Untitled
Clinical and Experimental Pharmacology and Physiology (2009)
36, 312– 318
DOPAMINE D2 RECEPTOR STIMULATION INHIBITS ANGIOTENSIN
II-INDUCED HYPERTROPHY IN CULTURED NEONATAL RAT
Hong Li,* Sa Shi,* Yi-Hua Sun,‡ Ya-Jun Zhao,* Quan-Feng Li,* Hong-Zhu Li,* Rui Wang†
and Chang-Qing Xu*
Departments of *
Pathophysiology and ‡
Clinical Laboratory, Second Affiliated Hospital of Harbin Medical University,
Harbin, China and †
Department of Biology, Lakehead University, Thunder Bay, Ontario, Canada
Key words: cardiomyocytes, dopamine D2 receptor,
1. Myocardial hypertrophy is a common pathological
change that accompanies cardiovascular disease. Dopamine D2
receptors have been demonstrated in cardiovascular tissues.
However, the pathophysiological involvement of D2 receptors
Dopamine is a very important catecholamine neurotransmitter in the
in myocardial hypertrophy is unclear. Therefore, the effects of
mammalian brain1 that has multiple roles in peripheral tissues.2–4 Its
the D2 receptor agonist bromocriptine and the D2 receptor
effects are exerted via stimulation of dopamine D1–D5 receptors.5–7
antagonist haloperidol on angiotensin (Ang) II- or endothelin
Binding of dopamine to D1 and D5 receptors stimulates adenylyl
(ET)-1-induced hypertrophy of cultured neonatal rat ventricular
cyclase and phospholipase C (PLC), as well as activating calcium
myocytes were investigated in the present study.
channels.8,9 Conversely, stimulation of D2, D3 and D4 receptors
2. Protein content and protein synthesis, determined by
inhibits adenylyl cyclase and calcium channels and activates the
examining [3H]-leucine uptake, were used as estimates of car-
opening of single K channels, resulting in an increase in K con-
diomyocyte hypertrophy. The expression of D2 receptor protein
ductance and associated membrane hyperpolarization.10,11
in neonatal rat ventricular myocytes was determined using
Myocardial hypertrophy, which is an adaptive response to various
western blotting. Changes in [Ca2] in cardiomyocytes were
mechanical changes and humoral stimuli, eventually leads to heart
observed by laser scanning confocal microscopy.
failure.12,13 Because cardiomyocytes rapidly lose their ability to
3. Angiotensin II and ET-1, both at 10 nmol/L, induced
divide under basal conditions both
in vivo and in culture, their
myocyte hypertrophy, as demonstrated by increased protein
growth response to various stimuli primarily involves the hypertrophy
content and synthesis, [Ca2] levels, protein kinase C (PKC)
of individual cells.14 Many studies have demonstrated that angio-
activity and phosphorylation of extracellular signal-regulated
tensin (Ang) II is a potent growth promoter of cardiomyocytes by
kinase, c-Jun N-terminal kinase and mitogen-activated protein
stimulating different signal transduction pathways,12,15 including the
kinase (MAPK) p38 (p38). Concomitant treatment of cells with
activity of G , which facilitates activation of the PLC/protein kinase
10 nmol/L AngII plus 10 mol/L bromocriptine significantly
C (PKC) pathway.16 Activated mitogen-activated protein kinase
inhibited cardiomyocyte hypertrophy, MAPK phosphorylation
(MAPK) may stimulate various transcriptors and induce increased
and PKC activity in the membrane, as well as [Ca2] signalling
gene expression and protein synthesis.17,18
pathways, compared with the effects of AngII alone. In addition,
In a previous study, we detected D2 receptor mRNA and protein
10 mol/L bromocriptine significantly inhibited cardiomyocyte
expression in normal rat cardiac tissues and reported, for the first
hypertrophy induced by 10 nmol/L ET-1. However, pretreatment
time, that expression decreased in an animal model of cardiac hyper-
with haloperidol (10 mol/L) had no significant effects on
trophy.19 Furthermore, we showed that the D2 receptor is also present
cardiomyocyte hypertrophy induced by either AngII or ET-1.
in cultured neonatal rat ventricular myocytes.20 Thus, the aim of the
4. In conclusion, D2 receptor stimulation inhibits AngII-
present study was to determine the effects of D2 receptor activation/
induced hypertrophy of cultured neonatal rat ventricular
inhibition on the hypertrophic response of cardiomyocytes to AngII
myocytes via inhibition of MAPK, PKC and [Ca2] signalling
and the mechanisms involved.
Cells and treatment
Correspondence: Chang-Qing Xu, Department of Pathophysiology of Harbin
Medical University, 194 XueFu Road, NanGang District, Harbin 150086,
Neonatal rat ventricular myocyctes were isolated from 2-day-old Wistar
rats by enzymatic digestion with 0.25% trypsin, as described previously.21
Received 10 June 2007; revision 17 August 2008; accepted 21 August 2008.
The culture medium was changed to serum-free medium for 24 h before
2008 The Authors
treatment. Dishes from each culture preparation were randomly assigned to
Journal compilation 2008 Blackwell Publishing Asia Pty Ltd
one of the following experimental groups, each comprising eight dishes:
D2 inhibits myocardial hypertrophy
(i) untreated controls; (ii) cells treated with 10 nmol/L AngII (Sigma, St Louis,MO, USA), 10 nmol/L ET-1 (Sigma), 10 mol/L bromocriptine (a D2 receptoragonist; Sigma) or 10 mol/L haloperidol (a D2 receptor antagonist; Sigma)alone; and (iii) cells pre-incubated with 10 mol/L bromocriptine or10 mol/L haloperidol for 10 h prior to the addition of 10 nmol/L AngII or10 nmol/L ET-1. All experiments were performed after cells had beenincubated for a further 72 h.
Cardiomyocyte purity and cell viability
Cardiomyocyte purity was monitored using an antibody to cardiac sarco-meric -actin (Boster Biological Technology, Wuhan, China) according to themanufacturer's instructions. Cell viability was analysed by the 3-(4,5-dimethyl-
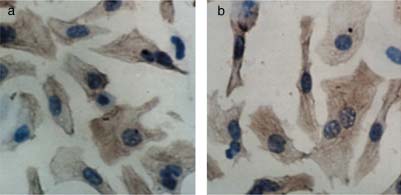
Immunohistochemical demonstration of the purity of cardiomyocytes.
2 thiazoyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT; Sigma) assay,
(a) Cardiomyocyte purity was monitored using an antibody to cardiac
performed in triplicate. Cells and dye crystals were solubilized with 200 L
sarcomeric -actin at 1:100 dilution. (b) The brown granule in the cytoplasm
dimethylsulphoxide and absorbance was measured at 490 nm, using a model
showed that the cell is a myocardial cell. The percentage of cardiomyocytes
ELX-800 microplate assay reader (One Lambda, Canoga Park, CA, USA).
was greater than 95%. (Original magnification 400.)
p-extracellular signal-regulated kinase (ERK; 1 : 2000 dilution; Promega),
Measurement of beating rate, myocyte diameter, protein
anti-p-c-Jun N-terminal kinase (JNK; 1 : 1000 dilution; Promega), anti-p-p38
content and protein synthesis
(1 : 1000 dilution; Neomarker, Fremont, CA, USA) or anti-actin (1 : 200dilution; Boster) antibodies overnight at 4C. Alkaline phosphatase-
The beating rate of myocardial cells was recorded with a JVC (Kanagawa-ku,
conjugated goat anti-mouse IgG (1 : 2500 dilution; Promega) antibodies
Japan) GZ-MG505AC digital recorder. Ten fields were chosen at random
were added and membranes were incubated at 37C for 1 h. Immunodetection
for every group and 10 cells were evaluated in each field. The number of
was performed using a BI2000 Imaging Analysis System (Chengdu Taimeng
beats over a 60 s period was counted manually.
Sci-Tec, Chengdu, China). -Actin was used as an internal control for
Total cell protein of 5 105 cells was measured using a modification of
the method of Lowry et al. and bovine serum albumin as a standard.22The total DNA content of each plate was quantified using ultraviolet spectro-photometry (DU-65 Spectrophotometer; Beckman, Woodland Hills, CA,
Measurement of intracellular Ca2
USA). Each experiment was repeated 12 times.
[3H]-Leucine uptake was used as an index of protein synthesis, as
Free intracellular calcium concentrations ([Ca2] ) in myocardial cells was
described previously.23 To correct for any minor differences in cell number
determined using the Fluo-3/AM (Dojindo Laboratories, Kumamoto, Japan)
between treatment groups, protein synthesis and protein content were
probe as follows. After treatment with AngII, bromocriptine or haloperidol
analysed by [3H]-leucine uptake/DNA content.
alone, cells were incubated with 5 mol/L Fluo-3/AM for 40 min at 37Cunder a 95 : 5 air : CO atmosphere, washed three times with phosphate-
buffered saline (PBS) and further incubated for 20 min in Dulbecco's
Preparation of PKC reagents and PKC activity assay
modified Eagle's medium (DMEM; Gibco Invitrogen, Carlsbad, CA, USA)in the presence of 10 nmol/L AngII. Dynamic changes in [Ca2] in myocardial
Cells were scraped into cool protein lysate (Hangzhou Sijiqing Biological
cells were measured after stimulation with AngII for 30 min by measuring
Engineering Materials, Hangzhou, China) containing 1% phenylmethyl-
Fluo-3 fluorescence (excitation at 488 nm and emission at 525 nm). Fluor-
sulphonyl fluoride (Amresco, Solon, OH, USA). Samples were centrifuged
escence intensity was measured to determine changes in [Ca2] . The
at 10 303 g for 15 min at 4C and the supernatant, representing the cytosolic
fluorescence intensity was observed in eight randomly chosen cells using a
fraction, collected. Pellets were suspended in protein lysate containing 0.1%
laser scanning confocal microscope (TCS SP2; Leica, Mannheim, Germany)
Triton X-100 (Amresco). After homogenization, samples were kept at 4C
to calculate average fluorescence intensity for all cells.
for 1 h, agitated every 20 min for 15 s and then centrifuged at 10 303 g forfor 20 min at 4C. Protein kinase C activity from cytosolic and membranefractions was determined according to the methods provided with the Protein
Kinase C Assay System (Promega, Madison, WI, USA). The incorporationof [-32P]-ATP (111 GBq/mmol, 0.37 MBq/L; Beijing Furei Biotechnology,
Data are presented as the meanSEM and were analysed using spss v. 11.5
Beijing, China) was determined by liquid scintillation and the activity of
software (SPSS, Chicago, IL, USA), with the number of observations
PKC was determined by subtracting the activity of the enzyme in the absence
indicated. Statistical significance was tested by post hoc analysis following
of phospholipids (control buffer) from that of the enzyme in the presence
one-way repeated-measures anova. Significance was set at P 0.05.
of phospholipids (activation buffer). In the present study, PKC activity wascalculated as pmol phosphate radioactivity transferred/min per mg protein.
Sodium dodecyl sulphate–polyacrylamide gel
Cardiomyocyte purity, viability and diameter
electrophoresis and western blotting
The percentage of -actin-positive cells was 95% of the total
Samples (50 g protein) from different experimental groups were separated
number of cells (Fig. 1). In preliminary experiments performed to
by 10% sodium dodecyl sulphate–polyacrylamide gel electrophoresis
determine the optimum concentration of all drugs, viability decreased
(SDS-PAGE) and transferred to polyvinylidene difluoride membranes
after treatment of cells with 100 and 1000 nmol/L AngII or ET-1, but
(Bio-Rad, Hercules, CA, USA) by electroblotting (100 V for 1.5 h). Mem-
no adverse effects were observed after treatment of cells with
branes were then blocked at 37C for 1 h in 5% (w/v) skimmed milk powderin TBS (Tris 10 mmol/L, NaCl 150 mmol/L, pH 8.0; Beijing Chemical
10 mol/L bromocriptine or haloperidol (Fig. 2a). At 10 nmol/L,
Reagents Factory, Beijing, China) and incubated with mouse anti-D2 receptor
AngII and ET-1 both increased cell diameter and there were no
(1 : 200 dilution; Santa Cruz Biotechnology, Santa Cruz, CA, USA), anti-
obvious differences in the hypertrophy induced by 10, 100 and
2008 The Authors
Journal compilation 2008 Blackwell Publishing Asia Pty Ltd


H Li et al.
Expression of the D2 receptor decreases in myocardial hypertrophy
induced by angiotensin (Ang) II or endothelin (ET)-1 (both at 10 nmol/L).
(a) Western blot analysis of D2 receptors. Each lane was loaded with 50 mgprotein. (b) Levels of D2 receptor protein were quantified by densitometryanalysis. Representative results from six different experiments are shown.
*P 0.05 compared with the control group.
At 10 nmol/L, treatment of cardiomyocytes with AngII and ET-1
alone increased cell diameter compared with the control group.
Pretreatment with 10 mol/L bromocriptine reduced the increasein cell diameter induced by both 10 nmol/L AngII and ET-1(Fig. 4b).
Protein content and synthesis were determined for each well
(containing 5 105 cells). Compared with the control group, theprotein content and synthesis of ventricular myocytes were signifi-cantly higher following treatment with AngII and ET-1 (both at
Cardiomyocyte (a) diameter and (b) viability. The effects of different
10 nmol/L). The D2 receptor agonist bromocriptine (10 mol/L)
concentrations of angiotensin (Ang) II and endothelin (ET)-1 were examined:
significantly decreased the AngII- or ET-1-induced increase in
(ⵧ), 1 nmol/L; ( ), 10 nmol/L; ( ), 100 nmol/L; (䊏), 1000 nmol/L. In
[3H]-leucine incorporation and, thus, protein content. In contrast, the
addition, the effects of different concentrations of bromocriptine (Bro) and
D2 receptor specific antagonist haloperidol had no significant effect
haloperidol (Hal) were evaluated: (ⵧ), 1 mol/L; ( ), 10 mol/L; ( ),
on the AngII- or ET-1-induced increases in protein content and
100 mol/L; (䊏), 1000 mol/L. Data are the meanSEM. *P 0.05 compared
synthesis (Fig. 4c,d).
with control; †P 0.05.
Phosphorylation of ERK1/2, JNK and p38
1000 nmol/L AngII or ET-1. At this concentration (10 nmol/L), neitherAngII nor ET-1 had any significant toxic effects, so this concentra-
There was no difference in non-phosphorylated ERK between
tion was used in subsequent exeperiments. Bromocriptine and
the different treatment groups. There was very little phosphorylation
haloperidol alone (both at 1, 10, 100 and 1000 mol/L) had no effect
of ERK, JNK and p38 MAPK in normal neonatal ventricular myo-
on cardiomyocyte dimater (Fig. 2b). Therefore, in all subsequent
cytes. When 10 nmol/L AngII was added to the medium, there was
experiments, 10 mol/L bromocriptine and haloperidol was used.
a marked increase in phosphorylation of ERK1/2, JNK and p38MAPK. However, in the bromocriptine-pretreated group, the AngII-induced phosphorylation of ERK and JNK was decreased signifi-
D2 receptor protein expression in neonatal rat
cantly (P 0.01; Fig. 5).
Expression of D2 receptor protein in neonatal rat ventricular myocytes
Measurement of [Ca2] and PKC activity
was detected with western blotting. Protein levels of the D2 receptor
were significantly lower in AngII- and ET-1-treated groups compared
From fluorescence images taken by a laser scanning confocal
with the control group (Fig. 3a,b).
microscope, we found that AngII markedly increased [Ca2] and that
bromocriptine significantly inhibited this increase. However, the D2receptor antagonist haloperidol had no effect on AngII-induced
Hypertrophy of neonatal rat ventricular myocytes
increase in [Ca2] (Fig. 6).
Cardiomyocytes were pretreated for 10 h with bromocriptine or
Treatment of cardiomyocytes with AngII increased PKC activity
haloperidol, then treated with AngII or Et-1; the beating rate was
in the membrane and cytosolic fractions compared with athe control
measured 72 h later. The beating rate of AngII-treated cardiomyo-
group. There was no difference in PKC activity in the cytosolic fraction
cytes was significantly higher than that of the control group. In contrast,
between the AngII alone, AngII bromocriptine and AngII
bromocriptine, haloperidol and ET-1 alone had no effect on the beating
haloperidol groups. However, PKC activity in the membrane fraction
rate of cardiomyocytes. Pretreatment of cells with bromocriptine
decreased in the bromocriptine-pretreated AngII-treated group
prior to AngII decreased the beating rate of myocardial cells (Fig. 4a).
2008 The Authors
Journal compilation 2008 Blackwell Publishing Asia Pty Ltd
D2 inhibits myocardial hypertrophy
we demonstrated that stimulation of dopamine D2 receptors inhibitedthe hypertrophic response. This inhibition was associated withactivation of MAPK and [Ca2] signalling pathways.
Both D1 and D2 receptors have been identified in cardiac
muscle.25–27 In a previous study, we detected the expression of D1and D2 receptor mRNA and protein in normal rat cardiac tissues;19interestingly, expression of D2 receptor mRNA and proteindecreased in an animal model of cardiac hypertrophy induced byexperimental aortic coarctation.19 In vivo, receptor downregulationmay be related to many humoral and neural factors. Culturedneonatal cardiac myocytes have been used extensively as an experi-mental model in which to investigate the mechanisms of myocytehypertrophy, avoiding interference from humoral and neural factors.
In this system, adrenoceptor stimulation, AngII, endothelin andpeptide growth factors cause myocyte hypertrophy without hyper-plasia. Some studies have shown that AngII plays an importantrole in the initiation of proto-oncogene expression and growth inmyocardial cells.28 In addition, angiotensin-converting enzymeinhibitors have been shown to prevent the development of cardiacremodelling after myocardial injury.29
In the present study, we observed D2 receptor mRNA and protein
expression in neonatal rat ventricular myocytes in vitro and foundthat expression decreased following treatment of cells with AngII.
In order to determine whether decreases in D2 receptor mRNA andprotein expression are unique to AngII, we examined D2 receptorexpression ventricular myocytes after treatment with ET-1 andobserved similar results. Therefore, we speculate that the decreasein the expression of D2 receptors is related to the reduction in therelative density of D2 receptors (as a proprotion of the increased cellsurface area resulting from hypertrophy).
Dopaminergic ligands easily discriminate between the different
dopamine receptor subtypes.30 In the present study, we chosebromocriptine and haloperidol as specific D2 receptor agonists andantagonists, respectively. The results showed that bromocriptinesignificantly inhibited hypertrophy of ventricular myocytes,with a decrease in protein content, cellular protein synthesis and celldiameter. These findings are consistent with recent observationsreported by Mejia-Rodriguez et al.24 In patients with end-stage renaldisease treated with continuous ambulatory peritoneal dialysis, therewas a 24.4% decrease in left ventricular mass index after treatmentwith bromocriptine compared with the control group. The authors
Dopamine D2 receptor receptor stimulation inhibits myocardial
conlcuded that bromocriptine inhibits noradrenaline release, ant-
hypertrophy induced by angiotensin (Ang) II or endothelin (ET)-1 (both at
agonizes aldosterone and downregulates angiotensin AT receptors,
10 nmol/L). (a) Beating rate, (b) cell diameter, (c) relative protein content
which may lead to regression of left ventricular hypertrophy.24 The
and (d) relative protein synthesis (normalized against DNA content) of
results of the present study indicate that D2 receptor activation
cardiomyocytes in the different experimental group. Data are the meanSEM.
*P 0.05. Bro, 10 mol/L bromocriptine; Hal, 10 mol/L haloperidol.
in vitro can also stimulate other pathways to inhibit myocardialhypertrophy, in addition to humoral and neural factors. Massonet al.31 have reported that CHF-1024, a D2 receptor agonist, bluntscardiac fibrosis in pressure overload and has no effect on cardiac
mass. One possible explanation for this is that CHF-1024 is not able
Cardiac hypertrophy is an independent risk factor for the development
to reduce levels of AngII, a major hypertrophic factor. Hussain et
of ischaemia, arrhythmia and sudden death.12,13 Evidence from
al.32 have proposed that bromocriptine (1 mmol/L) alone stimulates
in vivo studies suggests that both dopamine and its receptors are
Na/K-ATPase activity. They have also suggested that pre-activation
implicated in cardiac hypertrophy. The D2 receptor agonist bro-
of D2, D3 and D4 receptors by bromocriptine prior to AngII
mocriptine induces regression of left ventricular hypertrophy in peri-
treatment abolishes AngII-mediated stimulation of Na/K-ATPase
toneal dialysis patients.24 However, the mechanisms invovled after D2
activity and inhibition of cAMP accumulation.32
receptor activation were not determined in any of these previous studies.
In vivo, bromocriptine induces tachycardia.33 The results of
In the present study, using an experimental model of AngII- or
the present study show that the beating rate of isolated myocytes
ET-1-mediated hypertrophy of neonatal rat cardiac myocytes in vitro,
in vitro was not changed after treatment with bromocriptine, suggesting
2008 The Authors
Journal compilation 2008 Blackwell Publishing Asia Pty Ltd

H Li et al.
Western blot amplification
of phosphorylated (a,b) extracellularsignal-regulated kinase (ERK) 1/2,(c) c-Jun N-terminal kinase (JNK) and(d) p38 protein in neonatal ventricularmyocytes. Levels of ERK1/2, JNKand p38 phosphorylation protein werequantified by densitometric analysis.
The results are representative of sixexperiments. Data are the meanSEM.
*P 0.05 compared with the controlgroup. AngII, 10 nmol/L angiotensinII; ET-1, 10 nmol/L endothelin-1;Bro, 10 mol/L bromocriptine; Hal,10 mol/L haloperidol.
Continuous record of [Ca2] in neonatal ventricular myocytes.
(a) Dynamic changes in [Ca2] in myocardial cells were measured 1 h after
stimulation with 10 nmol/L angiotensin (AngII) II. (b) Fluorescence intensitychanges in [Ca2] were recorded continuously with a laser scanning confocal
Protein kinase C (PKC) activity in the (a) membrane and (b) cytosolic
microscope in the different treatment groups. Angiotensin II increased the
fraction of neonatal ventricular myocytes. Data are the meanSEM.
intracellular concentration of calcium. Bro, 10 mol/L bromocriptine; Hal,
*P 0.05. AngII, 10 nmol/L angiotensin II; Bro, 10 mol/L bromocriptine;
10 mol/L haloperidol.
Hal, 10 mol/L haloperidol.
2008 The Authors
Journal compilation 2008 Blackwell Publishing Asia Pty Ltd
D2 inhibits myocardial hypertrophy
that bromocriptine increases the beating rate of cardiomyocytes
cells. Further studies are needed to determine whether our findings
in vivo by changing cardiac vagal or sympathetic tone.
are relevant to pressure overload in other species, as well as in
Ganguly et al.34 have found evidence of a relationship between
humans with chronic cardiac hypertrophy.
D1 receptors and hypertrophy. They have shown that SCH 23390,
In summary, D2 receptor stimulation partly inhibited AngII-induced
a D1 receptor antagonist, partially regresses cardiac hypertrophic
hypertrophy in cultured neonatal rat ventricular myocytes via
changes after aortic constriction. D1 and D2 receptors have different
inhibition of MAPK, PKC and [Ca2] signalling pathways.
pharmacological characteristics in the PKC pathway: D1 receptor-stimulated adenylyl cyclase strongly stimulates cAMP accumulation,
whereas activation of D2 receptors inhibits adenylyl cyclase.35 Thefact that blockade of D1 receptors and stimulation of D2 receptors
This study was supported by grants from the Education Office
inhibit hypertrophy suggests that suppression of the PKC signalling
Project of Heilongjiang Province (No. 11511225) and the National
pathway may cause inhibition of cardiac hypertrophy in vivo and
Natural Science Foundation of China (No. 30470688).
in vitro. In addition, via a G -protein, the D2 receptor participates
in the activation of potassium conductance, resulting in inhibitionof voltage-gated calcium currents in melanotrophs and stimulation
of phospholipase D activity.36 In the present study, we found that
Bunzow JR, Van Tol HH, Grandy DK et al. Cloning and expression
the D2 receptor agonist alone had no effect on protein content,
of a rat D2 dopamine receptor cDNA. Nature 1988; 336: 783–7.
cellular protein synthesis or cell diameter. These results indicate
Dive A, Foret F, Jamart J, Bulpa P, Installé E. Effect of dopamine on
that activation of D2 receptors interferes with AngII-mediated
gastrointestinal motility during critical illness. Intens. Care Med. 2000;
26: 901–7.
Accumulating evidence from in vitro and in vivo studies suggests
Sealfon SC, Olanow CW. Dopamine receptors: From structure to
behavior. Trends Neurosci. 2000; 23: 34 –40.
that AngII participates in the development of hypertrophy by
Jose PA, Eisner GM, Felder R. Regulation of blood pressure by
activating many signalling pathways and that PKC, MAPK and
dopamine receptors. Nephron Physiol. 2003; 95: 19 –27.
phosphatidase–calcium pathways may be involved.29,37 In an attempt
Anita S, Hyman BN. Coupling of dopamine receptor subtypes to
to understand the mechanism through which D2 receptor activation
multiple and diverse G proteins. Int. J. Dev. Neurosci. 2000; 18: 669–
exerts its antihypertrophic effects, we investigated the role of the
aforementioned AngII-related signalling pathways in our culture
Lee KH, Blaha CD, Harris BT et al. Dopamine efflux in the rat striatum
system. The results showed that AngII stimulation significantly
evoked by electrical stimulation of the subthalamic nucleus: Potentialmechanism of action in Parkinson's disease. Eur. J. Neurosci. 2006;
increased [Ca2] . In contrast, 10 mol/L bromocriptine significantly
decreased [Ca2] , which was accompanied by inhibition of myocardial
Johanson CE, Frey KA, Lundahl LH et al. Cognitive function and
hypertrophy. The increase in [Ca2] in ventricular myocytes is
nigrostriatal markers in abstinent methamphetamine abusers. Psycho-
connected directly to cell hypertrophy and calcium modulation by
pharmacology 2006; 185: 327–38.
the D2 receptor may play a role in the inhibition of hypertrophy.
Noble EP. Addiction and its reward process through polymorphisms
Angiontensin II induced a marked increase in the phosphorylation
of the D2 dopamine receptor gene: A review. Eur. Psychiatry 2000;
of ERK and JNK. This was significantly reduced by 80% and 30%,
15: 79 – 89.
Missale C, Nash SR, Robinson SW, Jaber M, Caron MG. Dopamine
respectively, after treatment with D2 receptor agonist bromocriptine.
receptors: From structure to function. Physiol. Rev. 1998; 78: 189 –224.
Probably the most important finding of the present study is that
Vallone D, Picetti R, Borrelli E. Structure and function of dopamine
D2 receptor activation can induce changes in other messenger
receptors. Neurosci. Biobehav. Rev. 2000; 24: 125–32.
molecules (e.g. a decrease in cAMP or nitric oxide), which results
Maurice N, Mercer J, Chan CS et al. D2 dopamine receptor-mediated
in the inhibition of phosphorylation of MAPK and an increase in
modulation of voltage-dependent Na channels reduces autonomous
[Ca2] . In the present study, the D2 receptor antagonist haloperidol
activity in striatal cholinergic interneurons. J. Neurosci. 2004; 24:
10 289 –301.
did not promote the hypertrophic response or the changes in MAPK
Molkentin JD, Lu JR, Antos CL et al. A calcineurin dependent
and [Ca2] . This infers that the D2 receptor was inactive in normal
transcriptional pathway for cardiac hypertrophy. Cell 1998; 93:
cells and was activated by the agonist, thus showed no significant
changes following treatment with the D2 receptor antagonist
Martin P, Detlev G. The molecular basis of cardiovascular hypertrophy:
haloperidol. The activity of p38 MAPK did not differ significantly
The role of the rennin–angiotensin system. J. Cardiovasc. Pharmacol.
between the groups, likely because p38 MAPK is involved mainly
1992; 19: 51– 8.
in cell apoptosis. We investigated changes in PKC activity in
Ueyama T, Kawashima S, Sakada T et al. Requirement of activationof the extracellular signal-regulated kinase cascade in myocardial
cytosolic and membrane fractions of ventricular myocytes and the
cell hypertrophy. J. Mol. Cell. Cardiol. 2000; 32: 947– 60.
results indicate that PKC was translocated to the cell membrane
Oriji GK. Angiotensin II stimulates hypertrophic growth of cultured
in AngII-treated cells. After treatment with bromocriptine, the
neonatal rat ventricular myocytes: Roles of PKC and PGF-alpha. Pros-
translocation of PKC to the cell membrane was significantly
taglandins Leukot. Essent. Fatty Acids 2000; 62: 233–7.
reduced by 30%. Activated PKC inhibits L-type Ca2 channels and
Saito S, Frank GD, Motley ED et al. Metalloprotease inhibitor blocks
decreases Ca2 influx.38 We propose that bromocriptine inhibited
angiotensin II-induced migration through inhibition of epidermal growth
activation of PKC and attenuated the deleterious increase in [Ca2]
factor receptor transactivation. Biochem. Biophys. Res. Commun. 2002;
that occurred during AngII-mediated hypertrophy of neonatal rat
Page C, Doubell AF. Mitogen-activated protein kinase (MAPK) in
cardiac myocytes in vitro.
cardiac tissues. Mol. Cell. Biochem. 1996; 151: 49 –57.
It is important to note that the present study dealt specifically
Kodama H, Fukuda K, Pan J et al. Significance of ERK cascade
with the effects of a D2 receptor agonist on neonatal myocardial
compared with JAK/STAT and PI3-K pathway in gp130-mediated
2008 The Authors
Journal compilation 2008 Blackwell Publishing Asia Pty Ltd
H Li et al.
cardiac hypertrophy. Am. J. Physiol. Heart Circ. Physiol. 2000; 279:
29. Aoki H, Richmond M, Izumo S, Sadoshima J. Specific role of the
1635 – 44.
extracellular signal-regulated kinase pathway in angiotensin II-
Li H, Xu CQ, Sun YH et al. Expression profile of DR1, DR2 protein
induced cardiac hypertrophy in vitro. Biochem. J. 2000; 347: 275–
in rat pathological cardiac hypertrophy. J. Pathophysiol. 2006; 22:
Rafalowska U, Sulkowski G, Wasekiewicz J, Januszewski S,
Li HZ, Han LP, Jiang CM et al. Effect of dopamine receptor 1 on
Kapuosciñski A. Alteration of dopamine transport and dopamine D(2)
apoptosis of cultured neonatal rat cardiomyocytes in simulated
receptor binding in the brain induced by early and late consequences
ischaemia/reperfusion. Basic Clin. Pharmacol. Toxicol. 2008; 102:
of global ischaemia caused by cardiac arrest in the rat. Resuscitation
2000; 47: 195–201.
Chesley A, Lundberg MS, Asai T et al. The beta(2)-adrenergic receptor
Masson S, Chimenti S, Salio M et al. CHF-1024a DA2/alpha2 agonist,
delivers an antiapoptotic signal to cardiac myocytes through G(i)-
blunts norepinephrine excretion and cardiac fibrosis in pressure
dependent coupling to phosphatidylinositol 3-kinase. Circ. Res. 2000;
overload. Cardiovasc. Drugs Ther. 2001; 15: 131– 8.
Hussain T, Abdul-Wahab R, Kotak DK, Lokhandwala MF. Bromoc-
Hartree EF. Determination of protein: A modification of the Lowry
riptine regulates angiotensin II response on sodium pump in proximal
method that gives a linear photometric response. Anal. Biochem. 1972;
tubules. Hypertension 1998; 32: 1054– 9.
48: 422–7.
Lahlou S, Lima GC, Leão-Filho CS, Duarte GP. Effects of long-term
Masao T, Koichi N, Hironori N et al. Statins as antioxidant therapy
pretreatment with isoproterenol on bromocriptine-induced tach-
for preventing cardiac myocyte hypertrophy. J. Clin. Invest. 2001; 108:
ycardia in conscious rats. Can. J. Physiol. Pharmacol. 2000; 78:
Mejía-Rodríguez O, Alvarez-Aguilar C, Vega-Gómez HE, Belio-Caro
Ganguly PK, Mukherjee K, Sahai A. Renal dopamine receptors
F, Vargas-Espinosa JM, Paniagua-Sierra JR. Bromocriptine induces
are involved in the development of cardiac hypertrophy. Mol. Cell.
regression of left ventricular hypertrophy in peritoneal dialysis patients.
Biochem. 1995; 44: 81– 4.
Proc. West. Pharmacol. Soc. 2005; 48: 122–5.
Taraskevich PS, Douglas WW. Dopamine (D2) or gamma-aminobutyric
Basu S, Dasgupta PS. Decreased dopamine receptor expression and its
acid (GABA ) receptor activation hyperpolarizes rat melanotrophs and
second-messenger cAMP in malignant human colon tissue. Dig. Dis.
pertussis toxin blocks these responses and the accompanying fall in
Sci. 1999; 44: 916 –21.
[Ca2] . Neurosci. Lett. 1990; 112: 205– 9.
Jackson DM, Westlind-Danielsson A. Dopamine receptors: Molecular
Yan Z, Song WJ, Surmeier J. D2 dopamine receptors reduce N-type
biology, biochemistry and behavioural aspects. Pharmacol. Ther. 1994;
Ca2 currents in rat neostriatal cholinergic interneurons through a
membrane-delimited, protein-kinase-C-insensitive pathway. J. Neuro-
Miyazawa T, Matsumoto M, Kato S, Takeuchi K. Dopamine-induced
physiol. 1997; 77: 1003–15.
protection against indomethacin-evoked intestinal lesions in rats: Role
Sarkar C, Chakroborty D, Mitra RB, Banerjee S, Dasgupta PS, Basu
of anti-intestinal motility mediated by D2 receptors. Med. Sci. Monit.
S. Dopamine in vivo inhibits VEGF-induced phosphorylation of
2003; 9: 71–7.
VEGFR-2, MAPK, and focal adhesion kinase in endothelial cells.
Aplin M, Christensen GL, Schneider M et al. Differential extracellular
Am. J. Physiol. Heart Circ. Physiol. 2004; 287: 1554– 60.
signal-regulated kinases 1 and 2 activation by the angiotensin type 1
Miyawaki H, Zhao XB, Ashraf M. Calcium preconditioning elicits
receptor supports distinct phenotypes of cardiac myocytes. Basic Clin.
strong protection against ischemic injury via protein kinase C signaling
Pharmacol. Toxicol. 2007; 100: 296 –301.
pathway. Circ. Res. 1996; 79: 137– 46.
2008 The Authors
Journal compilation 2008 Blackwell Publishing Asia Pty Ltd
Source: http://doc.sciencenet.cn/upload/file/2011319155129903.pdf
Auswirkungen des Einsatzes von Antibiotika und Substanzen mit antibiotischer Wirkung in der Landwirtschaft und im Ein Literatur-Review Materialienband Nr. 4 Dr. med. M. Dettenkofer, M. Ackermann, M. Eikenberg, H. Merkel Unterauftrag des Instituts für Umweltmedizin und Krankenhaushygiene (Dir.: Prof. Dr. med. F.D. Daschner) am Universitätsklinikum Freiburg, im Rahmen des
SJIF Impact Factor 2.026 ejpmr, 2015,2(6), 141-146 Research Article EUROPEAN JOURNAL OF PHARMACEUTICAL Gopalakrishnan et al. European Journal of Pharmaceutical and Medical Resea N 3 294-3211 AND MEDICAL RESEARCH HEPATOPROTECTIVE ACTIVITY STUDIES OF CUCUMIS TRIGONUS ROXB.