Levitra enthält Vardenafil, das eine kürzere Wirkdauer als Tadalafil hat, dafür aber schnell einsetzt. Männer, die diskret bestellen möchten, suchen häufig nach levitra kaufen ohne rezept. Dabei spielt die rechtliche Lage in der Schweiz eine wichtige Rolle.
Secondary open-angle glaucomas

Thomas F. Freddo, O.D., Ph.D., F.A.A.O.
Professor of Optometry
University of Waterloo

Ocular injury; recent or old Secondary to Iritis Phacolytic Ghost cell Melanomalytic Steroid-induced Pigmentary glaucoma Exfoliative glaucoma

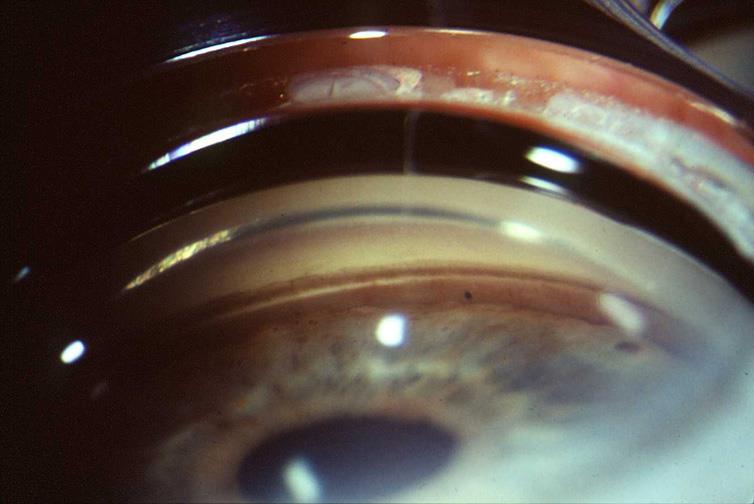
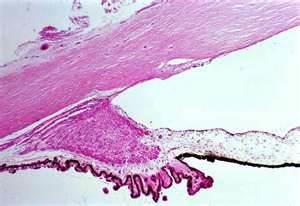
Note especially deep ciliary body band and posterior dislocation of iris root.
Follows non-penetrating blunt trauma – Important to remember that not all patients can document a traumatic event. Gonio all young patients with unilateral cataract.
Following blunt trauma recession develops often, especially if associated hyphemaR/O ruptured globe – no gonio for first 2-3 weeks. Gonio both eyes to compare. Use goniolens not prism.
Only 5-20% of those with recession will develop glaucoma, but it can occur at any time after the injury.
More common if recession greater than 180 degrees.
Prostaglandin analogs have a theoretical benefit because the trabecular meshwork is thought to be dysfunctional. Otherwise, initial med therapy similar to other OAGs



Aqueous inflow is always reduced but
the magnitude varies.
Episcleral venous pressure is rarely
Trabecular outflow is reduced by
variable amounts.
Uveoscleral outflow is increased
History: Presentation 5 days or more after pain and
redness begins can herald a bad prognosis.
Examination will reveal injection, corneal edema,
elevated IOP, anterior chamber reaction with or without pseudohypopyon, particles on the lens capsule and anterior capsule wrinkling.
Gonioscopy is essential; rule out angle closure due
to phacomorphic glaucoma (secondary angle closure due to lens intumescence) or neovascularization of the angle (e.g. DM or BRVO)
Assess posterior pole: rule out
vitreous hemorrhage (which can result in ghost-cell glaucoma) or vitritis (which may be associated with infectious endophthalmitis or panuveitis). If the view is obstructed, a B-scan should be performed.
!!!!!! When you dilate,
keep patient on their back until pupil has come back down a bit to prevent luxation into the AC!!!!!!
Luxation of hypermature lens into AC
Medical management is used to temporarily control
the glaucoma and inflammation. Hyperosmotic agents, aqueous suppressants, anti-inflammatory drugs and cycloplegics are used as indicated.
Definitive treatment is removal of lens – usually ECCE
with or without IOL and post-op management of inflammation and IOP. In cases with delayed diagnosis a combined ECCE with trab may be indicated to control post-op IOP.
Ghost cells usually look more khaki-colored
Usually history of vitreous hemorrhage in past 1-3 months
Intraocular pressure (IOP) spikes (can be 60 to 70 mmHg),
causing corneal edema.
"Cells" in the anterior chamber, usually tan in color
The anterior chamber will exhibit "cells and flare"
But the "cells" will seem disproportionate to the amount of
flare and with little or no ocular redness.
Symptoms
Less pain is reported than expected from a severely elevated
Patients with high IOP may also present with blurry vision
(corneal edema) headache, brow ache, nausea and/or vomiting.
Notice it looks like 3+ cells and flare
Management
Ghost cell glaucoma usually resolves once the vitreous hemorrhage has cleared,
but intervention is commonly required.
Medical Therapy
Aqueous suppressants are the first line approach. Monotherapy or a
combination of topical alpha agonists, beta blockers, prostanoids, and carbonic anhydrase inhibitors may be used.
Oral carbonic anyhdrase inhibitor may be added. Intravenous Mannitol or
Diamox may be used for extremely high IOPs in acute settings.
Surgery might be required to clear the cell load from the trabecular meshwork.
This can be accomplished by AC paracentesis and irrigation. It can be repeated if IOP rises again.
If AC washouts are unsuccessful because of the further entrance of ghost cells
from the vitreous, vitrectomy to remove the contents of the vitreous cavity, including the degenerating RBCs may be required.
Potent glucocorticoids will increase IOP more than 5
mmHg in 40% of the population and more than 15 mmHg in 4-6%. ("Steroid-responders")
Steroid-induced IOP elevation typically occurs within a
few weeks of beginning steroid therapy.
IOP usually lowers spontaneously within a few weeks to
months of stopping the steroid.
Patients with preexisting POAG, a family history of
POAG, diabetes, high myopia, or connective tissue diseases are at greater risk to be steroid responders.
Most from topically applied drops
(including topically applied creams to the periorbital area)
Intravitreal injection Intravenous Parenteral Inhalation
TOPICAL OCULAR STEROIDS
Prednisolone acetate
Pred Mild (Allergan)
Prednisolone sodium phosphate
Dexamethasone alcohol
Fluorometholone acetate
Fluorometholone alcohol
Rimexolone
Lotoprednol etabonate
Keep in mind that as an acute uveitis responds, the
ciliary epithelium may repair and begin producing aqueous at normal levels before the meshwork gets cleared of obstruction. This could result in a pressure rise that looks steroid-induced when its not.
Try to reduce or eliminate continued use of steroid If pressure rise is sustained, these patients are
treated identically to those with primary open-angle glaucoma. First beta-blockers, then Alpha agonists, then CAI, then prostanoids and mitotics.
Risk Factors
1) Gender: Strong male predominance for PG, with all case series
showing a male to female ratio of between 2:1 and 5:1. Lower male predominance is noted for PDS, with case series describing male to female ratios between 1:1 and 2:1.
2. Age. Males with PG and PDS most often present in their 30s, females
roughly a decade later in life. Cases of PDS have been identified in patients as young as early teens.
3) Myopia. The most common refractive error is in the range of -3 to -4
4) Race. Both pigment dispersion syndrome and PG occur infrequently
(<5% of patients identified in case series) in persons of African ancestry.
5) Flat corneas. Patients with PDS and PG have significantly flatter
corneas than control subjects of similar age and refractive error.
6) Family history. Likely autosomal dominant inheritance pattern with
incomplete penetrance. Thus, bilateral.
1) IOP > 21 - Age, refractive error and family
history were not associated with conversion to PG.
2) Degree of iridolenticular contact in
patients with asymmetric disease.
3) Greater trabecular meshwork
Acute elevations, producing vision reduction and colored
haloes from corneal edema can occur with vigorous exercise. Different from Uhthoffs sign.
African Americans may not demonstrate defects due to thicker irides and ability of dense pigmentation of anterior border layer to prevent light passing through defects on posterior iris surface from being seen in retro.
Work of Dr Jeff Liebman
Commonly more resistant to medical therapy than POAG but prognosis
overall is quite good.
Initial medical therapy is commonly Beta-blockers
Can be supplemented with miotics to relieve reverse pupillary block but
pilo should be used with caution in moderate to high myopes due to risk of RD.
Alpha-agonists can play a role but allergy rates can limit broad utility
Topical CAIs and be added or combi-durgs including them if
Prostanoids can be useful as well – increased iris pigmentation does not
effect course of disease (stromal vs epithelial)
LTP if effective but its beneficial effect is often not as lasting as in POAG.
Due to absorption of laser energy, by pigment, lower settings used.
More commonly progress to needing filter.
Iridotomy will reduce iridozonular contact and equilibrate AC and PC
pressure difference but may be more useful as prophy in PDS rather than TX for PG. More commonly progress to needing filter.
Increased risk for retinal detachment,
which may occur in as many as 6-7% of individuals.
2X risk of retinal breaks 2X risk of lattice degeneration when
compared to age and refraction-matched controls.
Extended exposure to an
infrared-emitting heat source or ocular trauma.
Transillumination defects commonly seen at pupil margin
This region swept clean by movement of pupil*
* It is this "scuffing" that produces transillumination defects at pupil
At EM level, this material is fibrillar and composed of elastin, elastin microfibrils, collagen and amyloid-P.
AGE, GENDER, LATERALITY Most common identifiable form of secondary open-angle glaucoma
within in the white population.
More common in females 3:1, rarely below age of 50.
Unilateral - Pseudoexfoliation syndrome usually affects one eye, but
typically the good eye will develop signs of pseudoexfoliation in 40% of cases within seven years.
RACE: Scandinavian countries: >50% of open-angle glaucoma is PEX
African-Americans and Eskimos – rare. Inuit -none
Oman: PEX accounts for half of all glaucoma
Bantu in South Africa: 19% of patients in a glaucoma clinic.
Spanish-Americans in New Mexico: 3-6%
PEX material was found in skin, heart, lungs, liver and
meninges of patients with PEX by Schloetzer-Schrehardt, et al. Arch Ophthalmol 110:1752, 1992.
PEX material found in the lung, heart, liver, and
gallbladder, in addition to the classic intraocular sites. The aggregates were in the fibrovascular septae and stroma of these organs, most frequently adjacent to elastic and oxytalan fibers. Streeten, et al in the Arch Ophthalmol 110:1757, 1992.
Treated same as primary open-angle glaucoma.
IOP in pseudoexfoliative glaucoma usually higher and
more difficult to lower.
As a result ALT/SLT often indicated earlier than with
primary open-angle glaucoma. ALT often has immediate pressure lowering effect, with reported initial success rates up to 80%.
SLT particularly effective but lower energy settings are
required due to the increased pigmentation found in eyes with pseudoexfoliation.
Medical management most often similar to POAG These conditions remind us of the importance of
doing baseline gonioscopy on every glaucoma suspect
Thanks for attending !
Source: http://www.gio.co.za/wp-content/uploads/2016/03/Secondary-Open-Angle-Glaucomas-GIO-2016.pdf
This page intentionally left blank Laurie Rozakis, Ph.D. The State University of New York Farmingdale State College New York Chicago San Francisco Lisbon London Madrid Mexico City Milan New Delhi San Juan Seoul Singapore Sydney Toronto Copyright © 2007, 1999 by The McGraw-Hill Companies, Inc. All rights reserved. Manufacturedin the United States of America. Except as permitted under the United States Copyright Act of1976, no part of this publication may be reproduced or distributed in any form or by any means,or stored in a database or retrieval system, without the prior written permission of the publisher.
Continuous Infusion of Beta-Lactam Antibioticsin Severe Sepsis: A Multicenter Double-Blind,Randomized Controlled Trial Joel M. Dulhunty,1 Jason A. Roberts,1 Joshua S. Davis,2 Steven A. R. Webb,3 Rinaldo Bellomo,4 Charles Gomersall,5Charudatt Shirwadkar,6 Glenn M. Eastwood,4 John Myburgh,7 David L. Paterson,8 and Jeffrey Lipman1 1Department of Intensive Care Medicine, Royal Brisbane and Women's Hospital, and Burns, Trauma and Critical Care Research Centre, University of








