Eecg.utoronto.ca
Final Report
Snap ‘n Dose
David Xue (Programmer)
Niraj Mistry (Apper)
Pooja Viswanathan (Programmer)
Total Report Word Count (excluding title page & sample projects page): 2485 (Penalty - 0)
Total Apper Context Word Count: 499 (Penalty - 0)
Final Report
Introduction
Fever is the most common and concerning reason for which parents bring their children
to medical attention, and is often caused by common infections (eg. cold). Over-the-counter (OTC) medications, Acetaminophen (Tylenol, Tempra) and Ibuprofen (Advil, Motrin), are the most commonly-used medications for fever and have severe consequences when overdose occurs including liver failure and kidney failure, respectively, or even death. Unfortunately, these errors occur frequently with greater than 50% of parents making errors when administering OTC medications to their children at home.
Barriers to correct dosing include OTC medications coming in a variety of dosage forms
(eg. tablet and liquid), a wide array of brand and generic names and single or multi-ingredient formulations. Furthermore, caregivers are exposed to a wide range of dosing instruments and complex and inconsistent package labeling, and are required to convert a weight-based dose to a volume (eg. milligrams to millilitres), which can be challenging for those with both high and low health literacy.
Despite the gravity of this problem, there have been limited studies on how to decrease
parental medication administration errors and maximize safety and efficacy. Thus, the goal of our app, Snap ‘n Dose, is to help caregivers administer the appropriate dose of OTC liquid medications to children.
This app directly addresses a common problem in the field of Pediatrics, the field of the
apper, who has seen many caregivers inappropriately dose their children with OTC and prescription medications, resulting in severe consequences. This app was developed with his insight into this problem and intimate understanding of the diverse user needs of both clinicians and caregivers. He has ensured that Snap ‘n Dose utilizes the latest evidence as it relates to patient safety, medication administration, and health literacy.
Final Report
Overall Design
The functionalities of the app can be broken into the following blocks:
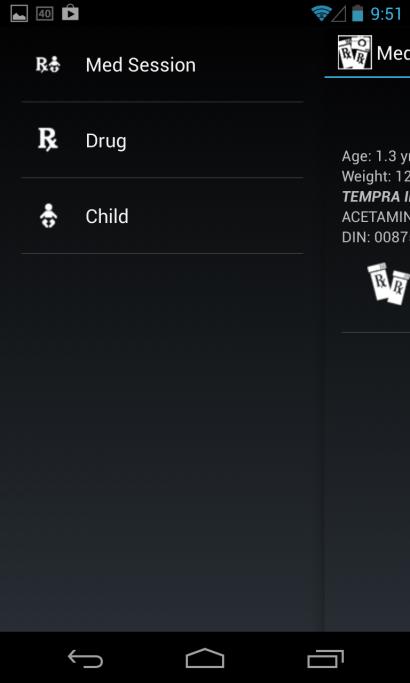
Drug Manager:
The Drug Manager has an optical character recognition (OCR) functionality that enables users to
capture images of the DIN (Drug Identifier Number) from the drug package using the phone's
camera, and recognizes the 8 digit DIN from those images. It then looks up the DIN Database,
which contains all the drug-relevant information. This information is then copied into a local
Drug Database which stores drugs added to the inventory by the user.
Child Manager:
The Child Manager maintains the children profiles. It enables the user to add a new child by
specifying the child's age and weight, and saves the profile to the database.
Medical Session Manager:
The Medical (Med) Session Manager maintains medical sessions that require the user to attach a
child together with a drug. The module queries the Child Database and Drug Database to get
child and drug info respectively, and saves it into the Med Session Database.
Dosage Calculator and Tracker:
This calculates the recommended dose based on the child's weight and the drug's strength. It
also uses the Formulary (containing dosage criteria based on the active ingredient and child's
age, and is usually referred to by clinicians when prescribing drugs) in the calculation. The
calculated dose is saved into the Med Session Database. This module also tracks administered
doses and saves them as observations into the Observation Database.
Reminder Manager:
The Reminder Manager takes care of reminder functionalities associated with a Med Session,
and maintains the Reminder Database. Four types of reminders are provided – dose
administration, or symptom checking such as pain, fever, or other symptoms. The user can set
the next reminder time (the system defaults to a time based on the recommended dosing interval).
The user can also snooze or dismiss reminders.
Observation Manager:
The Observation Manager allows the user to record observations into the Observation Database.
Observation records can be one of four types: dose administration, pain, fever, and other. When
the user enters a new record, a time stamp, an observation value, and a comment is recorded.
Depending on the observation type, the value recorded takes on different units: millilitres for
dose administration, an integer from 1-10 for pain, and a decimal number in degrees Celsius for
fever. No values are recorded for other observations (only free-form text is permitted).
History Manager: This module visualizes information in the Observation Database using graphs and text.
Final Report
Screen Shots & Functionality
Vertical Menu Bar Users can select where to initiate their interaction by selecting one of the items
Final Report
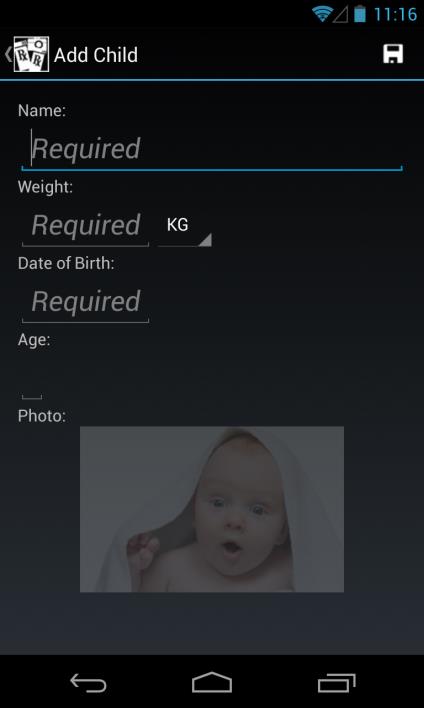
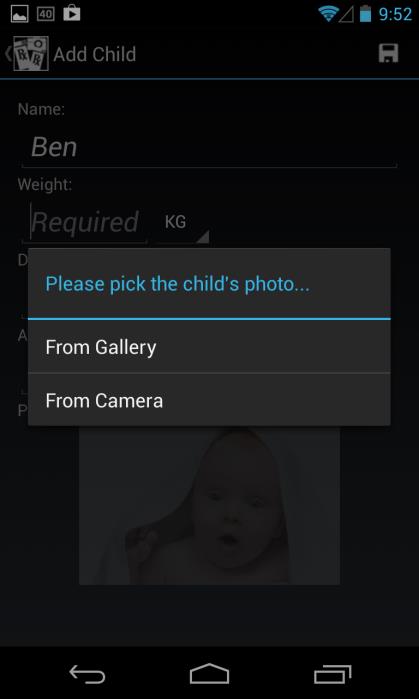
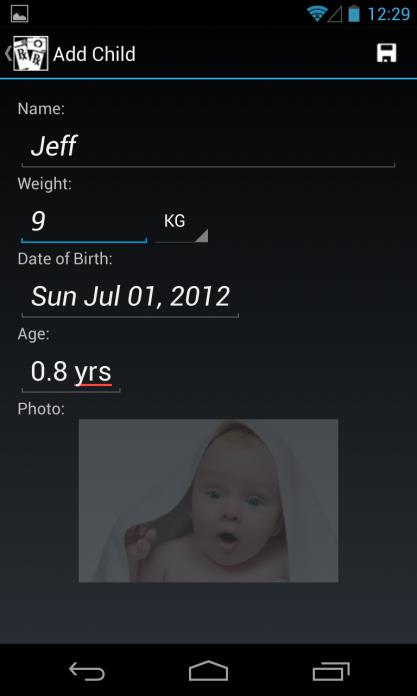
Add Child (Profile)
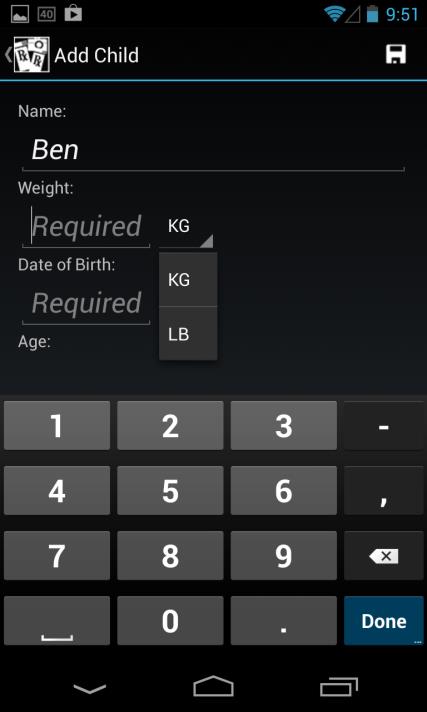
Enter a child's name, weight in either KG or LBS (values are automatically converted
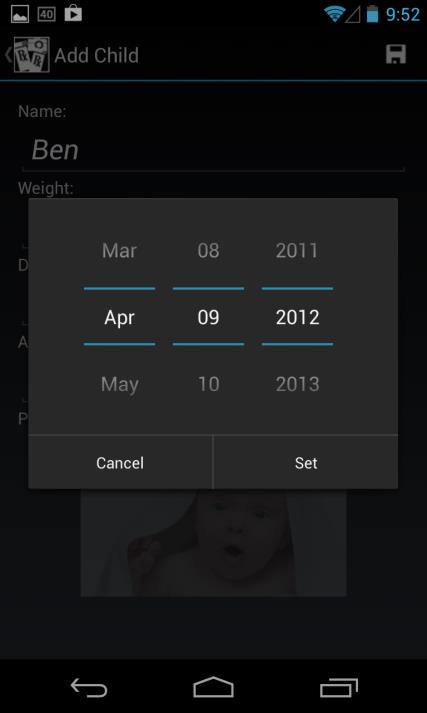
based on the unit selected, and the calculated dose is based on weight in KG), and date of birth using standard scroll wheel (age is automatically calculated and displayed)
Enter a child's picture either from Gallery (existing photo) or Camera (new photo)
Click on Save (Floppy Disk icon, top right corner) to save child profile
Final Report
After Saving a new child profile, the user is prompted to start a new Med Session
If they click on Yes, it takes them to the Add Med Session screen; if they click on Cancel,
they are taken to the Child Screen, which shows a list of the children in the database
The user has 3 options in the Add Med Session screen (right screenshot)
o Click on "Don't attach a drug" or "Cancel" if they do not wish to add a drug at
o Click on an existing drug that is displayed o Click on New Drug, which goes to the Add Drug Screen
Final Report
The user has 2 options to enter a new drug into the
Enter the Drug Identification Number (DIN)
manually, which once entered, shows the Drug Name, Strength and Package Image
Click on the Camera icon in the top right which takes
the user to the Snap ‘n Dose screen below
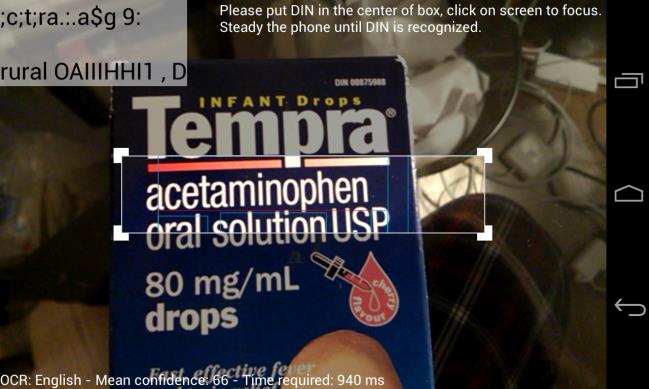
Snap ‘n Dose Screen
On-screen instructions prompt the
user: "Please put the DIN in the center of the (bounding) box, click on the screen to focus. Steady the phone until the DIN is recognized."
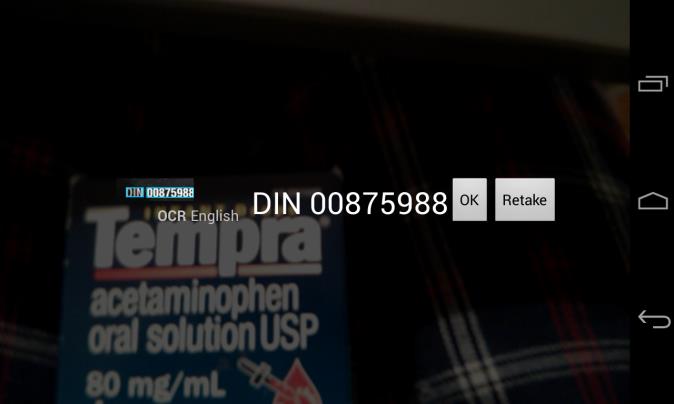
Once the DIN is recognized the
screen below appears
The user can now confirm that the
DIN is correct by comparing it to the product packaging
Tapping OK returns the user to the
Add Drug Screen where they click on Save, which leads them to the Drug or Add Med Session Screen (based on where they came from)
Tapping Retake if incorrect returns
the user to the Snap ‘n Dose Screen
Final Report
Add Med Session
The screen shows child/drug information if previously entered/selected
Calculate dose by clicking on the calculator icon on the top right, which takes the user to
the Dosage Administration Screen
If weight is modified on this screen before dosage calculation, the user can update the
child's profile with this new weight (right)
You have entered a new weight. Click OK to update…
Final Report
If you have not yet added a child, then the screen below (on the left) appears, allowing
o Click to select an existing child from the Child Database or Add a New Child or o Enter a child's age and weight (middle), without adding them to the Child
o Calculate dose by clicking on the calculator icon on the top right, which takes the
user to the Dosage Administration Screen
Dosage Administration Screen
The user can enter free-text Comments: (eg. looks
Clicking Dismiss takes the user to the Med Session
Clicking Administer triggers a prompt asking the
user "Would you like to set a new reminder?" to administer medication (see next)
Final Report
Set Dosage Reminder Screen
The user can set a reminder based on the last dosage
administered and tap Set; if they do not want a reminder, they can tap Cancel
The default time is selected based on the
recommended dosage interval
The last administered dose time is displayed at the
bottom of the screen
Dosage Reminder Screen
A toast prompts the user that "It is time for a dosage
administration for Sam"
They can dismiss the reminder Snoozing the reminder allows the user to set a time
interval after which to be reminded again
Tapping "See more" takes the user to the Dosage
Administration screen
Final Report
Med Session
This screen shows all of the active Med Sessions
The top Med Session (Age 1.4 years) shows a Med
Session that was initiated without adding a Child Profile from the database
The bottom Med Session (Sam) shows a
Medication Session that was initiated using child Sam from the Child Profile database
Within each Med Session there are 4 icons and the
subsequent actions that happen when clicking on each are as follows:
takes the user to the Dosage Administration
Screen (see above)
takes the user to the Symptom Observation
screen (see below)
takes the user to the Set Dosage Reminder
screen (see above)
takes the user to the Observation History
screen (see below)
Final Report
Symptom Observation
Users can enter an Observation Type
o Fever & Pain Observation is numerical o Other Observation is free text
Users can enter comments about the Symptom
Clicking record will Save the Symptom Observation to the Observation History (see
below) and will show the Set Symptom Reminder Screen (not shown), which is very similar to the Set Dosage Reminder Screen.
Final Report
Observation History
On the left, users can view a graphical display of Dose administrations (yellow dots),
Fever observations (purple line) and Pain observations (green line) on Y-axis with time on the X-axis.
Clicking the icon in the top right corner shows the screen on the right, which is a textual
list of the graphical display.
Final Report
Key Learnings
One programmer did the back-end while the other developed the front-end (GUI) for
initial (core) functionality. This process seemed to work very well initially. However, when time came for integration of the back and front ends, both programmers were required to work physically within the same space to ensure optimal function and debugging.
We also obtained informal feedback from parents and health professionals, which was
valuable and could have been of more benefit if obtained earlier in the process.
Finally, the drug formulary, which is a document that contains dosage recommendations
for medications, was not freely available and was not black & white. For example, the dosage
recommendations for acetaminophen from the formulary are 10 to 15 mg/kg every 4 to 6 hours
as required. As such, we realized that adding the ability to view and potentially edit the
formulary recommendations would be valuable (through a clinician interface).
Group Member Contributions
Niraj (Apper):
Recognized opportunity for app
Outlined drug formulary dosage recommendations
Contacted Health Canada Drug Product Database to obtain tailored database of required
Obtained sample medications for development and testing
Transcribed and formatted databases for formulary and drug products Provided feedback to programmers regarding GUI and sought feedback from health
professionals on format, features and function of app prototype
Testing of prototype for bugs
David (mainly Front-End)
Contributed in designing the screens and overall navigation of the app
Implemented most of the screens
Adapted the OCR sample project (see open source section) and integrated it into our app
Contributed in front-end back-end integration
Pooja (mainly Back-End)
Implemented the backend (database manipulation, alarm services, etc.)
Implemented the arithmetic (dosage calculation) Implemented the Observation screen
Did most of the front-end back-end integration
Future Work
Add ability to edit formulary
Optimize DIN recognition (eg. recognize italics fonts, control for ambient lighting and
Add decision support prompts & logic. For example, acetaminophen can be administered
every 4 hours and not sooner, thus the timer should not allow a user to set it sooner. Further, the maximum daily dose of acetaminophen is 75 mg/kg/day, thus the medication history should keep track of this to prevent overdosing.
Improve the GUI through user-centred design principles and engagement of a graphics
Final Report
Add ability to visualize medication dosage recommendation in appropriate medication
dispensing device (eg. medication cup, spoon or syringe)
Consider cloud-based data storage for the DIN database, versus local on-device storage
Consider integration of external thermometer temperature readings
Apper Context
I believe the Snap ‘n Dose app has enormous potential to reduce the burden of outpatient
(ambulatory) medication administration errors in pediatrics. As described in the introduction, medication administration errors are highly prevalence in pediatrics and are the result of a multitude of factors. Research suggests that a disproportionate burden of errors occur in families with low health literacy and that solutions should place fewer literacy demands on families. Snap ‘n Dose has the ability to overcome the literacy demands by addressing both, literacy skills (eg. drug concentration and dosage volume calculation) and task navigation (eg. visualization of proper dose, reminders for dose administration). Snap ‘n Dose will act as an intermediary in families' management of their child's illness as tool to safety administer and track their children's medication use and symptoms. This will alleviate the mental burden on caregivers to allow them to focus on caring for their child and will improve communication between caregivers and clinicians through the sharing of timely and accurate information.
From a research perspective, the process of this project, have been invaluable in
identifying knowledge gaps ripe for research. Preliminary feedback from users and the programmers helped me realize that what is prescribed to children, may not be necessarily what is actually administered. For example, it is recommended that acetaminophen be administered no more frequently than every 4 hours, however, other factors may lead to a more or less frequent administration (eg. bedtime of a child, access to medication etc.). Thus, it is important to understand caregiver and clinician perceptions of recommended dosing guidelines for acetaminophen and ibuprofen to compare what is written in the formulary to what actually happens in practice. For example, perhaps parents wish to be reminded at 3h 45min so they can measure their child's temperature and prepare the medication for administration at the 4h interval, as opposed to being reminded at the 4h mark exactly.
The final product of this project, Snap ‘n Dose app, will be invaluable in adding to the
usability and user-centred/patient-centred design clinical literature. There is a paucity of literature addressing the development of complex interventions, especially technology-based interventions, and poor design is often cited as a reason for lack of effect in clinical studies. Thus, this app will be used in a qualitative study to understand how clinicians and caregivers: (1) perceive and use the drug formulary recommendations for acetaminophen and ibuprofen; (2) perceive the Snap ‘n Dose prototype app; and (3) perceive the key features, functions and format important to include in a final version of the Snap ‘n Dose app. This final version will be tested in a usability evaluation to determine how it meets all end-user usability needs (outcome impact goals and end users' requirements and information needs) as well as determine facilitators and barriers to its use at the point-of-care (eg. in the home environment). Finally, the app will be evaluated in a randomized controlled trial to determine if it leads to appropriate dosage administration by caregivers. Note: we would be interested in a business proposal, and do not wish to release our code.
Final Report
Links to Sample Projects/Open Source Libraries/Tutorial Resources/Useful Websites
Open source UI widgets:
o ActionBarSherlock for the action bar
o ViewPagerIndicator for the sliding pages
o SlidingMenu for the sliding menu
Open source OCR engine and sample project:
o Tess-two (a fork of Tesseract Tools for Android)
o Android-ocr (sample project using tess-two)
Open source graphing engine:
References
Ogilvie JD, Rieder MJ, Lim R. Acetaminophen overdose in children. CMAJ. 2012 Sep
18;184(13):1492-6. doi: 10.1503/cmaj.111338. Epub 2012 Jun 4.
Yin HS, Mendelsohn AL, Wolf MS, Parker RM, Fierman A, van Schaick L, Bazan IS, Kline
MD, Dreyer BP. Parents' medication administration errors: role of dosing instruments and health
literacy. Arch Pediatr Adolesc Med. 2010 Feb;164(2):181-6.
doi:10.1001/archpediatrics.2009.269.
Yin HS, Wolf MS, Dreyer BP, Sanders LM, Parker RM. Evaluation of consistency in dosing
directions and measuring devices for pediatric nonprescription liquid medications. JAMA. 2010
Dec 15;304(23):2595-602. doi: 10.1001/jama.2010.1797. Epub 2010 Nov 30.
Zhou L, Maviglia SM, Mahoney LM, Chang F, Orav EJ, Plasek J, Boulware LJ, Bates DW,
Rocha RA. Supratherapeutic dosing of acetaminophen among hospitalized patients. Arch Intern
Med. 2012 Dec 10;172(22):1721-8.
Source: http://www.eecg.utoronto.ca/~jayar/ece1778.2013/Reports/SnapNDose.pdf
Cl i ni cal Coref erence Annot at i o n G ui del i ne s ( w i t h excerpt s f rom O DIE gui del i nes an d m odi f i ed f or SH ARP n/ TH Y ME) Last updated November 2, 2013 The following is a summary of medical coreference annotation guidelines designed for the medical annotation projects. We acknowledge the ODIE project and OntoNotes coreference annotation guidelines in the creation of these guidelines. Our goals are:
Doping Control Manual 2012 Anti-‐doping Rules Therapeutic Use Exemption (TUE) FIRS Sports Medicine Commission Viale Tiziano 74 00196, Roma, Italy Doping Control Manual TUE December 2009v1 General Information Doping control may occur during any FIRS Events. Athletes, who use medications that are included on the Prohibited List, must fill in the Therapeutic Use Exemption form with their physician. The completed TUE forms must be submitted to the FIRS doping control officer at least 30 days before the event. All documents (TUE form and medical evidence attachments) must be written in English or Spanish. Generic names of medication must be used. Information about Therapeutic Use Exemption (TUE) For substances and methods on the Prohibited List FIRS athletes, like other athletes, may have illnesses or conditions that require them to take particular medications. If the medication that an athlete is required to take to treat an illness or condition happens to fall under the Prohibited List, a Therapeutic Use Exemption will give the athlete the authorization to take the needed medicine. Athletes may apply for a TUE for any treatment involving a substance or method on the Prohibited List. Medical evidence needed to support the diagnosis There needs to be objective medical evidence to confirm the diagnosis and the need for treatment with a prohibited substance. This medical evidence must be supplied by the treating doctor and sent together with the TUE application. Medical evidence shall include complete medical history (for example onset of the disease, family history, triggers, severity, etc.), results of all examinations and laboratory investigations and/or the imaging studies. Required in official FIRS Events All international level athletes defined as athletes in the FIRS Registered Testing and/or players entering in an FIRS World Championship, or a Major Games, who use prohibited substances and/or methods, must fill the TUE Form and submit the Application to the FIRS. FIRS is responsible for accepting applications and granting the certificates. All TUE applications will be reviewed by the FIRS Medical Committee. If approved, the athlete can begin treatment after receiving the authorization notice from the FIRS. In rare cases of an acute life threatening condition retroactive approval will be considered. Reciprocal Approval of TUE FIRS medical commission will accept, by reciprocal arrangement, any TUE issued by a FIRS approved anti-doping organization (ADO) in conformity with the WADA International standards for TUE. ADOs include other International Federations and National anti-doping organizations. Sanction fee for late TUE applications The TUE applications need to be sent to FIRS at least 30 days in advance of the FIRS event. The sanction fee of $50 US may be applied for the late incoming TUE applications. The sanction fee, if applied, shall be paid by the National Federation of the applying athlete.








