Levitra enthält Vardenafil, das eine kürzere Wirkdauer als Tadalafil hat, dafür aber schnell einsetzt. Männer, die diskret bestellen möchten, suchen häufig nach levitra kaufen ohne rezept. Dabei spielt die rechtliche Lage in der Schweiz eine wichtige Rolle.
Consequences of acute stress and cortisol manipulation on the physiology, behavior, and reproductive outcome of female pacific salmon on spawning grounds

Contents lists available at
Hormones and Behavior
Consequences of acute stress and cortisol manipulation on the physiology, behavior,and reproductive outcome of female Pacific salmon on spawning grounds
Sarah H. McConnachie ,, Katrina V. Cook , David A. Patterson , Kathleen M. Gilmour , Scott G. Hinch Anthony P. Farrell , Steven J. Cooke
a Fish Ecology and Conservation Physiology Laboratory, Department of Biology, Carleton University, 1125 Colonel By Drive, Ottawa, Ontario, Canada K1S 5B6b Fraser Environmental Watch Program, Fisheries and Oceans Canada, Pacific Region, Science Branch, Cooperative Resource Management Institute,School of Resource and Environmental Management, Simon Fraser University, Burnaby, British Columbia, Canada V5A 1S6c Department of Biology, University of Ottawa, 30 Marie Curie, Ottawa, Ontario, Canada K1N 6N5d Department of Forest Sciences and Institute of Resources, Environment and Sustainability, University of British Columbia, Vancouver, British Columbia, Canada V6T 1Z4e Department of Zoology and Faculty of Land and Food Systems, University of British Columbia, Vancouver, British Columbia, Canada V6T 1Z4f Institute of Environmental Science, Carleton University, 1125 Colonel By Drive, Ottawa, Ontario, Canada K1S 5B6
Life-history theory predicts that stress responses should be muted to maximize reproductive fitness. Yet, the
Received 20 December 2011
relationship between stress and reproduction for semelparous salmon is unusual because successfully
Revised 30 April 2012
spawning individuals have elevated plasma cortisol levels. To tease apart the effects of high baseline cortisol
Accepted 2 May 2012
levels and stress-induced elevation of cortisol titers, we determined how varying degrees of cortisol elevation
Available online 8 May 2012
(i.e., acute and chronic) affected behavior, reproductive physiology, and reproductive success of adult femalepink salmon (Oncorhynchus gorbuscha) relative to different states of ovulation (i.e., ripe and unripe). Exhaus-
tive exercise and air exposure were applied as acute stressors to manipulate plasma cortisol in salmon either
confined to a behavioral arena or free-swimming in a spawning channel. Cortisol (eliciting a cortisol eleva-
tion to levels similar to those in post-spawn female salmon) and metyrapone (a corticosteroid synthesis in-
Oncorhynchus gorbuscha
hibitor) implants were also used to chemically manipulate plasma cortisol. Cortisol implants elevated plasma
Hormone injections
cortisol, and impaired reproductive success; cortisol-treated fish released fewer eggs and died sooner than
fish in other treatment groups. In contrast, acute stressors elevated plasma cortisol and the metyrapone im-
plant suppressed plasma cortisol, but neither treatment significantly altered reproductive success, behavior,
or physiology. Our results suggest that acute stressors do not influence behavior or reproductive outcome
when experienced upon arrival at spawning grounds. Thus, certain critical aspects of salmonid reproductioncan become refractory to various stressful conditions on spawning grounds. However, there is a limit to theability of these fish to tolerate elevated cortisol levels as revealed by experimental elevation of cortisol.
2012 Elsevier Inc. All rights reserved.
an emergency response and animals attempt to regain
Considerable evidence supports the notion that stress can impair
allostasis (). Yet, much of the existing
the reproductive outcome of a wide range of vertebrates, including
work on chronic stress/glucocorticoid elevation is focused on the
birds ), reptiles
long-term consequences for animals during non-reproductive pe-
riods rather than immediately before or during reproduction. For ex-
), and fish ). The
ample, many toxicological studies demonstrate direct long-term
acute stress response and associated elevation of glucocorticoids is
reproductive impairments (e.g., suppression of reproductive hor-
believed to be adaptive, while chronic elevation of glucocorticoids
mones) associated with emergency resource reallocation to mainte-
can have various negative tertiary effects, including impaired im-
nance and survival (e.g., reviewed in see
mune function and fitness whenever resources are directed towards
also ).
Furthermore, most of these studies consider iteroparous species (i.e.
repeat breeders), which have the life-history option of delaying a
⁎ Corresponding author.
reproductive event when challenged.
E-mail addresses: (S.H. McConnachie),
In contrast, semelparous species usually cannot delay the repro-
(K.V. Cook), (D.A. Patterson),
ductive event because they invest in reproduction only once in a life-
(K.M. Gilmour), (S.G. Hinch),(A.P. Farrell), (S.J. Cooke).
time. For semelparous fishes such as Pacific salmonids (Oncorhynchus
0018-506X/$ – see front matter 2012 Elsevier Inc. All rights reserved.
doi:
S.H. McConnachie et al. / Hormones and Behavior 62 (2012) 67–76
spp.), some argue that the spawning date is genetically fixed, which
successfully blocks cortisol synthesis in fish in the short-term
implies that it cannot be altered by external stressors
Curiously, virtually nothing is known about whether exposing
but has rarely been used with a cocoa butter carrier (but see
semelparous Pacific salmonids to stress on spawning grounds influ-
). Rainbow trout (O. mykiss), a congeneric of pink
ences their behavior and reproductive success. Yet, these fish routine-
salmon, weighing approximately 150 g were anesthetized with ben-
ly encounter many stressors that trigger a cortisol response as they
zocaine (0.05 mg ml−1 water; p-aminobenzoic acid ethyl ester;
approach their spawning date, suggesting that the acute stress re-
Sigma E1501, Sigma-Aldrich) and given an IP injection of metyrapone
sponse remains active during the reproductive period. For example,
mixed in heated liquid cocoa butter (200 mg kg−1 fish in l ml cocoa
plasma cortisol rises when fish encounter hydraulic challenges and
butter kg−1 fish); upon injection into the fish, the cocoa butter rapid-
elevated water temperature during the spawning migration (
ly cools to a thick paste, providing a slow-release metyrapone im-
). Furthermore, a progressive increase
plant. After 1 and 5 days, fish were subjected to 1 min of air
in baseline plasma cortisol levels of unknown etiology occurs as salm-
exposure as an acute stressor, and a blood sample was withdrawn
on swim to the spawning grounds
by caudal puncture 30 min later for assessment of plasma cortisol
levels. The expectation was that this 30-min delay would be sufficient
cortisol concentrations rise from 25 ng ml−1 in pink salmon (O.
for the maximum or near maximum rise in plasma cortisol level to be
gorbuscha) at river entry (), to 350 ng ml−1 on
arrival at the spawning ground (female sockeye salmon [O. nerka];), and 1287 ng ml−1 when the fish become mor-
Weaver Creek spawning channel
ibund (female sockeye salmon; ). Thus, an acutestressor can elevate plasma cortisol against a background of progres-
All field experiments were conducted at the Weaver Creek
sively increasing plasma cortisol levels during the spawning
spawning channel located in British Columbia, Canada (see
for detailed information). Each experiment involved
A stressed state should generally be incompatible with reproduc-
groups of naive fish (i.e., fish were not reused among experiments).
tion and, based on life-history theory, one could postulate that the
The artificial channel, 2.93 km long and 6.1 m wide, is composed of
cortisol stress response of semelparous salmon should be muted, or
a cobble (1.2–7.6 cm) substrate and has a consistent water depth of
physiologically irrelevant, during this period (
25–30 cm. Fish densities and flow conditions were monitored
) to mitigate any potential negative effects of cortisol
throughout the spawning period and manually operated gates were
elevation above the (high) baseline levels on spawning grounds.
used to regulate fish movements into the spawning channel
Thus, we postulate that reproductive drive in a semelparous salmon
(). Experiments were timed to coincide with peak
species will outweigh any cortisol-mediated mating inhibition.
pink salmon spawning activity in early October 2009.
Acute, stress-related increases in plasma cortisol suppress the normalincreases in plasma sex hormone concentrations for Pacific salmon
Reproductive physiology on arrival
during early phases of upriver migration (However,increases in plasma cortisol during migration are regarded as adap-
On arrival at the spawning channel in early October, female pink
tive and necessary for salmon to be able to return to their natal
salmon were individually removed from the raceway via dip net
streams and spawn (Complicating matters is
and immediately placed in a trough supplied with flow-through
the fact that spawning Pacific salmon also undergo senescence,
water from the raceway. Fish were categorized as either "unripe"
which alters many physiological processes, including hormone regu-
(N = 52, unovulated, where eggs are still confined to intact ovaries)
lation To address
or "ripe" (N = 60, ovulated, where eggs have been released into the
these issues, we experimentally determined how short-term changes
body cavity and gentle abdominal pressure near the vent easily ex-
in and experimental manipulation of plasma cortisol influenced the
pels eggs) and a blood sample was collected via caudal puncture
reproductive physiology, behavior, and spawning outcome of wild fe-
(2 ml blood sample; collected using 3 ml vacutainer and 1.5 in.,
male pink salmon (O. gorbuscha). We administered cortisol implants
18 ga needle, lithium heparin; Becton Dickson, NJ) within 30 s
and predicted that plasma cortisol elevation, lasting between 2 and
(Within 3 min the fish were released back into
5 days, would negatively affect reproductive behavior (e.g., less time
the spawning channel. Blood samples were stored in an ice-water
spent guarding eggs or fighting for a mate), physiology (i.e., suppres-
slurry and centrifuged (5 min at 10,000 g) within 45 min, after
sion of reproductive hormones), and outcome (i.e., number of eggs
which the plasma was frozen in liquid nitrogen immediately. Samples
released). We also predicted that the response to acute stressors
were subsequently stored at −80 °C until further analysis.
(i.e., exhaustive exercise or air exposure) would be muted in semel-
In addition, subsets of ripe (N= 6) and unripe (N= 12) salmon
parous salmon and would not alter these same responses. Conversely,
were given an intraperitoneal (IP) injection of either cortisol (hydrocor-
an intraperitoneal (IP) implant of metyrapone, which blocks the last
tisone 21-hemisuccinate; Sigma H4881, Sigma-Aldrich; 110 mg kg−1
step of glucocorticoid synthesis, was expected to lower plasma corti-
fish in 50 ml melted cocoa butter kg−1 fish; ) to el-
sol levels () and retard reproduction and senes-
evate cortisol levels for a short period (i.e., 2 to 5 days), or metyrapone
cence. To our knowledge, hormone manipulations of this type had
(200 mg kg−1 fish; 1 ml cocoa butter kg−1 fish) to block glucocorticoid
not before been performed on senescing Pacific salmon.
synthesis (), before being placed in individual,opaque, experimental chambers ( 50 l) situated on the bank of the
Materials and methods
channel and equipped with flow-through water. Fish were leftundisturbed for approximately 24 h, after which they were individually
Metyrapone validation
removed and blood was sampled immediately via caudal puncture.
All fish were handled in accordance with the guidelines of the Ca-
Longevity and reproductive status study
nadian Council on Animal Care (Carleton University, B09-12; Univer-sity of Ottawa, BL-228). A pilot laboratory experiment was carried out
On October 6th and 7th 2009, 120 unripe pink salmon that had
to determine the effectiveness of metyrapone (2-methyl-1, 2-di-3-
voluntarily entered the raceway were marked with unique individual
pyridyl-1-propanone; Sigma 85625, Sigma-Aldrich) at blocking corti-
Peterson disk tags placed in the dorsal musculature. The tags could be
sol synthesis when delivered in a cocoa butter implant. Metyrapone
read on free-swimming fish with binoculars, which allowed the fish
S.H. McConnachie et al. / Hormones and Behavior 62 (2012) 67–76
to be observed without any disturbances. Fish were randomly
fish was on the receiving end of an aggressive act (both were
assigned to one of six treatment groups (N = 20 per treatment
summed and divided by total observation minutes, and aggression re-
group): a) control fish (only tagged); b) sham injection-controls
ceived was subtracted from aggression given to yield an overall ag-
(tagged and given an IP injection of 50 ml kg−1 melted cocoa butter);
gression score). The daily duration of behavioral observations on
c) cortisol-treated (as described above); d) metyrapone-treated (as
each fish (i.e., 10 min) was consistent with other studies
described above); e) chased (acutely stressed by 3-min of being
and is believed to be representative of longer time periods
"chased" by hand around a circular tank supplied with flow-through
given the reasonable predictability and stability of behavioral reper-
channel water); and f) air-exposed (as in (e), followed by 1 min of
toires for this species. After 4 days, the fish were collectively culled
air exposure to increase the severity of the acute stressor). After-
in a process lasting b 10 min; fish were killed by cerebral percussion.
wards, fish were immediately released into the spawning channel
After immediate blood sampling, the percentage of eggs released
and closely monitored during daylight hours so that moribund or
was estimated (as described above).
dead fish could be collected daily.
Longevity in the spawning channel following release (i.e. time until
death after arrival) was calculated using the methods outlined inFork length, total mass, gonad mass, epidermal
Plasma glucose and cortisol concentrations were measured as in-
coverage by fungus, and general condition also were documented. Re-
dicators of stress Briefly, plasma glucose
productive status was reported as the percentage (%) of eggs released
values were determined using a YSI 2300 STAT Plus glucose analyzer
by each individual. The relationship between percentage of eggs
(YSI Inc., Yellow Springs, Ohio). Plasma cortisol levels were measured
remaining relative to percentage of eggs initially expected was deter-
using a commercial ELISA kit (Neogen Corporation # 402710, Lexing-
mined following the methods of . Briefly, the antic-
ton KY). For cortisol, the assay has 47% cross-reactivity with the drug
ipated initial gonad mass was determined from a known relationship
prednisolone, which would not be present in the samples.
between body mass and gonad mass established for a separate group
The assay also has 15% cross-reactivity with cortisone and 11-
of mature, unripe pink salmon sampled from the spawning channel
deoxycortisol. The analytical sensitivity (B/B
(N= 21; gonad mass= 10.1·body mass −297.9, R2=0.80, P=0.005).
0, 80%) for the cortisol
assay was at 0.04 ng ml−1. Testosterone and 17β-estradiol are both
Eggs were weighed and counted in whole ovaries and a linear body
major reproductive hormones and plasma concentrations of these hor-
mass to fork length relationship, together with a linear fork length to
mones also were measured by ELISA kits (Neogen Corporation,
gonad mass relationship, was used to interpolate the expected egg
, catalog numbers: 402110, 402510). Testosterone and
mass before ovulation for the experimental fish. Many fish had
17β-estradiol were extracted from plasma samples using ethyl ether
spawned all of their eggs (100% success), but any eggs remaining
according to the kit manufacturer's protocols. The assay manufacturer
were weighed first as five groups of 10 eggs, with any eggs remaining
states that the estradiol assay does not cross-react with any other estro-
thereafter being weighed collectively. Individual egg mass is known to
gens. Analytical sensitivity (B/B
be uniform within an individual (D. Patterson, personal communica-
0, 80%) was at 0.03 ng ml− 1. According
to the manufacturer, the testosterone assay is 100% cross-reactive
tion), and so this method provided an accurate estimate of the number
with dihydrotestosterone and the analytical sensitivity (B/B
of eggs retained by each fish without having to count every egg.
was at 0.006 ng ml−1. Cortisol, glucose, testosterone, and 17β-estradiol were assayed in duplicate at appropriate dilutions. Inter- and
Spawning behavior in enclosures
intra-assay variability was b10% for all assays. More detailed descrip-tions of the analytical techniques can be found in
Behaviors were studied in unripe and ripe salmon held in enclo-
sures that had been constructed within the spawning channel. Ablood sample (as described above) was withdrawn from 30 salmon(6 treatment groups as above; N = 5 for each treatment group) in
Statistical analysis
the raceway before placing them in a holding tank for transfer to asection of the spawning channel that housed a net-pen (2 m wide
Results from the metyrapone pilot study were analyzed using a
by 15 m long; constructed out of Vexar rigid mesh fencing; Master-
two-way analysis of variance (ANOVA) to determine whether cortisol
net, Mississauga, Ontario). Fish were treated according to their exper-
values varied by treatment and time. Results from the cortisol and
imental group before being placed into the enclosure. Twenty "ripe"
metyrapone validation study before and after 24 h were compared
male pink salmon (i.e. males that released sperm when squeezed
using two-way repeated measures ANOVA models with time and
gently near the vent) had been placed into the net-pen 12 h earlier.
treatment as effects. For the channel experiment, longevity among
Fewer males were placed in the pen than females to facilitate compe-
treatment groups was compared using a log-rank survival analysis
tition among females. Two trials were completed for unripe salmon in
to 50% mortality. The percentage of eggs released by each fish was
early October 2009, and two trials were completed for ripe fish in late
averaged within groups and compared using a one-way ANOVA. For
October 2009.
the enclosure experiments, all hormone and blood physiology values
Behavioral observations were carried out for 10 min daily on four
and behavioral metrics were compared before and after 4 days using
consecutive days. The order of observing each fish was randomized
two-way repeated measures ANOVA models with time and treatment
daily. Reproductive behaviors of pink salmon are well known, and
being the independent variables. Time until territory establishment
are similar to behaviors displayed by other semelparous Pacific salm-
was determined using log-rank survival analyses. The percentage of
on Females prepare their
eggs released by each fish was averaged for each treatment group and
nesting area, fend off intruders from their territory through aggres-
compared using a one-way ANOVA. Tukey's post-hoc tests were
sive action, and spend time with males to ensure fertilization occurs.
employed following significant one-way ANOVAs to determine differ-
We recorded on what day fish established a territory, how much time
ences among groups (where p b 0.05). The assumptions of equality of
the fish spent holding that territory (represented as a percent, aver-
variances and normal distribution were tested for all analyses and
aged over days on territory), what percentage of their time females
relevant transformations applied where assumptions could not be met.
spent with males (averaged across days on an established territory),
Percentage data were arcsine transformed prior to analysis. Where trans-
the number of nest construction digging behaviors that occurred (av-
formation of the data was not possible or effective, non-parametric anal-
eraged across days spent on a territory), how many times a fish made
yses were performed. All analyses were conducted using JMP, version
an aggressive display towards a conspecific, and how many times that
8.0.2 (SAS Institute Inc., Cary, NC). The level of significance (α) for all
S.H. McConnachie et al. / Hormones and Behavior 62 (2012) 67–76
tests was assessed at 0.05. All data are presented as mean ±standard
error unless otherwise noted.
Initial blood hormone and glucose values of ripe and unripe pink salmon(Oncorhynchus gorbuscha) removed from the Weaver Creek raceway in October,2009, presented as mean (± SE). N = 52 for unripe fish and N = 60 for ripe fish. All
data were analyzed using the Wilcoxon Rank-Sum Test, except for cortisol (*), whichwas analyzed using log-transformed data in a one-way ANOVA.
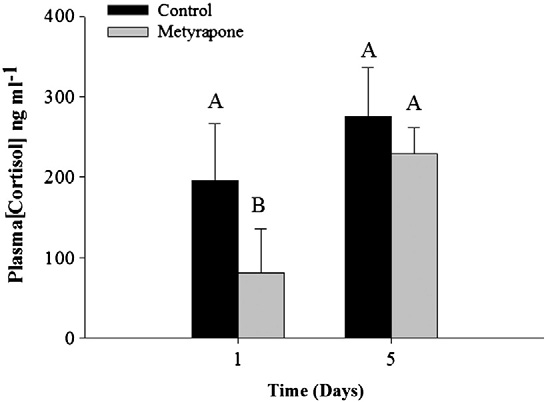
Effectiveness of metyrapone
Metyrapone-treated rainbow trout subjected to an acute stressor
exhibited significantly lower plasma cortisol concentrations than
Glucose (mmol l−1)
sham-treated fish 1 day following treatment (two-way ANOVA Time
Cortisol (ng ml−1)*
Estradiol (ng ml−1)
effect: F = 7.8, df = 1, p = 0.02; but not after 5 days (Treat-
Testosterone (ng ml− 1)
ment effect: F = 3.1, df = 1, p = 0.1; Interaction: F = 4.7, df = 3,p = 0.03; ). Therefore, we assumed that pink salmon would ex-perience a short-term depression of plasma cortisol during acute
measures ANOVA: Treatment effect: F= 1.0, df =1, p b 0.001; Time:
stress (i.e., for at least 24 h but not as long as 5 days) and used
F = 34, df =1, pb 0.001; Interaction: F=5.4, df=3, pb 0.001;
cocoa butter as a vehicle for metyrapone delivery.
Plasma glucose values increased 24 h after either treatment (Treatmenteffect: F =5.1, df =1, p= 0.3; Time: F =6.8, df =1, p= 0.02; Interaction:
Raceway blood physiology and hormone validations
F= 2.2, df =3, p=0.03; Estradiol was unaffected by either treat-ment (Treatment effect: F=0.69, df= 1, p=0.4; Time: F= 0.90, df =1,
Reproductive hormone titers were indicative of whether pink
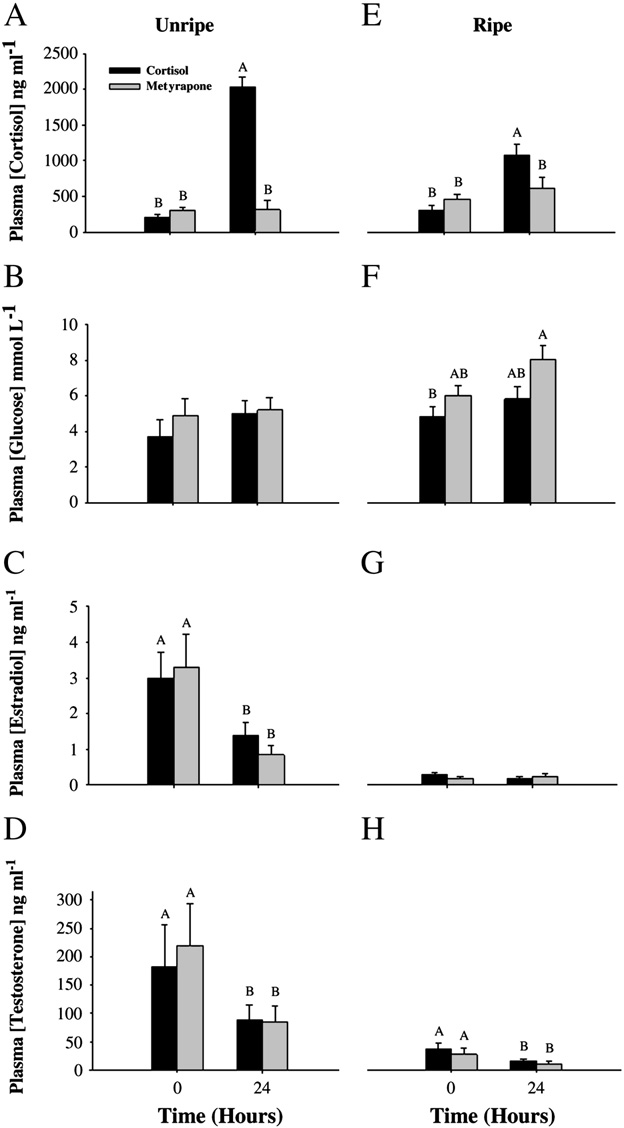
salmon in the spawning channel were ripe or unripe (). Plasma estradiol and testosterone were both signifi-cantly lower in ripe fish (estradiol: F = 70, df = 1, p b 0.001; testoster-one: F = 25, df = 1, p b 0.001; However, plasma cortisolconcentrations were similar (one-way ANOVA, F = 0.31, df = 1,p = 0.6; and plasma glucose concentrations were higher inripe fish (one-way ANOVA, F = 13, df = 1, p b 0.001; ) for arriv-ing pink salmon.
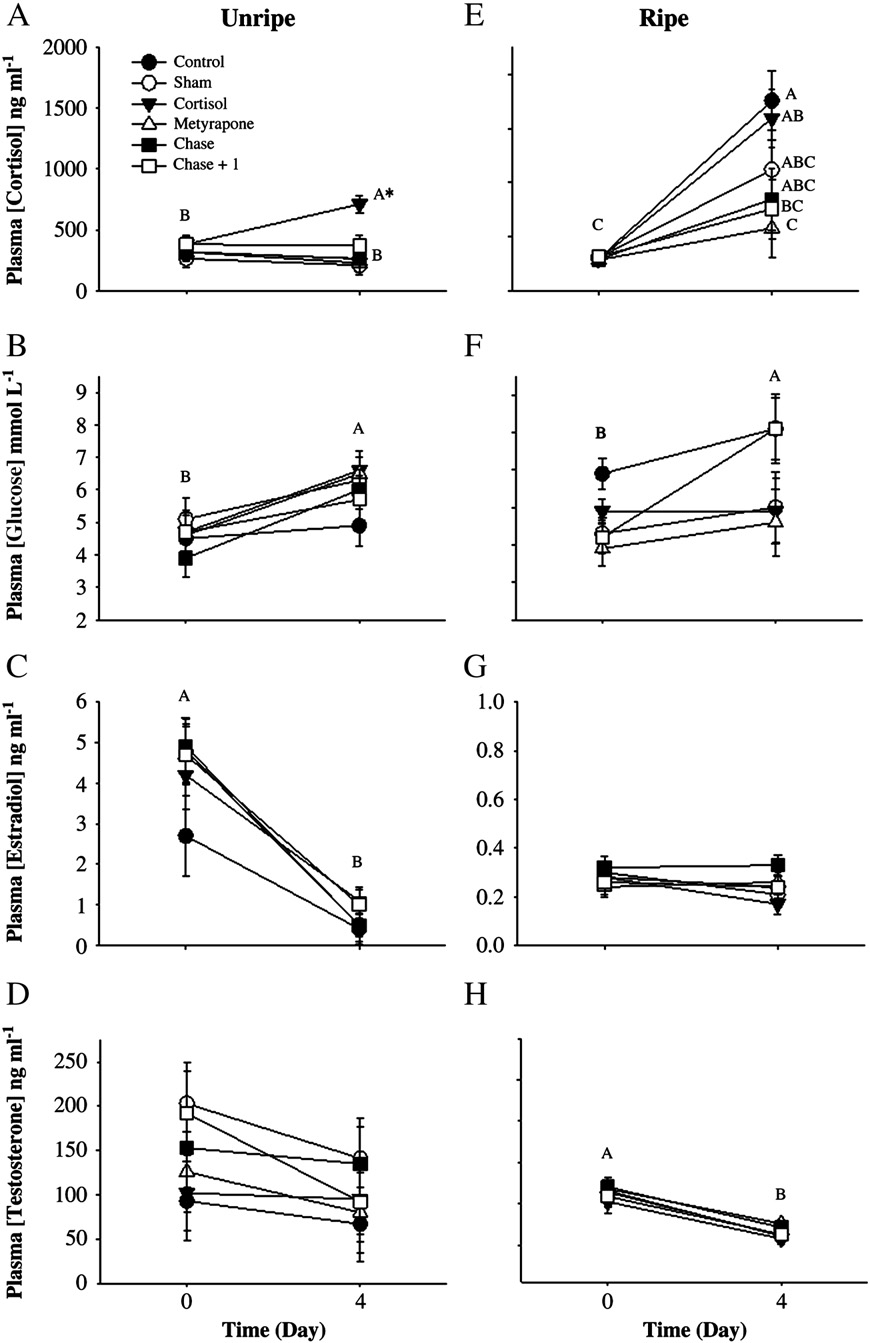
For unripe fish held in isolation chambers, cortisol implants signifi-
cantly elevated plasma cortisol by 10-fold, but metyrapone implantshad no effect on circulating cortisol levels after 24 h (two-wayrepeated-measures ANOVA: Treatment effect: F=55, df= 1, p b 0.001;Time: F=70, df= 1, p b 0.001; Interaction: F=15, df=3, pb 0.001;Plasma glucose was unchanged 24 h after either treatment(Treatment effect: F=0.69, df =1, p =0.4; Time: F= 0.90, df =1,p= 0.4; Interaction: F= 0.39, df =3, p =0.9; ). Plasma concentra-tions of both estradiol (Treatment effect: F =0.8, df= 1, p =0.8; Time:F= 8.5, df =1, p =0.02; Interaction: F=1.5, df= 3, p= 0.3; C)and testosterone (Treatment effect: F=0.13, df= 1, p =0.7; Time:F= 5.7, df =1, p=0.04; Interaction: F=1.7, df= 3, p =0.2; D) de-creased 24 h after either treatment.
For ripe fish held in isolation chambers, the cortisol implant again
increased plasma cortisol values, but the response was attenuated com-pared with that of unripe fish E). Plasma cortisol concentrationwas not affected by the metyrapone implant (two-way repeated-
Fig. 2. A–H. Summary of pink salmon (Oncorhynchus gorbuscha) plasma hormone and
Fig. 1. Mean (± SE) cortisol values for control and metyrapone-treated rainbow trout
glucose values for unripe (A–D) and ripe (E–H) fish both before and 24 h after treat-
(Oncorhynchus mykiss) subjected to an air-exposure stressor either 1 or 5 days after
ment with cortisol or metyrapone. Values are stated as mean (± SE). Dissimilar letters
treatment with metyrapone. Data were log-transformed and analyzed using a two-
denote significant differences among treatment groups and time periods (Tukey–
way ANOVA. Dissimilar letters denote a significant difference between treatment
Kramer HSD test, p b 0.05). N = 6 for each treatment for unripe fish; N = 12 for ripe
groups and/or time periods (Tukey–Kramer HSD test, p b 0.05). Sample sizes are as fol-
fish. All Ranked Sum data were analyzed using a two-way repeated-measures
lows: 1 day: control = 2, metyrapone = 5. 5 days: control = 4, metyrapone = 4.
ANOVA, with time and treatment as independent variables.
S.H. McConnachie et al. / Hormones and Behavior 62 (2012) 67–76
p= 0.4; Interaction: F=1.2, df= 3, p =0.3; Plasma testosteronewas decreased 24 h after both treatments (Treatment effect: F=0.83,df= 1, p= 0.4; Time: F =21, df =1, pb 0.001; Interaction: F=0.27,df= 3, p= 0.6;
Longevity and reproductive study
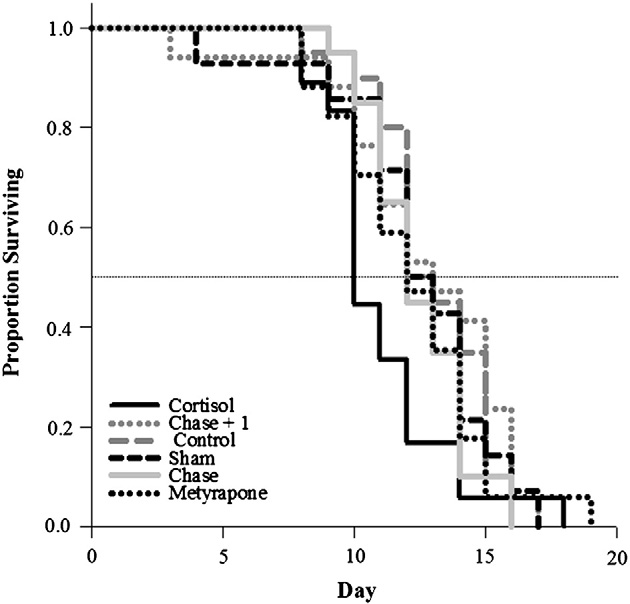
Pink salmon treated with cortisol exhibited reduced longevity rel-
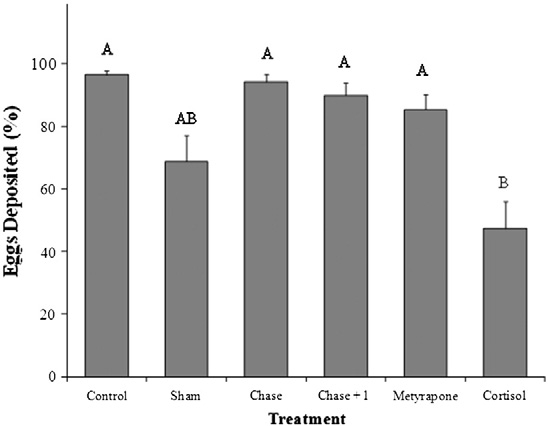
ative to all other treatment groups (log-rank survival time to 50%mortality; λ2 = 13.1, df = 5, p = 0.02; ). Cortisol-treated fishalso released fewer eggs during their time in the channel comparedwith all other treatment groups except the sham group [47% forcortisol-injected; 69% for sham-treated; >85% for all other groups(one-way ANOVA, F = 13, df = 5, p b 0.001; )].
Enclosure experiment: reproductive status
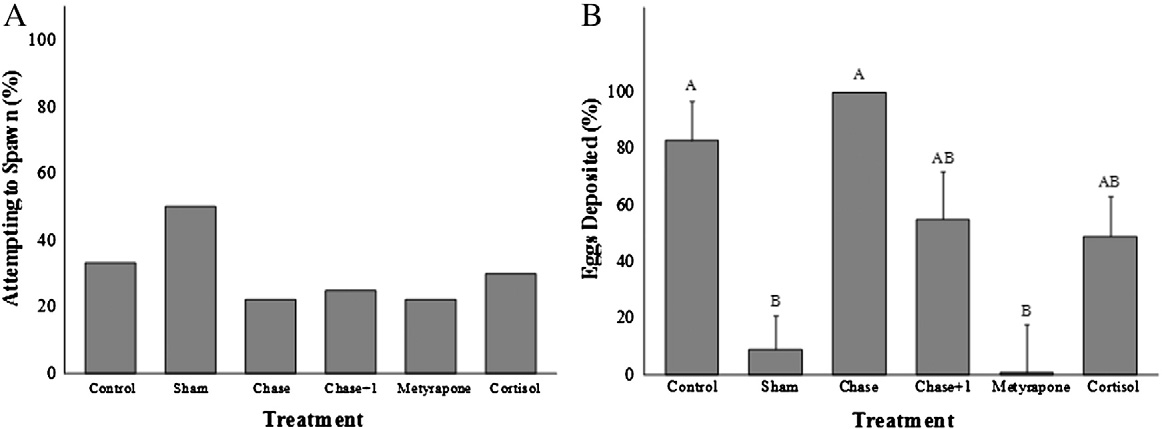
Fig. 4. A comparison across treatment groups (see text for details of treatment groups)of the percentage (%) of total possible eggs deposited by pink salmon (Oncorhynchus
Treatment with cortisol, metyrapone or acute stress did not influ-
gorbuscha) in the Weaver Creek spawning channel during early October, 2009. All
ence the extent to which fish ripened during the experiment
data were transformed into ArcSine (square root) values before analysis. Samplesizes were as follows; chase and control = 20, cortisol = 18, chase + 1 min air exposure
. For those fish that did ripen during the enclosure experiment,
and metyrapone = 17 and sham = 14. Dissimilar letters denote significant differences
differences in egg release (%) were observed (Wilcoxon Rank Sum;
among treatment groups (Tukey–Kramer HSD test, p b 0.05).
λ2=11.2, df=5, p=0.04; . Control and chased fish releasedmore than 80% of their eggs, chase + 1 min air exposure and cortisol-treated fish released approximately 50% of their eggs, and metyrapone
Time: F = 72, df = 1, p b 0.001; Interaction: F=0.6, df=5, p=0.7;
and sham-treated fish released the fewest eggs (b10%). For ripe fish,
C), whereas plasma testosterone concentrations remained
there were no statistically significant differences in egg release
unchanged over the 4 day experimentation period (Treatment effect:
among treatment groups (data not shown). However, cortisol-treated
F = 1.1, df = 1, p = 0.4; Time: F = 3.3, df = 1, p = 0.08; Interaction:
fish released 50% of their eggs, whereas all other treatment groups
F = 0.3, df = 5, p = 0.9; D).
released >70% of their eggs.
For ripe fish, plasma cortisol levels varied across treatment groups
after 4 days Control fish exhibited the highest levels (1756±
Enclosure experiment: hormone profiles
274 ng ml−1), and cortisol-treated fish displayed similar concentrations
(averaging 1592 ±207 ng ml−1). Values were similar among sham-
Among unripe fish, cortisol-treated fish exhibited elevated cortisol
treated (1118 ±211 ng ml−1), chased fish (846±289 ng ml−1), and
concentrations 4 days following treatment (two-way repeated-
chased+ 1 (757 ±186 ng ml−1) fish, whereas the lowest value (577±
measures ANOVA: Treatment effect: F = 4.6, df = 1, p = 0.002; Time:
193 ng ml−1) was observed in metyrapone-treated fish (two-way
F = 0.4, df = 1, p = 0.9; Interaction: F = 2.5, df = 5, p = 0.04; .
repeated-measures ANOVA: Treatment effect: F=2.7, df =1, p =0.03;
Plasma glucose concentration increased in all fish during the 4 day ex-
Time: F= 55, df =1, pb 0.001; Interaction: F=3.2, df=5, p=0.01;
periment (Treatment effect: F = 0.7, df = 1, p = 0.6; Time: F = 15.6,
E). Plasma glucose concentrations increased during the 4 day peri-
df= 1, p b 0.001; Interaction: F=0.6, df=5, p=0.7; B). Plasma
od, across treatments (Treatment effect: F=1.7, df= 1, p=0.1; Time:
estradiol levels decreased (Treatment effect: F = 0.5, df= 1, p = 0.8;
F= 5.4, df =1, p= 0.02; Interaction: F =0.93, df =5, p= 0.5; Plasma estradiol levels were low and did not change (Treatment effect:F= 1.2, df=1, p=0.3; Time: F=2.5, df =1, p =0.1; Interaction:F= 0.7, df =5, p =0.6; , whereas plasma testosterone concentra-tions decreased after 4 days (Treatment effect: F= 0.4, df= 1, p= 0.8;Time: F= 91, df= 1, p b 0.001; Interaction: F=0.2, df=5, p=0.9;H).
Enclosure experiment: behavior observations
Among unripe fish, treatment did not influence the rate of territory
establishment (log-rank survival analysis; λ2=2.4, df=5, p=0.8).
Based on behavioral observations for fish on territories, cortisol-treated fish spent 10% less time holding their territory comparedwith controls (one-way ANOVA: F = 12, df = 5, p = 0.03; Addi-tionally, cortisol-treated fish were less aggressive and experiencedmore aggressive acts by conspecifics (F=13, df=5, p=0.04;Among ripe fish, no differences were noted for territory estab-lishment (log-rank survival analysis; λ2=4.0, df=5, p=0.5). In addi-tion, no behavioral differences were observed among the treatmentsgroups ().
Fig. 3. Log-rank survival analysis to 50% mortality in each treatment group (see text for
By experimentally elevating plasma cortisol in unripe fish for be-
details of treatment groups), comparing longevity among pink salmon (Oncorhynchus
tween 2 and 5 days with a cortisol in cocoa butter implant, we negative-
gorbuscha) in the Weaver Creek spawning channel. Sample sizes were as follows:chase and control = 20, cortisol = 18, chase + 1 and metyrapone = 17 and sham = 14.
ly impacted the longevity, reproductive behavior, and reproductive
S.H. McConnachie et al. / Hormones and Behavior 62 (2012) 67–76
Fig. 5. (A and B). Figure A presents the percentage of pink salmon (Oncorhynchus gorbuscha) that became ripe during the behavior trials and thus were able to spawn during theenclosure experiment. Figure B presents the percentage of total eggs available (%) that were deposited during the 4 day trials by ripened fish across treatment groups. Sample sizeswere as follows: chase = 1/9, chase + 1 = 2/8, control = 3/9, cortisol = 3/10, metyrapone = 2/9, sham = 4/8. All data were transformed into ArcSine (square root) values beforebeing analyzed. Dissimilar letters denote significant differences among treatment groups (Tukey–Kramer HSD test, p b 0.05).
outcome of pink salmon on their spawning grounds. Conversely, acute
Nonetheless, collectively these data are consistent with the notion
stressors that also presumably elevated plasma cortisol, namely exercise
that semelparous salmon may be resilient to the effects of stress hor-
and air exposure, did not affect reproductive outcomes in either ripe or
mones during the final phases of reproduction
unripe fish. These results demonstrate that a sustained elevation of plas-
). However, in the case of Pacific salmon, it is unclear
ma cortisol carries significant reproductive costs for semelparous salmon
when such a transition takes place during the migration. In main-
on their spawning grounds (despite their high baseline cortisol levels),
stream riverine habitats, fish mount a cortisol response to a stressor
but that temporary elevations may not. In an ecologically relevant
and cortisol does, indeed, result in suppression of reproductive
context, events that could elicit a prolonged stress response that might
hormone titers ). Yet, our data indi-
last 2–5 days include periods of high water temperature (
cate that, upon arrival at spawning grounds, reproductive hormones are
), seasonally high (or low) river discharge ),
not altered by either certain acute stressors that are expected to elevate
river obstructions or regions that are hydraulically complex (
plasma cortisol levels (see below) or experimental cortisol manipulation.
), or disease ). In contrast, very short-
Because we did not observe any differences between ripe and unripe fish
term stressors, which might include fisheries interactions, failed preda-
with respect to the influence of cortisol elevation on hormone titers, the
tion events, and antagonistic interactions with conspecifics just prior to
onset of resistance to elevated cortisol appears to occur prior to ovulation,
or during spawning may result in fewer effects on reproduction.
a point that warrants investigation in a further study. The transition maybe associated with the decline from stable levels of reproductive hor-
Cortisol manipulation and reproductive hormones
mones as the fish move into an ovulated state. During ovulation, thereis a critical need to increase 17α-hydroxy-20 β-dihydroprogesterone
In a variety of fish species, elevation of glucocorticoids results in de-
(17α, 20β-P) to complete reproduction because this
creased reproductive hormone concentrations (see review by
hormone induces sexual maturation necessary for the ovulation process,
), which in iteroparous fish can lead to a postponed
whereas estradiol and testosterone mediate maturation and ovulation
reproductive event. Additionally, a stressful reproductive environment
(e.g., fish exposed to bleached kraft pulp mill effluent) negativelyimpacts reproductive fitness in various ways
Channel longevity and reproductive success
). In semelparous Pacific salmonexposed to a natural hydraulic challenge during their reproductive
Cortisol-treated fish exhibited decreased longevity and high egg
migration up the Fraser River system in BC (at the Hell's Gate fishway,
retention during the channel experiment, despite our finding that
in the Thompson Canyon, BC, ),
cortisol treatment did not change reproductive hormone titers.
reproductive hormone titers (i.e., 11-ketotestosterone, estradiol and
Therefore, chronic cortisol elevation on spawning grounds negatively
testosterone) fall dramatically while cortisol levels increase. Further
influences reproductive function and success. Even though egg re-
upstream, where the river is less challenging and perhaps less than
lease by metyrapone- and sham-treated fish during the unripe enclo-
1 day later in the migration, baseline values of cortisol are restored
sure experiment was reduced when compared to other treatment
( 100 ng ml−1) and reproductive hormones return to their elevated
groups, overall these fish still released the majority of their eggs and
levels Yet prior to the present study, the potential
longevity was comparable to control groups. As such, even if the
interactions between cortisol and reproductive hormone oscillations
cocoa butter implant did prevent some egg release in the sham, corti-
had not been investigated in terms of impacts on behavior at spawning
sol, and metyrapone treatments (see discussion below), the existence
grounds and reproduction for a semelparous species. The raceway
of differences among these treatments lends support to the notion
blood profiles and hormone validation data collected in the present
that the driver of the differences was of a physiological nature rather
study indicated that, even though cortisol titers in cortisol-treated
than an artifact of the use of cocoa butter.
fish were increased to levels observed in senescing salmon (
This suite of findings is particularly important because fisheries
managers are concerned with the largely unexplained phenomenon
cortisol treatment did not alter reproductive hormone titers in either
of "pre-spawn mortality"—fish that die on spawning grounds either
unripe or ripe fish.
without spawning or with significant egg retention
It is important to recognize that the experimental elevation of cor-
The eggs of such fishes are often still viable (
tisol titers with IP implants is not itself a stress response, but instead
so it appears that other factors are inhibiting reproductive be-
results in elevated cortisol that is consistent with a stress response.
havior and/or are advancing senescence. In a study of sockeye salmon
S.H. McConnachie et al. / Hormones and Behavior 62 (2012) 67–76
Fig. 6. (A–H). Blood hormone and glucose values of unripe (A–D) and ripe (E–H) pink salmon (Oncorhynchus gorbuscha) before experimentation and 4 days after treatment (seetext for treatment details), stated as mean values (± SE). All Ranked Sum data were analyzed using two-way, repeated-measures ANOVAs with time and treatment as the indepen-dent variables. Dissimilar letters denote significant differences among treatment groups and time periods (Tukey–Kramer HSD test, p b 0.05). Sample sizes were as follows for un-ripe fish: before; cortisol = 10, control, chase and metyrapone = 9, chase + 1 and sham = 8. After; cortisol = 10, chase and control = 9, chase + 1, sham and metyrapone = 8. Samplesizes were as follows for ripe fish: N = 10 for all groups except for the "after" chase group where N = 9.
Table 2Pink salmon (Oncorhynchus gorbuscha) behavior profiles for unripe fish during 4 day trials; values are stated as mean (±SE). All data were analyzed using Wilcoxon Rank-Sumtests, and Tukey's HSD test was used to determine where differences lay when a significant effect was obtained (noted by letter scores). All data that are expressed as percentageswere transformed into ArcSine (square root) values before being analyzed. Data for all variables except the aggression score were averaged over days that fish were on establishedterritories. Aggression scores were added for all days spent on territories and divided by number of observational min. Each fish had a similar score for aggressive attacks against,and this score, divided by number of observational min, was subtracted from the previous value to obtain the overall aggression score. Sample sizes were as follows: chase = 9,control and sham = 7, chase + 1 and cortisol = 6, metyrapone = 5.
% Time on territory
Average # of digs
S.H. McConnachie et al. / Hormones and Behavior 62 (2012) 67–76
Table 3Pink salmon (Oncorhynchus gorbuscha) behavior profiles for ripe fish during 4 day trials; values are stated as mean (±SE). All data were analyzed using Wilcoxon Rank-Sum testsand Tukey's HSD test was used to determine where differences lay when a significant effect was obtained (noted by letter scores). All data that are expressed as percentages weretransformed into ArcSine (square root) values before being analyzed. Data for all variables except the aggression score were averaged over days that fish were on established ter-ritories. Aggression scores were added for all days spent on territories and divided by number of observational min. Each fish had a similar score for aggressive attacks against, andthis score, divided by number of observational min was subtracted from the previous value to obtain the overall aggression score. Sample sizes were as follows: metyrapone = 10,sham, cortisol, control and chase + 1 = 9 and chase = 7.
% Time on territory
Average # of digs
at the Weaver Creek spawning channel, related
between the effects of baseline cortisol and stress-induced cortisol on
mortality to changes in physiological condition and activity levels,
providing a baseline of variables that change as Pacific salmon (spe-
There was evidence that metyrapone treatment caused some egg
cifically sockeye salmon) senesce. To complement that work, the pre-
retention and delayed senescence, as observed in the enclosure
sent study attempted to identify whether stressful conditions can
study (i.e., significantly lower cortisol values compared with other
cause pre-spawn mortality on spawning grounds. It seems plausible
treatment groups in ripe fish). If cortisol spikes immediately prior to
that since cortisol treatment in the present study increased cortisol
ovulation ), this process could have been inhibited
values to those found in senescing fish and at the same time reduced
through the action of metyrapone in blocking cortisol synthesis. Addi-
longevity, then the premature mortality we observed was a function
tionally, cortisol rises again during senescence (),
of this senescence-like physiological state, a state that was not
and this process also could have been inhibited by the action of
reached via the imposition of acute stressors, even though exposure
metyrapone. To examine these possibilities, more detailed time
to acute stressors was expected to acutely elevate circulating cortisol
course of plasma hormone levels is needed. Ideally, metyrapone-
treated fish should be monitored just prior to ovulation, immediatelyfollowing egg release and before morbidity.
Elevated cortisol levels on spawning grounds
Unripe cortisol-treated fish spent less time on their territory than
all other groups. In addition, cortisol-treated fish were significantly
One of the most notable findings of this study was that exposure of
less aggressive than fish in the other treatment groups, and were fre-
pink salmon to acute stressors on spawning grounds did not alter
quently subjected to aggressive attacks from conspecifics. A decrease
spawning ground longevity, reproductive success, or behavior, in ac-
in aggressiveness is detrimental to reproductive success because a fe-
cordance with theory that semelparous animals in general should resist
male benefits from guarding its territory from other females looking
stress (i.e. attenuate stress responses and/or exhibit resistance to the
for suitable habitat, and aggressive behavior is often associated with
effects of elevated stress hormone levels) in favor of allocating energy
reproductive success (). These re-
to their current, and only, reproductive opportunity
sults are supported by previous studies that found that cortisol treat-
). Behavioral and physiological profiles of spawning
ment increased the probability of individual fish (rainbow trout in
Pacific salmon are well documented, but the function of (baseline) cor-
these cases) experiencing increased fin damage indicative of both ag-
tisol elevation in semelparous fish in their natural spawning habitat is
gressive attacks (and becoming socially
not well understood. From a mechanistic standpoint, it has yet to be
subordinate (No behav-
determined how semelparous salmon successfully breed despite circu-
ioral differences were detected among treatments for ripe fish. This
lating cortisol being elevated to a level that would inhibit reproduction
finding suggests that even in the face of chronically elevated cortisol
in other species. However, our data indicate that there is a limit to this
levels, reproductively mature fish maintain key reproductive behav-
capacity because cortisol treatment did impair reproduction.
iors, further supporting the idea that fish with limited reproductive
The scope of the present study does not enable us to speculate
opportunity will still engage in spawning in what would be regarded
about the mechanism of cortisol elevation on spawning grounds. More-
as extreme situations during other life-history phases.
over, we did not measure cortisol receptor occupancy or sensitivity,factors that will affect the ability of (high) cortisol levels to mediate
Metyrapone treatment
target tissue responses, and an issue that ideally would be addressedin future studies. We can conclude, however, that acute elevation of
Metyrapone inhibits the enzyme 11-β hydroxylase, thereby
cortisol levels does not hinder reproductive behaviors and outcome.
preventing synthesis of cortisol from 11-deoxycortisol
In addition, it seems that the second spike in cortisol is an indicator
No significant changes in cortisol titers, reproductive behavior, re-
of impending senescence, as noted in previous studies (e.g.,
productive success, or hormone levels occurred as a result of metyrapone
). If high cortisol levels are evident before spawning is com-
treatment. determined that metyrapone inhibits the
plete, key reproductive behaviors and outcome can be negatively af-
cortisol response to a stressor but does not reduce baseline (non-stressed)
fected, as evidenced in this study by the use of semi-chronic cortisol
cortisol levels. There is also a suggestion that plasma cortisol does not turn
implants. It would have been useful to collect blood immediately fol-
over rapidly for semelparous salmon on spawning grounds
lowing exposure of fish to the acute stressors to assess the extent of
Therefore, it is possible that baseline (i.e. non-
the stress response elicited. In a similar study on stress responsiveness,
stressed) levels of cortisol were maintained, but increases in cortisol levels
observed an increase in cortisol levels from 333 ± 17
with stress were prevented (although this was not tested in the current
to 497 ± 22 ng ml−1 following 2 min of air exposure using Weaver
study). For future studies, responsiveness could be observed following in-
Creek sockeye salmon. Other Pacific salmonids (including sockeye,
jection to determine whether metyrapone-treated fish respond to acute
chum [O. keta], coho [O. kisutch] and Chinook [O. tshawtscha]), as well
stressors. This approach would provide a useful means of distinguishing
as pink salmon, all have been found to experience an acute stress
S.H. McConnachie et al. / Hormones and Behavior 62 (2012) 67–76
response when exposed to short-term stressors, with cortisol levels re-
covering within 2–4 h (Mike Donaldson, UBC, personal communica-tion). Therefore, the pink salmon in this study likely experienced an
Barry, T.P., Marwah, A., Nunez, S., 2010. Inhibition of cortisol metabolism by 17α,
20β-P: mechanism mediating semelparity in salmon? Gen. Comp. Endocrinol.
acute stress response with chasing and air exposure, but were not neg-
165, 53–59.
atively impacted by these acute stressors in terms of reproductive
Barton, B.A., 2002. Stress in fish: a diversity of responses with particular references to
physiology, behavior, or outcome.
changes in circulating corticosteroids. Integr. Comp. Biol. 42, 517–525.
Barton, B.A., Iwama, G.K., 1991. Physiological changes in fish from stress in aquaculture
with emphasis on the response end effects of corticosteroids. Annu. Rev. Fish Dis. 1,2–26.
Study limitations
Boonstra, R., Hik, D., Singleton, G.R., Tinnikov, A., 1998. The impact of predator-induced
stress on the snowshoe hare cycle. Ecol. Monogr. 79, 371–394.
Bowron, L.K., Munkittrick, K.R., McMaster, M.E., Tetrault, G., Hewitt, L.M., 2009.
Sham-treated fish were negatively affected by the administration
Responses of white sucker (Catostomus commersoni) to 20 years of process and
of a cocoa-butter implant alone. Although longevity was not altered,
water treatment changes at a bleached kraft pulp mill, and to mill shutdown.
sham-treated fish released only 70% of their eggs on average in the
Aquat. Toxicol. 95, 117–132.
Carruth, L.L., Jones, R.E., Norris, D.O., 2002. Cortisol and pacific salmon: a new look at
channel experiment, somewhat less (but not significantly so) than
the role of stress hormones in olfaction home-stream migration. Integr. Comp.
control fish, and released only 15% in the unripe enclosure trials, a
Biol. 42, 574–581.
value significantly lower than that of control fish. When fish were dis-
Cook, K.V., McConnachie, S.H., Gilmour, K.M., Hinch, S.G., Cooke, S.J., 2011. Fitness and
behavioral correlates of pre-stress and stress-induced plasma cortisol titers in pink
sected afterwards, some eggs were observed within the body cavity
salmon (Oncorhynchus gorbuscha) upon arrival at spawning grounds. Horm. Behav.
intermingled with the cocoa butter, creating a mass that might not
60, 489–497.
be easily expelled through the vent during spawning. This unexpect-
Cooke, S.J., Hinch, S.G., Crossin, G.T., Patterson, D.A., English, K.K., Shrimpton, J.M., Van
Der Kraak, G., Farrell, A.P., 2006. Physiology of individual late-run Fraser River
ed outcome might be prevented in future studies by using a vehicle
sockeye salmon (Oncorhynchus nerka) sampled in the ocean correlates with fate
with a lower melting point or by using less volume than used in the
during spawning migration. Can. J. Fish. Aquat. Sci. 63, 1469–1480.
present study. Indeed, a recent study on brown trout (Salmo trutta)
DeNardo, D.F., Sinervo, B., 1994a. Effects of corticosterone and activity and home range
size of free-ranging male lizards. Horm. Behav. 28, 53–65.
revealed that cocoa butter implants reduced egg and hatchling
DeNardo, D.F., Sinervo, B., 1994b. Effects of steroid hormone interaction on activity and
size () relative to controls, further empha-
home range size of male lizards. Horm. Behav. 28, 273–287.
sizing the need for additional research on improving the mecha-
Dibattista, J.D., Anisman, H., Whitehead, M., Gilmour, K.M., 2005. The effects of cortisol
nisms for experimental delivery of lipophilic hormones, a
administration on social status and brain monoaminergic activity in rainbow trout(Oncorhynchus mykiss). J. Exp. Biol. 208, 2707–2718.
technique that is becoming increasingly common in fish physiolo-
Donaldson, E.M., Fagerlund, U.H.M., 1972. Corticosteroid dynamics in Pacific salmon.
gy research (reviewed in ). In our study, be-
Gen. Comp. Endocrinol. 3, 254–265.
cause cortisol-treated fish exhibited high cortisol levels with
Doyon, C., Leclair, J., Trudeau, V.L., Moon, T.W., 2006. Corticotropin-releasing factor and
neuropeptide Y mRNA levels are modified by glucocorticoids in rainbow trout,
reduced longevity together with a decrease in the number of eggs
Oncorhynchus mykiss. Gen. Comp. Endocrinol. 146, 126–135.
released, we believe that our results support a real and significant
Dye, H.M., Sumpter, J.P., Fagerlund, U.H.M., Donaldson, E.M., 1986. Changes in repro-
effect of cortisol itself.
ductive parameters during the spawning migration of pink salmon, Oncorhynchusgorbuscha (Walbaum). J. Fish Biol. 29, 167–176.
Farrell, A.P., Gallaugher, P.E., Routledge, R., 2001a. Rapid recovery of exhausted adult
coho salmon after commercial capture by troll fishing. Can. J. Fish. Aquat. Sci. 58,
Farrell, A.P., Gallaugher, P.E., Fraser, J., Pike, D., Bowering, P., Hadwin, A.K.M.,
Parkhouse, W., Routledge, R., 2001b. Successful recovery of the physiological status
Because the migratory and spawning processes of Pacific salmon
of coho salmon on board a commercial gillnet vessel by means of a newly designed
are regarded as remarkable challenges, we strive to understand the
revival box. Can. J. Fish. Aquat. Sci. 58, 1931–1946.
Gamperl, A.K., Vijayan, M.M., Boutilier, R.G., 1994. Experimental control of stress hor-
links among physiology, behavior and fitness in these animals. Salm-
mone levels in fishes: techniques and application. Rev. Fish. Biol. Fish. 4, 215–255.
on migrations historically have shown a large degree of consistency,
Gilmour, K.M., DiBattista, J.D., Thomas, J.B., 2005. Physiological causes and conse-
but any environmental changes or anthropogenic perturbations are
quences of social status in salmonid fish. Integr. Comp. Biol. 45, 263–273.
considered a potential threat to reproduction, and thus survival, of a
Goetz, F.W., 1983. Hormonal control of oocyte final maturation and ovulation in fishes.
In: Hoar, W.S., Randall, D.J. (Eds.), Fish Physiology: Reproduction, Behavior and
given population. Our results suggest that acute stressors do not in-
Fertility Control. Academic Press, New York, NY, pp. 117–170. Volume 9 Part 2.
fluence behavior or reproductive outcome when experienced upon
Gregory, T.R., Wood, C.M., 1999. The effects of chronic plasma cortisol elevation on the
arrival at spawning grounds. However, there is a limit to the ability
feeding behavior, growth, competitive ability, and swimming performance ofjuvenile rainbow trout. Physiol. Biochem. Zool. 72, 286–295.
of these fish to tolerate elevated cortisol levels because experimental
Heard, W.R., 1991. Life history of pink salmon (Oncohynchus gorbuscha). In: Groot, C.,
cortisol elevation for several days negatively affected reproductive
Margolis, L. (Eds.), Pacific Salmon Life Histories. UBC Press, Vancouver, pp.
success and longevity. Collectively, our results address a void in cur-
Hinch, S.G., Bratty, J.M., 2000. Effects of swim speed and activity pattern on success of
rent research, explaining how varying degrees of cortisol elevation
adult sockeye salmon migration through an area of difficult passage. Trans. Am.
can influence reproductive behavior and spawning success of Pacific
Fish. Soc. 129, 604–612.
salmon. Finally, our study is among the first field studies conducted
Hinch, S.G., Cooke, S.J., Healey, M.C., Farrell, A.P., 2006. Behavioral physiology of fish mi-
grations: salmon as a model approach. In: Farrell, A.P., Brauner, C.J. (Eds.), Behavior
to investigate the ecological consequences of stress during reproduc-
and Physiology of Fish: Fish physiology, 24.
tion for a semelparous species.
Hoogenboom, M.O., Armstrong, J.D., Miles, S., Burton, T., Groothuis, T.G.G., Metcalfe,
N.B., 2011. Implantation of coca butter reduces egg and hatchling size in Salmotrutta. J. Fish Biol. 79, 587–596.
Hopkins, T.E., Wood, C.M., Walsh, P.J., 1995. Interactions of cortisol and nitrogen
metabolism in the ureogenic toadfish Opsanus beta. J. Exp. Biol. 198, 2229–2235.
Hruska, K.A., Hinch, S.G., Healey, M.C., Farrell, A.P., 2007. Electromyogram telemetry,
This research was supported by Natural Sciences and Engineering
non-destructive physiological biopsy, and genetic markers: linking recent tech-niques with behavioral observations for the study of reproductive successes in
Research Council of Canada Discovery and Strategic grants to S.J.C.,
sockeye salmon mating systems. Am. Fish. Soc. Symp. 54, 17–29.
S.G.H., A.P.F. and K.M.G. Research was also supported by the Depart-
Hruska, K.A., Hinch, S.G., Healey, M.C., Patterson, D.A., Larsson, S., Farrell, A.P., 2010. In-
ment of Fisheries and Oceans (Canada) Environmental Watch Program
fluences of sex and activity level on physiological changes in individual adultsockeye salmon during rapid senescence. Physiol. Biochem. Zool. 83, 663–676.
led by D.A.P. Field support was provided by Connie O'Connor, Alison
Janz, D.M., McMaster, M.E., Munkittrick, K.R., Van Der Kraak, G., 1997. Elevated ovarian
Colotelo, Mike Donaldson, Graham Raby, Charlotte Whitney, Kim
follicular apoptosis and heat shock protein-70 expression in white sucker exposed
Hruska, Juliette Mudra and Tim Clark. Jayme Hills and Vanessa Ives
to bleached kraft pulp mill effluent. Toxicol. Appl. Pharmacol. 147, 391–398.
Jardine, J.J., Van Der Kraak, G.J., Munkittrick, K.R., 1996. Capture and confinement stress
conducted plasma analyses. Rick Stitt and the Weaver Creek Spawning
in white sucker exposed to kraft pulp mill effluent. Ecotoxicol. Environ. Saf. 33,
Channel staff provided logistical and technical support.
S.H. McConnachie et al. / Hormones and Behavior 62 (2012) 67–76
Mathes, M.T., Hinch, S.G., Cooke, S.J., Crossin, G.T., Patterson, D.A., Lotto, A.G., Farrell,
Rodela, T.M., McDonald, M.D., Walsh, P.J., Gilmour, K.M., 2009. The regulatory role of
A.P., 2010. Effect of water temperature, timing, physiological condition, and lake
glucocorticoid and mineralocorticoid receptors in pulsatile urea excretion of the
thermal refugia on migrating adult Weaver Creek sockeye salmon (Oncorhynchus
gulf toadfish, Opsanus beta. J. Exp. Biol. 212, 1849–1858.
nerka). Can. J. Fish. Aquat. Sci. 67, 70–84.
Schreck, C.B., 2010. Stress and fish reproduction: the roles of allostasis and hormesis.
McBride, J.R., Fagerlund, U.H.M., Dye, H.M., Bagshaw, J., 1986. Changes in structure of
Gen. Comp. Endocrinol. 165, 549–556.
tissues and in plasma cortisol during the spawning migration of pick salmon,
Schreck, C.B., Contreras-Sanchez, W., Fitzpatrick, M.S., 2001. Effects of stress on fish re-
Oncorhynchus gorbuscha (Walbaum). J. Fish Biol. 29, 153–166.
production, gamete quality, and progeny. Aquacult. 197, 3–24.
Mehranvar, L., Healey, M., Farrell, A., Hinch, S., 2004. Social versus genetic measures of
Silverin, B., 1997. The stress response and autumn dispersal behavior in willow tits.
reproductive success in sockeye salmon, Oncorhynchus nerka. Evol. Ecol. Res 6,
Anim. Behav. 10, 451–459.
Stein-Behrens, B.A., Sapolsky, R.M., 1992. Stress, glucocorticoids, and aging. Aging Clin.
Milla, S., Wang, N., Mandiki, S.N.M., Kestemont, P., 2009. Corticosteroids: friends or foes
Exp. Res. 4, 197–210.
of teleost fish reproduction? Comp. Biochem. Physiol. A Mol. Integr. Physiol. 153,
Tierney, K.B., Patterson, D.A., Kennedy, C.J., 2009. The influence of maternal condition
on offspring performance in sockeye salmon Oncorhynchus nerka. J. Fish Biol. 75,
Milligan, C.L., 2003. A regulatory role for cortisol in muscle glycogen metabolism in
rainbow trout Oncorhynchus mykiss Walbaum. J. Exp. Biol. 206, 3167–3173.
Van Der Kraak, G., Munkittrick, K.R., McMaster, M.E., MacLatchy, D.L., 1998. A compar-
Mishra, A., Joy, K.P., 2006. Effects of gonadotropin in vivo and 2-hydroxyoestradiol-17
ison of bleached kraft mill effluent 17 beta-estradiol, and beta-sitisterol effects on
beta in vitro on follicular steroid hormone profile associated with oocyte maturation
reproductive function in fish. In: Kendall, R.J., Dickerson, D.L., Giesy, J.P., Suk, W.P.
in the catfish Heteropneustes fossilis. J. Endocrinol. 189, 341–353.
(Eds.), Principles and Processes for Evaluation Endocrine Disruption in Wildlife.
Mommsen, T.P., Vijayan, M.M., Moon, T.W., 1999. Cortisol in teleosts: dynamics, mech-
SETAC Press, Florida, pp. 249–265.
anisms of action, and metabolic regulation. Rev. Fish Biol. Fish. 9, 211–268.
Wagner, G.N., Hinch, S.G., Kuchel, L.J., Lotto, A., Jones, S.R.M., Patterson, D.A., Macdonald,
Morbey, Y.E., Brassil, C.E., Hendry, A.P., 2005. Rapid senescence in Pacific salmon. Am.
J.S., Van Der Kraak, G., Shrimpton, M., English, K.K., Larsson, S., Cooke, S.J., Healey,
Nat. 166, 556–568.
M.C., Farrell, A.P., 2005. Metabolic rates and swimming performance of adult Fraser
Negro-Vilar, A., 1993. Stress and other environmental factors affecting fertility in men
River sockeye salmon (Oncorhynchus nerka) after a controlled infection with
and women: overview. Environ. Health Perspect. 101, 59–64.
Parvicapsula minibicornis. Can. J. Fish. Aquat. Sci. 62, 2124–2133.
Pickering, A.D., Pottinger, T.G., Carragher, J., Sumpter, J.P., 1987. The effects of acute and
Wingfield, J.C., 1988. Changes in reproductive function of free-living birds in direct
chronic stress on the levels of reproductive hormones in the plasma of the mature
response to environmental perturbations. In: Stetson, M.H. (Ed.), Processing of Envi-
brown trout, Salmo trutta L. Gen. Comp. Endocrinol. 68, 249–259.
ronmental Information in Vertebrates. Springer-Verlag, Berlin, pp. 121–148.
Quinn, T.P., Foote, C.J., 1994. The effects of body size and sexual dimorphism on the repro-
Wingfield, J.C., 2003. Control of behavioral strategies for capricious environments.
ductive behavior of sockeye salmon, Oncorhynchus nerka. Anim. Behav. 48, 751–761.
Anim. Behav. 66, 807–816.
Quinn, T.P., Unwin, M.J., Kinnison, M.T., 2000. Evolution of temporal isolation in the
Wingfield, J.C., Sapolsky, R.M., 2003. Reproduction and resistance to stress: when and
wild: genetic divergence in timing of migration and breeding by introduced
how. J. Neuroendocrinol. 15, 711–724.
Chinook salmon populations. Evolution 54, 1372–1385.
Wingfield, J.C., Maney, D.L., Breuner, C.W., Jacobs, J.D., Lynn, S., Ramenofsky, M.,
Rand, P.S., Hinch, S.G., Morrison, J., Foreman, M.G.G., MacNutt, M.J., Macdonald, J.S.,
Richardson, R.D., 1998. Ecological bases of hormone–behavior interaction: the
Healey, M.C., Farrell, A.P., Higgs, D.A., 2006. Effects of river discharge, temperature,
emergency life history stage. Am. Zool. 38, 191–206.
and future climates on energetic and mortality of adult migrating Fraser River
Young, J.L., Hinch, S.G., Cooke, S.J., Crossin, G.T., Patterson, D.A., Farrell, A.P., Van Der
sockeye salmon. Trans. Am. Fish. Soc. 135, 655–667.
Krakk, G., Lotto, A.G., Lister, A., Healey, M.C., English, K.K., 2006. Physiological and
Robertson, O.H., Wexler, B.C., 1959. Hyperplasia of the adrenal cortical tissue in Pacific
energetic correlates of en route mortality for abnormally early migrating adult
salmon (genus Oncorhynchus) and rainbow trout (Salmo gairnerii) accompanying
socleye salmon (Oncorhynchus nerka) in the Thompson River, British Columba.
sexual maturation and spawning. Endocrinology 65, 225–238.
Can. J. Fish. Aquat. Sci. 63, 1067–1077.
Source: http://www.fecpl.ca/wp-content/uploads/2012/01/HB-McConnachie-et-al-2012.pdf
Personalised, Serendipitous and Diverse Linked Data Resource Recommendations Milan Dojchinovski and Tomas Vitvar Web Intelligence Research Group Faculty of Information Technology Czech Technical University in Prague Abstract. Due to the huge and diverse amount of information, the ac-tual access to a piece of information in the Linked Open Data (LOD)cloud still demands significant amount of effort. To overcome this prob-lem, number of Linked Data based recommender systems have been de-veloped. However, they have been primarily developed for a particulardomain, they require human intervention in the dataset pre-processingstep, and they can be hardly adopted to new datasets. In this paper, wepresent our method for personalised access to Linked Data, in particularfocusing on its applicability and its salient features.
Allergology International. 2007;56:37-43DOI: 10.2332! Awarded Article, Annual Meeting of JSA The Relationship between ExhaledNitric Oxide Measured with an Off-lineMethod and Airway ReversibleObstruction in Japanese Adults withAsthmaTakahiro Tsuburai1, Naomi Tsurikisawa1, Masami Taniguchi1, Sonoko Morita1, Emiko Ono1,Chiyako Oshikata1, Mamoru Ohtomo1, Yuji Maeda1, Kunihiko Ikehara2 and Kazuo Akiyama1







