Levitra enthält Vardenafil, das eine kürzere Wirkdauer als Tadalafil hat, dafür aber schnell einsetzt. Männer, die diskret bestellen möchten, suchen häufig nach levitra kaufen ohne rezept. Dabei spielt die rechtliche Lage in der Schweiz eine wichtige Rolle.
Human embryonic stem cells derived by somatic cell nuclear transfer
Please cite this article in press as: Tachibana et al., Human Embryonic Stem Cells Derived by Somatic Cell Nuclear Transfer, Cell (2013),http://dx.doi.org/10.1016/j.cell.2013.05.006
Human Embryonic Stem Cells Derivedby Somatic Cell Nuclear Transfer
Masahito Tachibana,Paula Michelle Sparman,Nuria Marti Rebecca Tippner-Hedges,Hong Eunju Alimujiang Fulati,Hyo-Sang Lee,Hathaitip Keith Masterson,Janine Larson,Deborah Karen David David Diana Jeffrey Jensen,Phillip Patton,Sumita Gokhale,Richard L. Stouffer,Don Wolf,and Shoukhrat 1Division of Reproductive & Developmental Sciences, Oregon National Primate Research Center, Oregon Health & Science University, 505NW 185th Avenue, Beaverton, OR 97006, USA2Division of Reproductive Endocrinology, Department of Obstetrics and Gynecology, Oregon Health & Science University, 3181 SW SamJackson Park Road, Portland, OR 97239, USA3Department of Oral Biology, Faculty of Dentistry, Mahidol University, Bangkok 10400, Thailand4Women's Health Research Unit, Oregon Health & Science University, 3303 SW Bond Avenue, Portland, OR 79239, USA5Boston University School of Medicine, 72 East Concord Street, Boston, MA 02118, USA6Present address: Laboratory Animal Center, Osong Medical Innovation Foundation, Chungbuk 363-951, Republic of Korea*Correspondence:
cloning, the nature of reprogramming oocyte factors and theirmechanism of action remain largely unknown.
Reprogramming somatic cells into pluripotent em-
In humans, SCNT was envisioned as a means of generating
bryonic stem cells (ESCs) by somatic cell nuclear
personalized embryonic stem cells from patients' somatic cells,
transfer (SCNT) has been envisioned as an approach
which could be used to study disease mechanisms and ulti-
for generating patient-matched nuclear transfer (NT)-
mately for cell-based therapies (;
ESCs for studies of disease mechanisms and for
). However, the derivation of human nuclear transfer-em-bryonic stem cells (NT-ESCs) has not been achieved despite
developing specific therapies. Past attempts to pro-
numerous attempts during the past decade. The roadblock
duce human NT-ESCs have failed secondary to early
responsible for failure is early embryonic arrest of human
embryonic arrest of SCNT embryos. Here, we identi-
SCNT embryos precluding derivation of stable NT-ESCs. Typi-
fied premature exit from meiosis in human oocytes
cally, SCNT embryos fail to progress beyond the eight-cell stage,
and suboptimal activation as key factors that are
presumably due to an inability to activate critical embryonic
responsible for these outcomes. Optimized SCNT
genes from the somatic donor cell nucleus (;
approaches designed to circumvent these limita-
). In a few cases, when SCNT embryos did reach
tions allowed derivation of human NT-ESCs. When
the blastocyst stage, either stable ESCs were not recovered or
applied to premium quality human oocytes, NT-
derivation was not attempted (
ESC lines were derived from as few as two oocytes.
). Though the underlying cause of early developmental
NT-ESCs displayed normal diploid karyotypes and
arrest remains unclear, most of these studies involving humanoocytes applied SCNT protocols developed for nonprimate spe-
inherited their nuclear genome exclusively from
cies. Previously, we demonstrated that SCNT procedures, when
parental somatic cells. Gene expression and differ-
adapted to primates, succeeded in reprogramming rhesus ma-
entiation profiles in human NT-ESCs were similar to
caque adult skin fibroblasts into NT-ESCs (;
embryo-derived ESCs, suggesting efficient reprog-
). Therefore, we reasoned that, similar to
ramming of somatic cells to a pluripotent state.
other mammals, human MII oocytes must contain reprogram-ming activity.
Several recent observations are relevant. Removal of human
oocytes' nuclear genetic material (chromosomes) negatively im-
Cytoplasmic factors present in mature, metaphase II (MII)-ar-
pacts the cytoplast's subsequent ability to induce reprogram-
rested oocytes have a unique ability to reset the identity of trans-
ming (However, when a somatic cell nucleus
planted somatic cell nuclei to the embryonic state. Since the
is transplanted into an intact oocyte containing its own chromo-
initial discovery in amphibians (), somatic cell nu-
somes, the resulting polyploid embryos are able to develop to
clear transfer (SCNT) success in a range of different mammalian
blastocysts and support ESC derivation. One possible explana-
species has demonstrated that such reprogramming activity in
tion for these observations is that critical reprogramming factors
enucleated or spindle-free oocytes (cytoplasts) is universal
in human MII oocytes are physically associated with the chromo-
somes or spindle apparatus and are depleted or critically dimin-
However, despite numerous applications of SCNT for animal
ished upon enucleation. Alternatively, it is possible that one or
Cell
153, 1–11, June 6, 2013 ª2013 Elsevier Inc. 1
Please cite this article in press as: Tachibana et al., Human Embryonic Stem Cells Derived by Somatic Cell Nuclear Transfer, Cell (2013),http://dx.doi.org/10.1016/j.cell.2013.05.006
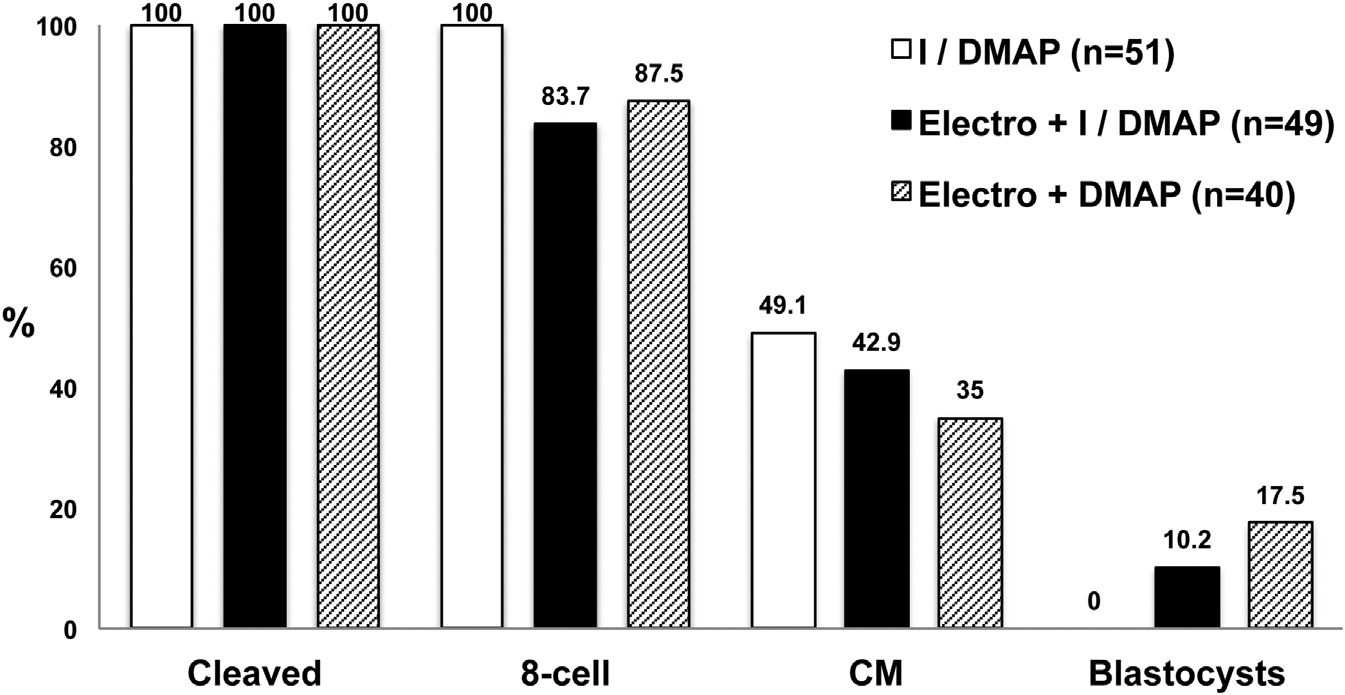
Figure 1. Development of Monkey SCNTEmbryos Reconstructed with OptimizedProtocolsAlthough HVJ-E fusion was efficient, SCNT con-structs required activation by electroporation forblastocyst formation. Elimination of ionomycinfrom the activation treatment further improvedblastocyst development. I, ionomycin; DMAP,6-DMAP; CM, compact morula.
See also and
virus of Japan (HVJ-E) to fuse nucleardonor cells with enucleated MII oocyteswhile maintaining cytoplasts in meiosis). Because oflimited oocyte availability, we first testedboth the feasibility and efficacy of HVJ-
more of the steps in SCNT—namely, oocyte enucleation, donor
E-based cell fusion on the development of rhesus macaque
cell nucleus introduction, or cytoplast activation—negatively
SCNT embryos.
impact cytoplast quality, rendering it incapable of inducing suffi-
The fusion rate of adult fibroblasts with cytoplasts was 100%
after HVJ-E treatment; however, and unexpectedly, the SCNT
In considering distinct biological features of human oocytes
embryos generated by HVJ-E fusion failed to progress beyond
that could be involved, we focused on our recent observation
the compact morula (CM) stage following standard ionomycin/
that meiotic arrest in human MII oocytes is unstable and can
DMAP (I/DMAP) activation. We previously demonstrated that
be easily perturbed by mechanical manipulations
monkey SCNT embryos produced by electrofusion developed
Earlier, we suggested that retention of meiosis-spe-
into blastocysts ;
cific activities in the cytoplast is critical for nuclear remodeling af-
Therefore, we postulated that exposure of the cytoplast to an
ter transplantation of an interphase-stage somatic cell nucleus
electropulse (electroporation) could be beneficial for SCNT re-
(). This remodeling is positively correlated
programming, perhaps as a supplemental activation stimulus.
with onward development of SCNT embryos after activation.
To test this possibility, we exposed HVJ-E-fused SCNT embryos
Therefore, we systematically evaluated modifications in oocyte
to electroporation before the standard I/DMAP activation treat-
enucleation and donor cell introduction that might work to retain
ment. Ten percent of SCNT embryos were capable of reaching
meiosis factors in human cytoplasts. We also determined that
the blastocyst stage Interestingly, this SCNT blasto-
routine cytoplast activation treatments were insufficient to sup-
cyst formation rate was unaffected even when exposure to
port subsequent human SCNT embryo development. We initially
ionomycin was omitted and SCNT embryos were activated
used rhesus macaque oocytes to evaluate factors affecting suc-
with electroporation followed by DMAP treatment
cessful SCNT reprogramming in a primate system. Subse-
Together, these results indicate that, although an electroporation
quently, we refined SCNT approaches with high-quality human
stimulus is not required for cell fusion, it is supportive of proper
oocytes donated by healthy volunteers and demonstrated that
cytoplast activation following SCNT.
methodological alterations significantly improve blastocyst for-
Histone deacetylase inhibitors, such as trichostatin A (TSA),
mation from human SCNT embryos. Moreover, we derived
have been associated with improved SCNT reprogramming in
several human NT-ESC lines from these embryos and validated
several mammalian species (;
that their nuclear DNA is an exclusive match to parental somatic
; We previously demonstrated enhanced
cells, whereas mitochondrial DNA originated almost exclusively
development of monkey SCNT embryos treated with 37.5 nM
from oocytes. We also conducted extensive pluripotency assays
TSA (from 4% up to 18% blastocyst development rate
on human NT-ESCs to verify reprogramming.
]). However, blastocyst quality and potential to giverise to stable ESCs remained unknown. Here, we plated 16 mon-
key SCNT blastocysts produced during TSA treatment on mitot-ically inactivated mouse embryonic fibroblast (mEF) feeders, but
SCNT Protocol Optimization in a Nonhuman Primate
none resulted in NT-ESC line isolation (We reasoned
that, although TSA treatment was promoting blastocyst forma-
Our recent studies demonstrated human MII oocyte sensitivity to
tion, high TSA concentrations may negatively affect blastocyst
premature activation induced by removal and reintroduction of
quality and epiblast lineage integrity. Therefore, we tested
meiotic spindles (and to the use of elec-
several lower TSA concentrations, as well as shorter exposure
trofusion in the context of cytoplast activation (
times, on monkey SCNT blastocyst development and ESC isola-
Consequently, our present investigation began with opti-
tion. Reducing the TSA concentration to 10 nM or shortening the
mizing the use of envelope from inactivated hemagglutinating
TSA exposure time from 24 to 12 hr did not affect blastocyst
2 Cell 153, 1–11, June 6, 2013 ª2013 Elsevier Inc.
Please cite this article in press as: Tachibana et al., Human Embryonic Stem Cells Derived by Somatic Cell Nuclear Transfer, Cell (2013),http://dx.doi.org/10.1016/j.cell.2013.05.006
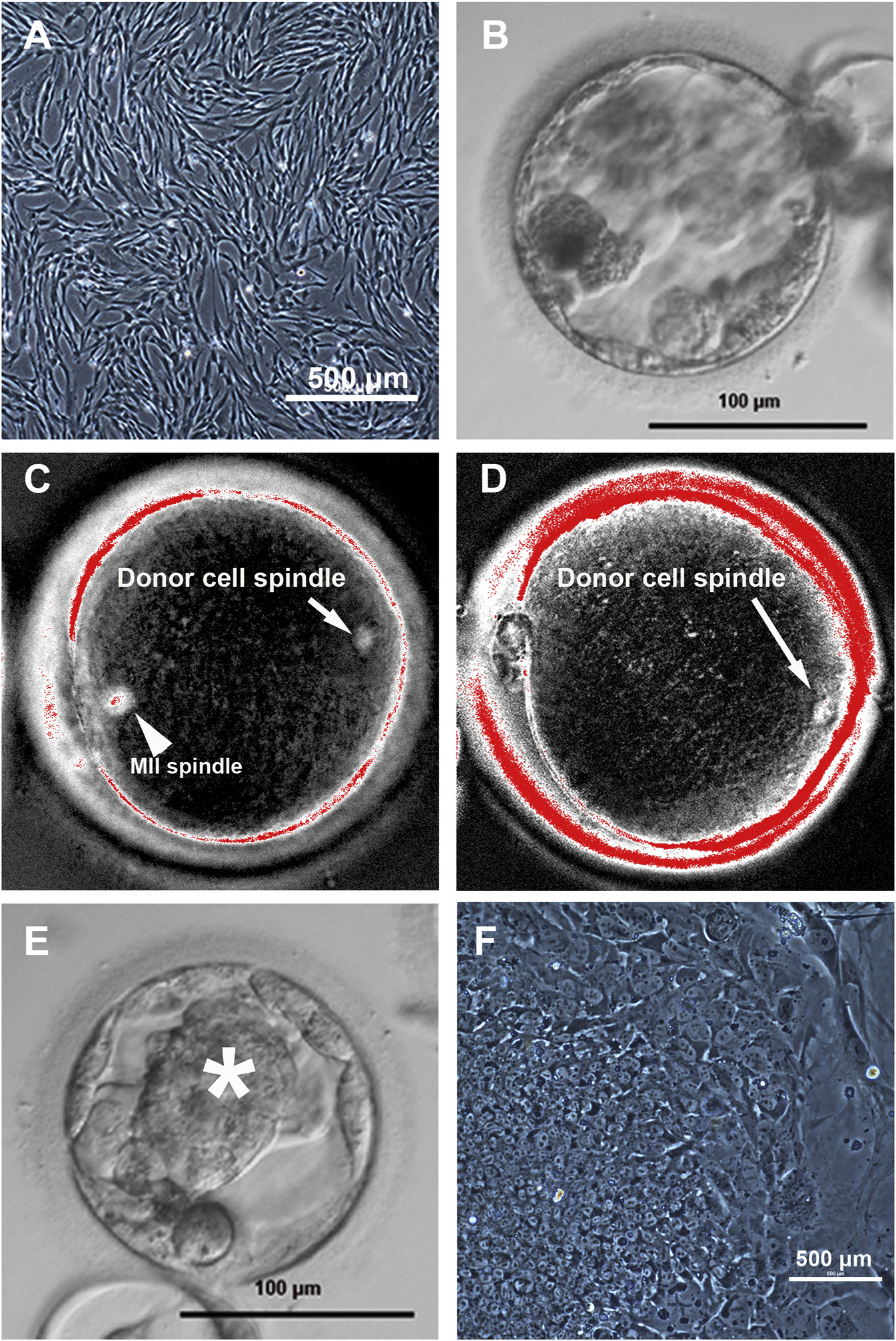
Figure 2. SCNT Blastocyst Development IsAffected by Premature Cytoplast Activation(A) Morphology of nuclear donor fetal fibroblastsbefore SCNT. (B) Poor-quality human SCNTblastocyst without distinct ICM produced withsuboptimal protocols; note the presence ofexcluded cells.
(C) Spindle-like structures detected when donornuclei were introduced into intact MII oocytes, butnot when introduction was conducted afterenucleation. Arrowhead and arrow point at thematernal MII spindle and somatic cell spindle,respectively.
(D) Somatic nuclear cell spindles were formed incytoplasts when oocyte enucleation and fusionwere conducted in the presence of caffeine.
(E) Human SCNT blastocyst with prominent ICM(asterisk) produced after caffeine treatment.
(F) NT-ESC colony with typical morphology derivedfrom a caffeine-treated SCNT human blastocyst.
dard ovarian stimulation protocols andtransvaginal follicular aspirations. Humandermal fibroblasts of fetal origin (HDF-f)synchronized in G0/G1 cell-cycle phasewere used as nuclear donors Spindle removal and HVJ-E-assisteddonor cell fusion were carried out within60 min of oocyte retrieval.
Most oocytes (95.2% [60 out of 63])
survived MII spindle removal conductedunder polarized microscopy (Oosight); ), and nuclear donor fibroblastswere introduced with 100% efficacy us-ing HVJ-E based fusion. Somatic cellnuclei did not form spindle-like structuresthat were detectable by noninvasive ex-amination under polarized microscopy.
Immediately after confirmation of fusion,oocytes were activated with electropora-tion/DMAP (4 hr) and exposed to 10 nMTSA for 12 hr. Most embryos (81.7% [49out of 60]; A, without caffeinegroup) formed one or two pronuclei atthe time of removal from TSA, whereasa slightly higher portion of embryoscleaved (86.7% [52 out of 60]), suggest-
rates (However, only SCNT blastocysts produced with
ing that some SCNT embryos did not exhibit visible pronuclei
10 nM TSA supported derivation of stable monkey NT-ESC lines,
at the time of examination A). Most cleaved embryos
though the number of plated blastocysts was small ().
developed to the eight-cell stage (61.5% [32 out of 52]), but
We concluded that these optimized protocols in a nonhuman pri-
few progressed to compact morula (13.5% [7 out of 52]) and
mate model were adequate to serve as a starting point for further
blastocyst (11.5% [6 out of 52]) stages Activation
testing with human oocytes.
of embryonic genes and transcription from the transplanted so-matic cell nucleus are required for development of SCNT em-
Producing Human SCNT Blastocysts and NT-ESC Lines
bryos beyond the eight-cell stage ;
Initially, human MII oocytes from healthy volunteers were
). Therefore, these results are consistent with the
exposed to the SCNT approach that produced the best results
premise that our modified SCNT protocol supports reprogram-
in the nonhuman primate. Oocytes were retrieved following stan-
ming of human somatic cells to the embryonic state.
Cell 153, 1–11, June 6, 2013 ª2013 Elsevier Inc. 3
Please cite this article in press as: Tachibana et al., Human Embryonic Stem Cells Derived by Somatic Cell Nuclear Transfer, Cell (2013),http://dx.doi.org/10.1016/j.cell.2013.05.006
during spindle removal but decline due to spontaneous activa-tion, we focused on noninvasive treatments to maintain meioticarrest during manipulation.
We reported previously that the exposure of monkey oocytes
to caffeine, a protein phosphatase inhibitor, was effective inprotecting the cytoplast from premature activation and improveddevelopment of SCNT embryos (). There-fore, human oocytes were maintained in 1.25 mM caffeine duringspindle removal and somatic cell fusion. As expected, somaticcell nuclei introduced into cytoplasts under these conditionsefficiently formed spindle-like structures that were detectableunder birefringence microscopy (83.3% [10 out of 12]) (D). More importantly, the blastocyst development rate ofcaffeine-treated embryos was notably enhanced (23.5%)compared to the standard SCNT group with caffeinegroup), and blastocysts were characterized by visible and prom-inent ICMs, similar to those observed for IVF-produced embryos(
Remarkably, when eight SCNT blastocysts produced with
caffeine exposure were utilized for ESCs isolation, all attachedto mEFs and four formed ICM outgrowths (whichgave rise to ESC-like colonies upon manual splitting onto freshmEF plates Subsequent passaging resulted in thepropagation of stable ESC colonies with typical morphologyand growth characteristics. This surprisingly high ESC derivationrate was similar to that reported in our previous study with humanIVF-derived blastocysts (50%) and was even higher than in
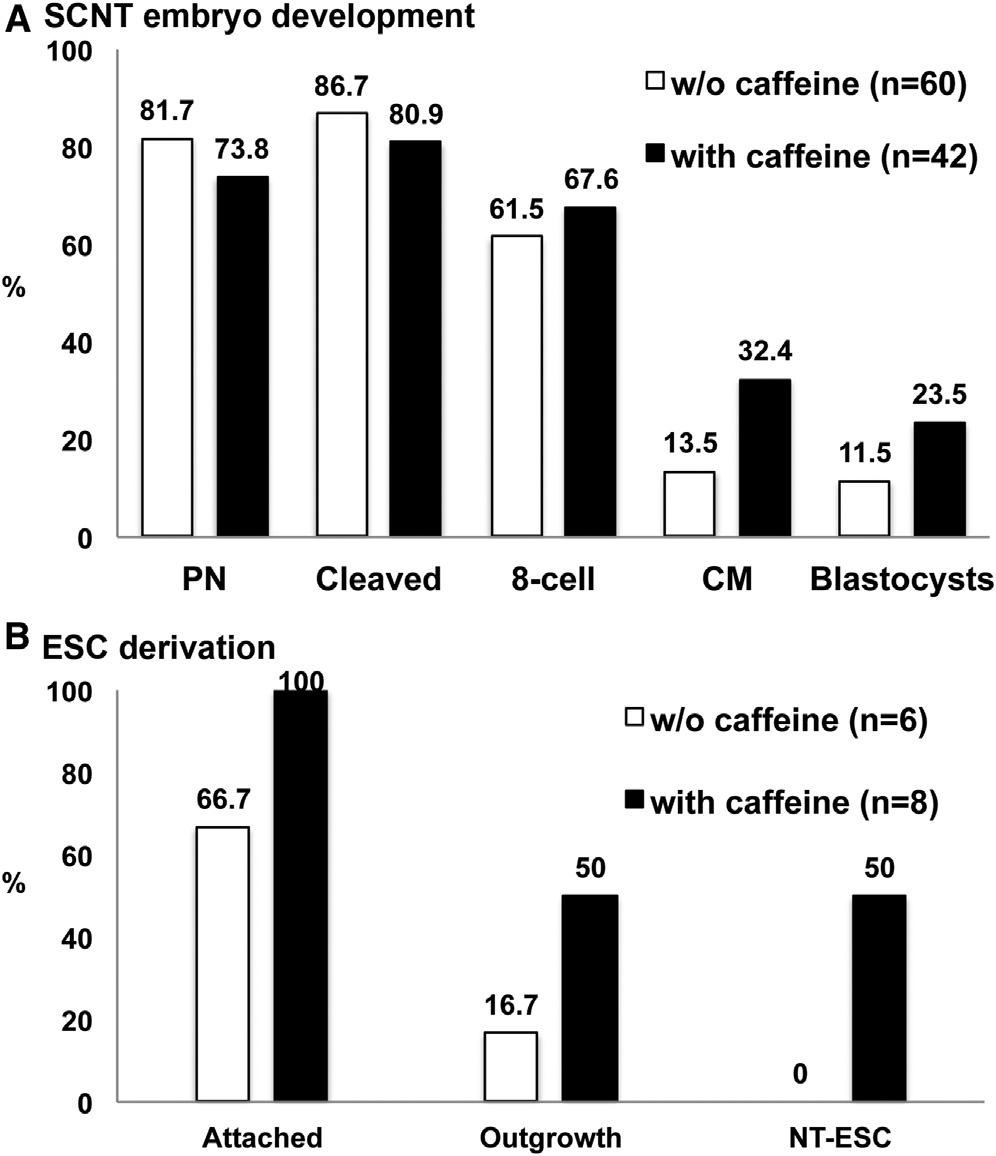
Figure 3. Development of Human SCNT Embryos and NT-ESC
manipulated spindle transfer embryos (38%) B)
Derivation after Caffeine Treatment
(A) Improved blastocyst development of human SCNT embryos treated with
Collectively, our findings indicate that a protocol developed in
caffeine. A total of 63 (five cycles) and 43 (three cycles) oocytes were utilizedfor SCNT without or with caffeine, respectively. Sixty (95.2%) and 42 (97.7%)
the monkey model supported blastocyst development for hu-
oocytes survived after SCNT micromanipulations.
man SCNT embryos. However, poor SCNT blastocyst quality
(B) NT-ESCs were derived only from blastocysts produced with caffeine.
precluded ESC isolation. Subsequent incorporation of caffeine
See also Figures S1, S2 and S3.
during enucleation and fusion allowed improved blastocystdevelopment and ESC line derivation.
Of note, human SCNT blastocysts exhibited poorly organized
Reproducibility of Human SCNT Results
trophectoderm and small or undetectable inner cell masses
Interestingly, all four human NT-ESC lines were derived from oo-
(ICMs). In addition, large blastomere-like cells were often
cytes retrieved from one egg donor (egg donor A). Eight mature
excluded B). Nevertheless, we plated six SCNT blasto-
MII oocytes were recovered after a single stimulation cycle. Us-
cysts onto feeder layers to examine their ability to support ESC
ing fetal dermal fibroblasts as nuclear donor cells and following
derivation. Though four blastocysts attached to mEFs, only
our caffeine-incorporated SCNT protocol, five blastocysts
one gave an outgrowth that, after further passaging, failed to
were produced (62.5%) that gave rise to four NT-ESC lines
produce stable ESC-like cells (
(80%) (Figure S1 available online). In the context of generating
Though the observed SCNT blastocyst development rate was
patient-specific pluripotent stem cells, reproducible results
encouraging, further optimization of the human protocol focused
with various patient-derived somatic cells and with different
on improvements in embryo quality. To assess whether spindle
egg donors are a necessity.
removal in human oocytes could cause spontaneous exit from
We therefore acquired a skin fibroblast culture from a patient
meiosis, we introduced somatic nuclei into intact MII oocytes
with Leigh syndrome. A total of 15 and 5 MII oocytes were
and examined their birefringence properties under polarized mi-
collected from two unrelated egg donor volunteers (B and C)
croscopy. All introduced somatic cell nuclei efficiently formed
and were used for SCNT with these fibroblasts. All oocytes sur-
spindle-like structures that were visible within 30 min of fusion
vived spindle removal and successfully fused with nuclear donor
(17 out of 17) Again, spindle formation was not
cells. Following activation and culture, four (27%, [4 out of 15])
observed when somatic cell nuclei were fused with manipulated,
and three (60% [3 out of 5]) blastocysts were produced from
spindle-free oocytes (0 out of 3). These observations are consis-
these egg donors After plating on mEFs and manual
tent with recent conclusions that human MII oocytes undergo
passaging, we established two stable NT-ESC lines—one from
premature activation secondary to spindle removal
each oocyte cohort (B). Thus, these outcomes confirm
). Assuming that meiosis-specific factors are retained
the reproducibility of our human SCNT protocols.
4 Cell 153, 1–11, June 6, 2013 ª2013 Elsevier Inc.
Please cite this article in press as: Tachibana et al., Human Embryonic Stem Cells Derived by Somatic Cell Nuclear Transfer, Cell (2013),http://dx.doi.org/10.1016/j.cell.2013.05.006
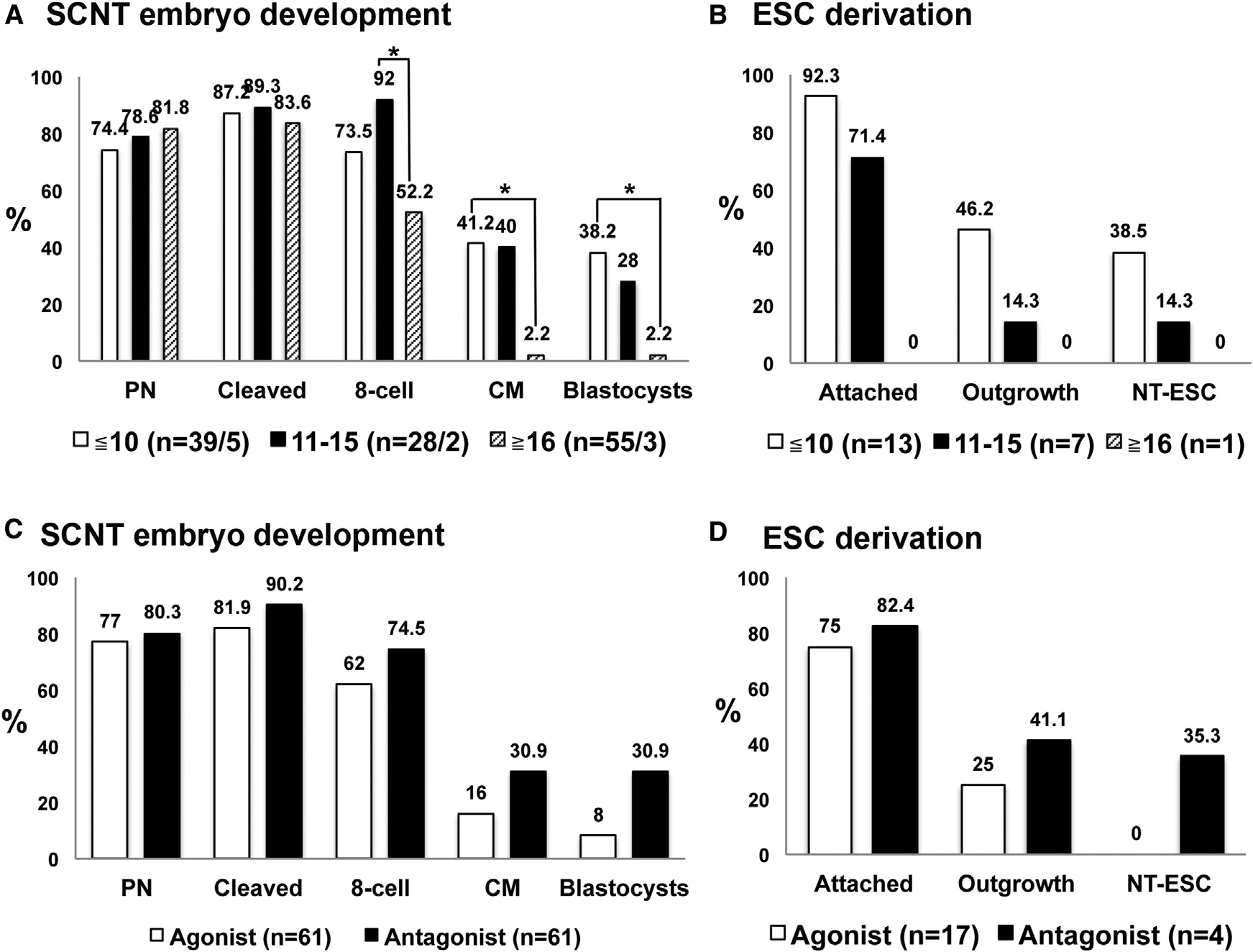
was similar between these groups, more embryos derived fromthe R16 MII oocytes/cycle group arrested after the eight-cellstage compared to the other two groups (A). In addition,the quality of recovered SCNT blastocysts correlated inverselywith the number of collected oocytes per cycle. Whereas fiveNT-ESC lines were derived from donors producing %10oocytes/cycle, only one was recovered from the 11–15 group,and no cell line was established from cycles with R16 oocytes(B). The peak systemic estradiol (E2) level in egg donorsprior to hCG priming positively correlated with the subsequentyield of oocytes (Figure S2). Thus, these observations implythat high numbers of oocytes collected from ovarian stimulationprotocols are associated with poor oocyte quality and reducedreprogramming ability in the context of SCNT.
In an effort to define optimal stimulation protocols that are
compatible with high-quality oocytes for SCNT, we analyzed theimpact of GnRH agonist and antagonist used to suppress pituitaryfunction in egg donors. Prior to ovarian stimulation, antimullerianhormone (AMH) levels and antral follicle counts (AFC) weremeasured for each egg donor (). Donors with higherAMH and AFC profiles indicative of high ovarian reserve receivedGnRH agonist (Lupron, four cycles), and the remaining donorsreceived GnRH antagonist (Ganirelix, six cycles) ). Theaverage number of MII oocytes (mean ± SD) collected per cyclewas not statistically different between the two groups (11.7 ± 5.6and 20.5 ± 11.9, respectively). However, SCNT embryo develop-ment beyond the eight-cell stage was suboptimal for oocytes pro-duced following GnRH agonist treatment (C). Moreover, allsix NT-ESC lines were derived exclusively from oocytes collectedfrom GnRH antagonist-treated cycles D). Based on theseobservations, it is possible that pituitary suppression with GnRH
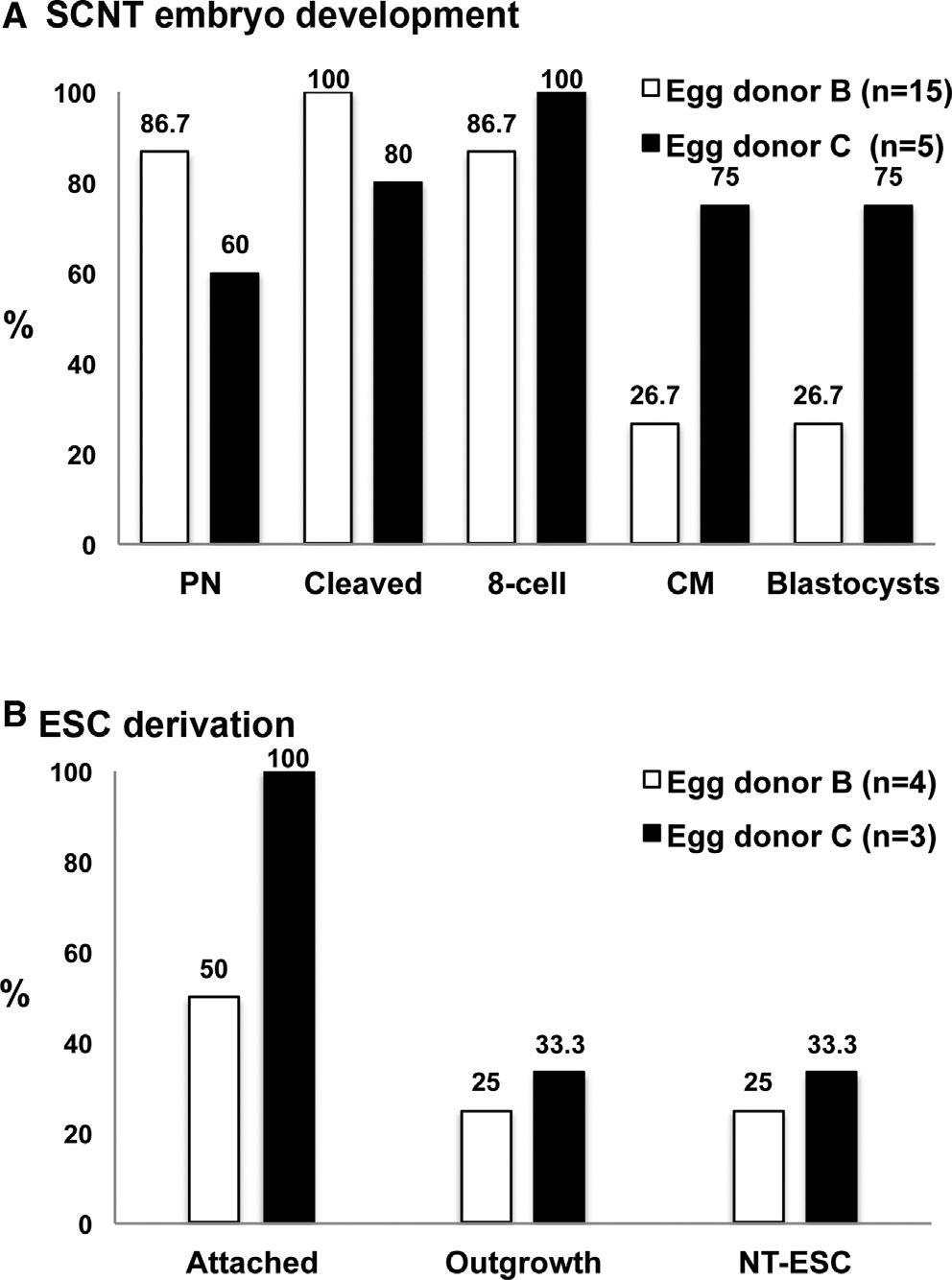
Figure 4. Validation of Human SCNT with Nuclear Donor Cells
antagonist during ovarian stimulation may positively impact
Derived from a Leigh's Disease Patient
oocyte reprogramming capability, making them compatible with
(A) In vitro development of SCNT embryos produced with skin fibroblast cellsfrom a Leigh's disease patient and two different egg donors (egg donors B
SCNT blastocyst development and ESC isolation.
and C). Fifteen MII oocytes were retrieved from egg donor B, whereas only five
Lastly, we looked at whether pronuclear formation can be used
oocytes were collected from donor C. SCNT blastocysts were generated from
as a predictive marker for SCNT outcomes. Most SCNT embryos
both oocyte cohorts.
formed a single pronucleus the day after nuclear transfer (56%
(B) NT-ESC derivation efficiency allowed isolation of one cell line per egg donor
[68 out of 122]), whereas a small portion (20% [24 out of 122]) dis-
played two pronuclei (Figure S3). As indicated above, pronuclear
See also Figures S2 and S3.
formation was not observed in some one-cell SCNT embryos(20% [24 out of 122]), whereas a minority (5% [6 out of 122])
Retrospective Analysis of Factors Affecting the Success
were already at the two-cell stage by the time of examination (Fig-
ure S3). After separate culture, we determined that cleavage and
Whereas SCNT manipulations and treatments were strictly
early preimplantation development were similar among these
controlled, the quality and quantity of human oocytes retrieved
groups, and although the blastocyst rate was higher in SCNT em-
from different egg donors varied significantly. High oocyte
bryos with two pronuclei (39%), stable NT-ESC lines were pro-
numbers retrieved from an ovarian stimulation cycle are gener-
duced from all four embryo types (Figure S3). Thus, despite the
ally associated with poor clinical IVF outcomes (
small number of SCNT embryos analyzed, it is reasonable to
conclude that visible pronuclear formation as conducted in these
Therefore, we conducted a retrospective analysis of ovarian
studies does not directly correlate with NT-ESC derivation.
stimulation procedures and the number of oocytes retrievedper cycle versus SCNT embryo development and NT-ESC deri-
Analysis of Human NT-ESCs
vation outcomes. We divided oocyte donation cycles into three
To confirm SCNT origin and define the degree of reprogram-
groups based on the range of collected mature MII oocytes—
ming, we expanded and extensively analyzed the four NT-ESC
specifically, 10 or fewer oocytes per cycle (five donors), between
lines derived from HDF-f fetal fibroblasts (designated as hE-
11 and 15 oocytes (two donors), and more than 16 oocytes per
SO-NT1, hESO-NT2, hESO-NT3, and hESO-NT4).
cycle (three donors). Although survival after spindle removal,
Initially, we employed microsatellite typing for 23 markers
fusion, pronuclear formation, and cleavage of SCNT embryos
mapping 22 human autosomal loci and one X-linked locus for
Cell 153, 1–11, June 6, 2013 ª2013 Elsevier Inc. 5
Please cite this article in press as: Tachibana et al., Human Embryonic Stem Cells Derived by Somatic Cell Nuclear Transfer, Cell (2013),http://dx.doi.org/10.1016/j.cell.2013.05.006
Figure 5. Ovarian Stimulation and Human SCNT Outcomes(A) Human SCNT development varied with the number of oocytes collected from each ovarian stimulation cycle. Cycles producing ten or fewer oocytes wereassociated with improved development of SCNT embryos.
(B) The efficacy of NT-ESC derivation also positively correlated with fewer numbers of oocytes collected in the ovarian stimulation cycle.
(C and D) SCNT embryo development from cycles treated with GnRH agonists or antagonists. Blastocyst development was higher for oocytes recovered fromdonors receiving a GnRH antagonist. NT-ESCs were derived only from oocytes recovered from donors receiving GnRH antagonist.
See also Figures S2 and S3 and
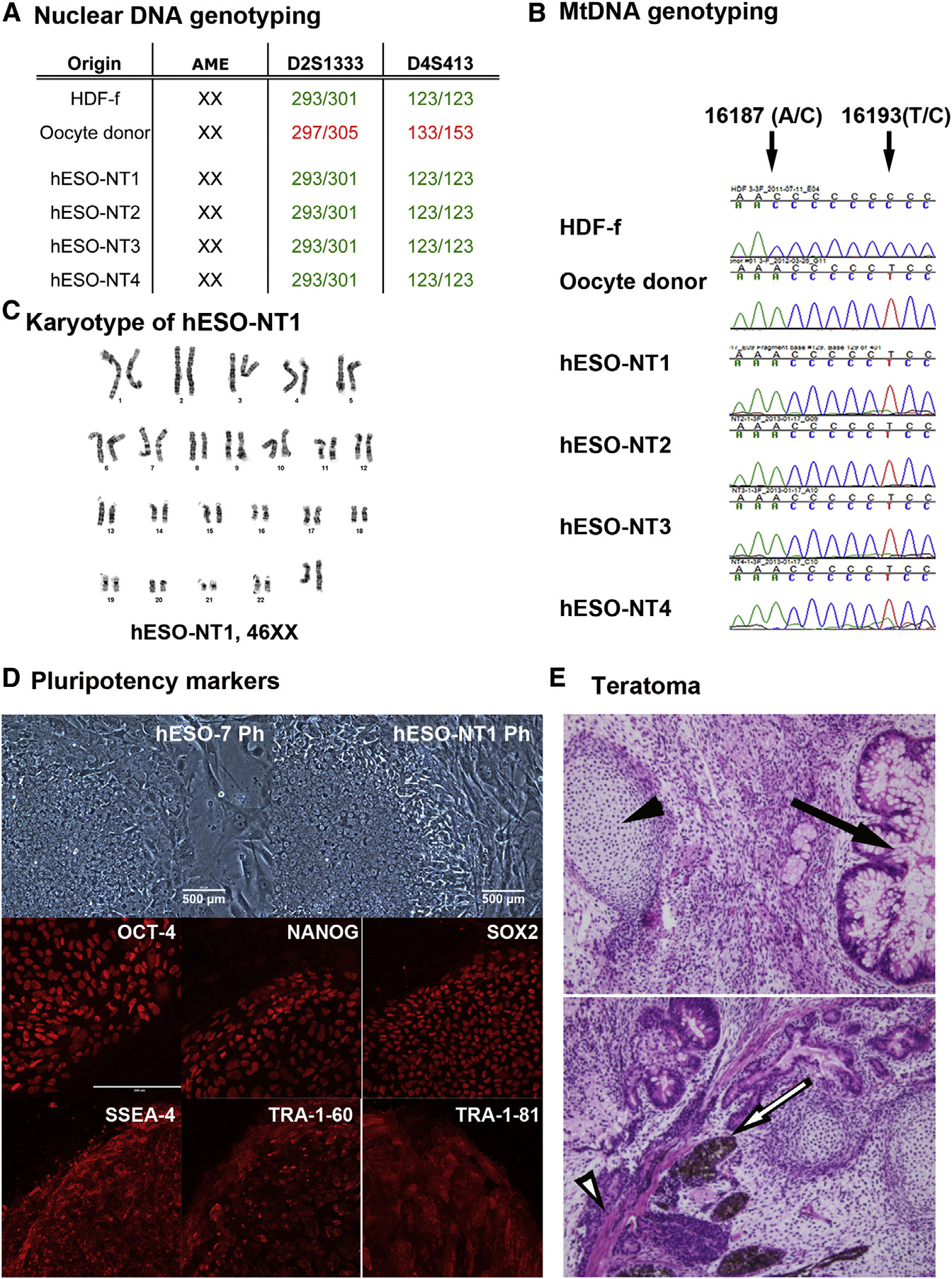
nuclear genome genotyping The results
euploid female karyotype (46XX) with no numerical or structural
unequivocally matched all four NT-ESC lines to the nuclear
abnormalities and S4).
donor fetal fibroblasts, with no detectable contribution of oocyte
To assess pluripotency in NT-ESC lines, we initially examined
alleles A and The defining feature of SCNT is
expression of generic stem cell markers by immunocytochem-
that the mitochondrial genome (mtDNA) in SCNT embryos and in
istry (ICC) and compared the results to two IVF-derived ESC lines
NT-ESCs is largely contributed by the oocyte. As expected, anal-
(hESO-7 and -8). These control ESC lines and the four NT-ESC
ysis of mtDNA sequences within the displacement loop (D loop)
lines were established from oocytes donated by the same donor
containing the hypervariable segment (HSV) confirmed that NT-
(egg donor A), and thus they carried identical mtDNA (
ESC lines inherited mainly oocyte mtDNA During
Similar to controls, all NT-ESC lines expressed
fusion of cytoplasts with nuclear donor fibroblasts, a small
OCT-4, NANOG, SOX2, SSEA-4, TRA-1-60, and TRA-1-81 (
amount of somatic cell mtDNA is cotransferred into the resultant
D and S5). Moreover, when injected into immunodeficient
embryos that may result in heteroplasmy. We employed sensi-
SCID mice, all NT-ESC lines produced tumors containing tissue
tive ARMS-qPCR (amplification refractory mutation system-
and cell types representing all three germ layers E). An
quantitative polymerase chain reaction) and detected a low level
in vitro differentiation assay demonstrated efficient formation of
of somatic cell mtDNA in all four NT-ESC lines (3.4% ± 1.7%;
embryoid bodies in suspension culture that, after attachment,
range 1.2%–4.9%) ). Interestingly, this carryover was
formed spontaneously contracting cardiomyocytes (
higher than what we had previously observed in ST-ESC lines
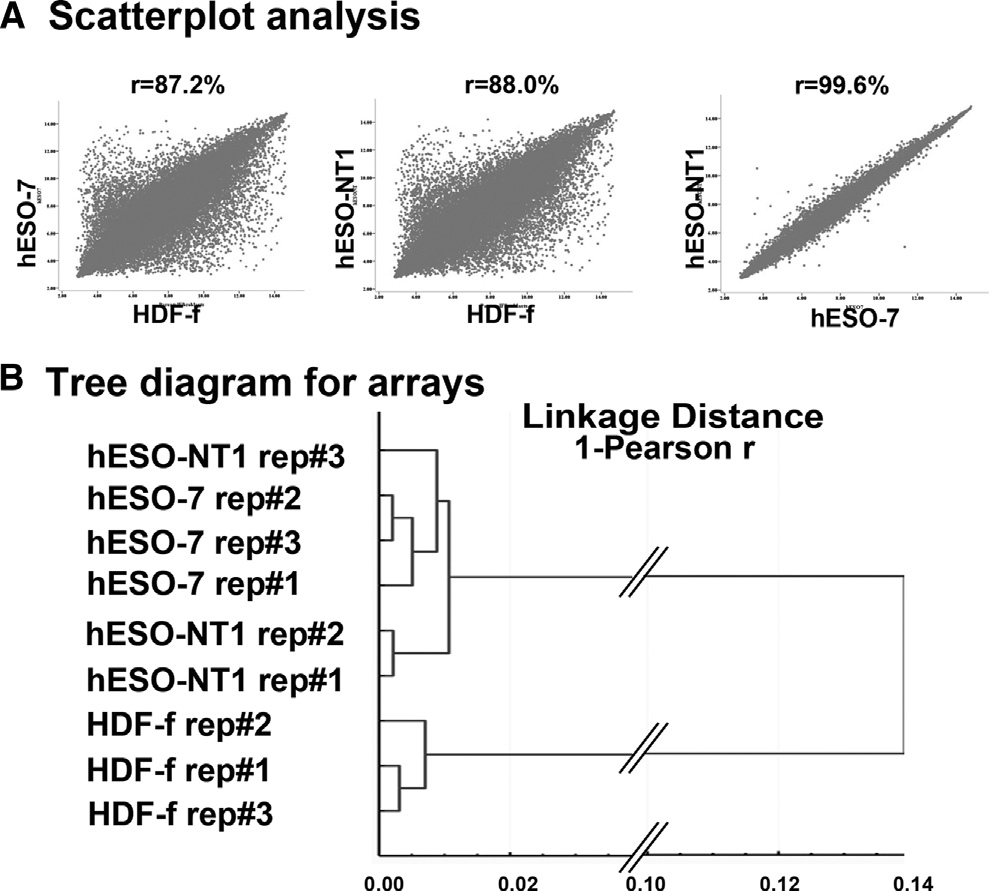
Lastly, we conducted microarray expression analysis of the
(0.6% ± 0.9%) derived after spindle transfer between oocytes
hESO-NT1 cell line and compared results to the IVF control,
(). Cytogenetic analysis by G-banding
hESO-7, and parental somatic cells HDF-f using the Affymetrix
analysis indicated that all four NT-ESC lines contained a normal
PrimeView platform. Initially, three biological replicates within
6 Cell 153, 1–11, June 6, 2013 ª2013 Elsevier Inc.
Please cite this article in press as: Tachibana et al., Human Embryonic Stem Cells Derived by Somatic Cell Nuclear Transfer, Cell (2013),http://dx.doi.org/10.1016/j.cell.2013.05.006
Figure 6. Genetic, Cytogenetic, and Plurip-otency Analysis of Human NT-ESCs(A) Nuclear DNA genotyping from four human NT-ESC lines (hESO-NT1, hESO-NT2, hESO-NT3,and hESO-NT4) determined by microsatelliteparentage analysis. A total of 24 microsatellitemarkers were used for each analysis. The repre-sentative markers for D2S1333 and D4S413 locidemonstrate that the nuclear DNA in these celllines was exclusively derived from the somaticHDF-f cell line. No contribution of oocyte nuclearDNA was detected.
(B) mtDNA genotyping by Sanger sequencingdemonstrated that all NT-ESC lines contain oocytemtDNA.
(C) Cytogenetic G-banding analysis confirmedthat all NT-ESCs exhibited a normal 46XX karyo-type (hESO-NT1 result is representative).
(D) Human NT-ESCs expressed standard plurip-otency markers detected by immunocytochem-istry for antibodies against OCT4, NANOG, SOX2,SSEA-4,
magnification, 3200; Ph, phase contrast.
(E) Histological analysis of teratoma tumors pro-duced after injection of human NT-ESCs into SCIDmice. An arrow and arrowhead in the top panelindicate intestinal-type epithelium with goblet cells(endoderm) and cartilage (mesodermal), respec-tively. An arrow and arrowhead in the lower paneldepict neuroecto-dermal (ectoderm) and muscle(mesoderm) tissues, respectively. Original magni-fication, 3200.
See also Figures S4 and S5 and and
in this list and Interest-ingly, the HLA-C major histocompatibilitygene was highly downregulated in hESO-NT1 compared with hESO-7 (79-fold)(
We demonstrate here for the first time thesuccessful reprogramming of human so-
each sample were compared against each other. For compari-
matic cells into ESCs following SCNT. By systematic analysis
sons, the detected signal for each probe set was plotted in a
of SCNT procedures, in some cases informed by studies in the
scattergraph, and the correlation value was calculated. This
rhesus monkey, we identified several steps, including spindle
assay demonstrated 99% transcriptional correlation within
removal, donor cell fusion, and cytoplast activation, that are crit-
each cell type, suggesting that minimal variations existed
ical for cellular reprogramming and SCNT blastocyst develop-
between biological replicates collected from different culture
ment. Previous studies have indicated that meiotic arrest in
plates (Figure S6). Each NT-ESC and IVF-ESC type was
human MII oocytes is unstable such that intrusive manipulations
compared against each other and against somatic cells (HDF-f).
can induce rapid exit from the metaphase stage (
As expected, both stem cell types displayed low transcriptional
). However, successful integration into and reprog-
correlation to fibroblasts and 7B). Among 50 genes
ramming of interphase (G0/G1) somatic cell nuclei in MII
with the highest fold change, many known pluripotency genes
cytoplasts are critically important and dependent on nuclear re-
were observed, including LIN28, POU5F1, NANOG, and SOX2
modeling events associated with high meiotic kinase activities
(In contrast, ESCs derived by IVF and SCNT were
present in cytoplasts ;
similar to each other (A and 7B). Some transcriptional
; ). Meiotic cytoplasts induce rapid nu-
differences between human NT-ESCs and IVF-ESCs were
clear envelope breakdown (NEBD) and premature chromosome
observed. However no known pluripotency genes were included
condensation (PCC) in transplanted interphase nuclei and
Cell 153, 1–11, June 6, 2013 ª2013 Elsevier Inc. 7
Please cite this article in press as: Tachibana et al., Human Embryonic Stem Cells Derived by Somatic Cell Nuclear Transfer, Cell (2013),http://dx.doi.org/10.1016/j.cell.2013.05.006
parthenogenetic development in intact MII oocytes ), and it is generally accepted that, if artificial activa-tion supports parthenogenetic embryo development to blasto-cysts and ESCs, such treatments should be sufficient to inducesimilar outcomes with reconstructed SCNT cytoplasts. How-ever, in the present studies, we found that supplemental electro-poration treatment was critical for cytoplast activation andsubsequent reprogramming was compatible with improvedSCNT development and ESC derivation. Thus, it is reasonableto speculate that the requirements for oocyte or cytoplast activa-tion in SCNT and parthenogenetic systems are different.
We also show that human SCNT reprogramming is dependent
on human oocyte quality. Particularly, larger numbers of oocytesretrieved following ovarian stimulation protocols with agonistwere negatively correlated with human SCNT blastocyst devel-opment. Thus, it is speculated that ovarian stimulation protocolsroutinely utilized for IVF treatments might be altered somewhat ifthe goal is obtaining ESC derivation by SCNT. Conversely, sub-optimal quality oocytes derived by in vitro maturation or othersources are likely to be unsuitable for SCNT (S.M., unpublisheddata). Clearly, further studies addressing gonadotropin dosage
Figure 7. Microarray Expression Analysis of Human NT-ESCs
and pituitary suppression regimens should be evaluated in the
(A) Scatterplot analysis comparing expression profiles of human NT-ESCs
context of recovering human oocytes suitable for SCNT.
(hESO-NT1) with IVF-derived ESC controls (hESO-7) and parental skin fibro-
It is also important to note that the oocyte quality is ultimately
blasts (HDF-f). Both IVF-ESCs and NT-ESCs displayed low transcriptional
linked to the genetic constitution of individual egg donors.
correlation to fibroblasts (left and middle) but were similar to each other (right).
(B) Tree diagram analysis linking NT-ESCs to IVF-ESCs.
Indeed, some oocyte cohorts did not support SCNT blastocyst
See also Figure S6 and , and .
development with the present protocol, whereas efficient blasto-cyst formation (23% [5 out of 21]) has been reported usingdifferent protocols involving electrofusion followed by activation
convert them to spindle-like structures. It has been proposed
with calcium ionophore and DMAP or DMAP/cytochalasin D
that NEBD and PCC are required for precise cell-cycle synchro-
(Nevertheless, reflecting what we presumed
nization between the incoming interphase donor nucleus and the
to be exceptional oocyte quality from one donor, five blastocysts
recipient mitotic cytoplast (Such drastic chro-
were produced from just eight oocytes, which subsequently re-
matin transformations are associated with efficient reprogram-
sulted in the derivation of four NT-ESC lines. Interestingly, prior
ming and improved development of SCNT embryos, whereas
egg donation from this donor was also associated with excep-
lack of or incomplete nuclear remodeling leads to early develop-
tional outcomes that supported derivation of four ESC lines
mental arrest (;
following ST procedures ). Although the
underlying genetic factors contributing to oocyte quality remain
Another issue is that somatic cell-specific transcription and
unknown, certain FMR-1 alleles, defined by the CGG nucleotide
epigenetic factors maintaining cellular identity become dissoci-
repeats, correlate with improved oocyte quality and IVF success
ated from the chromatin during NEBD and PCC and are more
(). Clearly, further studies are warranted to
actively replaced by oocyte-specific programs
elucidate the genetic and clinical parameters associated with
Here, we suggest that retention of meiotic activity in hu-
optimal oocyte quality for human SCNT.
man MII cytoplasts aided by enucleation in the presence of
Given past difficulties in achieving the ultimate goal of produc-
caffeine and HVJ-E-based fusion enhances conversion of so-
ing human NT-ESCs, it was generally assumed that derivation of
matic cell nuclei to spindle-like structures. Moreover, human
ESCs via SCNT would require an inordinate number of oocytes
SCNT embryos generated with our approach developed to blas-
and thus could not be scaled up for widespread therapeutic
tocysts and NT-ESCs.
use (). However, here, our revamped
Another important finding in our study is that standard activa-
SCNT protocols allowed derivation of at least one ESC line
tion treatments involving exposure to ionomycin and 6-DMAP
from each oocyte donation cycle.
are not sufficient for supporting development of human SCNT
A battery of pluripotency tests performed on human NT-ESCs
embryos. During normal fertilization, sperm entry triggers oocyte
demonstrated their similarities to genuine IVF-derived ESCs.
activation that is critical for completion of meiosis and for the
Transcriptional interrogation indicated that NT-ESCs departed
initiation of mitotic divisions. Activation is also critical for the oo-
from their parental somatic cell gene expression pattern with up-
cyte's cytoplasm to acquire the reprogramming and metabolic
regulation of pluripotency associated genes. In addition, NT-
activity that is necessary to support subsequent development
ESCs demonstrated the ability to differentiate into a variety
(Presently, the efficacy of artificial
of other cell types in teratoma tumors. In-vitro-directed differen-
activation protocols is commonly measured by induction of
tiation induced formation of contracting cardiomyocytes,
8 Cell 153, 1–11, June 6, 2013 ª2013 Elsevier Inc.
Please cite this article in press as: Tachibana et al., Human Embryonic Stem Cells Derived by Somatic Cell Nuclear Transfer, Cell (2013),http://dx.doi.org/10.1016/j.cell.2013.05.006
demonstrating their potential for regenerative medicine. Genetic
Products) or antagonist (Ganirelix, Merck) were given. Human chorionic
analyses showed that all four NT-ESC lines tested to date con-
gonadotropin (hCG) was prescribed to trigger oocyte maturation. Self-admin-
tained normal diploid karyotypes, with no detectable gross chro-
istration of injectable rFSH (sc, Follistim, Merck) commenced on cycle day 2or 3 and continued for �8–12 days. The starting gonadotropin dose was
mosomal abnormalities or contribution from the oocyte genome
75–125 IU/day and 1–2 A hMG (sc, Menopur, Ferring Pharmaceuticals). The
apart from mtDNA.
dose was adjusted per individual response using an established stepdown
An approach to patient-specific pluripotent stem cell deriva-
regimen until the day of hCG injection. Ovarian response and follicular growth
tion that precludes the use of embryos is based on somatic
were monitored by transvaginal ultrasound and measurement of serum estra-
cell reprogramming by induced expression of a few critical tran-
diol levels. When two or more follicles reached R 18 mm in diameter, subjects
scription factors (referred as induced pluripotent stem cells
received hCG (104 IU, sc, Ovidrel, EMDSerono) to trigger follicle and oocytematuration. Thirty-six hours following hCG injection, subjects underwent
oocyte retrieval via transvaginal follicular aspiration.
Recent studies have concluded that
Cumulus-oocyte complexes (COCs) were collected from aspirates and
human iPSCs are characterized by high frequencies of subchro-
placed in HTF w/HEPES medium (LifeGlobal, IVFonline) supplemented with
mosomal copy number alterations compared to IVF-derived
10% serum substitute supplement (SSS; Quinns Advantage Serum, Cooper-
ESCs Some of these genetic changes
Surgical) (HTF w/HEPES 10%) at 37�C. COCs were treated with hyaluronidase
were associated with the reprogramming process itself, whereas
to disaggregate cumulus and granulosa cells. Oocytes were isolated andclassified as germinal vesicle (GV), meiotic metaphase I (MI), and mature meta-
others could have been inherited from the parental somatic cells
phase II (MII) stage and were then placed in Global medium (LifeGlobal, IVFon-
(). In addition, iPSC-specific methylation and
line) supplemented with 10% SSS (Global 10%) at 37�C in 5% CO2 and
transcriptional abnormalities in imprinted regions and X chromo-
covered with tissue culture oil (Sage IVF, Cooper Surgical).
somes were also described ). Direct compar-isons between iPSCs and NT-ESCs in the mouse indicated that
Nuclear Donor Cell Preparations
such abnormalities are less frequent in the latter case,
Commercially available female dermal fibroblasts of fetal origin (HDF-f) were
concluding that SCNT-based reprogramming is more efficient
obtained from ScienCell Research Laboratories, and Leigh syndrome patientcells were acquired from the Coriell Cell Repositories. Cells were expanded
in resetting the epigenetic identity of parental somatic cells
in 75 cm3 cell culture flasks (Corning) containing DMEM/F12 supplemented
(). Further comparisons of the genetic, epige-
with 100 IU ml�1 penicillin, 100 mg ml�1 streptomycin (Invitrogen), 10% FBS
netic, and transcriptional characteristics of human NT-ESCs,
at 37�C in 5% CO2. Fibroblasts were then disaggregated with trypsin treat-
IVF-ESCs, and iPSCs are clearly justified.
ment and were frozen down in aliquots of 3 3 105 cells in medium containing
Lastly, one of the fundamental differences of SCNT-based
10% dimethyl sulphoxide (DMSO, Sigma). Cells were subsequently thawed
reprogramming is that NT-ESCs contain mtDNA almost exclu-
prior to SCNT and cultured in four-well dishes (Nunc) under standard condi-tions until they reached confluency. Confluent cells were synchronized in the
sively originating from the oocyte. This fact is generally underap-
G0/G1 phase of the cell cycle by culture in DMEM/F12 medium with 0.5%
preciated but may represent an advantage over iPSC derivation
FBS for 2–4 days before SCNT.
because it ensures that NT-ESCs acquire the potential to pro-duce metabolically functional cells and tissues for cell therapies,
Human SCNT Procedure and Embryo Culture
irrespective of the nuclear donor cell mtDNA. Thus, SCNT offers
Enucleations were performed as described previously ).
a strategy for correcting of mtDNA mutations and rescuing the
Oocytes were placed into a 50 ml manipulation droplet of HTF w/HEPES 10%
metabolic function of pluripotent cells from patients with in-
medium containing 5 mg/ml cytochalasin B and 1.25 mM caffeine in a glass-bottom dish. The droplet was covered with tissue culture oil, and oocytes
herited or acquired mtDNA diseases.
were maintained at 37�C for 10–15 min before spindle removal. The dishwas then mounted on the stage of an inverted microscope (Olympus IX71)
EXPERIMENTAL PROCEDURES
equipped with a stage warmer ), Narishige microma-nipulators, Oosight Imaging System (), and a laser
Rhesus Macaque SCNT
objective (An oocyte was positioned using
All animal procedures were approved by the Institutional Animal Care and Use
a holding pipette so that the spindle was situated close to the 2 to 4 o'clock
Committee at the Oregon National Primate Research Center. Oocyte collec-
position. The zona pellucida next to the spindle was drilled with laser pulses,
tions, SCNT, embryo culture, and NT-ESC isolation procedures were per-
and an enucleation pipette was inserted through the opening. A small amount
formed as previously described (,
of cytoplasm surrounded by plasma membrane and contacting the spindle
was aspirated into the pipette. Next, a disaggregated fibroblast was aspiratedinto a micropipette and was briefly transferred to the drop containing HVJ-E
Human Oocyte Donations
extract (Ishihara Sangyo Kaisha). The cell was then placed into the perivitelline
The study protocols were approved by both the OHSU Embryonic Stem Cell
space of the cytoplast on the side opposite the first polar body. This construct
Research Oversight Committee and the Institutional Review Board. Anony-
was rinsed with HTF w/HEPES 10%, transferred to global 10% medium, and
mous egg donors of ages 23–31 were recruited through the OHSU Women's
incubated at 37�C in 5% CO2 for 30 min until fusion occurred as confirmed
Health Research Unit via print and web-based advertising. Responding
visually by the disappearance of the donor cell from the perivitelline space.
women were screened with respect to their reproductive, medical, and psy-
Constructs were then subjected to artificial activation consisting of electropo-
chosocial health. Healthy nonobese (BMI < 28 kg/m2) women who passed
ration pulses (two 50 ms DC pulses of 2.7 kV cm�1) (Electro Square Porator
the initial medical and psychological evaluations were invited to participate
T-820, BTX) in 0.25 M d-sorbitol buffer containing 0.1 mM calcium acetate,
in a research egg donation program. Egg donors were financially compen-
0.5 mM magnesium acetate, 0.5 mM HEPES, and 1 mg ml�1 fatty-acid-free
sated for the time, effort, discomfort, and inconvenience associated with the
BSA. Activated SCNT constructs were then incubated in Global medium
donation process.
(without serum) containing 2 mM DMAP at 37�C in 5% CO2 for 4 hr. After
Ovarian stimulation protocols followed established clinical IVF guidelines as
DMAP, SCNT embryos were rinsed with HTF w/HEPES 10% and transferred
described previously (In brief, a combination of recom-
into four-well dishes containing Global medium supplemented with 10%
binant human-follicle-stimulating hormone (rFSH) and human menopausal go-
FBS, 12 mM b-mercaptoethanol (BME), and 10 nM Trichostatin A (TSA, Sigma)
nadotropins (hMG) and either GnRH agonist (Lupron, Tap Pharmaceutical
and cultured at 37�C in 5% CO2, 5% O2, and 90% N2 for 12 hr. Embryos were
Cell 153, 1–11, June 6, 2013 ª2013 Elsevier Inc. 9
Please cite this article in press as: Tachibana et al., Human Embryonic Stem Cells Derived by Somatic Cell Nuclear Transfer, Cell (2013),http://dx.doi.org/10.1016/j.cell.2013.05.006
then rinsed, checked for pronuclear formation, and cultured in Global medium
clear donor cells. We are also indebted to Vanessa Domush, Elizabeth Smo-
supplemented with 10% FBS and 12 mM b-mercaptoethanol (BME) at 37�C in
lens, Cathy Ramsey, Ying Li, Riffat Ahmed, Brittany Daughtry, and Erin
5% CO2, 5% O2, and 90% N2 for a maximum of 7 days. The medium was
Wolff for their technical support. The human oocyte/embryo research was sup-
changed only once, at day 3 of culture.
ported by OHSU institutional funds and by the grant from Leducq Foundation.
The nonhuman primate studies were supported by grants from the National
Isolation, Culture, and Characterization of Human NT-ESCs
Institutes of Health HD063276, HD057121, HD059946, EY021214, and
After zona pellucida removal via brief exposure to 0.5% protease (Sigma),
SCNT blastocysts were plated onto confluent feeder layers of mitomycin-C-inactivated mouse embryonic fibroblasts (mEFs) and were cultured for
Received: April 30, 2013
5–7 days at 37�C, 3% CO
Revised: May 3, 2013
2, 5% O2, and 92% N2 in ESC derivation medium
consisting of DMEM/F12 (Invitrogen) supplemented with 0.1 mM nonessential
Accepted: May 3, 2013
amino acids, 1 mM l-glutamine, 0.1 mM b-mercaptoethanol, 5 ng/ml basic
Published: May 15, 2013
fibroblast growth factor, 10 mM ROCK inhibitor (Sigma), 10% FBS, and 10%knockout serum replacement (KSR; Invitrogen). Before use, fresh ESC deriva-
tion medium was mixed (50%:50%, v/v) with derivation medium conditioned ina 24 hr culture with growing human ESCs. Outgrowths of the inner cell mass
Byrne, J.A., Pedersen, D.A., Clepper, L.L., Nelson, M., Sanger, W.G., Gokhale,
(ICMs) were manually dissociated into small clumps with a microscalpel and
S., Wolf, D.P., and Mitalipov, S.M. (2007). Producing primate embryonic stem
were replated on fresh mEF plates. After the first passage of ICM outgrowths,
cells by somatic cell nuclear transfer. Nature 450, 497–502.
FBS and ROCK inhibitor were omitted, and KSR was increased to 20%. Col-
Campbell, K.H., McWhir, J., Ritchie, W.A., and Wilmut, I. (1996). Sheep cloned
onies with ESC-like morphologies were selected for further propagation, char-
by nuclear transfer from a cultured cell line. Nature 380, 64–66.
acterization, and cytogenetic analyses.
Choi, I., and Campbell, K.H. (2010). Treatment of ovine oocytes with caffeine
Immunocytochemistry, in vivo and in vitro differentiation, and microarray an-
increases the accessibility of DNase I to the donor chromatin and reduces
alyses were performed as described ; ;
apoptosis in somatic cell nuclear transfer embryos. Reprod. Fertil. Dev. 22,
). Detailed protocols are also available in the
Daley, G.Q., and Solbakk, J.H. (2011). Stem cells: Triple genomes go far.
Nature 478, 40–41.
Nuclear DNA Genotyping and Cytogenetic Analyses
Danan, C., Sternberg, D., Van Steirteghem, A., Cazeneuve, C., Duquesnoy, P.,
Genotyping of NT-ESCs was performed by microsatellite typing using 23
Besmond, C., Goossens, M., Lissens, W., and Amselem, S. (1999). Evaluation
markers representing 22 human autosomal loci and one X-linked locus, as pre-
of parental mitochondrial inheritance in neonates born after intracytoplasmic
viously described Karyotyping was performed by
sperm injection. Am. J. Hum. Genet. 65, 463–473.
GWT banding on 20 metaphase cells from each human NT-ESC line at the Hu-
Ding, X., Wang, Y., Zhang, D., Wang, Y., Guo, Z., and Zhang, Y. (2008).
man Genetics Laboratory, University of Nebraska Medical Center, as previ-
Increased pre-implantation development of cloned bovine embryos treated
ously described ).
with 5-aza-20-deoxycytidine and trichostatin A. Theriogenology 70, 622–630.
Egli, D., Birkhoff, G., and Eggan, K. (2008). Mediators of reprogramming: tran-
Mitochondrial DNA Genotyping
scription factors and transitions through mitosis. Nat. Rev. Mol. Cell Biol. 9,
Genotyping of mtDNA was performed as previously described (
). The human mitochondrial displacement loop region (D loop)harboring the hypervariable segment 1(HSV-1) was amplified using published
Egli, D., Chen, A.E., Saphier, G., Ichida, J., Fitzgerald, C., Go, K.J., Acevedo,
primers ). PCR products were sequenced, and the informa-
N., Patel, J., Baetscher, M., Kearns, W.G., et al. (2011). Reprogramming within
tive single nucleotide polymorphic (SNP) sites were identified using
hours following nuclear transfer into mouse but not human zygotes. Nat. Com-
Sequencher v. 4.7 software (GeneCodes). Quantitative mtDNA analysis was
mun. 2, 488.
performed by ARMS-qPCR as described (The detailed
Fan, Y., Jiang, Y., Chen, X., Ou, Z., Yin, Y., Huang, S., Kou, Z., Li, Q., Long, X.,
protocol is also available in
Liu, J., et al. (2011). Derivation of cloned human blastocysts by histone deace-tylase inhibitor treatment after somatic cell nuclear transfer with b-thalassemia
SUPPLEMENTAL INFORMATION
fibroblasts. Stem Cells Dev. 20, 1951–1959.
French, A.J., Adams, C.A., Anderson, L.S., Kitchen, J.R., Hughes, M.R., and
Supplemental Information includes Extended Experimental Procedures, six
Wood, S.H. (2008). Development of human cloned blastocysts following so-
figures, eight tables, and one movie and can be found with this article online
matic cell nuclear transfer with adult fibroblasts. Stem Cells 26, 485–493.
Gleicher, N., Weghofer, A., Lee, I.H., and Barad, D.H. (2011). Association ofFMR1 genotypes with in vitro fertilization (IVF) outcomes based on ethnicity/
race. PLoS ONE 6, e18781.
Gurdon, J.B. (1962). The developmental capacity of nuclei taken from intesti-
The authors would like to acknowledge the OHSU Embryonic Stem Cell
nal epithelium cells of feeding tadpoles. J. Embryol. Exp. Morphol. 10,
Research Oversight Committee and the Institutional Review Board for
providing oversight and guidance with human embryo and ESC studies. We
Kim, K., Doi, A., Wen, B., Ng, K., Zhao, R., Cahan, P., Kim, J., Aryee, M.J., Ji,
thank egg donor volunteers and the staff at the Women's Health Research
H., Ehrlich, L.I., et al. (2010). Epigenetic memory in induced pluripotent stem
Unit at the Center for Women's Health, the Division of Reproductive Endocri-
cells. Nature 467, 285–290.
nology & Infertility of the Department of Obstetrics & Gynecology of Oregon
Kishigami, S., Mizutani, E., Ohta, H., Hikichi, T., Thuan, N.V., Wakayama, S.,
Health & Science University for their support and procurement of human gam-
Bui, H.T., and Wakayama, T. (2006). Significant improvement of mouse cloning
etes. The Division of Animal Resources, Surgery Team, Assisted Reproductive
technique by treatment with trichostatin A after somatic nuclear transfer. Bio-
Technology & Embryonic Stem Cell Core, Endocrine Technology Core, and
chem. Biophys. Res. Commun. 340, 183–189.
Imaging & Morphology Core at the Oregon National Primate Research Centerprovided expertise and services for the nonhuman primate studies. Hamilton
Lanza, R.P., Cibelli, J.B., and West, M.D. (1999). Prospects for the use of nu-
Thorne donated the XYClone laser system for this study. We are grateful to
clear transfer in human transplantation. Nat. Biotechnol. 17, 1171–1174.
Dr. Warren Sanger and Dianna Zaleski for karyotyping services, Dr. Cecilia Pe-
Laurent, L.C., Ulitsky, I., Slavin, I., Tran, H., Schork, A., Morey, R., Lynch, C.,
nedo for microsatellite analysis, and Dr. Cary Harding for assistance with nu-
Harness, J.V., Lee, S., Barrero, M.J., et al. (2011). Dynamic changes in the
10 Cell 153, 1–11, June 6, 2013 ª2013 Elsevier Inc.
Please cite this article in press as: Tachibana et al., Human Embryonic Stem Cells Derived by Somatic Cell Nuclear Transfer, Cell (2013),http://dx.doi.org/10.1016/j.cell.2013.05.006
copy number of pluripotency and cell proliferation genes in human ESCs and
Tachibana, M., Sparman, M., Sritanaudomchai, H., Ma, H., Clepper, L., Wood-
iPSCs during reprogramming and time in culture. Cell Stem Cell 8, 106–118.
ward, J., Li, Y., Ramsey, C., Kolotushkina, O., and Mitalipov, S. (2009). Mito-
Li, J., Svarcova, O., Villemoes, K., Kragh, P.M., Schmidt, M., Bøgh, I.B., Zhang,
chondrial gene replacement in primate offspring and embryonic stem cells.
Y., Du, Y., Lin, L., Purup, S., et al. (2008). High in vitro development after so-
Nature 461, 367–372.
matic cell nuclear transfer and trichostatin A treatment of reconstructed
Tachibana, M., Amato, P., Sparman, M., Woodward, J., Sanchis, D.M., Ma, H.,
porcine embryos. Theriogenology 70, 800–808.
Gutierrez, N.M., Tippner-Hedges, R., Kang, E., Lee, H.S., et al. (2013). To-
Mitalipov, S.M., Nusser, K.D., and Wolf, D.P. (2001). Parthenogenetic activa-
wards germline gene therapy of inherited mitochondrial diseases. Nature
tion of rhesus monkey oocytes and reconstructed embryos. Biol. Reprod.
Takahashi, K., and Yamanaka, S. (2006). Induction of pluripotent stem cells
Mitalipov, S.M., Zhou, Q., Byrne, J.A., Ji, W.Z., Norgren, R.B., and Wolf, D.P.
from mouse embryonic and adult fibroblast cultures by defined factors. Cell
(2007). Reprogramming following somatic cell nuclear transfer in primates is
dependent upon nuclear remodeling. Hum. Reprod. 22, 2232–2242.
Takahashi, K., Tanabe, K., Ohnuki, M., Narita, M., Ichisaka, T., Tomoda, K.,
Nakajima, N., Inomata, T., Ito, J., and Kashiwazaki, N. (2008). Treatment with
and Yamanaka, S. (2007). Induction of pluripotent stem cells from adult human
proteasome inhibitor MG132 during cloning improves survival and pronuclear
fibroblasts by defined factors. Cell 131, 861–872.
number of reconstructed rat embryos. Cloning Stem Cells 10, 461–468.
Nazor, K.L., Altun, G., Lynch, C., Tran, H., Harness, J.V., Slavin, I., Garitaonan-
van der Gaast, M.H., Eijkemans, M.J., van der Net, J.B., de Boer, E.J., Burger,
dia, I., Mu¨ller, F.J., Wang, Y.C., Boscolo, F.S., et al. (2012). Recurrent
C.W., van Leeuwen, F.E., Fauser, B.C., and Macklon, N.S. (2006). Optimum
variations in DNA methylation in human pluripotent stem cells and their differ-
number of oocytes for a successful first IVF treatment cycle. Reprod. Biomed.
entiated derivatives. Cell Stem Cell 10, 620–634.
Online 13, 476–480.
Noggle, S., Fung, H.L., Gore, A., Martinez, H., Satriani, K.C., Prosser, R., Oum,
Wakayama, T., Perry, A.C., Zuccotti, M., Johnson, K.R., and Yanagimachi, R.
K., Paull, D., Druckenmiller, S., Freeby, M., et al. (2011). Human oocytes repro-
(1998). Full-term development of mice from enucleated oocytes injected with
gram somatic cells to a pluripotent state. Nature 478, 70–75.
cumulus cell nuclei. Nature 394, 369–374.
Pellicer, A., Ruiz, A., Castellvi, R.M., Calatayud, C., Ruiz, M., Tarin, J.J., Miro´,
Wilmut, I., Schnieke, A.E., McWhir, J., Kind, A.J., and Campbell, K.H. (1997).
F., and Bonilla-Musoles, F. (1989). Is the retrieval of high numbers of oocytes
Viable offspring derived from fetal and adult mammalian cells. Nature 385,
desirable in patients treated with gonadotrophin-releasing hormone analogues
(GnRHa) and gonadotrophins? Hum. Reprod. 4, 536–540.
Wilmut, I., Beaujean, N., de Sousa, P.A., Dinnyes, A., King, T.J., Paterson, L.A.,
Santos, M.A., Kuijk, E.W., and Macklon, N.S. (2010). The impact of ovarian
Wells, D.N., and Young, L.E. (2002). Somatic cell nuclear transfer. Nature 419,
stimulation for IVF on the developing embryo. Reproduction 139, 23–34.
Solter, D. (2000). Mammalian cloning: advances and limitations. Nat. Rev.
Genet. 1, 199–207.
Wu, Y.G., Zhou, P., Lan, G.C., Wang, G., Luo, M.J., and Tan, J.H. (2007). Theeffects of delayed activation and MG132 treatment on nuclear remodeling and
Sparman, M., Dighe, V., Sritanaudomchai, H., Ma, H., Ramsey, C., Pedersen,
preimplantation development of embryos cloned by electrofusion are corre-
D., Clepper, L., Nighot, P., Wolf, D., Hennebold, J., and Mitalipov, S. (2009).
lated with the age of recipient cytoplasts. Cloning Stem Cells 9, 417–431.
Epigenetic reprogramming by somatic cell nuclear transfer in primates.
Stem Cells 27, 1255–1264.
Yang, X., Smith, S.L., Tian, X.C., Lewin, H.A., Renard, J.P., and Wakayama, T.
Sparman, M.L., Tachibana, M., and Mitalipov, S.M. (2010). Cloning of non-
(2007). Nuclear reprogramming of cloned embryos and its implications for
human primates: the road ‘‘less traveled by''. Int. J. Dev. Biol. 54, 1671–1678.
therapeutic cloning. Nat. Genet. 39, 295–302.
Susko-Parrish, J.L., Leibfried-Rutledge, M.L., Northey, D.L., Schutzkus, V.,
Yu, J., Vodyanik, M.A., Smuga-Otto, K., Antosiewicz-Bourget, J., Frane, J.L.,
and First, N.L. (1994). Inhibition of protein kinases after an induced calcium
Tian, S., Nie, J., Jonsdottir, G.A., Ruotti, V., Stewart, R., et al. (2007). Induced
transient causes transition of bovine oocytes to embryonic cycles without
pluripotent stem cell lines derived from human somatic cells. Science 318,
meiotic completion. Dev. Biol. 166, 729–739.
Cell 153, 1–11, June 6, 2013 ª2013 Elsevier Inc. 11
Source: http://www.explores.pl/files/Cell.pdf
Curr Oncol, Vol. 20, pp. e442-447; doi: http:/ dx.doi.org/10.3747/co.20.1497 DENOSUMAB AND GIANT CELL TUMOUR OF BONE R E V I E W A R T I C L EDenosumab and giant cell tumour of bone—a review and future management considerationsS.F. Xu md phd,* B. Adams md,† X.C. Yu md phd,* and M. Xu md* specimen revealed a tumour mass with histologic
Facultad de Ciencias Veterinarias -UNCPBA- Actualización sobre las bases terapéuticas para la Peritonitis Infecciosa Felina (PIF) y presentación de tres casos clínicos de PIF tratados con Talidomida. Alarcón, Gabriela Verónica; Paludi, Alejandro Esteban; Nejamkin, Pablo. Mayo, 2016







