Levitra enthält Vardenafil, das eine kürzere Wirkdauer als Tadalafil hat, dafür aber schnell einsetzt. Männer, die diskret bestellen möchten, suchen häufig nach levitra kaufen ohne rezept. Dabei spielt die rechtliche Lage in der Schweiz eine wichtige Rolle.
Pone.0064711 1.15
Increased Beta2-Adrenoceptors in Doxorubicin-InducedCardiomyopathy in Rat
Nolwenn Merlet1,2, Nicolas Piriou1,3, Bertrand Rozec1,4, Amandine Grabherr1, Benjamin Lauzier1,2, Jean-
Noe¨l Trochu1,2,3, Chantal Gauthier1,2*
1 l'institut du thorax, Unite´ Inserm UMR 1087/CNRS UMR 6291, Nantes, France, 2 Universite´ de Nantes, Nantes, France, 3 CHU Nantes, l'institut du thorax, Nantes, France,
4 CHU Nantes, Department of Anaesthesiology, Nantes, France
Background: The toxicity of doxorubicin, leading to an irreversible heart failure, limits its use as chemotherapeutic agent.
The beneficial effects of early administration of b-blocker were reported in patients with heart failure due to doxorubicin,suggesting an important role of b-adrenoceptors (b-ARs). This study aimed to identify a putative target (b-AR and/or itseffectors) at the early phase of a chronic doxorubicin-induced cardiomyopathy (Dox-CM) in a rat model.
Methodology: Dox-CM was induced by six doxorubicin injections (cumulative dose: 15 mg.kg21) and validated byechocardiography and left ventricle (LV) catheterization. The b-AR protein expressions in LV were evaluated by western-blotat days 35 (d35) and 70 (d70) after the first doxorubicin injection. Ex vivo cardiac contractility (dP/dtmax, dP/dtmin) wasevaluated on isolated heart in response to specific b-AR stimulations at d35.
Results: At d35, Dox-CM hearts were characterized by mild LV systolic and diastolic dysfunctions, which were exacerbated atd70. In Dox-CM hearts, b3-AR expression was only decreased at d70 (-3768%). At d35, b1-AR expression was decreased by6866%, but ex vivo b1-AR function was preserved due to, at least in part, an increased adenylyl cyclase response assessed byforskolin. b2-AR expression was increased both at d35 (+58622%) and d70 (+174635%), with an increase of ex vivo b2-ARresponse at d35. Inhibition of Gi protein with pertussis toxin did not affect b2-AR response in Dox-CM hearts, suggesting adecoupling of b2-AR to Gi protein.
Conclusion: This study highlights the b1/b2-AR imbalance in early Dox-CM and reveals the important role that b2-AR/Gicoupling could play in this pathology. Our results suggest that b2-AR could be an interesting target at early stage of Dox-CM.
Citation: Merlet N, Piriou N, Rozec B, Grabherr A, Lauzier B, et al. (2013) Increased Beta2-Adrenoceptors in Doxorubicin-Induced Cardiomyopathy in Rat. PLoSONE 8(5): e64711. doi:10.1371/journal.pone.0064711
Editor: Philippe Rouet, I2MC INSERM UMR U1048, France
Received January 29, 2013; Accepted April 17, 2013; Published May 31, 2013
Copyright: ß 2013 Merlet et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permitsunrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Funding: This work was supported by the ‘‘Association Franc¸aise contre les Myopathies'', the ‘‘Fe´de´ration Franc¸aise de Cardiologie'', the ‘‘Fondation de France''and the ‘‘Fondation Genavie''. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Competing Interests: The authors have declared that no competing interests exist.
* E-mail:
[email protected]
As in different HF etiologies, Dox-CM is characterized by an
alteration of adrenergic system [8]. However, at the present time,
Anthracyclines, like doxorubicin (Dox), epirubicin and dauno-
only few studies have examined the role of cardiac b1- and b2-
rubicin, are among the most effective drugs used in chemotherapy
adrenoceptor (b-AR) subtypes in the pathogenesis of Dox-
for cancer patients. Since the late 60s, Dox is frequently used
cardiotoxicity [9,10] and only one study, at late-onset Dox-CM,
against a variety of cancers including Hodgkin's lymphoma [1],
assessed b3-AR subtype [11], which is recently described as a new
soft-tissue sarcomas [2], leukemia and solid tumors. However, Dox
target for some b-blockers such as nebivolol [12,13]. Despite this
administration is limited due to severe cardiotoxic effects leading
lack in experimental data, some clinical studies investigated b-
to dilated cardiomyopathy [3]. Prognosis of heart failure (HF) due
blocker therapies in Dox-CM. Kalay et al., demonstrated that left
to Dox-cardiotoxicity is poor and even worse than ischemic or
ventricular (LV) diameters remained constant and diastolic
idiopathic dilated cardiomyopathy. Although several mechanisms
function was better preserved after Dox-treatment in patients
have been proposed to describe the mechanisms by which Dox
receiving carvedilol, compared to placebo [14]. However,
induces cardiotoxicity (generation of free radicals, mitochondrial
Georgakopoulos et al., demonstrated that metoprolol, a b-blocker
disruption, alteration of cellular energetic, and initiation of
without antioxidative properties, failed to give cardioprotection in
apoptotic cascades), these mechanisms are still not fully under-
lymphoma-treated doxorubicin patients [15]. It was reported that
stood [4–6] and there is no specific treatment for Dox-induced
an early start of treatment with angiotensin-converting enzyme
cardiomyopathy (Dox-CM) [6,7]; treatments classically used for
inhibitors (ACEIs), in association or not with b-blockers, both
other HFs with systolic dysfunction induce only limited beneficial
improves myocardial contractility [16] and patients' prognosis
effects in Dox-CM.
PLOS ONE www.plosone.org
May 2013 Volume 8 Issue 5 e64711
Beta-Adrenoceptors in Doxorubicin Cardiomyopathy
Table 1. Antibodies used for western-blot.
Secondary antibody
Rabbit polyclonal antibody, Sigma-Aldrich
Goat anti-rabbit immunoglobulin G, Santa-Cruz
Biotechnology (sc-2054)
Rabbit polyclonal antibody, AbCam Ltd
Goat anti-rabbit immunoglobulin G, Santa-Cruz
Biotechnology (sc-2054)
Rabbit polyclonal antibody, Santa Cruz
Goat anti-rabbit immunoglobulin G, Santa-Cruz
Biotechnology (sc-50436)
Biotechnology (sc-2054)
Rabbit polyclonal antibody, Merk Chemicals Ltd 1/1,000
Goat anti-rabbit immunoglobulin G, Santa-Cruz
Biotechnology (sc-2054)
Rabbit polyclonal antibody, Merk Chemicals Ltd 1/5,000
Goat anti-rabbit immunoglobulin G, Santa-Cruz
Biotechnology (sc-2054)
Mouse monoclonal antibody, Santa-Cruz
Goat anti-mouse immunoglobulin G, Santa-Cruz
Biotechnology (sc-32233)
Biotechnology (sc-2055)
Primary antibodies were diluted in TBS-T, excepted for Gia2 protein detection which was diluted in 5% non-fat dry milk in TBS-T. All secondary antibodies were diluted in1% non-fat dry milk in TBS-T.
doi:10.1371/journal.pone.0064711.t001
[17]. Although the exact mechanism is still poorly understood, theauthors highlighted the importance of an early diagnosis, becausea delayed treatment (.6 months after the end of chemotherapy) isinefficient [17].
The aim of the present study was to identify a putative target (b-
AR and/or its effectors) involved at the early phase of a chronicDox-CM in a rat model. This study demonstrated for the first timethat b2-AR expression was increased from 35 days after the firstDox-injection, this effect was maintained until 70 days after thefirst Dox-injection, resulting in an increase of b2-AR-inducedcontractility. In addition, b1-AR function was preserved, in spite ofdecreased b1-AR protein expression. This discrepancy could beexplained by an increase of adenylyl cyclase (AC) expression and/or activity as illustrated by an increased forskolin-induced responsein Dox-CM rats.
All experiments were performed in accordance with the 1996
Guide for the Care and Use of Laboratory Animals published bythe U.S. National Institute of Health. The protocol was approvedby the Direction De´partementale de la Protection des Populations(agreement number C-44 015) and all efforts were made tominimize suffering.
One hundred and fifty eight male Sprague-Dawley rats (225–
250 g) were purchased from Janvier (Le Genest St Isle, France)and were housed under standard conditions of room temperature,humidity (40–60%) and 12 h light/dark cycle. Food and waterwere available ad libitum.
Doxorubicin (AdriblastineH 50 mg/25 mL, Pfizer, France) was
administered intraperitoneally in six equal injections (eachcontaining 2.5 mg.kg21) over a period of two weeks, with a totalcumulative dose of 15 mg.kg21 body weight (Dox-CM: n = 100).
Age-matched rats injected with saline were used as controls (Ctrl:n = 58). Rats were then bred in animal housing until three weeksafter the last injection (day 35 (d35); Ctrl: n = 46, Dox-CM: n = 76)
Figure 1. Body weight (A) and survival curves (B) of Ctrl and
or until eight weeks after the last injection (day 70 (d70); Ctrl:
Dox-CM rats. Values are means 6 sem **: P,0.001 vs Ctrl. Ctrl:
n = 12, Dox-CM: n = 24). At d35, twenty Dox-CM rats and twenty
control, Dox-CM: Doxorubicin-induced cardiomyopathy.
Ctrl rats were randomly selected to be hemodynamically explored
PLOS ONE www.plosone.org
May 2013 Volume 8 Issue 5 e64711
Beta-Adrenoceptors in Doxorubicin Cardiomyopathy
Table 2. Cardiac morphological parameters in Ctrl and Dox-CM rats, at d35 and d70.
Heart wt/Body wt (mg.g21)
LV wt/Body wt (mg.g21)
Heart wt/Tibia length (mg.cm21)
LV wt/Tibia length (mg.cm21)
Ctrl: control; Dox-CM: Doxorubicin-induced cardiomyopathy; LV: left ventricle; wt: weight. Values are means 6 sem. *: P,0.05 vs respective Ctrl. **: P,0.001 vsrespective Ctrl.
doi:10.1371/journal.pone.0064711.t002
by echocardiography-Doppler. Then either rats were hemody-
2D speckle-tracking method on every medial myocardial segment
namically assessed by LV catheterization, or rat hearts were
removed either to test ex vivo cardiac contractile function or toperform biochemical studies. At d70, rats were used to perform
Left Ventricle Catheterization
echocardiography-Doppler and biochemical studies.
LV catheterization was performed, at d35, by a 2F microtip
pressure catheter (SPR 838, Millar instruments Inc, Houston,
Texas). Anaesthesia maintenance on spontaneously breathing rats
Transthoracic echocardiography was performed using a com-
was performed with an inhalational anaesthesia system for small
mercially available ultrasound system (VIVID7, GE Healthcare,
animal (TEM anaesthesia, Lormont, France). Isoflurane was
Horton, Norway) equipped with a 10 MHz sectorial probe. Rats
delivered through a nose mask at a concentration of 2% volume
were anaesthetized with a gas-mixture of 1% isoflurane (ForeneH,
and 1 L.min21 O2 flow to limit hemodynamic repercussion. Body
Abbott France, Rungis, France) in O2. The chest was shaved and
temperature was monitored by rectal probe and maintained
the animal was positioned on a heating pad in a supine position.
constant (37uC) by warming-blanket. The right carotid artery was
All recordings were monitored under a continuous single-channel
isolated, ligated at the proximal part and the pressure catheter was
electrocardiogram obtained on the imaging system by fixing the
inserted in. Signals were recorded using an Analogic/Digital
electrodes to the limbs. Using two-dimensional imaging, a short
converter (EMKA Technologies, Paris, France), stored and
axis view of the LV at the level of the papillary muscles was
displayed on a computer by the IOX1.5.7 Software System
obtained and the two-dimensionally guided M-mode recording
(EMKA Technologies). Data were analysed using Datanalyst
through the anterior and posterior walls of the LV was taken as
software (EMKA Technologies). The following parameters were
recommended by the American Society of Echocardiography [18].
obtained: LV end diastolic pressure (LVEDP), LV contraction and
Then, trans-mitral inflow in pulsed-wave Doppler from apical four
relaxation velocities (dP/dtmax and dP/dtmin, respectively), the
chamber view and tissue Doppler imaging (TDI) on basal
index of LV relaxation constant (Tau).
segments of septal and lateral walls in apical four chamber view
The animals were thereafter sacrificed by injection of a
were taken as previously described [19]. A cine-loop of LV
parasternal short axis view with high frame rate was obtained. Allacquisitions were performed by the same operator.
All images were digitally stored on hard disks for off-line analysis
The expressions of b1-AR, b2-AR, b3-AR, Gsa, Gia2 and
(EchoPac Q-analysis software, GE Healthcare). Measurements
GAPDH were examined by western-blot, at d35 and d70. Hearts
were made on five cardiac cycles and averaged for each data
were rapidly isolated and placed in a cold Tyrode solution
value. The following parameters were determined as recom-
composed as followed (in mM): NaCl, 137; KCl, 5.4; MgCl2, 1.2;
mended by the American Society of Echocardiography [18]: LV
Na2HPO4, 1.2; Hepes, 20; CaCl2, 1.0; pH 7.4 (Sigma-Aldrich, St
end diastolic and systolic diameters (LVEDD and LVESD),
Quentin Fallavier, France). LV and septum free walls were
diastolic posterior wall thicknesses (dPWth). LV end diastolic and
carefully separated and freezed in liquid nitrogen and stored at
systolic volumes (LVEDV and LVESV) were calculated from the
280uC until used. LV and septum free walls samples were
Teichholz method in order to assess LV ejection fraction (LVEF),
homogenized in 3 mL of Ripa buffer for 1 g of tissue plus 1X
whereas LV shortening fraction (LVSF) was calculated from
PMSF (Interchim, Montluc¸on, France) and 2X protease inhibitor
LVEDD and LVESD previously measured. LV diastolic function
cocktail (Roche, Mannheim, Germany). Protein samples (25 mg)
parameters were derived from pulsed-wave trans-mitral inflow
were submitted to electrophoresis on a 10% polyacrylamide/
pattern and TDI off-line analyses as previously described [20]: the
sodium dodecyl sulfate gel and then were run 150 min at 20 mA
peak of E wave velocities, the isovolumic relaxation time (IVRT),
per membrane in TG-SDS buffer (Interchim). Gels and nitrocel-
the mean of peak velocities of basal septal and lateral walls (pulsed
lulose membranes (Hybond C super membrane, Amersham, Saclay,
wave TDI) during systole (Sa) and in early diastole (Ea) to calculate
France) were equilibrated in TG-SDS buffer with 20% ethanol,
E/Ea ratio. Radial 2D strain analyses were performed using the
and protein fractions were transferred using an electroblottingapparatus (Bio-Rad, Marnes La Coquette, France). Nonspecific
PLOS ONE www.plosone.org
May 2013 Volume 8 Issue 5 e64711
Beta-Adrenoceptors in Doxorubicin Cardiomyopathy
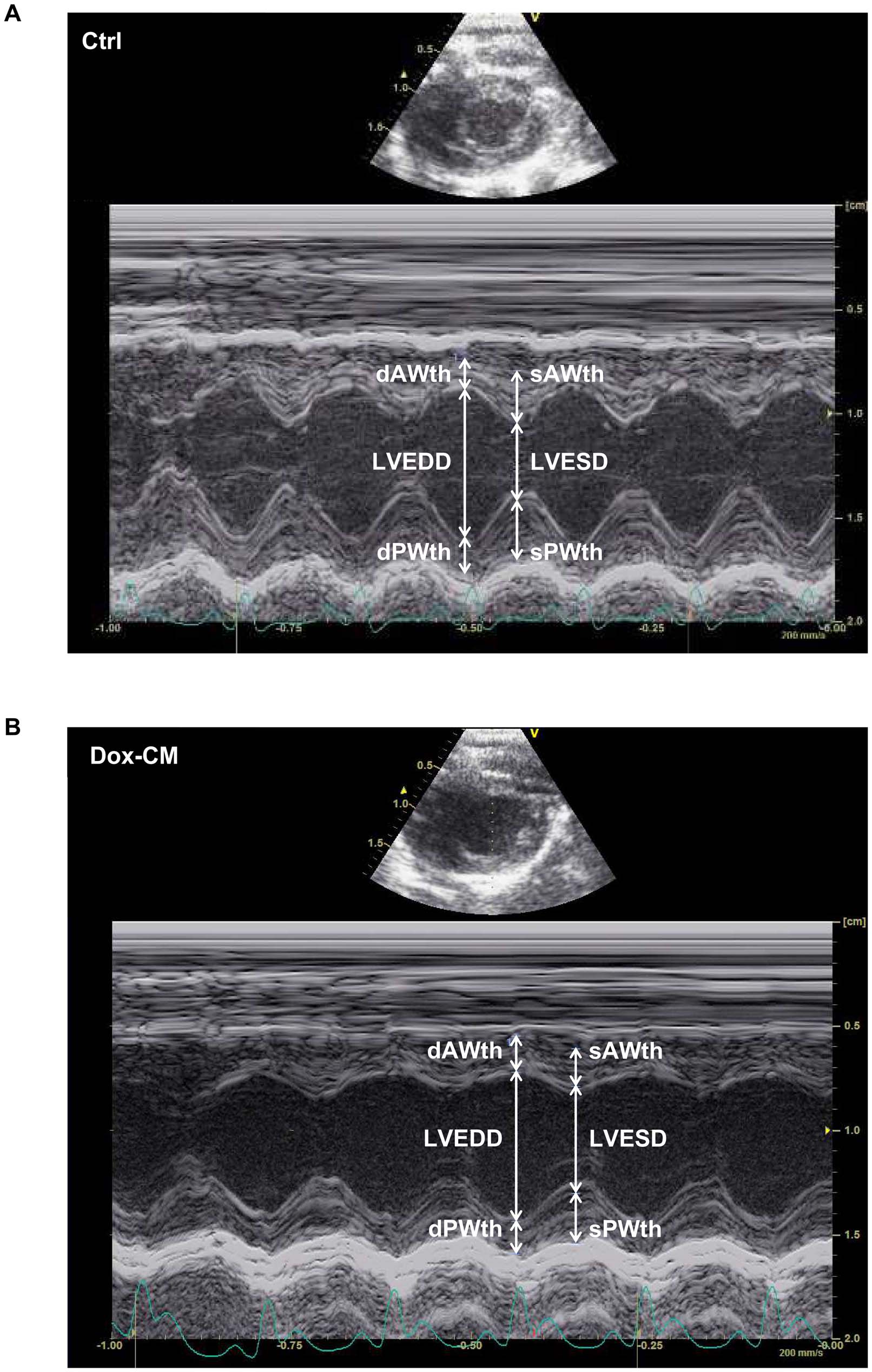
Figure 2. Representative images obtained by echocardiography at day 35 in Ctrl (A) and Dox-CM (B) rats. Images were obtained with ashort axis view of a two-dimensionally directed M-mode. Ctrl: control, dAWth: diastolic anterior wall thickness, Dox-CM: Doxorubicin-inducedcardiomyopathy, dPWth: diastolic posterior wall thickness, LVEDD: left ventricular end diastolic diameter, LVESD: left ventricular end systolic diameter,sAWth: systolic anterior wall thickness, sPWth: systolic posterior wall thickness.
doi:10.1371/journal.pone.0064711.g002
PLOS ONE www.plosone.org
May 2013 Volume 8 Issue 5 e64711
Beta-Adrenoceptors in Doxorubicin Cardiomyopathy
PLOS ONE www.plosone.org
May 2013 Volume 8 Issue 5 e64711
Beta-Adrenoceptors in Doxorubicin Cardiomyopathy
Figure 3. In vivo cardiac parameters obtained by echocardiography-Doppler in Ctrl and Dox-CM rats. Ctrl are showed in empty bar andDox-CM in full bar. Values are means 6 sem *: P,0.05 vs respective Ctrl **: P,0.001 vs respective Ctrl. d35: day 35, d70: day 70, Dox-CM: Doxorubicin-induced cardiomyopathy, dPWth: diastolic posterior wall thickness; E: E wave velocity; Ea: peak velocity of basal and lateral walls in early diastole;IVRT: isovolumic relaxation time; LV: left ventricle; LVEF: left ventricular ejection fraction; LVEV: left ventricular end volume; LVSF: left ventricularshortening fraction; Sa: peak velocity of basal and lateral walls in systole.
doi:10.1371/journal.pone.0064711.g003
binding was blocked by incubating membranes in 5% non-fat dry
period, specific b1-, b2- or b3-AR functions and AC stimulation
milk in Tris-buffered saline (TBS) (200 mM Trizma base, 1.4 M
were assessed by constructing concentration-response curves to
NaCl, pH = 7.5) with 0.1% Tween 20 added (TBS-T) and then
different pharmacological agents. Thus, b1- and b2-AR functions
membranes were incubated with the primary antibody. Mem-
were assessed by perfusing isoproterenol (a non selective b-AR
branes were washed in 5% non-fat dry milk in TBS-T and
agonist, 1029 to 1025 M) in the presence of 1026 M L-748,337 (a
hybridized with the secondary antibody in 1% non-fat dry milk in
selective b3-AR antagonist) associated to 1026 M ICI-118,551 (a
TBS-T. Finally, membranes were washed with 5% non-fat dry
selective b2-AR antagonist) or to 1026 M CGP-20712A (a
milk in TBS-T then TBS, and antibody complexes were revealed
selective b1-AR antagonist), respectively. b3-AR function was
by the enhanced chemiluminescence detection process (Bio-Rad).
assessed by perfusing growing concentrations (1029 to 1025 M) of
Chemiluminescence was visualized using an Amersham Image-
SR 58611A (a b3-AR agonist) and AC response was assessed by
Quant RT-ECL camera (GE Healthcare) and band signals were
perfusing growing concentrations (3.1028 to 1025 M) of forskolin
assessed by densitometry with ImageQuant TL software (GE
(an AC activator). In order to assess the role of Gi in the b2-AR
Healthcare). For each lane, a ratio to the corresponding GAPDH
pathway, we used pertussis toxin (PTX) (a selective Gi inhibitor).
band intensity was calculated; the use of GAPDH as reference was
Classically, PTX is administered to rats two or three days before
validated by checking the abundance stability of GAPDH protein
experiments [22]. However, due to high mortality in Dox-CM rats
between Ctrl and Dox-CM groups.
pre-treated with PTX, we were not able to apply this protocol and
Antibody references and conditions used in this study are
used another one consisting in pre-treating heart with PTX
summarized in Table 1.
(4 mg.L21) in a closed system during 30 min, as described by othergroups [23]. Data were analysed using Datanalyst software
Whole Heart Contractility
(EMKA Technologies). After Langendorff experiments, hearts
At d35, after removal, hearts were quickly flushed into a cold
were weighed, as well as LV free walls which were carefully
Tyrode buffer, and then were mounted on a cannula via the aorta
separated from the heart.
on a Langendorff apparatus (EMKA Technologies) and perfusedwith a Krebs-modified solution (in mM: NaCl, 116; KCl, 5;
Data Analysis and Statistics
Data are presented as means 6 standard error to the mean
1.1; NaH2PO4, 0.35; NaHCO3, 27; glucose, 10;
mannitol, 16; Na-Pyruvate, 2; CaCl
(sem) of n experiments obtained from n different animals. In vivo
2, 1.8) continuously oxygen-
ated with a 95% O
and ex vivo cardiac parameters in baseline as well as protein
2, 5% CO2 gas mixture to maintain a pH 7.4.
Hearts were perfused at a constant flow-rate of 14 mL.min21 at
expressions were compared using Student's t test for unpaired
37.060.4uC. After heart beatings became regular, left auricle was
data. Concentration-response curves were compared with a two-
gently cut off to insert a small latex balloon, connected to a
way ANOVA (concentration, treatments) for repeated measures
pressure transducer to record LV pressure, into the LV via mitral
followed, when appropriated, by a Bonferroni's multiple compar-
valves. Before starting record, the minimum pressure into the
isons test. Differences were considered significant if P,0.05.
balloon was adjusted to 8–15 mmHg. Cardiac parameters wererecorded using IOX1.5.7 software (EMKA Technologies): inotro-
Drugs and Chemicals
pism, lusitropism and chronotropism were respectively evaluated
by measuring the contraction velocity (dP/dt
max), the relaxation
min) and the heart rate (HR). After the stabilization
dro chloride, L-748,337, N-[[3-[(2S)-2-Hydroxy-3-[[2-[4-[(phenylsulfo-
Table 3. In vivo cardiac parameters obtained by LV
catheterization in Ctrl and Dox-CM rats, at d35.
forskolin and PTX were obtained from Tocris Bioscience (Bristol,UK), isoproterenol was obtained from Sigma-Aldrich (St QuentinFallavier, France) and SR 58611A, [(RS)-N-[(25)-7-ethxycarbonyl-
ethanamide hydrochloride] was a generous gift from Sanofi-
Synthe´labo (Montpellier, France). All drugs were dissolved in distilled
water, excepted isoproterenol which was solubilised in 1% acid
ascorbic and L-748,337 and forskolin which were solubilised in
LV diastolic function
dimethylsulfoxide (Sigma-Aldrich). The final concentration of the
solvent in the organ bath was less than 0.1% v.v21 and was used as
controls for the effect of the active drug.
LV loading conditions LVEDP (mmHg)
Ctrl: control; Dox-CM: Doxorubicin-induced cardiomyopathy; dP/dtmax: leftventricular contraction velocity; dP/dtmin: left ventricular relaxation velocity; LV:
Mortality and General Characteristics of Animals
left ventricle; LVEDP: left ventricular end diastolic pressure; Tau: index of leftventricular relaxation constant. Values are means 6 sem. *: P,0.05 vs Ctrl.
Dox-CM rats did not gain weight during the Dox-treatment,
whereas the body weight of Ctrl rats increased regularly. After the
PLOS ONE www.plosone.org
May 2013 Volume 8 Issue 5 e64711
Beta-Adrenoceptors in Doxorubicin Cardiomyopathy
Figure 4. b3-AR expression and function in Dox-CM hearts. A. Representative b3-AR immunoblotting at days 35 (d35) and 70 (d70). B. b3-ARprotein quantification at day 35. C. b3-AR protein quantification at day 70. Protein levels were quantified using Amersham ImageQuant RT-ECLcamera (GE Healthcare). The band signals were assessed by densitometry with ImageQuant TL software (GE Healthcare) and a ratio to thecorresponding GAPDH band intensity was calculated. D. Cardiac inotropic (dP/dtmax), E. lusitropic (dP/dtmin) and F. chronotropic (HR) effects ofincreasing concentrations of SR 58611A (1029 to 1025 M) were evaluated on isolated perfused hearts. **: P,0.001 vs Ctrl. Ctrl: control, Dox-CM:Doxorubicin-induced cardiomyopathy.
doi:10.1371/journal.pone.0064711.g004
PLOS ONE www.plosone.org
May 2013 Volume 8 Issue 5 e64711
Beta-Adrenoceptors in Doxorubicin Cardiomyopathy
end of Dox-treatment, the rat body weight increased again, but
intensity in Dox-CM and Ctrl (P = 0.192), whereas, at d70, b3-ARs
remained significantly lower than those of Ctrl rats, and reached a
were down-expressed in Dox-CM hearts compared to Ctrl hearts
plateau from 5 weeks after the end of Dox-treatment (day 49;
(237.267.9%; P,0.001) (Figures 4A–C). In Ctrl isolated heart, at
Figure 1A). In addition, Dox-CM rats became lethargic compared
d35, b3-AR stimulation performed by cumulative increasing
to Ctrl one.
concentrations of SR 58611A produced no significant effect on
The mortality rate was 25.0% at d35 and 58.3% at d70 in Dox-
heart rate and dP/dtmax at any concentration (P = 0.239 and
CM when no mortality was observed in Ctrl (Figure 1B). At d35,
P = 0.385, respectively) (Figures 4D, F) and decreased dP/dtmin
all surviving Dox-CM rats presented a hepatomegaly and 79.4%
only at the higher concentration of SR 58611A (1025M)
of them presented ascites (30.865.8 mL), whereas at d70 no
(P = 0.004) (Figure 4E). Compared to Ctrl hearts, b3-AR
hepatomegaly was observed and ascites was present in 38.9% of
stimulation in Dox-CM hearts produced a significant decrease in
Dox-CM rats (35.764.9 mL). Whole heart and LV weights from
dP/dtmax only at the higher concentration of SR 58611A (1025M)
Dox-CM rats were significantly smaller than those of Ctrl animals
(P,0.001) (Figure 4D) and had no effect on dP/dtmin nor heart
both at d35 and d70, and they were still smaller after
rate (P = 0.406 and P = 0.107, respectively) (Figures 4E, F).
normalization to the tibia length. However, heart/body weightratios and LV/body weight ratios were similar between Ctrl and
b1-adrenoceptor Expression and Function
Dox-CM rats at d35 when it was increased in Dox-CM group
By western-blot, the antibody directed against b1-AR detected a
compared to Ctrl at d70 (Table 2).
band at 72 kDa (Figure 5A) whose intensity was significantlydecreased in Dox-CM LV at d35 by 268.266.1% (P = 0.002 vs
Cardiac Function Obtained by Echocardiography-
Ctrl) (Figure 5B) and at d70 by 275.3614.0% (P,0.001 vs Ctrl)
(Figure 5C). In Ctrl heart, at d35, b1-AR stimulation induced a
Echocardiography-Doppler analyses were performed at d35
concentration-dependant increase in dP/dtmax, dP/dtmin and
and d70. Heart rate, measured before all echocardiographic
heart rate with a maximal effect observed at a concentration of
acquisitions, was monitored in order to be similar between both
1025 M (P,0.001, each) (Figures 5D–F). The heart rate and the
groups (d35: Ctrl: 36466 bpm, Dox-CM: 37166 bpm, P = 0.427;
positive inotropic and lusitropic effects were unchanged in Dox-
d70: Ctrl: 36866 bpm, Dox-CM: 337617 bpm, P = 0.079).
CM isolated hearts compared to Ctrl hearts (P = 0.544, P = 0.772
Representative images obtained by echocardiography in short
and P = 0.667, respectively) (Figures 5D–F).
axis view of a two-dimensionally directed M-mode at d35 areshown in Figure 2.
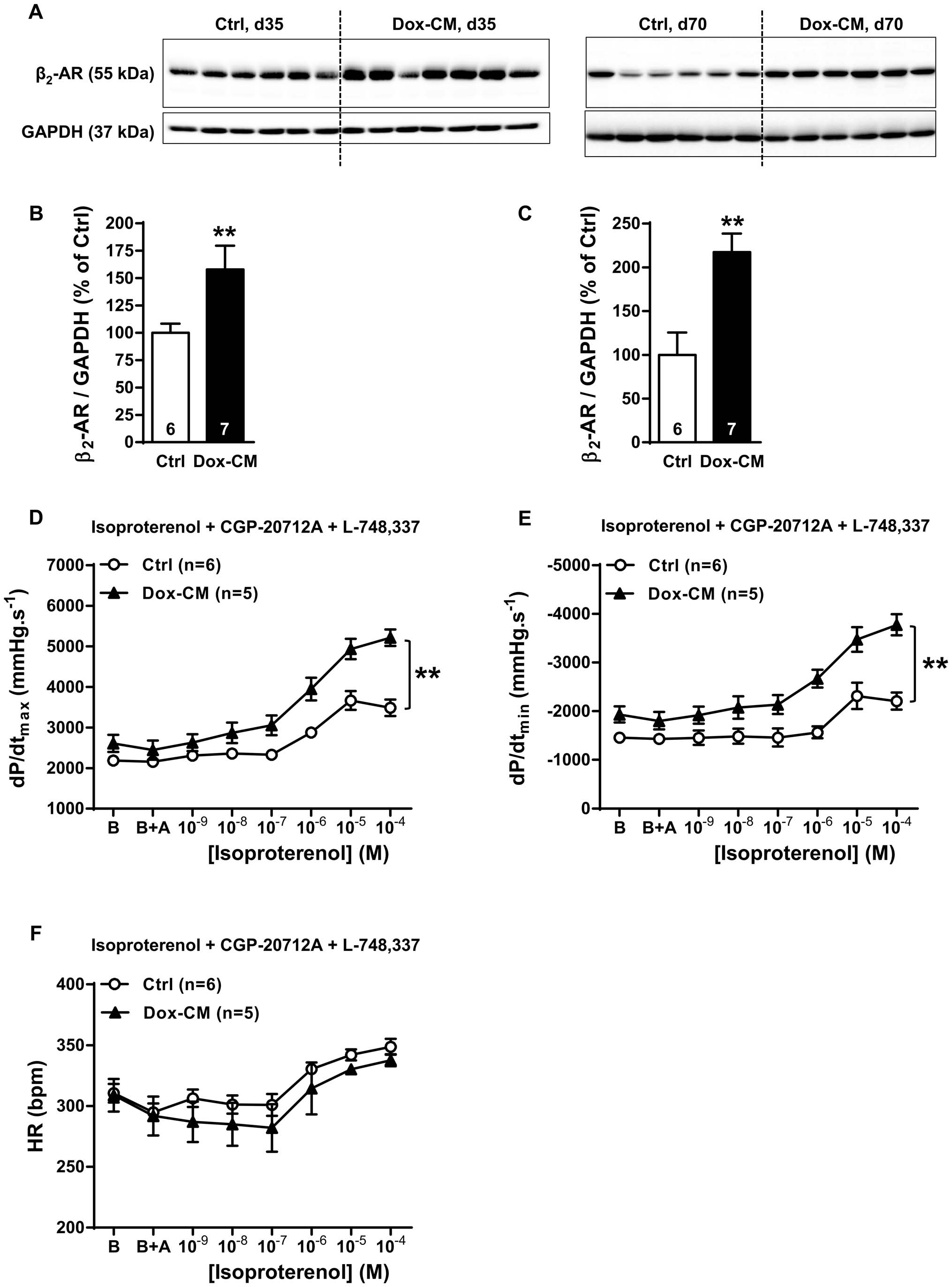
b2-adrenoceptor Expression and Function
The dPWth decreased in Dox-CM compared to Ctrl hearts at
By western-blot, the antibody directed against b2-AR detected a
d35 (29.563.3%, P = 0.055) and was significantly thinner at d70
band at 55 kDa (Figure 6A) whose intensity was significantly
(220.564.4%, P,0.001) (Figure 3A). LVESV was similar in both
increased in Dox-CM LV at d35 by +57.9621.6% (P = 0.039 vs
groups at d35 as at d70 (P = 0.199 and P = 0.116, respectively). On
Ctrl) (Figure 6B) and at d70 by +173.7635.1% (P,0.001 vs Ctrl)
the contrary, LVEDV was reduced in Dox-CM compared to Ctrl
(Figure 6C). At d35, b2-AR stimulation induced a similar
at d35 (212.661.9%, P,0.001) as at d70 (221.768.7%,
concentration-dependant increase of heart rate between Ctrl and
P = 0.050) (Figure 3B).
Dox-CM hearts (P = 0.695) (Figure 6F). In Ctrl isolated hearts, b2-
Compared to Ctrl, Dox-CM LVs were characterized at d35 by
AR stimulation induced a concentration-dependant increase of
mild but significant decreases in LVEF (-14.262.0%; P,0.001)
dP/dtmax and dP/dtmin (P,0.001 and P = 0.005, respectively)
and LVSF (223.462.7%; P,0.001), that were more pronounced
(Figures 6D, E) but at a lesser extent than b1-AR one. In Dox-CM
at d70 (225.266.2%, P,0.001 and 234.967.4%, P,0.001,
hearts, the maximal effects induced by b2-AR stimulation on dP/
respectively) (Figures 3C, D). Moreover, Dox-CM hearts presented
dtmax and dP/dtmin, compared to Ctrl, were both increased by
an alteration in longitudinal deformation, since Sa was decreased
+108.0618.2% (P,0.001) and +155.3626.8% (P,0.001), respec-
at d35 (231.266.5%; P = 0.001) as at d70 (248.6610.3%,
tively (Figures 6D, E), and reached a similar level than this
P,0.001) (Figure 3E) whereas radial deformation was only
produced by b1-AR stimulation.
observed at d70 (238.764.9%, P = 0.031) (Figure 3F). In Dox-CM rats, IVRT value was significantly increased at d35
Gs Protein Expression and Adenylyl Cyclase Stimulation
(+25.763.4%, P,0.001) as at d70 (+31.1610.6%, P = 0.003)
By western-blot, we detected two bands for Gsa protein
(Figure 3G) albeit the LV filling pressures, evaluated by E/Ea
expression: a short one at 45 kDa and a long one at 52 kDa
ratio, were similar in Ctrl and Dox-CM rats at both d35 and d70
(Figure 7A). The expression of both forms was unchanged in Dox-
(P = 0.776 and P = 0.071, respectively) (Figure 3H).
CM hearts at both d35 and d70 (Figures 7B, C). In Ctrl and Dox-CM isolated hearts, at d35, forskolin induced a similar concen-
Cardiac Function Obtained by Left Ventricle
tration-dependant increase of heart rate between Ctrl and Dox-
CM hearts (P = 0.941) (Figure 7F). In Ctrl group, forskolin induced
As our study consists to identify molecular target at early stage
a concentration-dependant increase of dP/dtmax (P,0.001) and
of Dox-CM, we only performed LV catheterization at d35. Basal
dP/dtmin (P = 0.010) (Figures 7D, E). In Dox-CM isolated hearts
heart rate, measured before all pressure acquisitions, was increased
compared to Ctrls, the forskolin effects on dP/dtmax and dP/dtmin
in Dox-CM rats compared to Ctrl (P = 0.007) (Table 3). Dox-CM
were increased by +43.2610.3% (P = 0.004) and +61.1610.7%
rats presented a decreased Tau (210.062.2%; P = 0.009) albeit
(P = 0.002), respectively (Figures 7D, E).
dP/dtmax and dP/dtmin were unchanged (Table 3). LVEDP wasunchanged between both groups (P = 0.216).
Gi Protein Expression and its Involvement in b2-ARCardiac Contractility
b3-adrenoceptor Expression and Function
Gia2 protein expression was detected by one band of 35 kDa
Using western-blot, we detected b3-AR protein at a band of
(Figure 8A) whose intensity was similar both in Ctrl and Dox-CM
68 kDa (Figure 4A). At d35, the detected band presented a similar
hearts at d35 (P = 0.184 vs Ctrl) (Figure 8B), but was increased in
PLOS ONE www.plosone.org
May 2013 Volume 8 Issue 5 e64711
Beta-Adrenoceptors in Doxorubicin Cardiomyopathy
Figure 5. Impaired b1-AR expression in Dox-CM hearts was not associated to an impaired cardiac b1-AR function. A. Representative b1-AR immunoblotting at days 35 (d35) and 70 (d70). B. b1-AR protein quantification at day 35. C. b1-AR protein quantification at day 70. Protein levelswere quantified using Amersham ImageQuant RT-ECL camera (GE Healthcare). The band signals were assessed by densitometry with ImageQuant TLsoftware (GE Healthcare) and a ratio to the corresponding GAPDH band intensity was calculated. D. Cardiac inotropic (dP/dtmax), E. lusitropic (dP/dtmin) and F. chronotropic (HR) effects of increasing concentrations of isoproterenol (1029 to 1025 M) in the presence of 1026 M ICI-118,551 a b2-ARantagonist and 1026 M of L-748,337 a b3-AR antagonist were evaluated on isolated perfused hearts. B: Baseline; B+A; Baseline in presence ofantagonists. **: P,0.001 vs Ctrl. Ctrl: control, Dox-CM: Doxorubicin-induced cardiomyopathy.
doi:10.1371/journal.pone.0064711.g005
PLOS ONE www.plosone.org
May 2013 Volume 8 Issue 5 e64711
Beta-Adrenoceptors in Doxorubicin Cardiomyopathy
PLOS ONE www.plosone.org
May 2013 Volume 8 Issue 5 e64711
Beta-Adrenoceptors in Doxorubicin Cardiomyopathy
Figure 6. b2-AR expression and function are increased in Dox-CM hearts. A. Representative b2-AR immunoblotting at days 35 (d35) and 70(d70). B. b2-AR protein quantification at day 35. C. b2-AR protein quantification at day 70. Protein levels were quantified using Amersham ImageQuantRT-ECL camera (GE Healthcare). The band signals were assessed by densitometry with ImageQuant TL software (GE Healthcare) and a ratio to thecorresponding GAPDH band intensity was calculated. D. Cardiac inotropic (dP/dtmax), E. lusitropic (dP/dtmin) and F. chronotropic (HR) effects ofincreasing concentrations of isoproterenol (1029 to 1024 M) in presence of 1026 M of CGP-20712A a b1-AR antagonist and 1026 M of L-748,337 a b3-AR antagonist were evaluated on isolated perfused hearts. B: Baseline; B+A; Baseline the in presence of antagonists. **: P,0.001 vs Ctrl; Ctrl: control,Dox-CM: Doxorubicin-induced cardiomyopathy.
doi:10.1371/journal.pone.0064711.g006
Dox-CM hearts at d70 by +118.1637.6% (P = 0.009 vs Ctrl)
compared to controls but a significantly reduced longitudinal
(Figure 8C). Ex vivo, at d35, Gi protein inhibition by PTX pre-
strain with preserved radial contractility [34].
treatment produced no effect in heart rate in both groups
In human, Dox administered to adults (for breast cancer, for
(P = 0.346) (Figure 8F). PTX-pretreatment induced a significant
example) is well known to induce chronic cardiotoxicity where
increase of b2-AR stimulation in Ctrl isolated hearts (dP/dtmax:
cardiac morphologic and functional alterations are close to those
+64.469.8%; P,0.001; dP/dtmin: +128.0620.8%; P,0.001)
of dilated cardiomyopathy [3]. However, when administered in
(Figures 8D, E) but induced no change of these two parameters
children, Dox can induce restrictive cardiomyopathy with normal
in Dox-CM hearts (P = 0.173 and P = 0.451, respectively)
LV dimensions [35]. Those findings, are consistent with those of
(Figures 8D, E).
other long-term follow-up studies conducted in other groups ofanthracycline-treated childhood cancer survivors [36]. In our
study, Dox was administered to young rats (7 week-old). At d35,we observed diastolic dysfunction with normal systolic function
This study demonstrated for the first time in Dox-cardiotoxicity,
that could support the hypothesis of a restrictive-cardiomyopathy.
b2-AR expression was increased at the early stage of the pathology,
At d70, the development of systolic dysfunction could suggest
with an increase of b2-AR-induced contractility. Furthermore, b1-
restrictive cardiomyopathy worsening with increase LV dimen-
AR function was preserved in spite of decreased b1-AR protein
sions. Indeed, although LVEDDs were not statistically different
expression and could be explained by an increase of AC
between Ctrl and Dox-CM rats at d70, it is important to note that
expression and/or activity as illustrated by an increased for-
LV dPWth was significantly decreased in Dox-CM compared to
skolin-induced contractility in isolated hearts from Dox-CM rats.
Ctrl rats, strengthening the notion of LV morphologic changes in
In our study, the body weight of Dox-CM rats was smaller than
those rats. We could hypothesize that a period of 56 days post-
in Ctrl one as previously described by other studies [8,24]. This
treatment was a too short duration of the disease evolution to
has been related to a decrease in food consumption [25,26]. In our
observe a significant LV dilation in Dox-CM rats. Indeed, in
experimental conditions, we have, 35 days after the beginning of
human HF, the dilation is considered as the last stage of ischemic
Dox-treatment, 25% of mortality and rats presented a large
or non ischemic cardiomyopathy evolution as in Dox-CM. In this
amount of ascites. Those observations were in agreement with
latter disease, it is well known that cardiomyopathy and thus LV
other studies that reported a mortality rate fluctuating between
dilation occur up to decades after exposure [37].
19% and 45% [27,28] and a volume of ascites generally varying
In our Dox-CM model, we reported a remodeling in b-AR
from 30 mL to 140 mL [24,28–30]. However, as Dox induced a
expression and function. Three weeks after last Dox-injection
multi-organ toxicity, it could be not excluded that mortality and
(d35), b3-AR protein expression was unchanged and a slight
ascites observed in rats could be due, at least in part, to liver and
negative inotropic effect was obtained in Dox-CM hearts at the
kidney damages. Seventy days after the beginning of Dox-
higher concentration of SR 58611A (1025 M). Our data suggest
treatment, mortality rate increased to 58% and ascites was still
that in early chronic Dox-cardiotoxicity, b3-AR could not play a
observed. Very few studies reported long term effects of Dox-
major role in the cardiac alterations. At a later stage (d70), we
treatment and data on mortality and ascites are very heteroge-
observed a decreased b3-AR protein expression in Dox-CM hearts
neous. Whereas a study observed 50% mortality rate 6 weeks after
albeit another team reported an overexpression of b3-AR protein
the last Dox-injection, associated with a very large amount of
at the same stage [11]. Several hypotheses could explain those
ascites [25] others did not observed mortality eight weeks after the
discrepancies. First, the rat strain used in the study is different
last Dox-injection [11]. Also, others did not reported mortality and
(Wistar vs Sprague-Dawley) and several studies reported that
observed a decrease in ascites amount between days 40 and 70
protein expression could be different between Sprague-Dawley
and Wistar rats [38,39] albeit no study compared b-AR subtypes
During echocardiographic acquisitions, heart rate was moni-
expression between these two rat strains. Second, in the Sun's
tored in order to have similar heart rate in both groups, allowing a
study, cardiac b-ARs expressions were assessed in Ctrl rats
comparison of echocardiographic parameters. At d35, Dox-CM
compared to Dox-treated rats which were also Sham-castrated
rats presented a slight alteration of systolic function as illustrated
(scrotum incision); no surgery being performed in Ctrl rats.
by a mild decrease in LVEF and LVSF and in LV longitudinal
Therefore, b-AR protein expressions were assessed in a Dox-CM
deformation. Dox-CM rats presented also a mild diastolic
model different from our one used in our study.
dysfunction as suggested by the increased IVRT and Tau values.
In our Dox-CM model, b1-AR protein expression was
At d70, decreases in LVEF, LVSF and LV longitudinal
decreased at both stages. This result is in agreement with those
deformation were exacerbated whereas diastolic dysfunction was
reported for b1-AR protein expression both in rabbits [40] and in
unchanged. As diastolic dysfunction frequently occurs before
rats [8,11]. Surprisingly, albeit a decreased b1-AR expression at
systolic dysfunction [31–33], we could suggest that in our animal
d35, the cardiac b1-AR responses were preserved. Several
model, at d35, systolic function just began to be altered, meaning
hypotheses could explain this feature. Firstly, a b1-AR reserve
that this model corresponds to an early chronic Dox-cardiotox-
could be present and recruited in such conditions and, secondly,
icity. It is important to note that our results are very close to that
an increase in b1-AR signaling pathway can be evoked. We
observed in women who received anthracycline-treatment for
showed that Dox-treatment did not change Gsa protein expres-
breast cancer. Indeed, these patients had preserved LVEF
sion, as it was reported in other study [8,11], but increased
PLOS ONE www.plosone.org
May 2013 Volume 8 Issue 5 e64711
Beta-Adrenoceptors in Doxorubicin Cardiomyopathy
Figure 7. Gsa protein expression and forskolin response. A. Representative Gsa immunoblotting at days 35 (d35) and 70 (d70). B. Gsa proteinquantification at day 35. C. Gsa protein quantification at day 70. Protein levels were quantified using Amersham ImageQuant RT-ECL camera (GE Healthcare).
The band signals were assessed by densitometry with ImageQuant TL software (GE Healthcare) and a ratio to the corresponding GAPDH band intensity wascalculated. D. Cardiac inotropic (dP/dtmax), E. lusitropic (dP/dtmin) and F. chronotropic (HR) effects of increasing concentrations of the adenylyl cyclase activatorforskolin (3.1028 to 1025 M) were evaluated on isolated perfused hearts. *: P,0.05 vs Ctrl. Ctrl: control, Dox-CM: Doxorubicin-induced cardiomyopathy.
doi:10.1371/journal.pone.0064711.g007
PLOS ONE www.plosone.org
May 2013 Volume 8 Issue 5 e64711
Beta-Adrenoceptors in Doxorubicin Cardiomyopathy
PLOS ONE www.plosone.org
May 2013 Volume 8 Issue 5 e64711
Beta-Adrenoceptors in Doxorubicin Cardiomyopathy
Figure 8. Gi protein expression and involvement in b2-AR cardiac contractility. A. Representative Gia2 immunoblotting at days 35 (d35)and 70 (d70). B. Gia2 protein quantification at day 35. C. Gia2 protein quantification at day 70. Protein levels were quantified using AmershamImageQuant RT-ECL camera (GE Healthcare). The band signals were assessed by densitometry with ImageQuant TL software (GE Healthcare) and aratio to the corresponding GAPDH band intensity was calculated. D. Cardiac inotropic (dP/dtmax), E. lusitropic (dP/dtmin) and F. chronotropic (HR)effects of increasing concentrations of isoproterenol (1029 to 1024 M) in the presence of 1026 M of CGP-20712A and 1026 M of L-748,337 wereevaluated on isolated perfused hearts pre-treated or not with 4 mg.L21 of pertussis toxin (PTX) a Gi protein inhibitor. B: Baseline; B+A; Baseline in thepresence of antagonists. *: P,0.05 vs Ctrl; **: P,0.001 vs Ctrl. Ctrl: control, Dox-CM: Doxorubicin-induced cardiomyopathy.
doi:10.1371/journal.pone.0064711.g008
forskolin response, suggesting an increase in AC expression and/or
HF [49–54]. Concerning a putative detrimental effect of b2-AR, it
activity. This surprising data contrasts with other studies which
could be proposed to use b2-AR blocker. However, it is important
reported a decreased AC activity [41,42] or no change [43].
to note that b2-AR antagonists by blocking other physiological
However, those differences seem to be due to the cardiotoxicity
functions regulated by b2-AR, could also have detrimental effects:
level since the Dox cumulative doses used were 24, 24.75 and
(i) a vasoconstriction because b2-AR is one of the most important
6 mg.kg21, respectively. Thus, the increased AC response to
vascular b-AR subtype whose activation induces a vasodilation in
forskolin observed in our study could be a compensatory
several vascular beds (including coronary vessels), (ii) a broncho-
mechanism at early stage of Dox-cardiotoxicity to maintain
constriction due to the reduction of bronchodilation induced by
cardiac contractility and could explain, at least in part, the
pulmonary b2-AR,…
preserved b1-AR response. However, as b1-ARs are involved in
Thus, we rather suggest that further experiments must be
pro-apoptotic effects, b1-AR could be also responsible for some
performed to identify the most suitable effector of the cardiac b2-
Dox cardiotoxic effects as previously suggested [9,10].
AR pathway. Then, according to the results of this next important
Finally, we reported, for the first time, in chronic Dox-
study, we hope that we could propose a new therapeutic target for
cardiotoxicity, a b2-AR protein overexpression. At d35, in Ctrl
isolated heart, specific b2-AR stimulation induced positive
In conclusion, we have shown for the first time in rat Dox-CM,
inotropic and lusitropic responses but to a lesser extent than b1-
an increase of b2-AR protein expression at early stage of the
AR one. This lower response could be explained by a more
pathology, associated to an increased contractility. Furthermore,
compartmentalized cAMP signaling of b2-AR [44]. Surprisingly,
we observed a preserved b1-AR function albeit b1-AR was
in Dox-CM hearts, b2-AR inotropic effect was increased and
reduced. Clinical studies have suggested no beneficial effect of
reached a similar level than b1-AR stimulation. This increase
could be due to several mechanisms. Firstly, we show that b
1-AR blocker, in Dox-treated lymphoma patients
[15] but a beneficial effect of nebivolol, a b
protein expression was increased in Dox-CM LV and could lead to
1-AR blocker associated
3-AR agonistic properties against anthracycline-induced
2-AR response. Secondly, cardiac b2-AR could be
cardiomyopathy in patients with breast cancer [55]. However, the
linked both to Gs and Gi proteins [45,46]. In Dox-CM LV, Gia2
clinical roles of b
protein expression was not modified at d35 but it was overex-
2-AR remain to elucidate in order to determine
pressed at d70 of Dox-CM, as reported previously at this latter
2-AR activation or blockade could prevent cardiac
alterations due to anthracycline.
stage by Sun et al. [11]. Surprisingly, at d35, in Ctrl hearts, the Giinhibition by PTX allowed to increase b2-AR response in order toreach the same level that obtained in Dox-CM in the absence of
PTX treatment, whereas Gi inhibition in Dox-CM hearts slightly
We thank Chrystelle Bailly, Ste´phanie Lemarchand-Minde´, Amandine
increased b2-AR response. These data suggest that, in Dox-CM,
Lefebvre and Patricia Charpentier for animal care, and Morte´za Erfanian
b2-AR was mainly linked to Gs.
for its technical assistance.
To know whether enhanced b2-AR/Gs signaling observed in
our study is a beneficial compensatory mechanism or a
Author Contributions
detrimental effect is still to be determined. To support the
Conceived and designed the experiments: NM BR CG. Performed the
hypothesis of a beneficial effect of b2-AR agonist, it has been
experiments: NM NP AG BL. Analyzed the data: NM NP AG BL BR CG.
shown that b2-AR/Gi signaling can mediate cardiac protective
Contributed reagents/materials/analysis tools: JNT. Wrote the paper: NM
effects by activating cell surviving pathway [10,47,48], and also
in vivo studies reported beneficial effects of b2-agonist therapy in
1. Connors JM (2005) State-of-the-art therapeutics: Hodgkin's lymphoma. J Clin
8. Kenk M, Thackeray JT, Thorn SL, Dhami K, Chow BJ, et al. (2010) Alterations
Oncol 23: 6400–6408.
of pre- and postsynaptic noradrenergic signaling in a rat model of adriamycin-
2. Grimer R, Judson I, Peake D, Seddon B (2010) Guidelines for the management
induced cardiotoxicity. J Nucl Cardiol 17: 254–263.
of soft tissue sarcomas. Sarcoma 2010: 506182.
9. Bernstein D, Fajardo G, Zhao M, Urashima T, Powers J, et al. (2005)
3. Ferreira AL, Matsubara LS, Matsubara BB (2008) Anthracycline-induced
Differential cardioprotective/cardiotoxic effects mediated by beta-adrenergic
cardiotoxicity. Cardiovasc Hematol Agents Med Chem 6: 278–281.
receptor subtypes. Am J Physiol Heart Circ Physiol 289: H2441–2449.
4. Nakamura T, Ueda Y, Juan Y, Katsuda S, Takahashi H, et al. (2000) Fas-
10. Fajardo G, Zhao M, Powers J, Bernstein D (2006) Differential cardiotoxic/
mediated apoptosis in adriamycin-induced cardiomyopathy in rats: In vivo
cardioprotective effects of beta-adrenergic receptor subtypes in myocytes and
study. Circulation 102: 572–578.
fibroblasts in doxorubicin cardiomyopathy. J Mol Cell Cardiol 40: 375–383.
5. Ueno M, Kakinuma Y, Yuhki K, Murakoshi N, Iemitsu M, et al. (2006)
11. Sun J, Fu L, Tang X, Han Y, Ma D, et al. (2011) Testosterone modulation of
Doxorubicin induces apoptosis by activation of caspase-3 in cultured
cardiac beta-adrenergic signals in a rat model of heart failure. Gen Comp
cardiomyocytes in vitro and rat cardiac ventricles in vivo. J Pharmacol Sci
Endocrinol 172: 518–525.
101: 151–158.
12. Dery AS, Hamilton LA, Starr JA (2011) Nebivolol for the treatment of heart
6. Takemura G, Fujiwara H (2007) Doxorubicin-induced cardiomyopathy from
failure. Am J Health Syst Pharm 68: 879–886.
the cardiotoxic mechanisms to management. Prog Cardiovasc Dis 49: 330–352.
13. Rozec B, Erfanian M, Laurent K, Trochu JN, Gauthier C (2009) Nebivolol, a
7. Chatterjee K, Zhang J, Honbo N, Karliner JS (2010) Doxorubicin cardiomy-
vasodilating selective beta(1)-blocker, is a beta(3)-adrenoceptor agonist in the
opathy. Cardiology 115: 155–162.
nonfailing transplanted human heart. J Am Coll Cardiol 53: 1532–1538.
PLOS ONE www.plosone.org
May 2013 Volume 8 Issue 5 e64711
Beta-Adrenoceptors in Doxorubicin Cardiomyopathy
14. Kalay N, Basar E, Ozdogru I, Er O, Cetinkaya Y, et al. (2006) Protective effects
35. Lipshultz SE, Lipsitz SR, Sallan SE, Dalton VM, Mone SM, et al. (2005)
of carvedilol against anthracycline-induced cardiomyopathy. J Am Coll Cardiol
Chronic progressive cardiac dysfunction years after doxorubicin therapy for
48: 2258–2262.
childhood acute lymphoblastic leukemia. J Clin Oncol 23: 2629–2636.
15. Georgakopoulos P, Roussou P, Matsakas E, Karavidas A, Anagnostopoulos N,
36. Brouwer CA, Gietema JA, van den Berg MP, Bink-Boelkens MT, Elzenga NJ, et
et al. (2010) Cardioprotective effect of metoprolol and enalapril in doxorubicin-
al. (2006) Long-term cardiac follow-up in survivors of a malignant bone tumour.
treated lymphoma patients: a prospective, parallel-group, randomized, con-
Ann Oncol 17: 1586–1591.
trolled study with 36-month follow-up. Am J Hematol 85: 894–896.
37. Steinherz LJ, Steinherz PG, Tan CT, Heller G, Murphy ML (1991) Cardiac
16. Senba M, Buziba N, Mori N, Morimoto K, Nakamura T (2011) Increased
toxicity 4 to 20 years after completing anthracycline therapy. JAMA 266: 1672–
prevalence of Kaposis sarcoma-associated herpesvirus in the Kaposis sarcoma-
endemic area of western Kenya in 1981–2000. Acta Virol 55: 161–164.
38. Kishida T, Muto S, Hayashi M, Tsutsui M, Tanaka S, et al. (2008) Strain
17. Cardinale D, Colombo A, Lamantia G, Colombo N, Civelli M, et al. (2010)
differences in hepatic cytochrome P450 1A and 3A expression between Sprague-
Anthracycline-induced cardiomyopathy: clinical relevance and response to
Dawley and Wistar rats. J Toxicol Sci 33: 447–457.
pharmacologic therapy. J Am Coll Cardiol 55: 213–220.
39. Jamesdaniel S, Ding D, Kermany MH, Jiang H, Salvi R, et al. (2009) Analysis of
18. Schiller NB, Shah PM, Crawford M, DeMaria A, Devereux R, et al. (1989)
cochlear protein profiles of Wistar, Sprague-Dawley, and Fischer 344 rats with
Recommendations for quantitation of the left ventricle by two-dimensional
normal hearing function. J Proteome Res 8: 3520–3528.
echocardiography. American Society of Echocardiography Committee on
40. Dhein S, Garbade J, Rouabah D, Abraham G, Ungemach FR, et al. (2006)
Standards, Subcommittee on Quantitation of Two-Dimensional Echocardio-
Effects of autologous bone marrow stem cell transplantation on beta-
grams. J Am Soc Echocardiogr 2: 358–367.
adrenoceptor density and electrical activation pattern in a rabbit model of
19. Liu J, Rigel DF (2009) Echocardiographic examination in rats and mice.
non-ischemic heart failure. J Cardiothorac Surg 1: 17.
Methods Mol Biol 573: 139–155.
41. Calderone A, de Champlain J, Rouleau JL (1991) Adriamycin-induced changes
20. Mulvagh S, Quinones MA, Kleiman NS, Cheirif J, Zoghbi WA (1992)
to the myocardial beta-adrenergic system in the rabbit. J Mol Cell Cardiol 23:
Estimation of left ventricular end-diastolic pressure from Doppler transmitral
flow velocity in cardiac patients independent of systolic performance. J Am Coll
42. Nagami K, Yoshikawa T, Suzuki M, Wainai Y, Anzai T, et al. (1997) Abnormal
Cardiol 20: 112–119.
beta-adrenergic transmembrane signaling in rabbits with adriamycin-induced
21. Popovic ZB, Benejam C, Bian J, Mal N, Drinko J, et al. (2007) Speckle-tracking
cardiomyopathy. Jpn Circ J 61: 249–255.
echocardiography correctly identifies segmental left ventricular dysfunction
43. Fu LX, Bergh CH, Hoebeke J, Liang QM, Sjogren KG, et al. (1991) Effect of
induced by scarring in a rat model of myocardial infarction. Am J Physiol Heart
metoprolol on activity of beta-adrenoceptor coupled to guanine nucleotide
Circ Physiol 292: H2809–2816.
binding regulatory proteins in adriamycin-induced cardiotoxicity. Basic Res
22. Rautureau Y, Toumaniantz G, Serpillon S, Jourdon P, Trochu JN, et al. (2002)
Cardiol 86: 117–126.
Beta 3-adrenoceptor in rat aorta: molecular and biochemical characterization
44. Nikolaev VO, Bunemann M, Schmitteckert E, Lohse MJ, Engelhardt S (2006)
and signalling pathway. Br J Pharmacol 137: 153–161.
Cyclic AMP imaging in adult cardiac myocytes reveals far-reaching beta1-
23. Bian JS, Zhang WM, Xia Q, Wong TM (1998) Phospholipase C inhibitors
adrenergic but locally confined beta2-adrenergic receptor-mediated signaling.
attenuate arrhythmias induced by kappa-receptor stimulation in the isolated rat
Circ Res 99: 1084–1091.
heart. J Mol Cell Cardiol 30: 2103–2110.
45. Pavoine C, Defer N (2005) The cardiac beta2-adrenergic signalling a new role
24. Lou H, Danelisen I, Singal PK (2004) Cytokines are not upregulated in
for the cPLA2. Cell Signal 17: 141–152.
adriamycin-induced cardiomyopathy and heart failure. J Mol Cell Cardiol 36:
46. Xiao RP, Zhu W, Zheng M, Cao C, Zhang Y, et al. (2006) Subtype-specific
alpha1- and beta-adrenoceptor signaling in the heart. Trends Pharmacol Sci 27:
25. Tong J, Ganguly PK, Singal PK (1991) Myocardial adrenergic changes at two
stages of heart failure due to adriamycin treatment in rats. Am J Physiol 260:
47. Zhu WZ, Zheng M, Koch WJ, Lefkowitz RJ, Kobilka BK, et al. (2001) Dual
modulation of cell survival and cell death by beta(2)-adrenergic signaling in adult
26. Ghibu S, Delemasure S, Richard C, Guilland JC, Martin L, et al. (2012) General
mouse cardiac myocytes. Proc Natl Acad Sci U S A 98: 1607–1612.
oxidative stress during doxorubicin-induced cardiotoxicity in rats: absence of
48. DeGeorge BR, Jr., Gao E, Boucher M, Vinge LE, Martini JS, et al. (2008)
cardioprotection and low antioxidant efficiency of alpha-lipoic acid. Biochimie
Targeted inhibition of cardiomyocyte Gi signaling enhances susceptibility to
94: 932–939.
apoptotic cell death in response to ischemic stress. Circulation 117: 1378–1387.
27. Iliskovic N, Singal PK (1997) Lipid lowering: an important factor in preventing
49. Ahmet I, Krawczyk M, Heller P, Moon C, Lakatta EG, et al. (2004) Beneficial
adriamycin-induced heart failure. Am J Pathol 150: 727–734.
effects of chronic pharmacological manipulation of beta-adrenoreceptor subtype
28. Morishima I, Matsui H, Mukawa H, Hayashi K, Toki Y, et al. (1998) Melatonin,
signaling in rodent dilated ischemic cardiomyopathy. Circulation 110: 1083–
a pineal hormone with antioxidant property, protects against adriamycin
cardiomyopathy in rats. Life Sci 63: 511–521.
50. Ahmet I, Krawczyk M, Zhu W, Woo AY, Morrell C, et al. (2008)
29. Danelisen I, Palace V, Lou H, Singal PK (2002) Maintenance of myocardial
Cardioprotective and survival benefits of long-term combined therapy with
levels of vitamin A in heart failure due to adriamycin. J Mol Cell Cardiol 34:
beta2 adrenoreceptor (AR) agonist and beta1 AR blocker in dilated
cardiomyopathy postmyocardial infarction. J Pharmacol Exp Ther 325: 491–
30. Iliskovic N, Panagia V, Slezak J, Kumar D, Li T, et al. (1997) Adriamycin
depresses in vivo and in vitro phosphatidylethanolamine N-methylation in rat
51. Ahmet I, Morrell C, Lakatta EG, Talan MI (2009) Therapeutic efficacy of a
heart sarcolemma. Mol Cell Biochem 176: 235–240.
combination of a beta1-adrenoreceptor (AR) blocker and beta2-AR agonist in a
31. Nagy AC, Cserep Z, Tolnay E, Nagykalnai T, Forster T (2008) Early diagnosis
rat model of postmyocardial infarction dilated heart failure exceeds that of a
of chemotherapy-induced cardiomyopathy: a prospective tissue Doppler imaging
beta1-AR blocker plus angiotensin-converting enzyme inhibitor. J Pharmacol
study. Pathol Oncol Res 14: 69–77.
Exp Ther 331: 178–185.
32. Stoddard MF, Seeger J, Liddell NE, Hadley TJ, Sullivan DM, et al. (1992)
52. Chakir K, Daya SK, Aiba T, Tunin RS, Dimaano VL, et al. (2009) Mechanisms
Prolongation of isovolumetric relaxation time as assessed by Doppler
of enhanced beta-adrenergic reserve from cardiac resynchronization therapy.
echocardiography predicts doxorubicin-induced systolic dysfunction in humans.
Circulation 119: 1231–1240.
J Am Coll Cardiol 20: 62–69.
53. Chakir K, Depry C, Dimaano VL, Zhu WZ, Vanderheyden M, et al. (2011)
33. Tassan-Mangina S, Codorean D, Metivier M, Costa B, Himberlin C, et al.
Galphas-biased beta2-adrenergic receptor signaling from restoring synchronous
(2006) Tissue Doppler imaging and conventional echocardiography after
contraction in the failing heart. Sci Transl Med 3: 100ra188.
anthracycline treatment in adults: early and late alterations of left ventricular
54. Zhu W, Petrashevskaya N, Ren S, Zhao A, Chakir K, et al. (2012) Gi-biased
function during a prospective study. Eur J Echocardiogr 7: 141–146.
beta2AR signaling links GRK2 upregulation to heart failure. Circ Res 110: 265–
34. Ho E, Brown A, Barrett P, Morgan RB, King G, et al. (2010) Subclinical
anthracycline- and trastuzumab-induced cardiotoxicity in the long-term follow-
55. Kaya MG, Ozkan M, Gunebakmaz O, Akkaya H, Kaya EG, et al. (2012)
up of asymptomatic breast cancer survivors: a speckle tracking echocardio-
Protective effects of nebivolol against anthracycline-induced cardiomyopathy: A
graphic study. Heart 96: 701–707.
randomized control study. Int J Cardiol.
PLOS ONE www.plosone.org
May 2013 Volume 8 Issue 5 e64711
Source: http://www.emkatechnologies.com/wp-content/uploads/2016/04/Merlet2013.pdf
Target cell availability and the successful suppression of HIV by hydroxyurea and didanosine Rob J. De Boer AIDS 1998, 12:1567–1570 Keywords: Hydroxyurea, immunosuppression, target cell availability, 72 weeks of ddI–HU treatment, three out of sixpatients had no detectable plasma virus, and that there
A Provider's Guide for the Care of Women with Physical Disabilities and Chronic Suzanne C. Smeltzer, RN, EdD, FAAN Professor & Director, Nursing Research Director, Health Promotion for Women with Disabilities Project Villanova University College of Nursing 800 Lancaster Avenue Villanova, PA 19085 Phone: 610-519-6828 Fax: 610-519-7650 Nancy C. Sharts-Hopko, RN, PhD, FAAN


