHKE, H.P. & LÜTTGE, U. Separa-
The only concrete evidence available for genuine
tion and purification of the tonoplast ATPase and pyro-
adaptive changes regards the H+ -transporting ATPase
phosphatase from plants with constitutive and inducible
of the tonoplast, at least in tobacco and in Mesembryan-
CAM. Planta, 175(4):465- 470, 1988.
themum crystallinum, and the H+ /Na+ -antiporters of
BREMBERGER, C. & LÜTGE, U. Dynamics of tonoplast
the plasmalemma and tonoplast. Clearly these may also
proton pumps and other tonoplast proteins of Mesem-
be the most important entities in the control of cytoplas-
bryanthemum crystallinum during the induction of cras-
mic Na+ load. The antiporters tend to eliminate Na+
sulacean ac id metabolism. Planta, 188(4):575-580,
from the cytoplasm. The tonoplast H+ -ATPase ener-
gizes the membrane of the vacuole, i.e. the most impor-
BREMBERGER, C. & LÜTTGE, U. Tonoplast ATPase of Me-
tant intracellular sink for solutes like Na+ and Cl- ions,
sembryanthemum crystallinum. Partial amino acid se-
R. Bras. Fisiol. Veg., 5(2):217-224,1993.
quence of subunits induced during the transition from
HILL, B.S. & HILL, A.E. ATP-driven chloride pumping and
C3-photosynthesis to Crassulacean Acid Metabolism.
ATPase activity in the Limonium salt gland. Journal of
Comptes Rendus Académie des Sciences Paris, 315
Membrane Biology, 12:145-158, 1973.
(Sér III):119-125, 1992b.
HURKMAN, W.J.; TANAKA, C.K. & DUPONT, F.M. The effects
BRÜGGEMANN, W. & JANIESCH, P. Characterization of
of salt stress on polypeptides in membrane fractions from
plasma membrane H+ -ATPase from salt-tolerant and
barley roots. Plant Physiology, 88(4):1263-1273, 1988.
salt-sensitive Plantago species. Journal of Plant Physi-
IKEDA, M. & OESTERHELT, D. A chloride translocating ade-
ology, 130 (4/5):395-411, 1987.
nosinetriphosphatase in Acetabularia acetabulum. 1. Pu-
BRÜGGEMANN, W. & JANIESCH, P. Properties of native and
rification and characterization of a novel type of adeno-
solubilized plasma membrane ATPase from the halo-
sine triphosphatase that differes from chloroplast F1
phyte Plantago crassifolia, grown under saline and non-
ad enosine triphosphatase. Biochemistry, 29:2065-
saline conditions. Physiologia Plantarum, 74(4):615-
IKEDA, M.; SCHMID, R. & OESTERHELT, D. A chloride
BRÜGGEMANN, W. & JANIESCH, P. Comparison of plasma
translocating adenosinetriphosphatase in Acelbularia
membrane ATPase from salt-treated and salt-free grown
acetabulum. 2. Rec o nstituio n o f the enzym e into
Plantago maritima L . Journal of Plant Physiology,
liposomes and effect of net charges of liposomes on
134(1):20-25, 1989.
chloride permeability and reconstitution. Biochemistry,
DOUGLAS, T.J. NaCl effects on 4-desmethylsterol composi-
29:2057-2065, 1990.
tion of plasma-membrane-enriched preparations from
JACOBY, B. & TEOMY, S. Assessment of Na+ /H+ antiport
citrus roots. Plant, Cell & Environment, 8(9):687-692,
in ATP-depleted red beet slices and barley roots. Plant
Science, 55 (2):103-106, 1988.
DOUGLAS, T.J. & SYKES, S.R. Phospholipid, galactolipid
JOHNSON, K.D. & CHRISPEELS, M.J. Tonoplast-bound
and free sterol composition of fibrous roots from citrus
protein kinase phosphorylates tonoplast intrinsic protein.
genotypes differing in choride exclusion ability. Plant,
Plant Physiology, 100(4):1787-1795, 1992.
Cell & Environment, 8(9):693-699, 1985.
KATZ, A.; PICK, U. & AVRON, M. Modulation of Na+ /H+
FORGAC, M. Structure and function of vacuolar class of
antiporter by extreme pH and salt in the halotolerant alga
ATP-d riven p ro to n p um p s. Physiological Review,
Dunaliella salina. Plant Physiology, 100(3):1224-1229,
GARBARINO, J. & DUPONT, F.M. NaCl induces a Na+ /H+
KLIEMCHEN, A.; SCHOMBURG, M.; GALLA, H.-J.; LÜTTGE,
antiport in tonoplast vesicles from barley roots. Plant
U. & KLUGE, M. Phenotypic changes in the fluidity of the
Physiology, 86(1):231-236, 1988.
tonoplast membrane of crassulacean acid metabolism
GARBARINO, J. & DUPONT, F.M. Rapid induction of
plants in response to temperature and salinity stress.
Na+ /H+ exchange activity in barley root tonoplast. Plant
Planta, 189 (3):403-409,1993.
Physiology, 89(1):1-4, 1989.
KLINK, R.; HASCHKE, H.-P.; KRAMER, D. & LÜTTGE, U.
GIMMLER, H.; SCHNEIDER, L. & KAADEN, R. The plasma
Membrane particles, proteins and ATPase activity of
membrane ATPase of Dunaliella parva. Zeltschrift für
tonoplast vesicles of Mesembryanthemum crystallinum
in the C3 and CAM state. Botanica Acta, 103(1):24-31,
GOLDFARB, V. & GRADMANN, D. ATPase activities in par-
tially purified membranes of Acetabularia. Plant Cell
KLINK, R. & LÜTTGE, U. Quantification of visible structural
Reports, 2:152- 155, 1983.
changes of the VOV1-ATPase in the leaf tonoplast of
GOLDFARB, V.; SANDERS, D. & GRADMANN, D. Reversal
Mesembryanthemum crystallinum by freeze-fracture rep-
of electrogenic Cl- pump in Acetabularia increases level
licas prepared during the C3-photosynthesis to CAM
and 32P labelling of ATP. Journal of Experimental Bot-
transition. Botanics Acta, 105(6):414-420, 1992.
any, 35(154):645-658, 1984.
KOYRO, H.W.; STELZER, R. & HUCHZERMEYER, B. ATPase
GRONWALD, J.W.; SUHAYDA, C.S.; TAL, M. & SHANNON,
activities and membrane fine structure of rhizodermal
M.C. Reduction in plasma membrane ATPase activity of
cells from Sorghum and Spartina roots grown under mild
tomato roots by salt stress. Plant Science, 66(2):145-
salt stress. Botanica Acta, 106(2):110-119, 1993.
KUIPER, D. & KUIPER, P.J.C. Lipid composition of the roots
HASCHKE, H.-P.; KAISER, G.; MARTINOIA, E.; HAMMER,
of Plantago species: Response to alteration of the level
U.; TEUCHER, T.; DORNE, A.J. & HEINZ, E. Lipid profiles
of mineral nutrition and ecological significance. Physi-
of leaf tonoplasts from plants with different CO
ologia Plantarum, 44:81-86, 1978.
mechanisms. Botanica Acta, 103(1):32-38, 1990.
KUIPER, P.J.C. Lipids in grape roots in relation to chloride
HASSIDIM, M.; BRAUN, Y.; LERNER, H.R. & REINHOLD, L.
transport. Plant Physiology, 43:1367-1371, 1968.
Studies on H+ -translocating ATPases in plants of varying
LEACH, R.P.; ROGERS, W.J.; WHEELER, K.P.; FLOWERS,
resistance to salinity. II. K+ strongly promotes develop-
T.J. & YEO, A.R. Molecular markers for ion compartmen-
ment of membrane potential in vesicles from cotton roots.
tation in cells of higher plants. I. Isolation of vacuoles of
Plant Physiology, 81(4):1057-1061, 1986.
HASSIDIM, M. BRAUN, Y.; LERNER, H.R. & REINHOLD, L.
41(230):1079-1087, 1990 a.
Na+ /H+ and K+ /H+ antiport in root membrane vesicles
LEACH, R.P.; WHEELER, K.P.; FLOWERS, T.J. & YEO, A.R.
isolated from the halophyte Atriplex and the glycophyte
Molecular markers for ion compartmentation in cells of
cotton. Plant Physiology, 94(4):1795-1801, 1990.
higher plants. II. Lipid composition of the tonoplast of the
halophyte Suaeda maritima (L.) Dum. Journal of Experi-
mental Botany, 41(230):1089-1094, 1990 b.
R. Bras. Fisiol. Veg., 5(2):217-224,1993.
LERNER, H.R.; REINHOLD, L.& GUY, R.; BRAUN, Y.; HAS-
location of nucleotide-binding subunits. Biochimica et
SIDIM, M. & POLJKOFF-MAYBER, A. Salt activation and
Biophysica Acta, 904(1):1-12, 1987.
inhibition of membrane ATPase from roots of the halo-
REUVENI, M. Utilization of metabolic energy under saline
phyte Atriplex nummularia. Plant, Cell & Environment,
conditions: changes in properties of ATP dependent en-
6(6):501-506, 1983.
zymes in plant cells grown under saline conditions. Bi-
LUDEVID, D.; HÖFTE, H.; HIMELBLAU, E. & CHRISPEELS,
ologia Plantarum, 34(3/4):181-191, 1992.
M.J. The expression pattern of the tonoplast intrinsic
REUVEN I, M.; B EN N ETT, A .B .; BRESSAN , R.A. &
protein γ-TIP in Arabidopsis thaliana is correlated with cell
HASEGAWA, P.M. Enhanced H+ -transport capacity and
enlargement. Plant Physiology, 100(4):1633-1639,
ATP hydrolysis activity of the tonoplast H+ -ATPase after
NaCl adaptation. Plant Physiology, 94 (2):524-530,
LÜTTGE, U. The role of crassulacean acid metabolism
(CAM) in the adaptation of plants to salinity. New Phy-
RICHTER, J. Die biochemische Anpassung der Tonoplasten-
tologist, in press, 1993.
ATPase an die Stoffwechselumstellung von der C3-Pho-
MAATHUIS, F.J.M.; FLOWERS, T.J. & YEO, A.R. Sodium
tosynthese zum CAM bei den Pflanzen Mesembryanthe-
chloride compartmentation in leaf vacuoles of the halo-
mum c rystallinum u n d Kalanc hoe blossfeldiana.
phyte Suaeda maritima (L.) Dum. and its relation to
tonoplast permeability. Journal of Experimental Bot-
Darmstadt, Dissertation D17, 1993.
any, 43(254):1219-1223, 1992.
SANCHEZ-AGUAYO, I.; GONZALES-UTOR, A.L. & MEDINA,
MAATHUIS, F.J.M. & PRINS, H.B.A. Patch-clamp studies on
A. Cytochemical localization of ATPase activity in salt-
root cell vacuoles of a salt-tolerant and a salt-sensitive
treated and salt-free grown Lycopersicon esculentum
Plantago species. Plant Physiology, 92(1):23-28,1990.
roots. Plant Physiology, 96(1):153-158, 1991.
MANDALA, S. & TAIZ, L. Characterization of the subunit
SCHACHTMAN, D. P.; TYERMAN, S. D. & TERRY, B. R. The
structure of the maize tonoplast ATPase. Journal of
K+ /Na+ selectivity of a cation channel in the plasma
Biological Chemistry, 261(27):12850-12855, 1986.
membrane of root cells does not differ in salt tolerant and
MENNEN, H.; JACOBY, B. & MARSCHNER, H. Is sodium
salt -sen sit ive w h eat sp ec ies. Pla nt Physiology,
proton antiport ubiquitous in plant cells? Journal of Plant
Physiology, 137(2):180-183, 1990.
SINGH, N.K.; HANDA, A.K.; HASEGAWA, P.M. & BRESSAN,
MILLS, D. & HODGES, T.K. Characterization of plasma mem-
R.A. Proteins associated with adaptation of cultured to-
brane ATPase from roots of Atriplex nummularia. Journal
bacco cells to NaCl. Plant Physiology, 79(1):126-137,
of Plant Physiology, 132(5):513-519, 1988.
MUNNS, R. Physiological processes limiting plant growth in
SMAHEL, M.; HAMANN, A. & GRADMANN, D. The prime
saline soils: some dogmas and hypotheses. Plant, Cell
plasmalemma ATPase of the halophilic alga Dunaliella
& Environment, 16(1):15-24, 1993.
bioculata: purification and characterization. Planta,
NARASIMHAN, M.L.; BINZEL, M.L.; PEREZ-PRAT, E.;
CHEN, Z.; NELSON, D.E.; SINGH, N.K.; BRESSAN, R.B.
SMAHEL, M.; KLIEBER, H.G. & GRADMANN, D. Vanadate-
& HASEGAWA, P.M. NaCl regulation of tonoplast ATPase
sensitive ATPase in the plasmalemma of Acetabularia:
70-kilo-dalton subunit mRNA in tobacco cells. Plant
biochemical and kinetic characterization. Planta, 188
NELSON, N. & TAIZ, L. The evolution of H+ -ATPases.
STAAL, M.; HOMMELS, C. & KUIPER, D. Characterization of
Trends in Biochemical Science, 14(3):113-116, 1989.
the plasmalemma ATPase activity from roots of Plantago
NIU, X.; ZHU, J.K.; NARASIMHAN, M.L.; BRESSAN, R.A. &
major ssp. pleiosperma, purified by the two-phase parti-
HASEGAWA, P.M. Plasma-membrane H+ -ATPase gene
tioning method. Physiologia Plantarum, 70(3):461-466,
expression is regulated by NaCl in cells of the halophyte
Atriplex nummularia L. Planta, 190(4):433-438, 1993.
STAAL, M.; MAATHUIS, F.J.M.; ELZENGA, J.T.M.; OVER-
PEDERSON, P.L. & CARAFOLI, E. Ion motive ATPases. I.
BEEK, J.H.M. & PRINS, H.B.A. Na+ /H+ antiport activity
Ubiquity, properties and significance to cell function.
in tonoplast vesicles from roots of the salt-tolerant Plan-
Trends in Biochemical Science, 12:146-150, 1987.
tago maritima and the salt- sensitive Plantago media.
PRUD'HOMME, M.P.; LE SAOS, J.; BOUCAUD, J.; OURSEL,
Physiologia Plantarum, 82(2):179-184, 1991.
A. & TREMOLIERES, A. Effect of NaCl on lipid metabolism
STUIVER, C.E.E.; DEKOK, L.J.; SANTENS, J.M.O. &
in roots of the halotolerant species Cochlearia anglica.
KUIPER, P.J.C. The effect of Na2SO4 on the lipid compo-
Plant Physiology and Biochemistry, 28:71-78, 1990.
sition of sugar beet plants. Zeitschrift für Pflanzen-
PUOPOLO, K.; SCZEKAN, M.; MAGNER, R. & FORGAC, M.
physiologie, 114(2):187-191, 1984.
The 40-kDa subunit enhances but is not required for
SZE, H. H+ -translocating ATPases: Advances using mem-
activity of the coated vesicle proton pump. Journal of
brane vesicles. Annual Review of Plant Physiology,
Biological Chemistry, 267(8):5171-5176, 1992.
36:175-208, 1985.
REA, P.A.; GRIFFITH, C.J.; MANOLSON, M.F. & SANDERS,
WARD, J.M.; REINDERS, A.; HSU, H.T. & SZE, H. Dissocia-
D. Irreversible inhibition of H+ -ATPase of higher plant
tion and reassembly of the vacuolar H+ -ATPase complex
tonoplast by chaotropic anions: evidence for peripheral
from oat roots. Plant Physiology, 99(1):161-169,1992.
R. Bras. Fisiol. Veg., 5(2):217-224,1993.
Source: http://www.cnpdia.embrapa.br/rbfv/pdfs/v5n2p217.pdf
How To Use Your HandiHaler It's important to remember to take SPIRIVA every day and use your SPIRIVA HandiHaler correctly as prescribed by your doctor. This quick overview may help. For complete information on using the SPIRIVA HandiHaler, please see the Instructions for Use attached to your SPIRIVA HandiHaler prescription. Familiarize yourself with the parts of your HandiHaler device:
TEST POTENZMITTEL schmerzen hervorrufen als die berühmte blaue Vorgängerpille. Ob allerdings auch das Herz- Kreislauf-Risiko geringer ist, das immer wieder zu Schlagzeilen und Beunruhigung führte, lässt sich heute noch nicht sagen. Im Zusammenhang mit Viagra war in den vergangenen Jahren eine Viel- zahl von Zwischenfällen bekannt geworden, in der Regel allerdings bei nicht bestimmungsgemä-ßem Gebrauch und durch Überanstrengung.

