Doi:10.1016/j.bcp.2008.08.008


Biological activities of curcumin and its analogues(Congeners) made by man and Mother Nature
Preetha Anand Sherin G. Thomas Ajaikumar B. Kunnumakkara Chitra Sundaram Kuzhuvelil B. Harikumar Bokyung Sung Sheeja T. Tharakan Krishna Misra Indira K. Priyadarsini , Kallikat N. Rajasekharan , Bharat B. Aggarwal
a Cytokine Research Laboratory, Department of Experimental Therapeutics, Unit 143, The University of Texas M.D. Anderson Cancer Center,1515 Holcombe Boulevard, Houston, TX 77030, USAb Department of Chemistry, University of Kerala, Thiruvananthapuram, Indiac Bio-informatics division, Indian Institute of Information Technology, Allahabad, Indiad Radiation and Photochemistry Division, Bhabha Atomic Research Centre, Mumbai-400085, India
Curcumin, a yellow pigment present in the Indian spice turmeric (associated with curry
Received 27 June 2008
powder), has been linked with suppression of inflammation; angiogenesis; tumorigenesis;
Accepted 7 August 2008
diabetes; diseases of the cardiovascular, pulmonary, and neurological systems, of skin, andof liver; loss of bone and muscle; depression; chronic fatigue; and neuropathic pain. Theutility of curcumin is limited by its color, lack of water solubility, and relatively low in vivo
bioavailability. Because of the multiple therapeutic activities attributed to curcumin, how-
ever, there is an intense search for a ‘‘super curcumin'' without these problems. Multiple
Synthetic analogues
approaches are being sought to overcome these limitations. These include discovery of
natural curcumin analogues from turmeric; discovery of natural curcumin analogues made
by Mother Nature; synthesis of ‘‘man-made'' curcumin analogues; reformulation of curcu-
min with various oils and with inhibitors of metabolism (e.g., piperine); development ofliposomal and nanoparticle formulations of curcumin; conjugation of curcumin prodrugs;and linking curcumin with polyethylene glycol. Curcumin is a homodimer of feruloyl-methane containing a methoxy group and a hydroxyl group, a heptadiene with two Michaelacceptors, and an a,b-diketone. Structural homologues involving modification of all thesegroups are being considered. This review focuses on the status of all these approaches ingenerating a ‘‘super curcumin.''.
# 2008 Elsevier Inc. All rights reserved.
b-diketone that exhibits keto-enol tautomerism. Curcuminhas been shown to exhibit antioxidant, anti-inflammatory,
Curcumin, commonly called diferuloyl methane, is a hydro-
antimicrobial, and anticarcinogenic activities. It also has
phobic polyphenol derived from the rhizome (turmeric) of the
hepatoprotective and nephroprotective activities, suppresses
herb Curcuma longa. Turmeric has been used traditionally for
thrombosis, protects against myocardial infarction, and has
many ailments because of its wide spectrum of pharmacolo-
hypoglycemic and antirheumatic properties. Moreover, cur-
gical activities. Curcumin has been identified as the active
cumin has been shown in various animal models and human
principle of turmeric; chemically, it is a bis-a, b-unsaturated
studies to be extremely safe even at very high doses In
* Corresponding author. Tel.: +1 713 7921817; fax: +1 713 7456339.
E-mail address: (B.B. Aggarwal).
0006-2952/$ – see front matter # 2008 Elsevier Inc. All rights reserved.
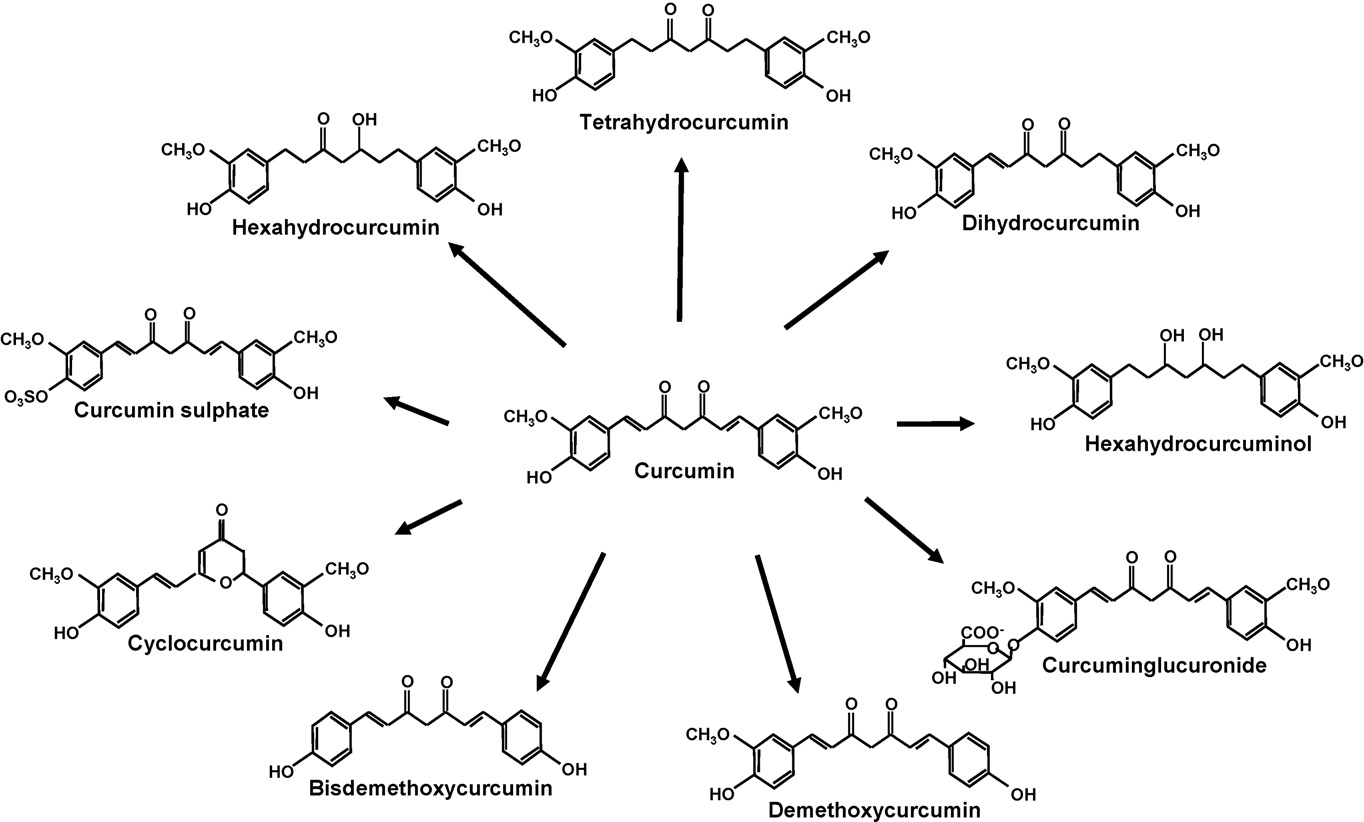
spite of its efficacy and safety, curcumin has not yet been
has an E-configuration (trans C C bonds). The aryl rings may
approved as a therapeutic agent. The poor aqueous solubility,
be symmetrically or unsymmetrically substituted; the most
relatively low bioavailability, and intense staining color of
prevalent natural substituents are of the oxy type, such as
curcumin have been highlighted as major problems; and
hydroxy or methoxy elements. In this review, the curcumin
consequently search for a ‘‘super curcumin'' without these
analogues are classified in three groups: analogues from
problems and with efficacy equal to or better than that of
turmeric, analogues from Mother Nature, and synthetic
curcumin is ongoing. This review presents the current status
of the efforts toward finding this ‘‘super curcumin.''
The strategies used in the search for ‘‘super curcumin'' can
Natural analogues from turmeric and its metabolites
be categorized under two broad headings, namely (1) syntheticanalogues or derivatives and (2) formulations. The most
The natural analogues of curcumin from turmeric and the
explored of these two is the analogues and derivatives. The
important metabolites of curcumin are depicted in The
literature describes numerous synthetic curcumin analogues
bioactivities of these analogues are summarized in
with a wide range of applications. This review analyzes thecurcumin analogues with special reference to their biological
Natural analogues from turmeric
activity. The formulation part of this review describes the
Turmeric contains three important analogues, curcumin,
adjuvant, nanoparticle, liposomal and micellar delivery
demethoxycurcumin (DMC), and bisdemethoxycurcumin
systems, phospholipid complexes, prodrugs and PEGylation
(BDMC). Collectively called curcuminoids, the three com-
of curcumin.
pounds differ in methoxy substitution on the aromatic ring.
While curcumin has two symmetric o-methoxy phenols linkedthrough the a,b-unsaturated b-diketone moiety, BDMC, also
Analogues and derivatives
symmetric, is deficient in two o-methoxy substitutions, andDMC has an asymmetric structure with one of the phenyl rings
Curcumin is a member of the linear diarylheptanoid class of
having o-methoxy substitution. Of the three curcuminoids,
natural products in which two oxy-substituted aryl moieties
curcumin is the most abundant in turmeric, followed by DMC
are linked together through a seven-carbon chain (The
and BDMC. Commercially available curcumin mixture contain
C7 chain of linear diarylheptanoids is known to have
77% curcumin, 17% DMC, and 3% BDMC.
unsaturation, oxo functions, enone moiety, and a 1,3-diketone
A lesser known curcuminoid from turmeric is cyclocurcu-
group. Except for the oxo and hydroxy functions, the C7 chain
min, first isolated and characterized by Kiuchi et al.
is generally unsubstituted. This unsaturation in the linker unit
Structurally, cyclocurcumin differs from curcumin in the b-
Fig. 1 – Natural analogues from turmeric and curcumin metabolites.
Table 1 – Activities of curcumin analogues derived from turmeric and of curcumin metabolites
� BDMC is more active than DMC or curcumin for cytotoxicity against ovarian cancer cells � BDMC is less active than curcumin or DMC as an antioxidant and as an oxidative DNA cleaving agent � BDMC is less active than curcumin or DMC as an inhibitor of peroxynitrite scavenger � BDMC was most active when compared with DMC or curcumin for antimutagenic and anticarcinogenic activity � BDMC is more active than curcumin or DMC for antitumor and antioxidant activity � BDMC is more active than curcumin or DMC for suppression of carcinogenesis � BDMC was more active than curcumin for reducing nicotine-induced oxidative stress � BDMC improved innate immunity and transcription of MGAT-III and Toll-like receptors in AD pts � BDMC is more active than curcumin for modulation of MDR1 gene � BDMC is less active than curcumin or DMC in inhibiting singlet oxygen-induced DNA damage � BDMC is less active than curcumin or DMC in binding and inhibiting Pgp and sensitizing cells to vinblastin � BDMC is less active than curcumin or DMC in binding and inhibiting MRP1 and sensitizing cells to etoposide � BDMC was more active than curcumin or DMC in protecting nerve and endothelial cells from beta amyloid-induced oxidative stress � BDMC prevents DMH induced colon carcinogenesis � BDMC is as active as curcumin in preventing DMH induced colon carcinogenesis � BDMC is more active than curcumin in preventing alcohol and PUFA-induced oxidative stress � BDMC is more active than curcumin in preventing CCL4-induced hepatotoxicity in rats � BDMC is more active than curcumin in preventing alcohol and PUFA-induced cholesterol, TGs, PLs and FFA � BDMC, curcumin, and DMC exhibit equivalent activity in suppression of blood glucose levels in diabetic mice through binding to PPAR-g � BDMC is less active than curcumin and DMC in protecting rats from lead-induced neurotoxicity � BDMC is less active than curcumin and DMC in suppressing NF-kB activation � BDMC is more active than DMC or curcumin in inducing NRF2-mediated induction of heme oxygenase-1 � BDMC is least active than DMC or curcumin in inducing p38 MAPK mediated induction of heme oxygenase-1 � BDMC is least active than DMC or curcumin in inhibiting H2O2-induced lipid peroxidation and hemolysis of eythrocytes � BDMC is least active than DMC or curcumin in inhibiting the proliferation of VSMC induced by ox-LDL and induction of LDL-R � BDMC is least active than DMC or curcumin in inhibiting the liposomal peroxidation; and of COX1 and COX2 activity � DMC is more potent than curcumin, BDMC and cyclocurcumin in inhibiting proliferation of breast cancer cells � DMC is more potent than curcumin and BDMC in inducing nematocidal activity � THC is less potent than curcumin in inhibiting the activity of 5-LOX; but more potent than curcumin in inhibiting COX-dependent
arachidonic acid metabolism
� THC is more active than curcumin in preventing DMH-induced ACF formation in mice � THC does not induces ROS production and membrane mobility coefficient but curcumin does � THC is less active than curcumin in preventing PMA-induced skin tumor promotion in mice � THC is more active than curcumin as an antioxidant THC is less active than curcumin as an antioxidant � THC is less active under aerated condition than curcumin but under N2O purged conditions, THC is more active than curcumin in
suppressing radiation-induced lipid peroxidation
� THCwas less active than curcumin, DMC or BDMC in suppressing NF-kB activation � THC, HHC, OHC are less active than curcumin in suppressing NF-kB activation � THC is more active than curcumin in suppressing nitrilotriacetate-induced oxidative renal damage � THC is more active than curcumin in protecting from chloroquine-induced hepatotoxicity in rats � THC is more active than curcumin in preventing brain lipid peroxidation in diabetic rats � THC is more potent than curcumin for antioxidant and antidiabetic effects in rats � THC is more potent than curcumin for modulation of renal and hepatic functional markers in diabetic rats � THC is more potent than curcumin for modulation of blood glucose, plasma insulin and erythrocyte TBARS in diabetic rats � THC is more potent than curcumin in decreasing blood glucose and increasing plasma insulin in diabetic rats � THC is less potent than curcumin in modulation of ABC drug transporters � THC's effect was comparable with curcumin on reduction of accummulation and cross-linking of collagen in diabetic rats � THC exhibits stronger antioxidant activity than HHC OHC > curcumin > DMC > BDMC � THC was more potent than curcumin in suppressing LDL oxidation � THC is more active than curcumin in suppressing lipid peroxidation of erythrocyte membrane ghosts � Cyclocur exhibits week anticancer activity
Note: BDMC, bisdemethoxycurcumin; COX, cyclooxygenase; DMC, demethoxycurcumin; HHC, hexahydrocurcumin; LDL, low-densitylipoproteins; NF-kB, nuclear factor kappa B; OHC, octahydrocurcumin; ROS, reactive oxygen species; THC, tetrahydrocurcumin.
diketone link. In this molecule, the a,b-unsaturated b-
Curcuma zedoaria, and Curcuma aromatica. Several research
diketone moiety of curcumin is replaced by an a,b-unsatu-
groups have investigated and compared their antioxidant,
rated dihydropyranone moiety. To date, not many biological
cardioprotective, neuroprotective, antidiabetic, antitumor,
studies on cyclocurcumin have been reported; in one study,
and chemopreventive activities, employing them either
Simon et al. reported that this analogue was ineffective in
individually or as mixtures. The curcuminoids have been
inhibiting MCF-7 tumor cell proliferation and arrest of cell
shown to be scavengers of free radicals and reactive oxygen
cycle progression.
species (ROS), such as hydroxyl radicals, superoxide radicals,
In the last few decades, efforts have been made to isolate
singlet oxygen, peroxyl radicals, and peroxynitrite, whose
curcuminoids from different sources, including Curcuma longa,
production is implicated in the induction of oxidative stress
They efficiently neutralized the stable free radical 1,1-
most effective, DMC moderately effective, and BDMC the least
diphenyl-2-picryl-hydrazyl (DPPH), and this reaction is often
effective. Curcumin and DMC, but not BDMC, reduced Pb(II)-
used in comparing the antioxidant activities of different
induced memory deficits in rats. BDMC, on the other hand,
compounds . Although all three are highly reactive in
exhibited potent immunostimulatory effects and was able to
these scavenging reactions, curcumin is more efficient than
correct immune defects of Alzheimer's disease patients by
DMC or BDMC.
enhancing phagocytosis of b-amyloid and regulation of the
Curcuminoids exhibit differential antioxidant activity in
transcription of b-1,4-mannosyl-glycoprotein 4-b-N-acetyl
several in vitro and in vivo models. They inhibited lipid
gluosaminyl transferase and toll-like receptors
peroxidation in a variety of models such as rat brain
Several in vitro and in vivo comparisons of the anti-
homogenates, rat liver microsomeks, erythrocytes, liposomes,
inflammatory and antitumor properties of curcuminoids have
and macrophages, where peroxidation is induced by Fenton
been reported. The activities varied depending on the type of
reagent, as well as metals, H2O2, and 2,20-azo-bis(2-amidino-
tumor and carcinogen employed. Curcumin, DMC, BDMC, and
propane) hydrochloride (AAPH) They pre-
a curcumin mix inhibited proliferation of a wide variety of
vented singlet oxygen-stimulated DNA cleavage in plasmid
tumor cells, including leukemia, lung cancer, head and neck
pBR322 DNA , significantly reduced H2O2- and AAPH-
cancer, pancreatic cancer, breast cancer, and prostate cancer
induced hemolysis of erythrocytes and attenuated H2O2-
Under identical experimental conditions, individual
mediated endothelial cell viability . Curcuminoids were
curcuminoids exhibited similar antiproliferative effects in
able to inhibit cyclo-oxygenase (COX)-1 and (COX)-2 enzymes
all these cell lines . In a separate study, however, DMC was
and reduce AAPH-induced conjugated diene formation
found to be more potent than curcumin or BDMC in inhibiting
during linoleic acid oxidation In most of these actions,
proliferation of MCF-7 breast cancer cells .
BDMC was less active than the other two, and curcumin was
Curcuminoids show antimutagenic and anticarcinogenic
the most potent of the three.
activity. They inhibited the mutagenic activity of 2-acetami-
In a different in vivo study, BDMC was found to be more
dofluorene and prevented crotean oil-induced skin tumor and
effective than curcumin and DMC in increasing the life span of
papilloma formation in mice . They significantly reduced
Swiss albino mice bearing Ehrlich ascites and in reducing lipid
tumor size in Swiss albino mice implanted with solid tumors
peroxidation and superoxide generation in their macrophages
Under identical treatment conditions, BDMC showed
. Interestingly, curcuminoids could also act as pro-
greater antitumor, antipromoter, and anticarcinogenic activ-
oxidants. A report by Ahsan et al. compared pro-oxidant
ities than curcumin or DMC. Similarly, in another study, the
activities of the curcuminoids by measuring their abilities to
cytotoxicity of BDMC against human ovarian cancer cell line
enhance Cu(II)-induced cleavage of plasmid pBR322 DNA
OVCAR-3 was more pronounced than that of curcumin or DMC
through production of ROS. Of the three curcuminoids
Curcumin and DMC had approximately the same potency
examined, curcumin was more effective than DMC and BDMC
in inhibiting 12-O-tetradecanoylphorbol-13-acetate (TPA)-
in inducing DNA cleavage.
induced inflammation of mouse ears as well as TPA-induced
Curcumin, DMC, and BDMC exhibit cardioprotective,
transformation of cultured JB6 (P+) cells, while the activity of
antidiabetic, and nematocidal activities. The three com-
BDMC was less .
pounds inhibited proliferation of bovine vascular smooth
P-glycoprotein (Pgp) is a member of the ATP-dependent
muscle cells stimulated by oxidized low-density lipoproteins
drug efflux protein pump (ABC transporter protein) super-
(LDL) and delayed development of arteriosclerosis Again,
family, linked to multidrug resistance (MDR) in cancer cells.
curcumin was the most efficient cardioprotective agent of the
Curcumin, DMC, and BDMC had the ability to modulate the
three. Turmeric extract containing the three curcuminoids
function of Pgp in multidrug-resistant human cervical
could cause lowering of the blood glucose level in type 2
carcinoma cell line KB-V1. The three curcuminoids were not
diabetic KK-Ay mice, and its hypoglycemic effect improved
effluxed by the Pgp transporter protein. At non-toxic doses,
when administered in combination with sesquiterpenes .
the curcuminoids increased the sensitivity of cells to the
It is the binding of curcuminoids to peroxisome proliferator-
chemotherapeutic agent vinblastine. Of the three, curcumin
ativated receptor-g (PPAR-g) and their acting as PPAR-g
was the most effective in retaining the drug ; it also is an
agonists that are responsible for their hypoglycemic effect.
effective MDR modulator The few and mild side effects
The three curcuminoids individually did not show nemato-
associated with curcuminoids make them attractive alter-
cidal activity against Toxocara canis, but their nematocidal
natives for better MDR modulation. Current research is
activity increased remarkably when they were combined,
investigating how these structurally related curcuminoids
suggesting a synergistic action .
modulate antioxidant, anti-inflammatory, and antiprolifera-
The neuroprotective effects of curcuminoids have been
tive responses, with the principal aim of evaluating their
investigated by various groups. Curcumin, DMC, and BDMC
mechanisms of action.
protected PC12 rat pheochromocytoma and normal human
Curcumin and DMC were more effective than BDMC in
umbilical vein endothelial cells against b-amyloid-induced
inducing p38 MAPK-mediated heme oxygenase-1 (HO-1)
oxidative stress even better than a-tocopherol Curcumi-
expression and activity in human endothelial cells . On
noids have been found to be inhibitors of lead acetate (Pb(II))-
the other hand, another related study reported that BDMC was
induced neurotoxicity in primary hippocampal neurons .
more active than either curcumin or DMC in inducing NRF-2-
They decreased lipid peroxidation, improved neuron viability,
mediated induction of HO-1
and prevented decrease in glutathione levels in rat brain.
A recent study by Sandur et al. reported that curcumin,
Under similar treatment concentrations, curcumin was the
DMC, and BDMC exhibited differential abilities in regulation of
anti-inflammatory and antiproliferative responses and ROS
THC was ineffective in producing intracellular ROS in
generation in chronic myeloid leukemia cell line KBM-5. Their
human gingival fibroblasts, human submandibular gland
relative potencies for suppression of tumor necrosis factor
carcinoma cells , and KBM-5 cells . THC is less potent
(TNF)-mediated nuclear factor-kB (NF-kB) activation are
than curcumin in modulating ABC drug transporters . It
curcumin > DMC > BDMC. Under similar experimental con-
failed to inhibit TNF-induced NF-kB activation in KBM-5 and
ditions, a mixture of curcuminoids showed better activity than
RAW cells . THC is less active than the curcuminoids in
any of the individual curcuminoids. However, the ROS-
preventing TPA-induced tumor promotion in mouse skin and
generating ability of curcuminoids in the same cells did not
inflammation of mouse ears and less active than curcumin in
correlate with either anti-inflammatory or antioxidant activ-
preventing phorbol 12-myristate 13-acetate (PMA)-induced
ity, and BDMC generated the highest quantities of ROS.
skin tumor promotion in mice On the other hand, THC
Curcumin and DMC induced glutathione level to a similar
was as effective as curcumin in inhibiting the release of
extent, whereas BDMC was the least effective in inducing
arachidionic acid and its metabolites, formation of prosta-
glutathione, indicating that the anti-inflammatory and anti-
glandin E2, and lipopolysaccharide (LPS)-induced COX-2
proliferative activities of curcuminoids are independent of
expression in RAW cells . THC exhibited chemopreventive
their redox-modulatory property.
activity by inhibiting 1,3-dimethylhydrazine-induced putativepreneoplastic aberrant crypt foci development in colons of
Curcumin metabolites
Various metabolites of curcumin have been reported, includ-ing dihydrocurcumin (DHC), tetrahydrocurcumin (THC), hex-
ahydrocurcumin (HHC), octahydrocurcumin (OHC), curcumin
Although curcumin, DMC, and BDMC differ in their chemical
glucuronide, and curcumin sulfate (see ). THC, a partially
structures only with regard to methoxy substitution, they
reduced derivative of curcumin not found in turmeric, is one of
exhibit significantly different antioxidant, antitumor, and
the major metabolites of curcumin. Other reduced forms of
anti-inflammatory activities. To date there has been no
curcumin, HHC and OHC, have also been considered curcumin
systematic study that clearly correlates the physicochemical
metabolites, but have not been examined as extensively as
and molecular properties of the three curcuminoids with their
THC. THC is obtained by partial hydrogenation of curcumin; it
biological activities. However, the existing literature provides
is colorless and more hydrophilic than curcumin. THC exhibits
some clues to understanding which group is actually
greater antioxidant potential than curcumin in most models
responsible for a given biological activity of the curcuminoids.
and presently is considered to be one of the factors responsible
Since many reports suggest that curcumin has better
for the in vivo antioxidant activity of curcumin (see
radical scavenging and antioxidant ability than the other two,
THC scavenged several free radicals, such as t-butoxyl
and that DMC is superior to BDMC in this activity, the o-
radicals, peroxyl radicals, and DPPH radical, better than the
methoxy substitutions are certainly involved in this activity.
curcuminoids and was more effective in inhibiting AAPH-
The hydrogen bonding interaction between the phenolic OH
induced red blood cell hemolysis and lipid peroxidation in
and the o-methoxy groups in curcumin markedly influences
rabbit erythrocyte membrane ghosts and rat liver microsomes
the O–H bond energy and H-atom abstraction by free radicals,
The relative activities of THC and curcumin in
thus making it a better free radical scavenger than BDMC
inhibiting gamma radiation-induced lipid peroxidation in
The ability of curcuminoids to act as antioxidants or pro-
rat liver microsomes varied depending on the level of oxygen
oxidants in the presence of metals such as Cu(II), Fe(II), or Pb(II)
present THC is useful as a functional food factor because
arises mainly from their chelating power . Although
of its cardioprotective ability, which is even greater than that
transition metal-chelation by curcumin can take place
of curcumin . It inhibited oxidative modification of LDL and
through either the diketone moiety or the o-methoxy phenol
showed protective effects against oxidative stress in choles-
moiety, in most cases chelation is observed only through the
terol-fed rats The ability of THC to suppress nitrolotria-
diketo group. Since the three curcuminoids possess similar
cetate-induced oxidative renal damage was greater than that
diketone moieties, their effects on metal-induced toxicity
should be similar. The o-methoxy group may influence the
Administration of THC to mice at an oral dose of 80 mg/kg
electron density on the diketo group, however, which in turn
body weight for nearly 15 days reduced hepatotoxicity induced
can affect their chelating ability.
by the commonly used antibiotic erythromycin estolate and
The a,b-unsaturated diketone moiety in the curcuminoids
the antimalarial drug chloroquine At the same dose for
is a Michael reaction acceptor, which belongs to the major
nearly 45 days, THC showed an antihyperlipidemic effect in
class of phase-II enzyme inducers Therefore, this
streptozotocin–nicontinamide-induced oxidative stress in
property may be responsible for inducing HO-1 and NF-kB
diabetic rats The membrane-bound antioxidant
suppression in cells by curcuminoids. Methoxy substitution
enzymes, which were decreased in these mice, increased
on the aromatic ring can significantly influence the interac-
significantly on THC treatment. Oral administration of THC
tions of curcuminoids with nucleophiles in the Michael
also prevented changes in the levels of fatty acids, glucose,
reaction. The reasons and the actual mechanism of the
and insulin in the blood of diabetic rats . These studies
antitumor activities of the curcuminoids are still far from
reported that THC significantly decreased lipid peroxidation in
understood. It is still not known why the o-methoxy-deficient
different tissues of these rats. All these studies confirmed that
BDMC is a more potent ROS inducer and the o-methoxy-
THC, when compared with similar treatment doses of
substituted curcumin is a more potent suppressor of NF-kB
curcumin, had much greater antidiabetic effects.
activation The effect of change in the lipophilicity of the
curcuminoids with methoxy substitution in influencing some
the curcumin molecule, and a large number of synthetic
of these activities also cannot be ignored.
analogues are known. The curcumin molecule is unique in its
Hydrogenation of the heptadiene moiety in curcumin to
physiological effects, however, having a greater number of
produce THC markedly increased the antioxidant activity but
molecular targets than any other molecule so far reported. In
significantly reduced the antitumor and anti-inflammatory
order to define a drug profile of this ‘‘wonder'' molecule, it is
abilities. It is clear that the o-methoxy phenol groups, when
necessary that, along with its synthetic analogues, its
not linked through conjugation with the b-diketone moiety,
naturally occurring analogues should be analyzed exhaus-
make the molecule a better antioxidant. This lack of
tively. shows a number of naturally occurring bioactive
conjugation in THC also can cause C–C bond cleavage at the
compounds having some structural similarity to the curcumin
active methylene carbon of the b-diketone group during
molecule, or at least having a pharmacophore containing one
oxidation, yielding smaller o-methoxy phenol derivatives that
aryl function with 3,4 substitution, i.e., either a methoxylated
also act as antioxidants . Lack of NF-kB activity and ROS-
phenol or catechol. These include ferulic acid, cinnamic acid,
generating ability in THC clearly confirms that the a,b-
caffeic acid, chlorogenic acid, capsaicin, gingerol, paradol
unsaturated b-diketone moiety in conjugation with the
zingerone, eugenol, dibenzoylmethane, dehydrozingerone,
aromatic rings is definitely involved in these activities.
cassumuin and yakuchinone.
Although no comparative studies on the antioxidant
Natural analogues made by Mother Nature
potential of different naturally occurring analogues of curcu-min are available, a look at and indicates that an
Structural variations in any lead compound are important for
ortho-methoxylated phenolic chromophore is desirable
its physiological activity, especially if these affect its receptor-
, which may be present in a single aromatic ring (e.g., ferulic
binding interactions. Structural variations also alter its
acid, caffeic acid, chlorogenic acid, capsaicin, gingerols,
pharmacokinetics, i.e., how easily the drug is absorbed,
zingerone, eugenols) or in two aromatic rings (e.g., oregonin,
distributed, metabolized, and excreted. Extensive structure-
the potent nitric oxide synthase (iNOS) inhibitor, dehydro-
activity relationship studies have been carried out on
guairetic acid, yakuchinones, cassumunins). The same chro-
Fig. 2 – Curcumin analogues from Mother Nature.
Table 2 – Relative potency of curcumin and its analogues made by Mother Nature
� Caffeic acid and ferulic acid but not cinnamic acid are more potent than curcumin in inhibiting lipid peroxidation � Caffeic acid, ferulic acid, and chlorogenic acid are less potent than curcumin in inhibiting TPA-induced inflammation and promotion of
� Dibenzoylmethane is several times more potent (10-fold) than curcumin in inducing phase II enzymes, in inhibiting DMBA-induced
mammary tumors in rodents and in inhibiting TPA-induced skin inflammation and tumor promotion
� 6-gingerol is more potent (107-fold) mutagen than curcumin whereas less potent in inhibiting TPA-induced inflammation, epidermal
ornithine decarboxylase activity, and skin tumor promotion in mice
� Capsaicin is more potent than curcumin in lowering acidic glycoprotein and inflammation in arthritic rats � Capsaicin and curcumin are more potent (1000-fold) than eugenol in inhibiting superoxide radical generation � Capsaicin and curcumin are equally potent in inhibiting arachidonic acid metabolism � Dehydrozingerone is less active than curcumin in inhibiting formation of conjugated dienes and spontaneous lipid peroxidation � Dehydrozingerone is as active as curcumin but less active than isoeugenol in inhibiting Epstein–Barr virus antigen early antigen activation � Yakuchinone A and B are as potent as curcumin in inhibiting LPS-induced nitric oxide production, TPA-induced superoxide production
and lipid peroxidation
� Cassumunins A and B are more active than curcumin in protecting thymocytes from H2O2-induced toxicity
Note: DMBA: 7,12-dimethylbenz[a]anthracene; H2O2, hydrogen peroxide; LPS, lipopolysachharide;TPA, 12-O-tetradecanoylphorbol-13-acetate.
mophore is responsible for both the antioxidant and pro-
tural alteration on curcumin are shown in Scheme 1.
oxidant properties of curcumin and its analogues, which may
Alterations of structure at all these molecular architectural
be due to its radical-generating or hydrogen bond donor/
sites have been attempted. The modification of the basic
structure of curcumin to access related compounds bychemical synthesis may be classified into three broad groups.
Synthetic analogues made by man
These are termed ‘‘curcumin derivatives,'' ‘‘curcumin analo-gues,'' and ‘‘metal complexes of curcumin'' in this review.
Curcumin and its analogues have been the subject of
Compounds that retain the basic structural features of
computational studies, mostly with the intention of unravel-
curcumin, such as the two dioxy-substituted benzene rings,
ing its unique structural features and exploiting the informa-
the –C C–CO–CH2–CO–C C-linker, and the oxy substituents
tion for further molecular design. depicts the
on the benzene rings, are designated as curcumin derivatives.
representative members of synthetic curcumin analogues
The second group, the curcumin analogues, which encompass
and summarizes the relative bioactivities of synthetic
all other compounds with some perceived or claimed
curcumin analogues. Recent high-level, ab initio, and compu-
structural analogy to curcumin, now vastly outnumber the
tationally intensive calculations have shown that the opti-
first group. The members of the third group are metal
mized structure of curcumin is planar and linear The
complexes of curcumin and its analogues.
enol form has been found to be the stable ground state, and in
The curcumin derivatives are generally synthesized by
the optimized structure the methoxy groups are seen pointing
derivatization, starting from curcumin. For example, the
in the opposite direction with respect to the 1,3-keto-enol
phenolic hydroxy group may be acylated, alkylated, glycosy-
group, as shown in Scheme 1 This study showed that
lated, and amino acylated (Scheme 2,
the phenolic and enolic groups provide areas of high polarity
The methoxy groups may be demethylated to hydroxy groups
and the C7 bridge region is quite hydrophobic. Suggestions
. The reactive methylene group of the linker may be
based upon computational chemistry regarding redesign of
acylated or alkylated or substituted by an arylidene group (Ar-
curcumin to enhance its bioactivities have appeared in the
CH ) , thereby introducing susbtituents on the C7 chain.
literature . In several recent studies that involve compu-
A battery of molecular tinkering has been applied to
tations of energy-minimized structures and subsequent
curcumin with a view to preparing analogues. The more
docking studies, only the b-diketo form has been investigated,
common strategies are indicated in Scheme 3 (C). The so-
despite the fact that curcumin exists mostly in the enol form.
called analogues of curcumin vary on a wide scale in their
The single crystal X-ray diffraction studies on curcumin
structural resemblance to curcumin, spanning a spectrum
and its derivatives reported by several groups indicate the
from structures such as (ferrocenyl-CH CH–CO)2 CH2 to
enol form as the preferred tautomer. The crystal structure
methyl ferulate.
studies show that curcumin in solid state has a perfectly
The hydrogenation of the C7 linker double bonds and the
delocalized central keto-enol unit coplanar with one trans-Ar-
carbonyl groups affords the simplest of the analogues, such as
CH CH-moiety. The plane of the second trans-CH CH-unit
DHC, THC, HHC, and OHC, which are obtained by the reduction
is twisted about 178 with respect to the former, planar,
of curcumin (Scheme 4, D) .
Ar-CH CH-unit. This second unit is also not coplanar with
Analogues that are sourced from curcumin also include
its attached aryl unit. Thus the computationally derived
those obtained by exploiting the reactivity of the central b-
structure differs somewhat with that seen in the solid state
diketone unit with hydrazine, its substituted derivatives, and
hydroxylamine. Such heterocyclizations lead to bisstyrylpyr-
The characteristic structural features of curcumin include
azoles and isoxazoles in which the central 1,3-diketone ? 1,3-
two o-methoxy phenol units, two enone moieties, and a 1,3-
keto-enol system has been masked and rigidized (Scheme 5,
diketone Ð 1,3-keto-enol system. The possibilities for struc-
More recently, monosemicarbazone
Table 3 – Relative activities of man-made curcumin analogues
� Diacetyl, diglycinoyl, diglycinoyl-di-piperoyl, dipiperoyl, and dialanoyl derivatives and curcumin-4,40-di-O-b-D glucopyranoside have
more potent antibacterial and antifungal activities than curcumin
� Pyrazole analogues and a curcumin Knoevenagel condensate have more potent antimalarial, antioxidant and COX-1- and COX-2-
inhibitory activities than curcumin
� Hydrazinocurcumin is a more potent inhibitor of endothelial cell proliferation than curcumin and it inhibits the cell cycle progression
of colon cancer cells via antagonism of Ca2/CaM functions
� Semicarbazone of curcumin has greater antioxidant and antiproliferative activities but less antiradical activity than curcumin � Compounds with ortho-diphenoxyl functionality exhibit greater antioxidant activity than curcumin � Cinnamoyl derivatives are more active than curcumin in inhibiting p300 enzyme � Symmetrical curcuminoids BJC005 and CHC002 have greater potency than curcumin in inhibiting Fos-Jun, tumor-induced angiogenesis,
migration, and invasion
� Synthetic analogues with a modified aromatic ring and/or modified enone/dienone bridge between rings have more potent antiangiogenic
and COX-1 inhibiting activity than curcumin
� Curcumin analogues that retain the 7-carbon spacer between the aryl rings, with a 5-carbon spacer and with a 3-carbon spacer, are
more active than curcumin in inhibiting TPA-induced AP-1 and TNF-induced NF-kB activation and are more active antioxidantsthan curcumin
� Cyclic curcumin analogues have more potent cytostatic, antitumor and radical-scavenging activities than curcumin � Synthesized EF24 and other related compounds have greater anticancer and antiangiogenic activities than curcumin � Fused pyridine analogues of curcumin have more potent antioxidant activity than curcumin � 2,6-dibenzylidenecyclohexanone, 2,5-dibenzylidenecyclopentanone, and 1,4-pentadiene-3-one substituted analogues of curcumin
have more potent human cytochrome P450-inhibitory activity than curcumin
� Cinnamoyl derivatives of curcumin are more potent than curcumin in inhibiting HIV-1 integrase � Mono-carbonyl analogues have the same or greater anti-inflammatory and antibacterial activity than curcumin � Symmetrical analogues with aromatic rings having an alkoxy substitution are more potent in suppressing tumor growth than curcumin � Aromatic enonic analogues are as or more potent than curcumin in inhibiting cell growth and proliferation � Synthetic analogues with asymmetrical units such as a phenyl group with alkyl amide, chloro-substituted benzamide, or heteroaromatic
amide moieties are more potent inhibitors of growth and tube formation than curcumin
� Symmetrical bis-alkynyl or alkyl pyridine and thiophene derivatives have more potent antiangiogenic activities than curcumin � Curcumin–boron complexes are more potent than curcumin in inhibiting HIV-l and HIV-2 proteases � Synthetic copper(II)-curcumin complexes have greater SOD mimicking, radiation-induced lipid peroxidation, and radical-scavenging
activities than curcumin
� Manganese complexes of curcumin and diacetylcurcumin are more potent in preventing excitotoxicity and kainic acid-induced nitric
oxide levels and neuronal cell damage in rats and are more potent nitric oxide radical scavengers and neuroprotectors than curcumin
� Copper(II) conjugate of a synthetic analogue with non-enolizable diketone is more potent than curcumin in inhibiting TNF-induced
NF-kB activation and proliferation
� Cyclopalladated complexes of curcumin have more potent antiproliferative effects than curcumin � Vanadium complex of curcumin has antidiabetic and hypolipidemic effects and improves the cardiovascular complications associated
� Vanadium, gallium, and indium complexes of curcumin and its derivatives have more potent cytotoxic activity than curcumin � Curcumin derivatives with a modified aromatic ring and a cyclohexanone bridge between rings are more potent than curcumin in
increasing mitochondrial membrane permeability
� Glycosylated derivatives of ciurcumin have more potent water-solubility and iron-chelating properties than curcumin � BDMC-A is more active than curcumin in suppressing nicotine, alcohol and polyunsaturated fatty acid-induced oxidative stress,
CCl4-induced hepatotoxicity and alcohol- and polyunsaturated fatty acid hyperlipidemia in rats
Note: AP-1, activator protein-1; BDMC, bisdemethoxycurcumin;; BJC005, 1,7-bis(4-hydroxy-5-methoxy-3-nitrophenyl)-1,6-heptadiene-3,5-dione; Ca2/CaM, calcium 2+/calmodulin; CHC002, 1,7-bis(3,4,5-trimethoxyphenyl)-1,6-heptadiene-3,5-dione; COX, cyclooxygenase; EF24, 2,6-bis(2-fluorobenzylidene)piperidone; HIV, human immunodeficiency virus; NF-kB, nuclear factor kappa B; ROS, reactive oxygen species; SOD,superoxide dismutase; TNF, tumor necrosis.
, bisthiosemicarbazone and an ethylene diamine
the C7 linker moiety (Scheme 7,
adduct of curcumin have also appeared in the literature.
Most of the analogues of curcumin are not obtained from
A further elaboration of this approach involves the use of b-
curcumin but rather have been synthesized from smaller
diketones other than acetylacetone derivatives. For example,
synthons. Curcumins are usually assembled from aralde-
the use of 2-acetylcycloalkanones has afforded analogues that
hydes and acetylacetone, and this route enables synthesis of
are conformation restricted. The C7 linker unit in these
a diverse set of curcumin analogues starting from aralde-
analogues now bears a cyclic structure (Scheme 8,
hydes; a few typical examples are shown in Scheme 6 (F).
This assembly of curcuminoids from araldehydes and
Yet another strategy has been alteration of the number of the
acetylacetone has produced a large number of analogues.
carbons in the middle linker chain, resulting in analogues that
The use of acetylacetone derivatives bearing substituents on
are further removed from the native curcumin structure.
the central carbon further extends this route, leading to
Reports show that deletion of one or both of the C C bonds
analogues with alkyl substituents on the middle carbon of
in the parent structure, omission of one C C and C O group
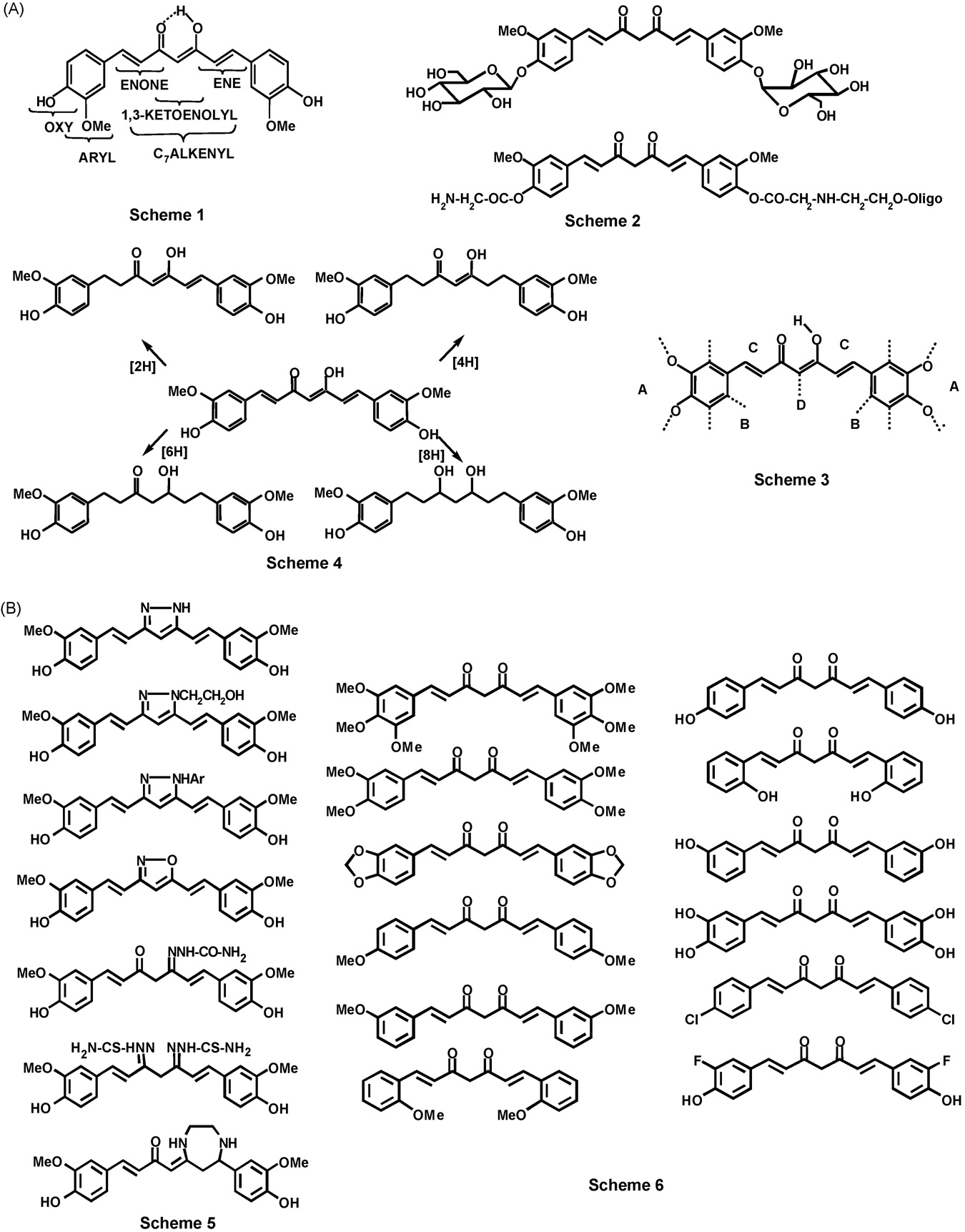
Fig. 3 – Curcumin analogues made by man. (A) Scheme 1: possible sites for structural modifications on curcumin; (B) Scheme2: curcumin derivatives; (C) Scheme 3: strategies for curcumin analogue preparation. (A) Modify –OMe and –OH groups;remove oxy groups; replace oxy groups. (B) Introduce/remove atoms/groups on aromatic rings; replace aromatic ring byhetero aromatic rings; or by multirings. (C) Alter number of –C C– and C O; incorporate –C C– in cyclic structure. (D)Replace 1,3-diketone by ketone; alter number of enone units; mask 1,3-diketone; convert 1,3-diketone to cyclic structures
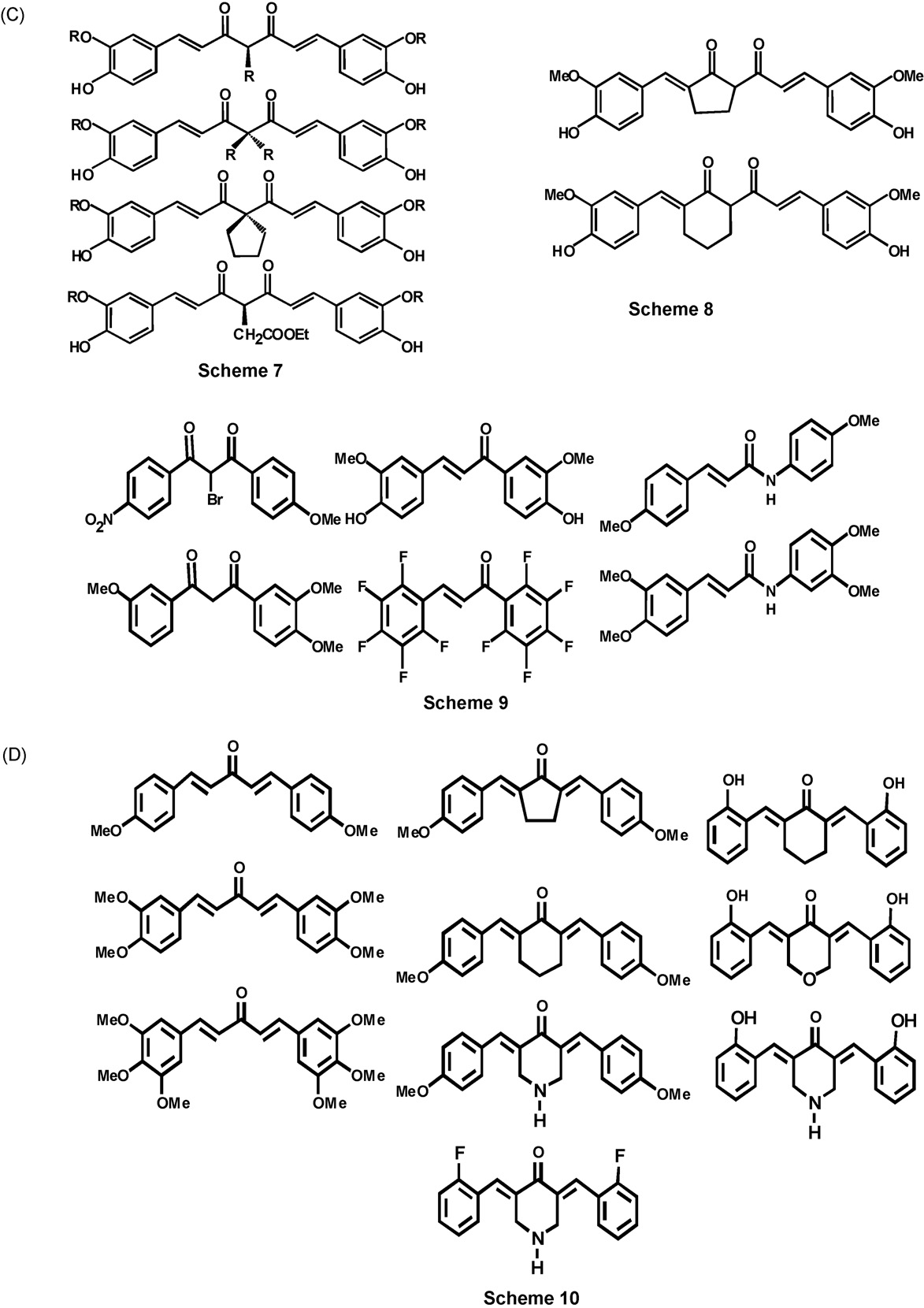
Fig. 3. (Continued ).
like pyrazole or isoxazole. (D) Scheme 4: analogues synthesized by reduction of curcumin; (E) Scheme 5: analoguessynthesized by masking the central 3-diketone unit; (F) Scheme 6: typical examples of analogues from araldehydes; (G)Scheme 7: Typical examples of analogues from substituted acetylacetones; (H) Scheme 8: conformationally restrictedanalogues; (I) Scheme 9: C3 bridged analogues; (J) Scheme 10: C5 bridged analogues; (K) Scheme 11: C7, C9, C11, and longerbridged analogues. (L) Scheme 12: exotic analogues.
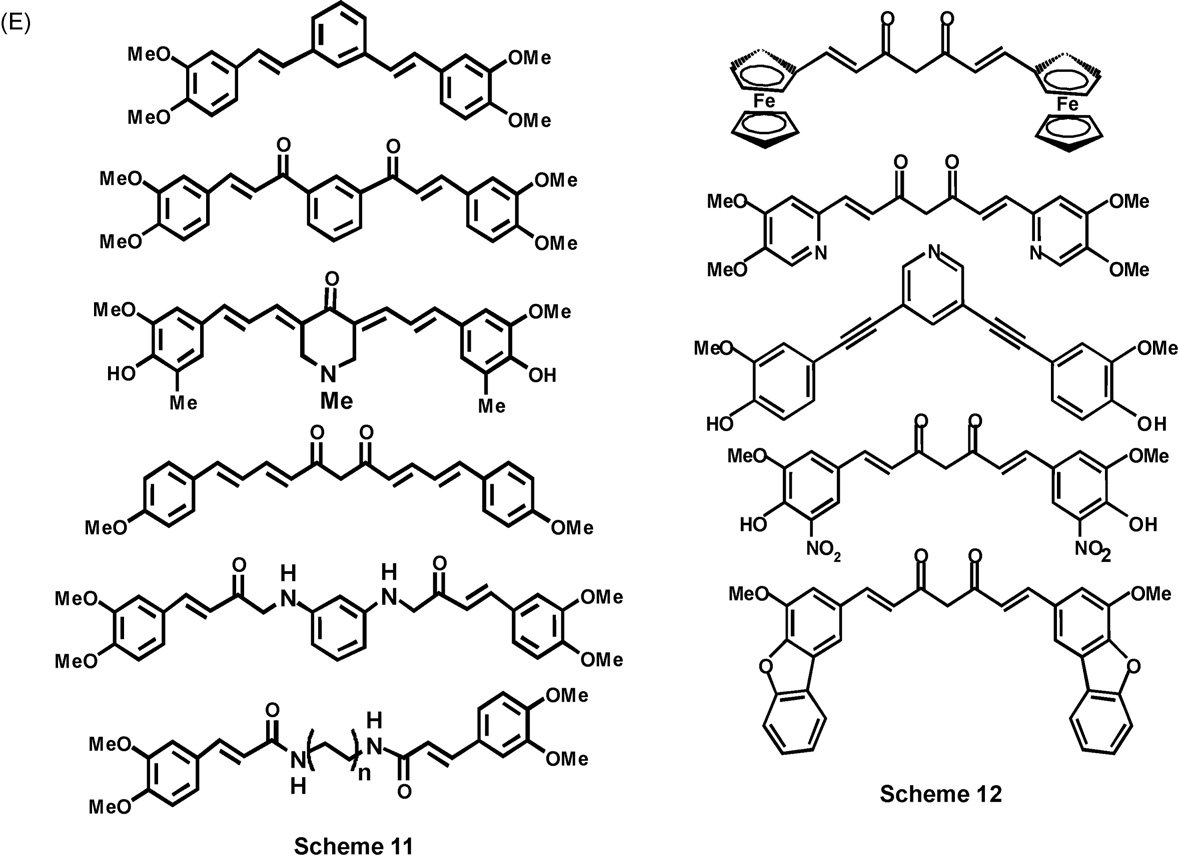
Fig. 3. (Continued ).
each (Scheme 9, I), avoidance of the –CH2–CO-unit (Scheme
Antioxidant activity
10, J), or addition of two more C C bonds (Scheme 11,
The antioxidant activities of curcumin and related compounds
all have been attempted, leading to C3, C5, C9, C11 or
have been investigated by a variety of assay systems, in both in
longer linkers in addition to the natural C7 linker unit. A few
vitro and in vivo conditions. The disparity in assay conditions
randomly selected, nonprioritized, representative structures
makes exact comparisons rather difficult. The general trends
are shown in , as the total numbers of such analogues now
that emerge are discussed in this section.
synthesized are too many to depict conveniently
In one of the early papers on the antioxidant activity of
curcumin and its derivatives, Sharma observed that the
Incorporation of the shortened linker unit carbons in carbo-
phenolic hydroxyl groups are needed for antioxidant activity
cyclic rings has been attempted
and that the presence of more than one of these groups,
Analogues with only one-half of the basic curcumin
as in the curcumin derivative bis(3,4-dihydroxycinnamoyl)-
skeleton embedded in the structure also have been synthe-
methane, confers better activity than that of curcumin itself
sized. These include esters and amides of ferulic acid and
. The mechanistic aspects of curcumin antioxidant activity
other similar cinnamic acids Further structural
have been more recently investigated at length, and the recent
alterations based on exotic modifications and more drastic
studies by Wright Sun et al. , Priyadarsini et al.
molecular surgery of curcumin appear in the literature
Ligeret et al. , Suzuki et al. , and Chen et al. seem
to suggest that the phenolic OH groups are important in the
Several metal complexes of curcumin, derivatives of
antioxidant activity, as was earlier surmised by Barclay et al.
curcumin, and analogues of curcumin have been reported.
and Venkatesan and Rao A possible role for the b-
These have generally been obtained by the reaction of
diketone moiety was suggested by Sugiyama et al. based
curcumin or one of its analogues with a metal salt. Boron
on their observations using dimethyltetrahydrocurcumin and
has long been known to form a complex with curcumin .
further advocated by the work of Jovanovic et al.
The complex resulting from combination of a molecule of
The presence of an ortho alkoxy group seems to potentiate
curcumin, oxalic acid, and a boron atom, sourced from boric
the antioxidant activity as does an additional hydroxy
oxide or acid, is known as rubrocurcumin. The complexation
group as in bis(3,4-dihydroxy)cinnamoylmethane . The
of two curcumin molecules with a boron atom affords
effect of the position of the hydroxy group has been investigated
rosocyanin. Complexes of copper , iron, manga-
under in vivo conditions and it seems that the 2-
nese , palladium vanadyl gallium, and
hydroxyphenyl group, as seen in bis(2-hydroxycinnmoyl)-
indium have been reported.
methane, yields better antioxidant activity than the 4-hydro-
xyphenyl group, as present in curcumin. The reduction of the
nyl]benzoate dimethyl ester, were more potent COX-1
C C bonds of the C7 linker leading to THC is apparently not
inhibitors than curcumin. Even the presence of the b-diketone
deleterious to antioxidant activity . Telomere repeat
moiety per se was not a must; its replacement by a pyrazole or
amplification protocol assays have shown that, though phe-
isoxazole unit did not abolish the COX-inhibitory activity of
nolic hydroxy groups are desirable, the enone and b-diketone
curcumin. Further, the pyrazole replacement provides better
moieties are not unavoidable . The desirability of the b-
COX-1/COX-2 selectivity The architectural change of the
diketo unit has been studied by Sardijiman et al. using
‘‘ene-[1,3-dioxo]-ene'' C7 linker in curcumin to a C5 ‘‘ene-oxo-
ene,'' as in 1,4-pentadiene-3-ones and their cyclopenta- and
hexanones, and cyclopentanones having a C5 linker. These
cyclohexa-analogues, has been reported to improve the inhibi-
workers report that the 4-hydroxyphenyl group confers potent
tion of LPS-induced TNF-a and interleukin-6 expression .
antioxidant activity, which is much enhanced by one, or two,methoxy susbstituents ortho to the hydroxy group. These C5-
Anticancer and anticarcinogenic activity
The anticarcinogenic properties of classical Michael acceptors,
greater antioxidant activity than curcumin. In a similar
recognized by Talalay et al. , have been demonstrated in
observation among 2,6-bis-benzylidenepiperidones, cyclohep-
curcumin and it has been suggested that the presence of a
tanones and acetones, Youssef et al. demonstrated greater
hydroxyphenyl group in compounds analogous to curcumin,
antioxidant activity in those examples that bear a 3-alkoxy-4-
especially in the 2-position, is supportive of the chemopro-
hydroxyphenyl unit The enhancement of antioxidant
tective activity through the ability to induce Phase II
activity offered by additional hydroxy substituents on the
detoxification enzymes. The necessity of the ‘‘ene-[1,3-
phenyl rings of curcumin-type compounds has been further
dioxo]-ene'' C7 linker, however, could not be firmly estab-
demonstrated by Venkateswarlu et al.
lished; Dinkova-Kostova et al. observed activity in dibenzoyl
The antioxidant potential of curcumin complexes has been
and di(2-hydroxybenzoyl)methanes, which are not examples
investigated by another approach. The manganese complexes
of classic Michael acceptors. An early report by Markaverich
of curcumin and its diacetyl derivative were found to show
et al. suggests that the Michael acceptor type 2,6-bis(3,4-
greater superoxide dismutase (SOD) activity HO radical-
scavenging activity and nitric oxide radical-scavenging
nones, having only a ‘‘ene-oxo-ene'' motif, could inhibit
activity than the parent molecules. The copper complex
cancer cell proliferation in vitro and in vivo. Dinkova-Kostova
of curcumin also has been found to exhibit antioxidant,
et al. investigated a large set of Michael acceptors and
superoxide-scavenging, and SOD enzyme-mimicking activ-
concluded that the shortened C5 ‘‘ene-oxo-ene'' version, as
ities superior to those of curcumin itself . In an
present in 2,6-bis(2 hydroxybenzylidene)cyclopentanone as a
investigation based on the trolox-equivalent antioxidant
typical example, is sufficient to confer potent quinone
capacity assay, Mohammadi et al. found that the vandyl,
reductase inducer activity, and the presence of a 2-hydro-
indium, and gallium complexes of curcumin I and curcumin III
xyphenyl unit in the bisbenzylidenealkanones and biscy-
were more potent than the respective ligands. In summary,
cloalkanones profoundly increases inducer potency. In a study
antioxidant activity seems to require, minimally, two hydro-
of the inhibition of formation of the Fos-Jun-DNA complex, the
xyphenyl units connected together through a linker unit, and
presence of a 4-hydroxyphenyl, flanked by an adjacent
the activity increases with additional oxy groups, especially if
methoxy or nitro group on the phenyl ring in curcumin
these are adjacent to one another. Whether the linker unit
analogues, conferred better potency . Interestingly, the 4-
should contain an unsaturation and/or an oxo group has not
nitrophenyl analogue also was active. It is tempting to
been conclusively established yet.
speculate that the ability of the phenyl ring substituent toaccept hydrogen bonds, either intramolecularly or intermo-
lecularly, is a structural factor possibly leading to bioactivity.
Saturation of the alkene and reduction of the carbonyl
In a study encompassing a large collection of curcumin
functions in the C7 linker of curcumin appear to reduce its
analogues of diverse structural types, Ishida et al.
anti-inflammatory activity by suppressing activation of NF-kB
observed that diarylheptanoids of curcumin type with 3,4-
through inhibition of IkB kinase activity . An early study
dihydroxyphenyl, 3,4-dimethoxyphenyl, 2-fluorophenyl, and
pointed to the fact that the hydroxyphenyl unit in curcumin
the pyrazole analogue of curcumin-I were cytotoxic, whereas
confers anti-inflammatory activity since acylation and alkyla-
the reduced curcumin types were inactive. These workers also
tion of the phenolic hydroxy group of curcumin were found to
examined a panel of 1,3-diarylpropan-1,3-diones that are
drastically reduce its anti-inflammatory activity Nurfina
examples of the C3 linker type, and the most active compound
et al. suggested that the presence of a 4-hydroxyphenyl unit is
happens to be a –CO–CHBr–CO– derivative whose structure, by
required for anti-inflammatory activity and that this activity
virtue of the very reactive bromo substituent, is quite remote
seems to increase if additional small-sized alkyl or methoxy
from that of curcumin. Other work done in the same
groups are present on the adjacent 3- and 5-positions on the
laboratories showed that bis(3,4-dimethoxyphenyl) units
phenyl ring Hong et al. found that the phenolic
and the ‘‘ene-[1,3-dioxo]-ene'' segment in curcumin analogues
hydroxyl groups are required for inhibition of COX-1 activity.
are important structural factors that confer antiandrogenic
However, Handler et al. recently observed that many
activity, with possible application in prostate cancer therapy
analogues of curcumin that lack a 4-hydroxyphenyl unit, such
The observation of Shim et al. that the so-called
hydrazinocurcumin analogues, which are formulated more
correctly as 3,5-bisstyrylpyrazoles, are more antiangiogenic
than curcumin also seems to point to the importance of the
seems to be desirable. The recent report by Ohori et al.
1,3-diketo unit or its masked version as a pyrazole or isoxazole
seems to support this very general surmise. The presence of a
moiety. Extension of this work to more curcumin analogues
halo substituent such as F does not provide much enhance-
has been reported by Ohtsu et al. who found that the
ment, the case of EF24 being a very successful exception.
presence of a methoxyphenyl or fluorophenyl and introduc-tion of a CH2CH2COOEt group into the 1,3-diketo unit affords anovel set of curcuminoid-type antiandrogens. More recently,
Dutta et al. showed that the monosemicarbazone ofcurcumin has greater cytotoxic activity than curcumin itself.
Apart from the synthetic analogues, several other strategies
In one of the more significant findings on the anticancer
have been evaluated to enhance the biological activity of
activity of compounds inspired by curcumin, Adams et al.
curcumin. These strategies include adjuvants, nanoparticles,
announced the superior activity of 2,6-bis(2-fluor-
liposomes, micelles, and phospholipid complexes. The adju-
obenzylidene)piperidone (EF24) in antiangiogenesis, cell cycle
vants were selected on the basis of their ability to prevent the
arrest, and apoptosis of cancer cells. These authors observed
rapid metabolism of curcumin by interfering with the
that the bis-benzylidenepiperidone, pyrone, and cyclohex-
enzymes that catalyze the metabolism of curcumin. All other
anone derivatives, containing the a,b-unsaturated ketone
formulations mentioned are designed primarily to increase
unit, exhibit much greater anticancer and antiangiogenesis
absorption of curcumin into tissues. Nanoparticles can
activities than curcumin, with its 1, 3-diketone unit. They also
provide more penetration to membrane barriers because of
observed that hydroxyl susbtituent in position 2 generally
their small size. Besides their size, their potential for
confers good activity, and concluded that incorporation of the
modification for targeting specific organs makes them
a,b-unsaturated keto group into a heteroatom-containing ring
excellent drug carriers. Liposomes, micelles, and phospholipid
was desirable. The improved cytotoxicity of bis-(3-alkoxy-4-
complexes can reduce the hydrophobicity of curcumin; these
hydroxybenzylidene) piperidones has been reported by Yous-
carriers also can increase the permeability of membrane
sef and El-Sherbeny . In this connection, it is notable that
barriers by interacting with the membrane components.
the increased cytotoxicity provided by more than one
Recently it was also reported that the water solubility of
hydroxyl substituent on the phenyl ring of curcuminoids is
curcumin could be 12-fold by the use of heat .
further exemplified by the analogues reported by Venkates-warlu et al.
The question of the essentiality of the b-keto unit in the
bioactivity of curcuminoids has been addressed recently by
Piperine is known to inhibit hepatic and intestinal glucur-
Lin et al. Their work seems to suggest that the enol-
onidation. When combined with piperine, the elimination
keto moiety is responsible for the antiandrogenic activity and
half-life and clearance of curcumin were significantly
that the di-keto form probably is not an active form. In an
decreased, resulting in an increase of bioavailability to 154%
ambitious study, Weber et al. investigated the inhibition of
that of curcumin alone in rats. In contrast, the increase in
TNF-a-induced activation of NF-kB by a large collection of
bioavailability was 2000% in humans, clearly showing that the
curcumin analogues, including those with C7, C5, or C3 linkers
effect of piperine on bioavailability of curcumin is much
between the aromatic rings. They observed that activity did
greater in humans than in rats. A human volunteer trial
not depend on linker length, except that compounds with the
conducted by our group revealed the enhancing effect of
a,b-unsaturated keto unit were more generally active, 1,5-
piperine on serum curcumin level. Six healthy adult male
bis(3-pyridyl)-1,4-pentadien-3-one being the most active
human volunteers took 2 g of curcumin with or without 5 mg
among the 72 compounds tested. Those without the enone
piperine (as Bioperine1) in this cross-over design study. Three
unit also exhibited activity, however, and the inhibitory
subjects were randomized to receive curcumin only, while the
activity of the activation of NF-kB did not correlate with the
remaining three received the curcumin + piperine combina-
antioxidant activity of the compounds tested. Many of the
tion. One week following initial drug administration, volun-
active compounds bore hydroxyl and/or methoxyphenyl
teers were crossed over to the other therapy and blood
groups, including the simple 4-hydroxy-3-methoxybenzala-
samples were obtained for evaluation. The presence of
ceophenone. Extending their search for a compound with better
piperine was found to double the absorption of curcumin
antiandrogen activity, Lin et al. examined a set of 50
The effect of piperine on tissue uptake of a radiolabeled
curcumin analogues, encompassing monophenyl and hetero-
fluoropropyl-substituted curcumin was evaluated in mice.
aryl curcumin analogues, curcumin analogues diversely sub-
Mice that received piperine had 48% greater brain uptake of
stituted on the phenyl rings, and curcumin analogues with
curcumin after 2 min than mice that did not receive piperine.
various linkers. Most of the active compounds had methoxy
However, the uptake in other organs was not found to be
substituents and several were C7 curcumin analogues with a
significantly improved by piperine in this study; the authors
substituted methylene carbon of the 1,3-diketo moiety.
think this observation can be explained by the poor solubility
Overall, it seems that shortening of the C7 linker to a C5
of piperine in 10% ethanolic saline (injection medium)
linker results in compounds that are more active than
Some other agents that showed a synergistic effect when
curcumin, with the caveat that the substituent groups and
used in combination with curcumin in various in vitro studies
their distribution pattern on the phenyl ring should be kept in
look promising for further evaluation. Five patients with
view. Alkoxy and hydroxy substituents are, in general, activity
familial adenomatous polyposis who had undergone colect-
promoting, and the presence of unsaturation and an oxo group
omy received curcumin 480 mg and quercetin 20 mg orally 3
times a day. The number and size of polyps were assessed at
that containing free curcuminoids . Sou et al. very
baseline and after therapy. All five patients had decreases in
recently reported that lipid-based nanoparticles provide
polyp number and size, 60.4% and 50.9%, respectively, from
improved intravenous delivery of curcumin to tissue macro-
baseline after a mean of 6 months of this treatment.
phages. At 6 h after intravenous injection in rats via the tail
Though the authors did not compare the effects of this
vein, curcumin in a nanoparticle delivery system was
combination treatment with those of the single agents,
massively distributed in macrophages of the bone marrow
this study at least throws light on the therapeutic value of
and spleen. Overall, nanoparticle-based systems for curcu-
this combination .
min delivery are still in their infancy, and much progress is
The synergistic inhibitory effect of curcumin and genistein
expected in this area.
against pesticide-induced growth of estrogen-dependentMCF-7 breast carcinoma cells has been reported. It was
Liposomes, micelles, and other delivery systems
showed that a combination of curcumin and genisteincompletely inhibited the cellular proliferation induced by an
Liposomes are excellent drug delivery systems since they
individual pesticide or a mixture of pesticides, and that the
can carry both hydrophilic and hydrophobic molecules. The
inhibitory effect was superior to the individual effects of either
in vitro and in vivo antitumor activity of liposomal curcumin
curcumin or genistein. Curcumin uptake within rat skin after
against human pancreatic carcinoma cells was evaluated
topical application of a curcumin hydrogel, with or without
and demonstrated that liposomal curcumin not only
eugenol or terpeneol pretreatment, was evaluated in an in vivo
inhibited pancreatic carcinoma growth but also exhibited
study. The effects of eugenol and terpeneol as enhancers of
antiangiogenic effects. Liposomal curcumin suppressed
skin curcumin absorption were demonstrated; 8 h after
pancreatic carcinoma growth in murine xenograft models
application, curcumin levels in skin were 2.2- and 2.5-fold
and inhibited tumor angiogenesis. In the in vivo part of this
greater, respectively, in mice that received eugenol or
study, the effect of liposomal curcumin was evaluated in
terpeniol pretreatment than in mice that received curcumin
comparison to no treatment or to treatment with a
alone. These observations indicate that these absorption-
liposomal vehicle in mice. Comparison of the effects of
enhancing agents may also be effective as adjuvants. Epigallo-
liposomal curcumin with those of free curcumin and
catechin-3-gallate, a component of green tea, could counteract
biodistribution profiles of liposomal curcumin and free
certain activities attributed to curcumin. BCM-95 (also called
curcumin have yet to be reported.
Biocurcumax) curcuminoids combined with turmeric oil
The preclinical anticancer activity of a liposomal curcu-
(turmerons) in a specific proportion enhanced the bioavail-
min formulation in colorectal cancer was recently evaluated.
ability and showed better absorption into blood and had longer
This study also compared the efficacy of liposomal curcumin
retention time than curcumin alone. Currently a multicenter,
with that of oxaliplatin, a standard chemotherapeutic agent
phase II, randomized, double-blinded, placebo-controlled clin-
for colorectal cancer. There was synergism between liposo-
ical study is ongoing to assess the efficacy and safety of BCM-95
mal curcumin and oxaliplatin at a ratio of 4:1 in LoVo cells in
in oral premalignant lesions or cervical cancer
vitro. In vivo, significant tumor growth inhibition wasobserved in Colo205 and LoVo xenografts, and the growth
inhibition by liposomal curcumin was greater than that byoxaliplatin in Colo205 cells. This study established that
Targeted and triggered drug delivery systems employing
liposomal curcumin has comparable or greater growth-
nanoparticle technology have emerged as solutions to the
inhibitory and apoptotic effects than oxaliplatin in colorectal
problems of enhancing the bioavailability of therapeutic
cancer both in vitro and in vivo. This group is currently
agents and reducing their unwanted side effects. The
developing liposomal curcumin for introduction into the
synthesis, physicochemical characterization, and cancer-
clinical setting .
related applications of a polymer-based nanoparticle of
Ruby et al. reported the antitumor and antioxidant
curcumin named ‘‘nanocurcumin'' was reported recently.
activities of neutral unilamellar liposomal curcuminoids in
Nanocurcumin was found to have in vitro activity similar to
mice. The in vitro cellular uptake studies of liposomal and
that of free curcumin in pancreatic cancer cell lines,
albumin-loaded curcumin showed that liposomal vehicle is
inhibiting activation of the transcription factor NF-kB and
capable of loading more curcumin into cells than either
reducing steady-state levels of pro-inflammatory cytokines
human serum albumin or aqueous dimethyl sulfoxide, and
such as interleukins and TNF-a. The authors determined
lymphoma cells showed greater uptake of curcumin than
neither the in vivo effect of nanocurcumin in mice nor its
lymphocytes. Nevertheless, in vivo preclinical studies are
biodistribution, which would show any potential increase of
warranted to verify that liposomal curcumin has greater
in vivo efficacy of nanocurcumin over that of free curcumin.
bioavailability and efficacy than free curcumin. A 13 � 105-fold
Curcuminoid-loaded solid lipid nanoparticles for topical
greater solubility of curcumin in a polymeric micellar
application were found to be stable for 6 months at room
temperature and gave prolonged in vitro release of curcumi-
block-polycaprolactone diblock copolymers (MePEG-b-PCL)
noids for up to 12 h. Furthermore, the light and oxygen
was also reported indicating the possibility of further
sensitivities of curcuminoids were strongly reduced by their
exploration on this micellar formulation
incorporation into this unique type of formulation. An in vivo
Another study compared the phototoxic effects of curcu-
study revealed the improved efficiency of this topical cream
min formulations in cyclodextrin and liposomes. Liposomes
containing curcuminoid-loaded solid lipid nanoparticles over
were proved to be a more suitable curcumin carrier system,
since as much as 30% of the phototoxic effect caused by
Curcumin–phospholipid complex significantly protected the
curcumin in cyclodextrin was obtained with about 1/30 of the
liver from carbon tetrachloride-induced acute liver damage
curcumin concentration in liposomes. Furthermore, curcumin
in rats by restoring levels of the enzymes of the liver
prepared in cyclodextrin yielded a significantly greater rate of
glutathione system and of SOD, catalase, and thiobarbituric
cell death than curcumin alone
acid reactive substances. Yet another study explored
The intestinal absorption of curcumin and a micellar
whether formulation with phosphatidylcholine increases
curcumin formulation with phospholipid and a bile salt was
the oral bioavailability or affects the metabolite profile of
evaluated using an in vitro model consisting of everted rat
curcumin in vivo. Male Wistar rats received 340 mg/kg of
intestinal sacs. This study suggested that curcumin is
either unformulated curcumin or curcumin formulated with
biologically transformed during absorption. Further, the in
phosphatidylcholine (Meriva) by oral gavage. Curcumin, the
vitro intestinal absorption of curcumin was found to increase
accompanying curcuminoids desmethoxycurcumin and bis-
from 47% to 56% when it was prepared in micelles.
desmethoxycurcumin, and the metabolites THC, HHC,
Pharmacokinetic studies demonstrated that curcumin in a
curcumin glucuronide, and curcumin sulfate were identified
polymeric micellar formulation had a 60-fold higher biological
in plasma, intestinal mucosa, and liver of rats that had
half-life in rats than curcumin solubilized in a mixture of
received Meriva. Peak plasma levels for parent curcumin
dimethylacetamide, polyethylene glycol (PEG), and dextrose
after administration of Meriva were fivefold higher than
those after administration of unformulated curcumin.
Monoesters of curcumin with valine and glycine and
Similarly, liver levels of curcumin were higher after
diesters with valine, glutamic acid, and demethylenated
administration of Meriva than after administration of
piperic acid have been prepared and assessed for their
unformulated curcumin. In contrast, curcumin concentra-
antimicrobial and anticancer activities. The results of this
tions in the gastrointestinal mucosa after ingestion of
study suggested that diesters of curcumin are relatively more
Meriva were somewhat lower than those observed after
active than curcumin itself because of their increased
administration of unformulated curcumin. These results
solubility, slow metabolism, and better cellular uptake.
suggest that curcumin formulated with phosphatidylcho-
Moreover, monoesters of curcumin had better antimicrobial
line furnishes higher systemic levels of the parent agent
activity than their corresponding diesters, indicating the
than unformulated curcumin .
significant role of a free phenolic group .
In an attempt to reduce the color staining effect and
Curcumin prodrugs
enhance the stability of curcumin, which are its principallimitations in dermatological applications, the curcumin was
Two curcumin prodrugs, N-maleoyl-L-valine-curcumin and N-
microencapsulated in gelatin. The results of this study
maleoyl-glycine-curcumin, were synthesized and evaluated
revealed that microencapsulation resolved the color-staining
for the selective inhibition of growth of bladder cancer cell
problem and enhanced the flow properties and photostability
lines. This study revealed that activation of curcumin
of curcumin .
prodrugs via hydrolysis functions of cellular esterase could
Gal et al. demonstrated the antioxidant effect of
inhibit the growth of tumor cells and reduce the side effects of
liposomal curcumin against copper-induced lipid peroxida-
these drugs on normal diploid cells .
tion. Very recently, the feasibility of a curcumin microemul-
A DNA-curcumin-tetraglycine was prepared by a deoxy
sion containing ethyl oleate, lecithin, and Tween80 as an
11-mer oligonucleotide, 50-GTTAGGGTTAG-30, complemen-
ultrasonic drug delivery carrier was evaluated Further-
tary to a repeat sequence of human telomerase RNA
more, Thangapazham et al. reported that a liposomal
template and linked through phosphate and a C-2 linker
curcumin formulation had 10-fold higher antiproliferative
to a bioactive tetraglycine conjugate of curcumin. This
activity in human prostate cancer cell lines than free
molecule, targeted by an antisense mechanism to telomer-
ase, has been found to act as a prodrug affecting cell growth.
Phospholipid complexes
In a study, curcumin (100 mg/kg) or curcumin–phospholipidcomplex (corresponding to 100 mg/kg curcumin) was admi-
PEGylation is used mainly to increase the solubility and
nistered orally to rats. Curcumin–phospholipid complex
decrease the degradation of drug molecules. The aqueous
produced a maximum plasma curcumin level of 600 ng/ml
solubility of curcumin was increased by formulating it with
2.33 h after oral administration, while free curcumin yielded
MePEG-b-PCL . A recent study by Salmaso et al.
a maximum plasma concentration of 267 ng/ml 1.62 h after
reported significant increase in solubility of curcumin in a
oral administration. The curcumin–phospholipid complex
bioconjugate with PEG and cyclodextrin. A bioconjugate with
yielded a curcumin half-life about 1.5-fold greater than that
beta-cyclodextrin and PEG was prepared and folic acid was
yielded by free curcumin. These results indicate that a
incorporated for targeting purposes. This bioconjugate, CD-
curcumin–phospholipid complex can significantly increase
(C6-PEG)5-FA, formed a complex with curcumin and increased
circulating levels of presumably active curcumin in rats.
curcumin solubility by about 3200-fold as compared to
Another study showed that a curcumin–phospholipid
native beta-cyclodextrins; this bioconjugation reduced the
complex yielded a threefold greater aqueous solubility
degradation rates of curcumin at pH 6.5 and 7.2 by 10- and 45-
and a better hepatoprotective effect than free curcumin.
fold, respectively. In vitro studies using folic acid receptor-
overexpressing and -non-expressing cells demonstrated that
unravel curcumin analogues that would be more suitable for
the new carrier possesses potential selectivity for the folic acid
human clinical trials.
receptor-overexpressing tumor cells. Two conjugates ofcurcumin with PEGs of different molecular weights exhibitedgreater cytotoxicity than unconjugated curcumin .
Although not meant to evaluate the effect of PEGylation,researchers used a PEG derivative to make nanocurcumin,
Dr. Aggarwal is the Ransom Horne, Jr., Professor of Cancer
which is described in section D2 of this review.
Research. This work was supported by grants from the ClaytonFoundation for Research. The authors thank Ms. Kathryn Halefor carefully reviewing this manuscript.
The fast growing research on curcumin, curcuminoids,
and natural and synthetic curcumin analogues clearlyconfirms the versatility and flexibility of curcumin for
[1] Aggarwal BB, Kumar A, Bharti AC. Anticancer potential of
structural modifications. However the actual role of differ-
curcumin: preclinical and clinical studies. Anticancer Res
ent functionalities in curcumin in influencing its special
physico-chemical properties and pleiotropic effects of
[2] Jagetia GC, Aggarwal BB. ‘‘Spicing up'' of the immune
natural and synthetic curcuminoids is far from understood.
system by curcumin. J Clin Immunol 2007;27:19–35.
Such structure-activity studies are still rewarding and
[3] Aggarwal BB, Sundaram C, Malani N, Ichikawa H.
would definitely provide a proper basis for unraveling the
Curcumin: the Indian solid gold. Adv Exp Med Biol
wide variety of biological actions of the age old spice.
[4] Shishodia S, Chaturvedi MM, Aggarwal BB. Role of
This review describes various approaches that have been
curcumin in cancer therapy. Curr Problems Cancer
undertaken to solve the problems associated with curcumin
by searching for molecules that are better than curcumin in
[5] Shishodia S, Sethi G, Aggarwal BB. Curcumin: getting back
bioactivity, solubility, bioavailability and being non-staining.
to the roots. Ann NY Acad Sci 2005;1056:206–17.
Overall, one finds a complex structural variations either
[6] Goel A, Kunnumakkara AB, Aggarwal BB. Curcumin as
among the natural analogues from turmeric and curcumin
‘‘Curecumin'': from kitchen to clinic. Biochem Pharmacol
metabolites or among the analogues made by Mother Nature
[7] Anand P, Kunnumakkara AB, Newman RA, Aggarwal BB.
and man. Surveying this large collection of molecules and the
Bioavailability of curcumin: problems and promises. Mol
associated reports on bioactivities, a few generalizations can
be made regarding the design of a molecule mimicking the
[8] Aggarwal BB, Harikumar KB. Potential therapeutic effects
curcumin scaffold and emulating its bioactivities. Albeit with
of curcumin, the anti-inflammatory agent, against
some exceptions, curcumin in general appears to be better
neurodegenerative, cardiovascular, pulmonary, metabolic,
than either DMC or BDMC in many bioactivity related screens.
autoimmune and neoplastic diseases. Int J Biochem CellBiol 2008.
The antioxidant activity seems to require one or more
[9] Kunnumakkara AB, Anand P, Aggarwal BB. Curcumin
oxysubstituents on aryl rings, preferably in an ortho
inhibits proliferation, invasion, angiogenesis and
orientation, adjacent to or connected by a carbon–carbon
metastasis of different cancers through interaction with
unit to a carbonyl function, flanking the latter. A similar
multiple cell signaling proteins. Cancer Lett 2008.
conclusion seems warranted in the case of antidiabetic
[10] Kunnumakkara AB, Diagaradjane P, Guha S, Deorukhkar
activity, though such studies are not as numerous as
A, Shentu S, Aggarwal BB, et al. Curcumin sensitizes
antioxidant studies. The picture regarding antitumor and
human colorectal cancer xenografts in nude mice togamma-radiation by targeting nuclear factor-kappaB-
cancer cell cytotoxic activities are much more diffuse. In
regulated gene products. Clin Cancer Res 2008;14:
general, oxyaryl substituent with an adjacent, unsaturated –
C C–CO-unit seems to offer antitumor and cancer cell
[11] Anand P, Sundaram C, Jhurani S, Kunnumakkara AB,
cytotoxicity. Antiinflammatory activity also seems to be
Aggarwal BB. Curcumin and cancer: an ‘‘old-age'' disease
better with the presence of such a molecular unit. The C7
with an ‘‘age-old'' solution. Cancer Lett 2008;267:133–64.
linker unit connecting the two oxyaryl rings in an ‘‘ene-[1,3-
[12] Goel A, Jhurani S, Aggarwal BB. Multi-targeted therapy by
dioxo]-ene'' fashion appears to be replaceable with a smaller
curcumin: how spicy is it? Mol Nutr Food Res 2008.
[13] Kiuchi F, Goto Y, Sugimoto N, Akao N, Kondo K, Tsuda Y.
carbon bridge such as ‘‘ene-oxo-ene'' or ‘‘ene-oxo-aryl''
Nematocidal activity of turmeric: synergistic action of
motifs. Further, the incorporation of the linker unit between
curcuminoids. Chem Pharm Bull (Tokyo) 1993;41:
the aryl moieties into a cyclic structure does not extinguish
[14] Simon A, Allais DP, Duroux JL, Basly JP, Durand-Fontanier
Whether using structural analogues or reformulations of
S, Delage C. Inhibitory effect of curcuminoids on MCF-7
curcumin, most studies have been done in vitro. Unlike native
cell proliferation and structure-activity relationships.
Cancer Lett 1998;129:111–6.
curcumin, these novel preparations have been subjected to
[15] Ahsan H, Parveen N, Khan NU, Hadi SM. Pro-oxidant, anti-
very few animal studies. Whether these analogues have the
oxidant and cleavage activities on DNA of curcumin and
same molecular targets as curcumin is also not clear at
its derivatives demethoxycurcumin and
present. Thus neither the bioavailability nor their activity in
bisdemethoxycurcumin. Chem Biol Interact 1999;121:
animal models is known. Future studies are expected to
[16] Kim JE, Kim AR, Chung HY, Han SY, Kim BS, Choi JS. In
vitro peroxynitrite scavenging activity of diarylheptanoids
promotion. Carcinogenesis 1995;16:2493–7.
from Curcuma longa. Phytother Res 2003;17:481–4.
[34] Bonte F, Noel-Hudson MS, Wepierre J, Meybeck A.
[17] Somparn P, Phisalaphong C, Nakornchai S, Unchern S,
Protective effect of curcuminoids on epidermal skin cells
Morales NP. Comparative antioxidant activities of
under free oxygen radical stress. Planta Med 1997;63:
curcumin and its demethoxy and hydrogenated
derivatives. Biol Pharm Bull 2007;30:74–8.
[35] Chearwae W, Anuchapreeda S, Nandigama K, Ambudkar
[18] Subramanian M, Sreejayan, Rao MN, Devasagayam TP,
SV, Limtrakul P. Biochemical mechanism of modulation of
Singh BB. Diminution of singlet oxygen-induced DNA
human P-glycoprotein (ABCB1) by curcumin I, II, and III
damage by curcumin and related antioxidants. Mutation
purified from Turmeric powder. Biochem Pharmacol
[19] Sreejayan N, Rao MN. Free radical scavenging activity of
[36] Devasena T, Rajasekaran KN, Gunasekaran G,
Viswanathan P, Menon VP. Anticarcinogenic effect of bis-
[20] Ramsewak RS, DeWitt DL, Nair MG. Cytotoxicity,
antioxidant and anti-inflammatory activities of curcumins
curcumin analog on DMH-induced colon cancer model.
I–III from Curcuma longa. Phytomedicine 2000;7:303–8.
Pharmacol Res 2003;47:133–40.
[21] Toda S, Ohnishi M, Kimura M, Nakashima K. Action of
[37] Chearwae W, Wu CP, Chu HY, Lee TR, Ambudkar SV,
curcuminoids on the hemolysis and lipid peroxidation of
Limtrakul P. Curcuminoids purified from turmeric powder
mouse erythrocytes induced by hydrogen peroxide. J
modulate the function of human multidrug resistance
protein 1 (ABCC1). Cancer Chemother Pharmacol
[22] Sreejayan, Rao MN. Curcuminoids as potent inhibitors of
lipid peroxidation. J Pharm Pharmacol 1994;46:1013–6.
[38] Pugazhenthi S, Akhov L, Selvaraj G, Wang M, Alam J.
[23] Jeong GS, Oh GS, Pae HO, Jeong SO, Kim YC, Shin MK, et al.
Regulation of heme oxygenase-1 expression by
Comparative effects of curcuminoids on endothelial heme
demethoxy curcuminoids through Nrf2 by a PI3-kinase/
oxygenase-1 expression: ortho-methoxy groups are
Akt-mediated pathway in mouse beta-cells. Am J Physiol
essential to enhance heme oxygenase activity and
Endocrinol Metab 2007;293:E645–55.
protection. Exp Mol Med 2006;38:393–400.
[39] Osawa T, Sugiyama Y, Inayoshi M, Kawakishi S.
[24] Ruby AJ, Kuttan G, Babu KD, Rajasekharan KN, Kuttan R.
Antioxidative activity of tetrahydrocurcuminoids. Biosci
Anti-tumour and antioxidant activity of natural
Biotechnol Biochem 1995;59:1609–12.
curcuminoids. Cancer Lett 1995;94:79–83.
[40] Sugiyama Y, Kawakishi S, Osawa T. Involvement of the
[25] Liu Y, Hong XQ. Effect of three different curcumin
beta-diketone moiety in the antioxidative mechanism of
pigmens on the prdiferation of vascular smooth muscle
tetrahydrocurcumin. Biochem Pharmacol 1996;52:519–25.
cells by ox-LDL and the expression of LDL-R. Zhongguo
[41] Khopde SM, Priyadarsini KI, Guha SN, Satav JG,
Zhong Yao Za Zhi 2006;31:500–3.
Venkatesan P, Rao MN. Inhibition of radiation-induced
[26] Nishiyama T, Mae T, Kishida H, Tsukagawa M, Mimaki Y,
lipid peroxidation by tetrahydrocurcumin: possible
Kuroda M, et al. Curcuminoids and sesquiterpenoids in
mechanisms by pulse radiolysis. Biosci Biotechnol
turmeric (Curcuma longa L.) suppress an increase in blood
glucose level in type 2 diabetic KK-Ay mice. J Agric Food
[42] Naito M, Wu X, Nomura H, Kodama M, Kato Y, Kato Y,
et al. The protective effects of tetrahydrocurcumin on
[27] Kim DS, Park SY, Kim JK. Curcuminoids from Curcuma
oxidative stress in cholesterol-fed rabbits. J Atheroscler
longa L. (Zingiberaceae) that protect PC12 rat
pheochromocytoma and normal human umbilical vein
[43] Okada K, Wangpoengtrakul C, Tanaka T, Toyokuni S,
endothelial cells from betaA(1–42) insult. Neurosci Lett
Uchida K, Osawa T. Curcumin and especially
tetrahydrocurcumin ameliorate oxidative stress-induced
[28] Dairam A, Limson JL, Watkins GM, Antunes E, Daya S.
renal injury in mice. J Nutr 2001;131:2090–5.
Curcuminoids, curcumin, and demethoxycurcumin
[44] Murugan P, Pari L. Effect of tetrahydrocurcumin on
reduce lead-induced memory deficits in male Wistar rats. J
erythromycin estolate-induced lipid peroxidation in rats. J
Agric Food Chem 2007;55:1039–44.
Basic Clin Physiol Pharmacol 2005;16:1–15.
[29] Fiala M, Liu PT, Espinosa-Jeffrey A, Rosenthal MJ, Bernard
[45] Pari L, Amali DR. Protective role of tetrahydrocurcumin
G, Ringman JM, et al. Innate immunity and transcription of
(THC) an active principle of turmeric on chloroquine
MGAT-III and Toll-like receptors in Alzheimer's disease
induced hepatotoxicity in rats. J Pharm Pharm Sci
patients are improved by bisdemethoxycurcumin. Proc
Natl Acad Sci USA 2007;104:12849–54.
[46] Pari L, Murugan P. Protective role of tetrahydrocurcumin
[30] Sandur SK, Pandey MK, Sung B, Ahn KS, Murakami A,
against erythromycin estolate-induced hepatotoxicity.
Sethi G, et al. Curcumin, demethoxycurcumin,
Pharmacol Res 2004;49:481–6.
bisdemethoxycurcumin, tetrahydrocurcumin and
[47] Pari L, Murugan P. Tetrahydrocurcumin: effect on
turmerones differentially regulate anti-inflammatory and
chloroquine-mediated oxidative damage in rat kidney.
anti-proliferative responses through a ROS-independent
Basic Clin Pharmacol Toxicol 2006;99:329–34.
mechanism. Carcinogenesis 2007;28:1765–73.
[48] Murugan P, Pari L. Antioxidant effect of
[31] Anto RJ, George J, Babu KV, Rajasekharan KN, Kuttan R.
tetrahydrocurcumin in streptozotocin-nicotinamide
Antimutagenic and anticarcinogenic activity of natural
induced diabetic rats. Life Sci 2006;79:1720–8.
and synthetic curcuminoids. Mutation Res 1996;370:127–
[49] Murugan P, Pari L. Effect of tetrahydrocurcumin on lipid
peroxidation and lipids in streptozotocin-nicotinamide-
[32] Syu WJ, Shen CC, Don MJ, Ou JC, Lee GH, Sun CM.
induced diabetic rats. Basic Clin Pharmacol Toxicol
Cytotoxicity of curcuminoids and some novel compounds
from Curcuma zedoaria. J Nat Prod 1998;61:1531–4.
[50] Murugan P, Pari L. Effect of tetrahydrocurcumin on plasma
[33] Huang MT, Ma W, Lu YP, Chang RL, Fisher C, Manchand
antioxidants in streptozotocin-nicotinamide experimental
PS, et al. Effects of curcumin, demethoxycurcumin,
diabetes. J Basic Clin Physiol Pharmacol 2006;17:
bisdemethoxycurcumin and tetrahydrocurcumin on 12-O-
[51] Pari L, Murugan P. Antihyperlipidemic effect of curcumin
[67] Dinkova-Kostova AT, Talalay P. Relation of structure of
and tetrahydrocurcumin in experimental type 2 diabetic
curcumin analogs to their potencies as inducers of
rats. Ren Fail 2007;29:881–9.
Phase 2 detoxification enzymes. Carcinogenesis
[52] Pari L, Murugan P. Changes in glycoprotein components in
streptozotocin–nicotinamide induced type 2 diabetes:
[68] Lin CC, Lu YP, Lou YR, Ho CT, Newmark HH, MacDonald C,
influence of tetrahydrocurcumin from Curcuma longa.
et al. Inhibition by dietary dibenzoylmethane of
Plant Foods Hum Nutr 2007;62:25–9.
mammary gland proliferation, formation of DMBA-DNA
[53] Pari L, Murugan P. Influence of tetrahydrocurcumin on tail
adducts in mammary glands, and mammary
tendon collagen contents and its properties in rats with
tumorigenesis in Sencar mice. Cancer Lett 2001;168:
streptozotocin-nicotinamide-induced type 2 diabetes.
Fundam Clin Pharmacol 2007;21:665–71.
[69] Nakamura H, Yamamoto T. The active part of the [6]-
[54] Pari L, Murugan P. Tetrahydrocurcumin prevents brain
gingerol molecule in mutagenesis. Mutation Res
lipid peroxidation in streptozotocin-induced diabetic rats.
J Med Food 2007;10:323–9.
[70] Park KK, Chun KS, Lee JM, Lee SS, Surh YJ. Inhibitory
[55] Murugan P, Pari L. Influence of tetrahydrocurcumin on
effects of [6]-gingerol, a major pungent principle of ginger,
erythrocyte membrane bound enzymes and antioxidant
on phorbol ester-induced inflammation, epidermal
status in experimental type 2 diabetic rats. J
ornithine decarboxylase activity and skin tumor
promotion in ICR mice. Cancer Lett 1998;129:139–44.
[56] Murugan P, Pari L. Influence of tetrahydrocurcumin on
[71] Joe B, Rao UJ, Lokesh BR. Presence of an acidic glycoprotein
hepatic and renal functional markers and protein levels in
in the serum of arthritic rats: modulation by capsaicin and
experimental type 2 diabetic rats. Basic Clin Pharmacol
curcumin. Mol Cell Biochem 1997;169:125–34.
[72] Joe B, Lokesh BR. Role of capsaicin, curcumin and dietary
[57] Atsumi T, Tonosaki K, Fujisawa S. Comparative
n � 3 fatty acids in lowering the generation of reactive
cytotoxicity and ROS generation by curcumin and
oxygen species in rat peritoneal macrophages. Biochim
tetrahydrocurcumin following visible-light irradiation or
Biophys Acta 1994;1224:255–63.
treatment with horseradish peroxidase. Anticancer Res
[73] Joe B, Lokesh BR. Effect of curcumin and capsaicin on
arachidonic acid metabolism and lysosomal enzyme
[58] Limtrakul P, Chearwae W, Shukla S, Phisalphong C,
secretion by rat peritoneal macrophages. Lipids
Ambudkar SV. Modulation of function of three ABC drug
transporters, P-glycoprotein (ABCB1), mitoxantrone
[74] Rajakumar DV, Rao MN. Antioxidant properties of
resistance protein (ABCG2) and multidrug resistance
dehydrozingerone and curcumin in rat brain
protein 1 (ABCC1) by tetrahydrocurcumin, a major
homogenates. Mol Cell Biochem 1994;140:73–9.
metabolite of curcumin. Mol Cell Biochem
[75] Motohashi N, Yamagami C, Tokuda H, Konoshima T,
Okuda Y, Okuda M, et al. Inhibitory effects of
[59] Pan MH, Lin-Shiau SY, Lin JK. Comparative studies on the
dehydrozingerone and related compounds on 12-O-
suppression of nitric oxide synthase by curcumin and its
hydrogenated metabolites through down-regulation of
virus early antigen activation. Cancer Lett 1998;134:37–42.
IkappaB kinase and NFkappaB activation in macrophages.
[76] Oh S, Jang S, Kim D, Han IO, Jung JC. Synthesis and
Biochem Pharmacol 2000;60:1665–76.
evaluation of biological properties of
[60] Hong J, Bose M, Ju J, Ryu JH, Chen X, Sang S, et al.
benzylideneacetophenone derivatives. Arch Pharmacal
Modulation of arachidonic acid metabolism by curcumin
and related beta-diketone derivatives: effects on cytosolic
[77] Chun KS, Sohn Y, Kim HS, Kim OH, Park KK, Lee JM, et al.
phospholipase A(2), cyclooxygenases and 5-lipoxygenase.
Anti-tumor promoting potential of naturally occurring
diarylheptanoids structurally related to curcumin.
[61] Kim JM, Araki S, Kim DJ, Park CB, Takasuka N, Baba-
Mutation Res 1999;428:49–57.
Toriyama H, et al. Chemopreventive effects of carotenoids
[78] Kumar S, Dubey KK, Tripathi S, Fujii M, Misra K. Design
and curcumins on mouse colon carcinogenesis after 1,2-
and synthesis of curcumin-bioconjugates to improve
dimethylhydrazine initiation. Carcinogenesis 1998;19:
systemic delivery. Nucl Acids Symp Ser 2000;75–6.
[79] Mishra S, Kapoor N, Mubarak Ali A, Pardhasaradhi BV,
[62] Shoskes D, Lapierre C, Cruz-Correa M, Muruve N, Rosario
Kumari AL, Khar A, et al. Differential apoptotic and redox
R, Fromkin B, et al. Beneficial effects of the bioflavonoids
regulatory activities of curcumin and its derivatives. Free
curcumin and quercetin on early function in cadaveric
Radic Biol Med 2005;38:1353–60.
renal transplantation: a randomized placebo controlled
[80] Mukhopadhyay A, Basu N, Ghatak N, Gujral PK. Anti-
trial. Transplantation 2005;80:1556–9.
inflammatory and irritant activities of curcumin
[63] Fujisawa S, Kadoma Y. Anti- and pro-oxidant effects of
analogues in rats. Agents Actions 1982;12:508–15.
oxidized quercetin, curcumin or curcumin-related
[81] Mishra S, Karmodiya K, Surolia N, Surolia A. Synthesis and
compounds with thiols or ascorbate as measured by the
exploration of novel curcumin analogues as anti-malarial
induction period method. In Vivo 2006;20:39–44.
agents. Bioorg Med Chem 2008;16:2894–902.
[64] Venkateswarlu S, Ramachandra MS, Subbaraju GV.
[82] Selvam C, Jachak SM, Thilagavathi R, Chakraborti AK.
Synthesis and biological evaluation of
Design, synthesis, biological evaluation and molecular
polyhydroxycurcuminoids. Bioorg Med Chem
docking of curcumin analogues as antioxidant,
cyclooxygenase inhibitory and anti-inflammatory agents.
[65] Sharma OP. Antioxidant activity of curcumin and related
Bioorg Med Chem Lett 2005;15:1793–7.
compounds. Biochem Pharmacol 1976;25:1811–2.
[83] Shim JS, Kim DH, Jung HJ, Kim JH, Lim D, Lee SK, et al.
[66] Conney AH, Lysz T, Ferraro T, Abidi TF, Manchand PS,
Hydrazinocurcumin, a novel synthetic curcumin
Laskin JD, et al. Inhibitory effect of curcumin and some
derivative, is a potent inhibitor of endothelial cell
related dietary compounds on tumor promotion and
proliferation. Bioorg Med Chem 2002;10:2987–92.
arachidonic acid metabolism in mouse skin. Adv Enzyme
[84] Shim JS, Lee J, Park HJ, Park SJ, Kwon HJ. A new curcumin
derivative, HBC, interferes with the cell cycle progression
of colon cancer cells via antagonization of the Ca2+/
antioxidant bis-o-hydroxycinnamoyl methane, analogue
calmodulin function. Chem Biol 2004;11:1455–63.
of natural curcuminoid in experimental diabetes. J Pharm
[85] Dutta S, Padhye S, Priyadarsini KI, Newton C. Antioxidant
Pharm Sci 2003;6:327–33.
and antiproliferative activity of curcumin semicarbazone.
[102] Adams BK, Cai J, Armstrong J, Herold M, Lu YJ, Sun A, et al.
Bioorg Med Chem Lett 2005;15:2738–44.
EF24, a novel synthetic curcumin analog, induces
[86] Chen WF, Deng SL, Zhou B, Yang L, Liu ZL. Curcumin and
apoptosis in cancer cells via a redox-dependent
its analogues as potent inhibitors of low density
mechanism. Anticancer Drugs 2005;16:263–75.
lipoprotein oxidation: H-atom abstraction from the
[103] Al-Omar MA, Youssef KM, El-Sherbeny MA, Awadalla SA,
phenolic groups and possible involvement of the 4-
El-Subbagh HI. Synthesis and in vitro antioxidant activity
hydroxy-3-methoxyphenyl groups. Free Radic Biol Med
of some new fused pyridine analogs. Arch Pharm
[87] Hahm ER, Cheon G, Lee J, Kim B, Park C, Yang CH. New and
[104] Sui Z, Salto R, Li J, Craik C, Ortiz de Montellano PR.
known symmetrical curcumin derivatives inhibit the
Inhibition of the HIV-1 and HIV-2 proteases by curcumin
formation of Fos-Jun-DNA complex. Cancer Lett
and curcumin boron complexes. Bioorg Med Chem
[88] Hahm ER, Gho YS, Park S, Park C, Kim KW, Yang CH.
[105] Robinson TP, Ehlers T, Hubbard IR, Bai X, Arbiser JL,
Synthetic curcumin analogs inhibit activator protein-1
Goldsmith DJ, et al. Design, synthesis, and biological
transcription and tumor-induced angiogenesis. Biochem
evaluation of angiogenesis inhibitors: aromatic enone and
Biophys Res Commun 2004;321:337–44.
dienone analogues of curcumin. Bioorg Med Chem Lett
[89] Handler N, Jaeger W, Puschacher H, Leisser K, Erker T.
Synthesis of novel curcumin analogues and their
[106] Woo HB, Shin WS, Lee S, Ahn CM. Synthesis of novel
evaluation as selective cyclooxygenase-1 (COX-1)
curcumin mimics with asymmetrical units and their
inhibitors. Chem Pharm Bull (Tokyo) 2007;55:64–71.
anti-angiogenic activity. Bioorg Med Chem Lett
[90] Furness MS, Robinson TP, Ehlers T, Hubbard RBt, Arbiser
JL, Goldsmith DJ, et al. Antiangiogenic agents: studies on
[107] Robinson TP, Hubbard RBt, Ehlers TJ, Arbiser JL, Goldsmith
fumagillin and curcumin analogs. Curr Pharm Des
DJ, Bowen JP. Synthesis and biological evaluation of
aromatic enones related to curcumin. Bioorg Med Chem
[91] Weber WM, Hunsaker LA, Abcouwer SF, Deck LM, Vander
Jagt DL. Anti-oxidant activities of curcumin and related
[108] Ahn CM, Shin WS, Bum Woo H, Lee S, Lee HW. Synthesis
enones. Bioorg Med Chem 2005;13:3811–20.
of symmetrical bis-alkynyl or alkyl pyridine and
[92] Weber WM, Hunsaker LA, Gonzales AM, Heynekamp JJ,
thiophene derivatives and their antiangiogenic activities.
Orlando RA, Deck LM, et al. TPA-induced up-regulation of
Bioorg Med Chem Lett 2004;14:3893–6.
activator protein-1 can be inhibited or enhanced by
[109] Barik A, Mishra B, Shen L, Mohan H, Kadam RM, Dutta S,
analogs of the natural product curcumin. Biochem
et al. Evaluation of a new copper(II)-curcumin complex as
superoxide dismutase mimic and its free radical
[93] Weber WM, Hunsaker LA, Roybal CN, Bobrovnikova-
reactions. Free Radic Biol Med 2005;39:811–22.
Marjon EV, Abcouwer SF, Royer RE, et al. Activation of
[110] Sumanont Y, Murakami Y, Tohda M, Vajragupta O,
NFkappaB is inhibited by curcumin and related enones.
Matsumoto K, Watanabe H. Evaluation of the nitric oxide
Bioorg Med Chem 2006;14:2450–61.
radical scavenging activity of manganese complexes of
[94] Youssef D, Nichols CE, Cameron TS, Balzarini J, De Clercq
curcumin and its derivative. Biol Pharm Bull 2004;27:
E, Jha A. Design, synthesis, and cytostatic activity of novel
cyclic curcumin analogues. Bioorg Med Chem Lett
[111] Sumanont Y, Murakami Y, Tohda M, Vajragupta O,
Watanabe H, Matsumoto K. Prevention of kainic acid-
[95] Youssef KM, El-Sherbeny MA, El-Shafie FS, Farag HA, Al-
induced changes in nitric oxide level and neuronal cell
Deeb OA, Awadalla SA. Synthesis of curcumin analogues
damage in the rat hippocampus by manganese complexes
as potential antioxidant, cancer chemopreventive agents.
of curcumin and diacetylcurcumin. Life Sci 2006;78:
Arch Pharm (Weinheim) 2004;337:42–54.
[96] Youssef KM, El-Sherbeny MA. Synthesis and antitumor
[112] Sumanont Y, Murakami Y, Tohda M, Vajragupta O,
activity of some curcumin analogs. Arch Pharm
Watanabe H, Matsumoto K. Effects of manganese
complexes of curcumin and diacetylcurcumin on kainic
[97] Lin L, Shi Q, Nyarko AK, Bastow KF, Wu CC, Su CY.
acid-induced neurotoxic responses in the rat
Antitumor agents. 250. Design and synthesis of new
hippocampus. Biol Pharm Bull 2007;30:1732–9.
curcumin analogues as potential anti-prostate cancer
[113] Vajragupta O, Boonchoong P, Berliner LJ. Manganese
agents. J Med Chem 2006;49:3963–72.
complexes of curcumin analogues: evaluation of hydroxyl
[98] Mazumder A, Neamati N, Sunder S, Schulz J, Pertz H, Eich
radical scavenging ability, superoxide dismutase activity
E, et al. Curcumin analogs with altered potencies against
and stability towards hydrolysis. Free Radic Res
HIV-1 integrase as probes for biochemical mechanisms of
drug action. J Med Chem 1997;40:3057–63.
[114] Zambre AP, Kulkarni VM, Padhye S, Sandur SK, Aggarwal
[99] Rukkumani R, Aruna K, Varma PS, Rajasekaran KN, Menon
BB. Novel curcumin analogs targeting TNF-induced NF-
VP. Comparative effects of curcumin and an analog of
kappaB activation and proliferation in human leukemic
curcumin on alcohol and PUFA induced oxidative stress. J
KBM-5 cells. Bioorg Med Chem 2006;14:7196–204.
Pharm Pharm Sci 2004;7:274–83.
[115] Pucci D, Bloise R, Bellusci A, Bernardini S, Ghedini M,
[100] Sardijiman SS, Reksohadiprodjo MS, van der Groot H,
Pirillo S, et al. Curcumin and cyclopalladated complexes: a
Timmerman H. 1.5-Diphentyl-1,4-pentadiene-3-ones and
recipe for bifunctional biomaterials. J Inorg Biochem
cyclic analogues as antioxidative agents. Synthesis and
structure activity relationships. Eur J Med Chem
[116] Majithiya JB, Balaraman R, Giridhar R, Yadav MR. Effect of
bis[curcumino]oxovanadium complex on non-diabetic
[101] Srinivasan A, Menon VP, Periaswamy V, Rajasekaran KN.
and streptozotocin-induced diabetic rats. J Trace Elem
Protection of pancreatic beta-cells by the potential
Med Biol 2005;18:211–7.
[117] Mohammadi K, Thompson KH, Patrick BO, Storr T,
[134] Mishra S, Narain U, Mishra R, Misra K. Design,
Martins C, Polishchuk E, et al. Synthesis and
development and synthesis of mixed bioconjugates of
characterization of dual function vanadyl, gallium and
piperic acid-glycine, curcumin-glycine/alanine and
indium curcumin complexes for medicinal applications. J
curcumin-glycine-piperic acid and their antibacterial and
Inorg Biochem 2005;99:2217–25.
antifungal properties. Bioorg Med Chem 2005;13:1477–86.
[118] Thompson KH, Bohmerle K, Polishchuk E, Martins C,
[135] Shi W, Dolai S, Rizk S, Hussain A, Tariq H, Averick S, et al.
Toleikis P, Tse J, et al. Complementary inhibition of
Synthesis of monofunctional curcumin derivatives,
synoviocyte, smooth muscle cell or mouse lymphoma cell
clicked curcumin dimer, and a PAMAM dendrimer
proliferation by a vanadyl curcumin complex compared to
curcumin conjugate for therapeutic applications. Org Lett
curcumin alone. J Inorg Biochem 2004;98:2063–70.
[119] Benassi R, Ferrari E, Grandi R, Lazzari S, Saladini M.
[136] Suzuki M, Nakamura T, Iyoki S, Fujiwara A, Watanabe Y,
Synthesis and characterization of new beta-diketo
Mohri K, et al. Elucidation of anti-allergic activities of
derivatives with iron chelating ability. J Inorg Biochem
curcumin-related compounds with a special reference to
their anti-oxidative activities. Biol Pharm Bull
[120] Devasena T, Rajasekaran KN, Menon VP. Bis-1,7-(2-
[137] Takeuchi T, Ishidoh T, Iijima H, Kuriyama I, Shimazaki N,
analog) ameliorates DMH-induced hepatic oxidative stress
Koiwai O, et al. Structural relationship of curcumin
during colon carcinogenesis. Pharmacol Res 2002;46:39–
derivatives binding to the BRCT domain of human DNA
polymerase lambda. Genes Cells 2006;11:223–35.
[121] Kalpana C, Sudheer AR, Rajasekharan KN, Menon VP.
[138] Tong QS, Zheng LD, Lu P, Jiang FC, Chen FM, Zeng FQ, et al.
Comparative effects of curcumin and its synthetic
Apoptosis-inducing effects of curcumin derivatives in
analogue on tissue lipid peroxidation and antioxidant
human bladder cancer cells. Anticancer Drugs
status during nicotine-induced toxicity. Singapore Med J
[139] Ishida J, Ohtsu H, Tachibana Y, Nakanishi Y, Bastow KF,
[122] Kamalakkannan N, Rukkumani R, Varma PS, Viswanathan
Nagai M, et al. Antitumor agents. Part 214: synthesis and
P, Rajasekharan KN, Menon VP. Comparative effects of
evaluation of curcumin analogues as cytotoxic agents.
curcumin and an analogue of curcumin in carbon
Bioorg Med Chem 2002;10:3481–7.
tetrachloride-induced hepatotoxicity in rats. Basic Clin
[140] Ohtsu H, Xiao Z, Ishida J, Nagai M, Wang HK, Itokawa H,
Pharmacol Toxicol 2005;97:15–21.
et al. Antitumor agents. 217. Curcumin analogues as novel
[123] Balasubramanian S, Eckert RL. Green tea polyphenol and
androgen receptor antagonists with potential as anti-
curcumin inversely regulate human involucrin promoter
prostate cancer agents. J Med Chem 2002;45:5037–42.
activity via opposing effects on CCAAT/enhancer-binding
[141] Poma P, Notarbartolo M, Labbozzetta M, Maurici A, Carina
protein function. J Biol Chem 2004;279:24007–14.
V, Alaimo A, et al. The antitumor activities of curcumin
[124] Wright JS. Predicting the antioxidant activity of curcumin
and of its isoxazole analogue are not affected by multiple
and curcuminoids. J Mol Struct 2002;591:207–17.
gene expression changes in an MDR model of the MCF-7
[125] Ishigami YG, Masuda M, Takizawa T, Suzuki Y. The crystal
breast cancer cell line: analysis of the possible molecular
structure and the fluorescent properties of curcumin.
basis. Int J Mol Med 2007;20:329–35.
Shikizai Kyokaishi 1999;72:71–7.
[142] Vajragupta O, Boonchoong P, Watanabe H, Tohda M,
[126] Tonnesen HHK, Mostad J. Structural studies of
Kummasud N, Sumanont Y. Manganese complexes of
curcuminoids. I. The crystal structure of curcumin. Acta
curcumin and its derivatives: evaluation for the radical
Chem Scand B 1982;36:475–80.
scavenging ability and neuroprotective activity. Free Radic
[127] Skrzypczak-Jankun E, Zhou K, McCabe NP, Selman SH,
Biol Med 2003;35:1632–44.
Jankun J. Structure of curcumin in complex with
[143] Venkatesan P, Rao MN. Structure-activity relationships for
lipoxygenase and its significance in cancer. Int J Mol Med
the inhibition of lipid peroxidation and the scavenging of
free radicals by synthetic symmetrical curcumin
[128] Barthelemy S, Vergnes L, Moynier M, Guyot D, Labidalle S,
analogues. J Pharm Pharmacol 2000;52:1123–8.
Bahraoui E. Curcumin and curcumin derivatives inhibit
[144] Costi R, Di Santo R, Artico M, Miele G, Valentini P,
Tat-mediated transactivation of type 1 human
Novellino E, et al. Cinnamoyl compounds as simple
immunodeficiency virus long terminal repeat. Res Virol
molecules that inhibit p300 histone acetyltransferase. J
Med Chem 2007;50:1973–7.
[129] Kumar S, Misra A, Tripathi S, Misra K. Study on curcumin-
[145] Jankun J, Aleem AM, Malgorzewicz S, Szkudlarek M,
oligonucleotide conjugate as a probable anticancer agent:
Zavodszky MI, Dewitt DL, et al. Synthetic curcuminoids
its hybridisation with telomere target sequence 50-
modulate the arachidonic acid metabolism of human
GGGATTGGGATT-30. Nucleic Acids Res Suppl 2001;137–8.
platelet 12-lipoxygenase and reduce sprout formation of
[130] Kumar S, Narain U, Tripathi S, Misra K. Syntheses of
human endothelial cells. Mol Cancer Ther 2006;5:
curcumin bioconjugates and study of their antibacterial
activities against beta-lactamase-producing
[146] Ligeret H, Barthelemy S, Bouchard Doulakas G, Carrupt PA,
microorganisms. Bioconjug Chem 2001;12:464–9.
Tillement JP, Labidalle S, et al. Fluoride curcumin
[131] Mishra S, Tripathi S, Misra K. Synthesis of a novel
derivatives: new mitochondrial uncoupling agents. FEBS
anticancer prodrug designed to target telomerase
sequence. Nucleic Acids Res Suppl 2002;277–8.
[147] Wei QY, Chen WF, Zhou B, Yang L, Liu ZL. Inhibition of
[132] Mohri K, Watanabe Y, Yoshida Y, Satoh M, Isobe K,
lipid peroxidation and protein oxidation in rat liver
Sugimoto N, et al. Synthesis of glycosylcurcuminoids.
mitochondria by curcumin and its analogues. Biochim
Chem Pharm Bull (Tokyo) 2003;51:1268–72.
Biophys Acta 2006;1760:70–7.
[133] Mizushina Y, Ishidoh T, Takeuchi T, Shimazaki N, Koiwai
[148] Nurfina AN, Reksohadiprodjo MS, Timmerman H, Jenie
O, Kuramochi K, et al. Monoacetylcurcumin: a new
UA, Sugiyanto D, van der Goot H. Synthesis of some
inhibitor of eukaryotic DNA polymerase lambda and a
symmetrical curcumin derivatives and their
new ligand for inhibitor-affinity chromatography.
antiinflammatory activity. Eur J Med Chem 1997;32:
Biochem Biophys Res Commun 2005;337:1288–95.
[149] Nichols CE, Youssef D, Harris RG, Jha A. Microwave-
survival signaling pathway: potential for prostate cancer
assisted synthesis of curcumin analogs. ARKIVOC
management. Neoplasia 2003;5:255–66.
[166] Annaraj J, Srinivasan S, Ponvel KM, Athappan P. Mixed
[150] Adams BK, Ferstl EM, Davis MC, Herold M, Kurtkaya S,
ligand copper(II) complexes of phenanthroline/bipyridyl
Camalier RF, et al. Synthesis and biological evaluation of
and curcumin diketimines as DNA intercalators and their
novel curcumin analogs as anti-cancer and anti-
electrochemical behavior under Nafion and clay modified
angiogenesis agents. Bioorg Med Chem 2004;12:3871–83.
electrodes. J Inorg Biochem 2005;99:669–76.
[151] Appiah-Opong R, de Esch I, Commandeur JN, Andarini M,
[167] Sun YM, Zhang HY, Chen DZ, Liu CB. Theoretical
Vermeulen NP. Structure-activity relationships for the
elucidation on the antioxidant mechanism of curcumin: a
inhibition of recombinant human cytochromes P450 by
DFT study. Org Lett 2002;4:2909–11.
curcumin analogues. Eur J Med Chem 2007.
[168] Priyadarsini KI, Maity DK, Naik GH, Kumar MS,
[152] Artico M, Di Santo R, Costi R, Novellino E, Greco G, Massa
Unnikrishnan MK, Satav JG, et al. Role of phenolic O–H
S, et al. Geometrically and conformationally restrained
and methylene hydrogen on the free radical reactions and
cinnamoyl compounds as inhibitors of HIV-1 integrase:
antioxidant activity of curcumin. Free Radic Biol Med
synthesis, biological evaluation, and molecular modeling.
J Med Chem 1998;41:3948–60.
[169] Barclay LR, Vinqvist MR, Mukai K, Goto H, Hashimoto Y,
[153] Chandru H, Sharada AC, Bettadaiah BK, Kumar CS,
Tokunaga A, et al. On the antioxidant mechanism of
Rangappa KS, Sunila. et al. In vivo growth inhibitory and
curcumin: classical methods are needed to determine
anti-angiogenic effects of synthetic novel dienone
antioxidant mechanism and activity. Org Lett 2000;2:
cyclopropoxy curcumin analogs on mouse Ehrlich ascites
tumor. Bioorg Med Chem 2007;15:7696–703.
[170] Jovanovic SV, Steenken S, Boone CW, Simic MG. H-atom
[154] Du ZY, Bao YD, Liu Z, Qiao W, Ma L, Huang ZS, et al.
transfer is a preferred antioxidant mechanism of
Curcumin analogs as potent aldose reductase inhibitors.
curcumin. J Am Chem Soc 1999;121:9677–81.
Arch Pharm (Weinheim) 2006;339:123–8.
[171] Talalay P. The importance of using scientific principles in
[155] Du ZY, Liu RR, Shao WY, Mao XP, Ma L, Gu LQ, et al. Alpha-
the development of medicinal agents from plants. Acad
glucosidase inhibition of natural curcuminoids and
curcumin analogs. Eur J Med Chem 2006;41:213–8.
[172] Kurien BT, Singh A, Matsumoto H, Scofield RH. Improving
[156] Liang G, Li X, Chen L, Yang S, Wu X, Studer E, et al.
the solubility and pharmacological efficacy of curcumin
Synthesis and anti-inflammatory activities of mono-
by heat treatment. Assay Drug Dev Technol 2007;5:567–76.
carbonyl analogues of curcumin. Bioorg Med Chem Lett
[173] Sou K, Inenaga S, Takeoka S, Tsuchida E. Loading of
curcumin into macrophages using lipid-based
[157] Liang G, Yang S, Jiang L, Zhao Y, Shao L, Xiao J, et al.
nanoparticles. Int J Pharm 2008;352:287–93.
Synthesis and anti-bacterial properties of mono-carbonyl
[174] Bruzell EM, Morisbak E, Tonnesen HH. Studies on
analogues of curcumin. Chem Pharm Bull (Tokyo)
curcumin and curcuminoids. XXIX. Photoinduced
cytotoxicity of curcumin in selected aqueous
[158] Ligeret H, Barthelemy S, Zini R, Tillement JP, Labidalle S,
preparations. Photochem Photobiol Sci 2005;4:523–30.
Morin D. Effects of curcumin and curcumin derivatives on
[175] Dubey SK, Sharma AK, Narain U, Misra K, Pati U. Design,
mitochondrial permeability transition pore. Free Radic
synthesis and characterization of some bioactive
Biol Med 2004;36:919–29.
conjugates of curcuminwith glycine, glutamic acid, valine
[159] Lin L, Shi Q, Su CY, Shih CC, Lee KH. Antitumor agents 247.
and demethylenated piperic acid and study of their
New 4-ethoxycarbonylethyl curcumin analogs as
antimicrobial and antiproliferative properties. Eur J Med
potential antiandrogenic agents. Bioorg Med Chem
Chem 2007 (in press).
[176] Aziz HA, Peh KK, Tan YT. Solubility of core materials in
[160] Markaverich BM, Schauweker TH, Gregory RR, Varma M,
aqueous polymeric solution effect on microencapsulation
Kittrell FS, Medina D, et al. Nuclear type II sites and
of curcumin. Drug Dev Ind Pharm 2007;33:1263–72.
malignant cell proliferation: inhibition by 2,6-bis-
[177] Gal S, Lichtenberg D, Bor A, Pinchuk I. Copper-induced
benzylidenecyclohexanones. Cancer Res 1992;52:
peroxidation of phosphatidylserine-containing liposomes
is inhibited by nanomolar concentrations of specific
[161] Ohori H, Yamakoshi H, Tomizawa M, Shibuya M, Kakudo
antioxidants. Chem Phys Lipids 2007;150:186–203.
Y, Takahashi A, et al. Synthesis and biological analysis of
[178] Lee MH, Lin HY, Chen HC, Thomas JL. Ultrasound
new curcumin analogues bearing an enhanced potential
mediates the release of curcumin from microemulsions.
for the medicinal treatment of cancer. Mol Cancer Ther
[179] Thangapazham RL, Puri A, Tele S, Blumenthal R,
[162] Park S, Chung S, Kim KM, Jung KC, Park C, Hahm ER, et al.
Maheshwari RK. Evaluation of a nanotechnology-based
Determination of binding constant of transcription factor
carrier for delivery of curcumin in prostate cancer cells.
myc-max/max-max and E-box DNA: the effect of
Int J Oncol 2008;32:1119–23.
inhibitors on the binding. Biochim Biophys Acta
[180] Lu P, Tong Q, Jiang F, Zheng L, Chen F, Zeng F, et al.
Preparation of curcumin prodrugs and their in vitro anti-
[163] Park CH, Lee JH, Yang CH. Curcumin derivatives inhibit
tumor activities. J Huazhong Univ Sci Technol Med Sci
the formation of Jun-Fos-DNA complex independently of
2005;25. p. 668–70, 78.
their conserved cysteine residues. J Biochem Mol Biol
[181] Kapoor N, Sharma AK, Dwivedi V, Kumar A, Pati U, Misra
K. Telomerase targeted anticancer bioactive prodrug by
[164] Reinke AA, Gestwicki JE. Structure-activity relationships
antisense-based approach. Cancer Lett 2007;248:
of amyloid beta-aggregation inhibitors based on
curcumin: influence of linker length and flexibility. Chem
[182] Letchford K, Liggins R, Burt H. Solubilization of
Biol Drug Des 2007;70:206–15.
hydrophobic drugs by methoxy poly(ethylene glycol)-
[165] Kumar AP, Garcia GE, Ghosh R, Rajnarayanan RV, Alworth
block-polycaprolactone diblock copolymer micelles:
WL, Slaga TJ. 4-Hydroxy-3-methoxybenzoic acid methyl
theoretical and experimental data and correlations. J
ester: a curcumin derivative targets Akt/NF kappa B cell
Pharm Sci 2008;97:1179–90.
[183] Salmaso S, Bersani S, Semenzato A, Caliceti P. New
tetrahydrocurcuminoids on the tumorpromoter-induced
cyclodextrin bioconjugates for active tumour targeting. J
reactive oxygen species generation in leukocytes in vitro
Drug Target 2007;15:379–90.
and in vivo. Jpn J Cancer Res 1998;4:361–70.
[184] Safavy A, Raisch KP, Mantena S, Sanford LL, Sham SW,
[187] Singletary K, MacDonald C, Iovinelli M, Fisher C, Wallig M.
Krishna NR, et al. Design and development of water-
Effect of the beta-diketones diferuloylmethane (curcumin)
soluble curcumin conjugates as potential anticancer
and dibenzoylmethane on rat mammary DNA adducts
agents. J Med Chem 2007;50:6284–8.
and tumors induced by 7,12-dimethylbenz[a]anthracene.
[185] Atsumi T, Fujisawa S, Tonosaki K. Relationship between
intracellular ROS production and membrane mobility in
[188] Nagano T, Oyama Y, Kajita N, Chikahisa L, Nakata M,
curcumin- and tetrahydrocurcumin-treated human
Okazaki E, et al. New curcuminoids isolated from
gingival fibroblasts and human submandibular gland
Zingiber cassumunar protect cells suffering from
carcinoma cells. Oral Dis 2005;4:236–42.
oxidative stress: a flow-cytometric study using rat
[186] Nakamura Y, Ohto Y, Murakami A, Osawa T, Ohigashi H.
thymocytes and H2O2. Jpn J Pharmacol 1997;75:
Inhibitory effects of curcumin and
Source: http://curcumin.co.nz/pdf/Biological_Activities_Of_Curcumin.pdf
Issue 5, March 2016 OQNHE 3rd INTERNATIONAL OQNHE conference, Benchmark, Test Blueprint and more. Hello friends.Oman Quality Network in Higher Education is proud to announce the publication of the fifth issue of its e-newsletter. This issue provides a glimpse into the activities conducted by the Network through some articles related to the higher
Mem. S.A.It. Suppl. Vol. 8, 64 Two platform independent versions of ATLAS12 Institut f¨ur Astronomie, Universit¨at Wien, T¨urkenschanzstraße 17, 1180 Vienna, Austriae-mail: [email protected] Abstract. ATLAS12 makes use of some non-standard features of the VAX/VMS compiler,which is highly appreciable if one uses a VMS workstation, but causes problems of portabil-ity that make it difficult to compile ATLAS12 on various non-VMS operating systems. Thisarticle describes the modifications to ATLAS12 that became necessary in order to get thiscode running with the open source GNU compiler (g77), which is easily available on mostLinux/Unix based systems (including Mac OS X). We also present our parallel and modu-larised Ada95 version of ATLAS12 and give an overview of CAMAS, our new magneticstellar atmosphere code.






