Physical properties of escherichia coli spheroplast membranes

Physical Properties of Escherichia coli Spheroplast Membranes
Yen Sun,Tzu-Lin Sun,and Huey W. Huang1Department of Physics & Astronomy, Rice University, Houston, Texas
We investigated the physical properties of bacterial cytoplasmic membranes by applying the method of micropi-
pette aspiration to Escherichia coli spheroplasts. We found that the properties of spheroplast membranes are significantlydifferent from that of laboratory-prepared lipid vesicles or that of previously investigated animal cells. The spheroplasts canadjust their internal osmolality by increasing their volumes more than three times upon osmotic downshift. Until the spheroplastsare swollen to their volume limit, their membranes are tensionless. At constant external osmolality, aspiration increases thesurface area of the membrane and creates tension. What distinguishes spheroplast membranes from lipid bilayers is that thearea change of a spheroplast membrane by tension is a relaxation process. No such time dependence is observed in lipidbilayers. The equilibrium tension-area relation is reversible. The apparent area stretching moduli are several times smallerthan that of stretching a lipid bilayer. We conclude that spheroplasts maintain a minimum surface area without tension by a mem-brane reservoir that removes the excessive membranes from the minimum surface area. Volume expansion eventually exhauststhe membrane reservoir; then the membrane behaves like a lipid bilayer with a comparable stretching modulus. Interestingly, themembranes cease to refold when spheroplasts lost viability, implying that the membrane reservoir is metabolically maintained.
Direct probing of the physical properties of cell membranes
(and daptomycin (To understand the in situ activ-
has been performed on various animal cells with the
ities of membrane-acting antibiotics, we need to know the
methods of micropipette aspiration and tether pulling
physical properties of the target membranes
). These physical studies have provided invaluable
As inhabitants of natural environments, bacteria have
insight into the mechanical properties of eukaryotic cell
abilities to survive very large changes of external osmolality
membranes and thus a better understanding of the mem-
(In E. coli, the cytoplasm responses to the changes of
brane's functions in cell biology In comparison,
external osmolality by adjusting its solute content and the
the cytoplasmic membranes of bacteria are much less ac-
amount of water ). As a result, the cytoplasmic os-
cessible to experimental study because they are normally
motic pressure could exceed that of the surrounding medium
shielded by outer membranes. Experimentalists have made
by 0.5 to 3 atm, as the external osmolality decreases from
use of spheroplasts, the cells from which the outer mem-
0.5 to 0.03 osmole/kg (Osm) (The cytoplasmic mem-
branes have been removed, for patch-clamp, fusion, and
brane cannot sustain such a large pressure drop. Cayley
other experiments and also for antibiotic studies
et al. () have showed that the periplasmic solutes and vol-
). However the physical properties of spheroplast mem-
ume also changed with external osmolality, and concluded
branes have not been studied. In this study we apply the
that the periplasm and cytoplasm are iso-osmotic (
method of micropipette aspiration to probe the stretching
Hence the turgor pressure is supported by the peptido-
elasticity of Escherichia coli (E. coli) spheroplast mem-
glycan-cell wall complex, rather than by the cytoplasmic
branes. The measurements reveal the basic properties of
membrane ). However, the physical properties of
bacterial cytoplasmic membranes, which are significantly
cytoplasmic membranes are otherwise unknown.
different from that of red cell membranes, or of labora-
Accordingly, we investigated E. coli speheroplasts over a
tory-prepared lipid vesicles. It is generally believed that
range of external osmolality. We found that, except at very
the tension in the cytoplasmic membrane determines the
low external osmolalities, the spheroplast membranes are
actions of mechanosensitive channels and osmoregula-
tensionless. However, increasing the area of a spheroplast
tors and perhaps other integral proteins as well
membrane by micropipette aspiration creates tension. As
The cytoplasmic membranes are also the target of
the tension changes by aspiration, the area of the spheroplast
naturally produced membrane-acting antibiotics, as has
membrane changes to a new equilibrium value by a relaxa-
been demonstrated recently with LL37 (), cecropin
tion process. And the relaxation is loading-rate dependent.
The equilibrium tension-area relation is reversible. Theseresults indicate that the cell maintains a minimum surface
Submitted May 8, 2014, and accepted for publication September 30, 2014.
area without a tension, and the surface area is controlled
*Correspondence:
by a membrane reservoir equivalent to membrane folds.
Editor: Hagan Bayley.
Ó 2014 by the Biophysical Society0006-3495/14/11/2082/9
E. coli Spheroplast Membrane
At constant external osmolality, unfolding and refolding of
water-filled U tube manometer and a negative pressure in the pipette was
the membranes are reversible, thus providing an apparent
produced by adjusting the height of the water level reference to the atmo-sphere pressure (). Although the technique for measuring spheroplasts
elasticity of stretching. However, when the cell lost its
and giant unilamellar vesicles (GUVs) is the same, the small size of sphe-
viability, the area increase loses its reversibility, implying
roplasts imposes some restrictions on spheroplast measurements. For GUV
that the reversible membrane reservoir is metabolically
experiments, the typical dimensions are 5 mm for the micropipette radius
maintained. Our results can be understood by assuming
ðRpÞ and 15 mm for the GUV radius ðRvÞ. The applied membrane tension
that the membrane reservoirs are mediated by reversible
is in the range of 1 to 7 mN/m, which requires a suction pressure Dp of
0:27 � 1:9 � 103 Pa, equivalent to 2:7 � 18:9 cm of water [calculated
noncovalent chemical bonds.
from the Laplace equation Dp ¼ 2tð1=Rp � 1=RvÞ ] (see Fora spheroplast of radius 2.5 mm, using a micropipette of radius 1.1 mm,the same tension would require three times as much water pressure.
MATERIALS AND METHODS
Thus the maximum applicable membrane tension is limited to 2 mN/m
Bacterial strains and culture
by the 20 cm height of the water column.
If an aspirated spheroplast consisted of a spherical part and a cylindri-
E. coli K-12 strain MG1655 (ATCC 700926) was purchased from ATCC
cal part (a protrusion into the micropipette) (similar to the GUV shown
(Manassas, VA). Luria-Bertani (LB) medium (5 g/L yeast extract, 10 g/L
in Lp the length of the protrusion, Rp the radius of the micropi-
peptone from casein, and 10 g/L sodium chloride) containing 15g/L agar
pette, and Rv the radius of the spherical part were carefully measured.
from EMD Millipore (Billerica, MA) was used for the growth of colonies
Then it was straightforward to show DA ¼ 2pRpDLp þ 8pRvDRv and
of E. coli. The medium was autoclaved before used for sterility.
DV ¼ pR2pDLp þ 4pR2vDRv (). As long as the osmolality balance wasmaintained, there should be no change of volume (the effect of the pressurechange by suction was so small that its contribution to the chemical poten-
Chemicals and media
tial change was 10�3 that of osmolality). Under the condition DV ¼ 0, DAwas directly proportional to DL
Sucrose, Tris, hydrochloric acid, lysozyme, DNase, EDTA, magnesium
p: DA ¼ 2pRpð1 � Rp=RvÞDLp. The frac-
tional area change DA=A was calculated from the change of the protrusion
chloride, sodium hydroxide, cephalexin, carboxyfluorescein, and carbonyl
cyanide m-chlorophenylhydrazone (CCCP) were purchased from Sigma
p. If aspiration created a spherical protrusion () (the
Aldrich (St. Louis, MO). FM 4-64 and Sytox green were purchased from
volume was V ¼ ph2ðr � ðh=3ÞÞ þ pH2ðRv � ðH=3ÞÞ, and the area
Life Technologies (Grand Island, NY). E. coli total lipid extract was pur-
A ¼ 2prh þ 2pRvH (the area change was calculated at constant
chased from Avanti Polar Lipids (Alabaster, AL).
volume and the tension was calculated by the Laplace equationDp ¼ 2tð1=r � 1=RvÞ.
The experiment of the GUVs of E. coli total lipid extract followed the
Preparation of E. coli spheroplasts
previously established method (
We prepared giant spheroplasts of E. coli by following the detailed pro-cedure described by Renner et al. (Briefly, cells were grown in LB me-
dium by shaking at 37�C overnight to the stationary phase. A small aliquot(1:100 dilution) was added into the LB medium and incubated in 37�C
Giant spheroplasts of E. coli
while shaking at 200 rpm until the absorbance at l ¼ 600 nm reached0.5 to 0.7. One-half ml of the cell culture was diluted to 5 ml in LB medium.
The methods for growing E. coli spheroplasts are well estab-
To grow long filamentous cells, cephalexin (60 mg/ml) was added and the
lished (). First, cephalexin was added to the cell cul-
culture was grown with shaking (200 rpm) at 42�C for 2 h. After cells
ture. In the presence of cephalexin that blocks septation,
reached an average length of 50 mm, cells were harvested by centrifuga-tion at 3000 � g for 1 min. The pellet was suspended in 500 mL of 800 mM
E. coli grew into long filaments (A). We have experi-
sucrose solution. Spheroplasts from filamentous cells were formed by add-
mented with the growth time and temperature () to
ing the reagents in the following order: 30 mL 1 M Tris � HCl (pH 8.0),
achieve longer filaments (T 50 mm). To remove the outer
24 mL 0.5 mg/mL lysozyme, 6 mL 5 mg/mL DNase, and 6 mL 125 mM
membrane and the peptidoglycan, we added lysozyme and
EDTA-NaOH (pH 8.0). After 5 to 20 min at room temperature, 100 mL
EDTA to the cell suspension, that converted filaments into
of STOP solution (10 mM Tris � HCl at pH 8, 0.7 M sucrose, 20 mMMgCl
spheroplasts A). Then a STOP solution ) was
2) was added to stabilize the spheroplasts. We did not find noticeable
differences in the results by different time duration of lysozyme treatments.
added to terminate the digestion. The osmolality of the
Spheroplasts were stored in liquid nitrogen for no longer than 2 weeks.
STOP solution was 0.845 Osm. It has been shown previously
Frozen spheroplasts were thawed slowly on ice before each use. Thirty mL
that the ratio of DNA/protein in filaments was about the
of spheroplasts was injected into an observation chamber containing a certain
same as in untreated cells (Thus 30 bacterial genomes
concentration of STOP solution. The spheroplasts were either directlyimaged with phase contrast microscopy or labeled with FM 4-64 (8 mM)
were present in a long filament and also in a resulting giant
for fluorescence imaging. We were able to measure the membrane area
spheroplast. We confirmed that the spheroplasts could revert
changes with either phase contrast or fluorescence images, although it was
to normal form of E. coli when returned to growth medium
easier with the latter. We did not notice any difference in the membrane prop-
((data not shown). We did not know if there were rem-
erties of spheroplasts whether they were labeled with FM 4-64 or not. The
nants of outer membrane or peptidoglycan attached to the
majority of aspiration measurements were performed with FM 4-64 label.
cytoplasmic membranes of spheroplasts (If therewere, we did not detect the effect of their presence.
Method of micropipette aspiration
To experiment with the cytoplasmic membranes, we
This method was a modification of the original method of Kwok and Evans
diluted the STOP solution by adding pure water. As the
) as described in Sun et al. (A micropipette was connected to a
STOP solution was diluted, the water influx enlarged the
Biophysical Journal 107(9) 2082–2090
Micropipette aspiration of spheroplastmembranes
The method of aspiration serves two purposes: one is toapply tension to the membrane and another is to measurethe membrane area changes. Previous studies have shownthat the membrane area changes by tension (by peptidebinding ), or by structural events (such as pore forma-tion) ) can provide a quantitative description for thephysical event taking place in the membrane. In this studywe used a micropipette to apply a small negative pressure(aspiration) to a spheroplast. When a spheroplast in the100% STOP solution was aspirated by a micropipette,the whole cell deformed and flowed into the pipette(). This could be because of the size of the micropi-pette being too close to the size of the spheroplast, or itcould be what was described as a liquid drop model inthe micropipette-aspiration experiments of human bloodcells (). We did not analyze the spheroplasts from100% STOP solution.
When the osmolality of the external medium was
reduced, the cell volume visibly enlarged because of wa-ter influx. The response of the enlarged spheroplasts tomicropipette aspiration was similar to the response of aGUV of lipids. In this case, aspiration reshaped the sphe-roplast surface to include a protrusion in the micropipette,leaving a larger spherical part outside the pipette (The equal osmolality inside and outside shouldkeep the volume of the spheroplast constant. Then anysurface area change would result in a change in the lengthof the protrusion, from which we measured the fractionalarea change DA=A (see Materials and Methods ()).
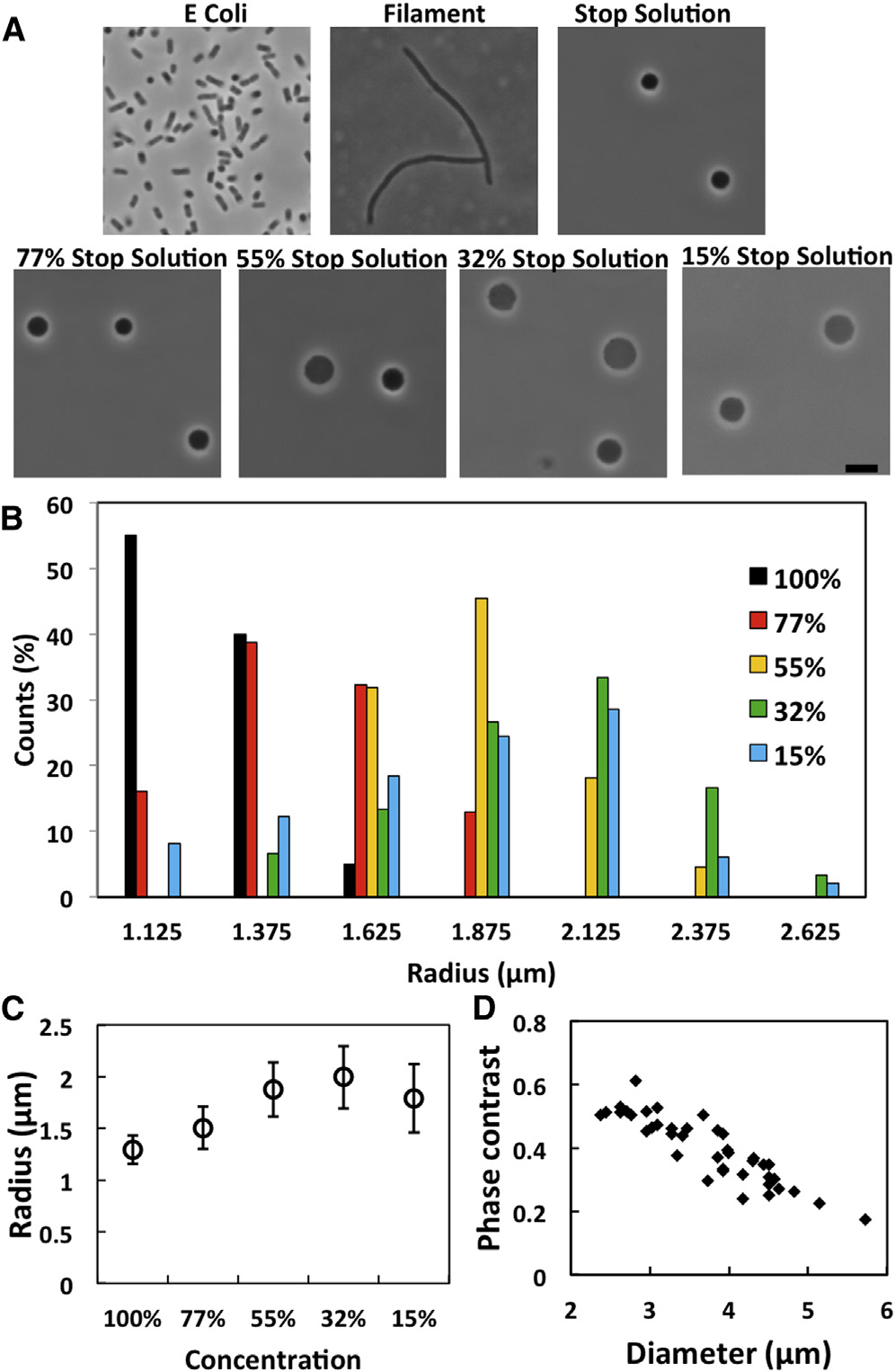
E. coli spheroplasts. (A) E. coli cells, filamentous cells, sphe-
We systematically investigated the spheroplast mem-
roplasts in 100% STOP solution, and in diluted STOP solutions. Scale bar ¼
branes by the micropipette-aspiration method and found
5 mm. (B) Size distribution among a population in different concentrations
the following general properties for E. coli spheroplast
of STOP solution. As the external concentration decreased below 50%,
more spheroplasts appeared to have lost the interior phase contrast. Thespheroplasts without interior phase contrast were not included in the radiussurvey. (C) The average radius of the population in different concentrations.
Membrane area expansion is a relaxation process
(D) Correlations of size versus phase contrast ðI0 � IÞ=I0 where I was theaverage phase contrast intensity of cytosol, I
We found that the initial surface tension of a spheroplast was
0 that of the outside solution.
Spheroplasts without phase contrast had ðI0 � IÞ=I0(0:01. To see this
always zero, except for very swollen spheroplasts that will
figure in color, go online.
be discussed later. When a tension was applied to the sphe-roplast surface, its surface area increased in a manner
spheroplasts, indicating that there was a membrane reservoir
describable as a relaxation process (Depending on
in the original state of spheroplast. The degree of swelling
the amount of area change, it could take as long as 100s
was correlated to the reduction of the interior phase contrast
for the surface area to reach equilibrium. If the tension
of the spheroplast against the external medium. It is impor-
was then reduced to the initial near zero value, the apparent
tant to note that the swelling and the phase contrast changes
surface area recovered the original value also in a relaxation
were reversible (). The size distribution of the sphe-
manner. This is very different from stretching a lipid bilayer
roplasts is plotted for different external osmolalities in
(), where the area change follows the tension with a
B and the average size in C. The correlation between
speed of sound in the material ).
the size and the interior phase contrast is shown in D.
The relaxation process gave rise to a loading-rate depen-
In general swelling increased with decreasing external
dence (which is well known in single-protein pulling
osmolality and swelling reduced phase contrast. However,
experiments ). When the tension was loaded in
in a given external osmolality, there was a range of degrees
different rates, the surface area expansion followed different
of swelling among the spheroplast population.
relaxation paths ().
Biophysical Journal 107(9) 2082–2090
E. coli Spheroplast Membrane
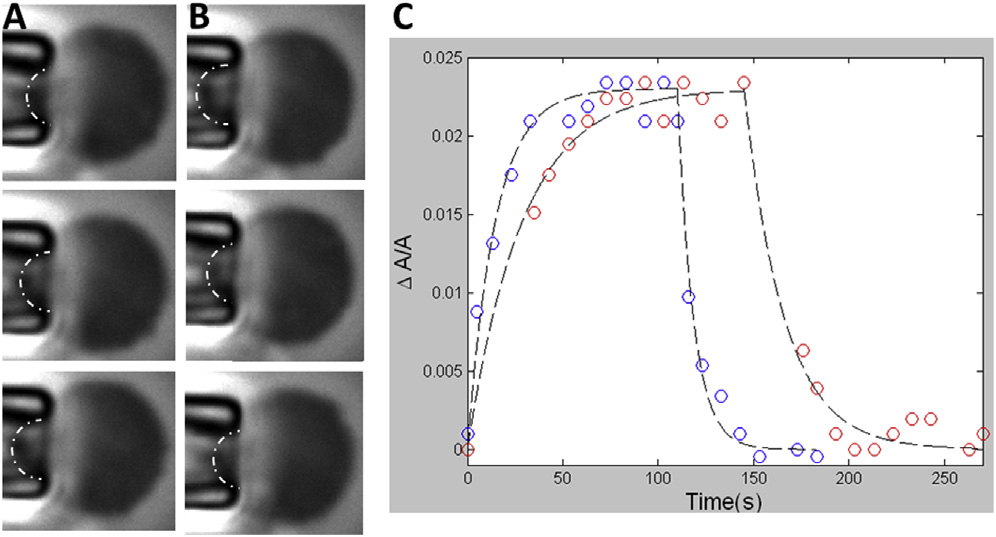
Time dependent area change of spheroplast membrane under
a change of tension. (A) At time 0, the tension was increased from 0.25 to0.83 mN/m. The images from top to bottom were at t ¼ 0, 5, and 70 s. Scalebar ¼ 2.5 mm. (B) After the membrane area reached equilibrium, the tensionwas decreased to 0.25 mN/m. The images from top to bottom were at t ¼ 0,5, and 70 s. The dash-dot lines indicate the geometric features used to calcu-late the area changes. (C) Two area versus time curves were performedat different rates of applying tension: red, the tension (from 0.25 to0.83 mN/m) was applied by lowing the water column at 2 mm/s; blue, at10 mm/s (see Materials and Methods). The curve fitting used the formuladescribed in Discussion; the time constants for the blue curve are 14.6and 8.4 s for area increase and decrease, respectively. 28.8 and 20.9 s forthe red curve, respectively. To see this figure in color, go online.
Surface area expansion by tension is reversible
Every spheroplast in a constant external osmolality gave awell-defined equilibrium tension t versus DA=A curve.
The curve is approximately linear for a small range of t.
(The range of t was limited by the height of the manometerwater column used to generate the aspiration pressure—see
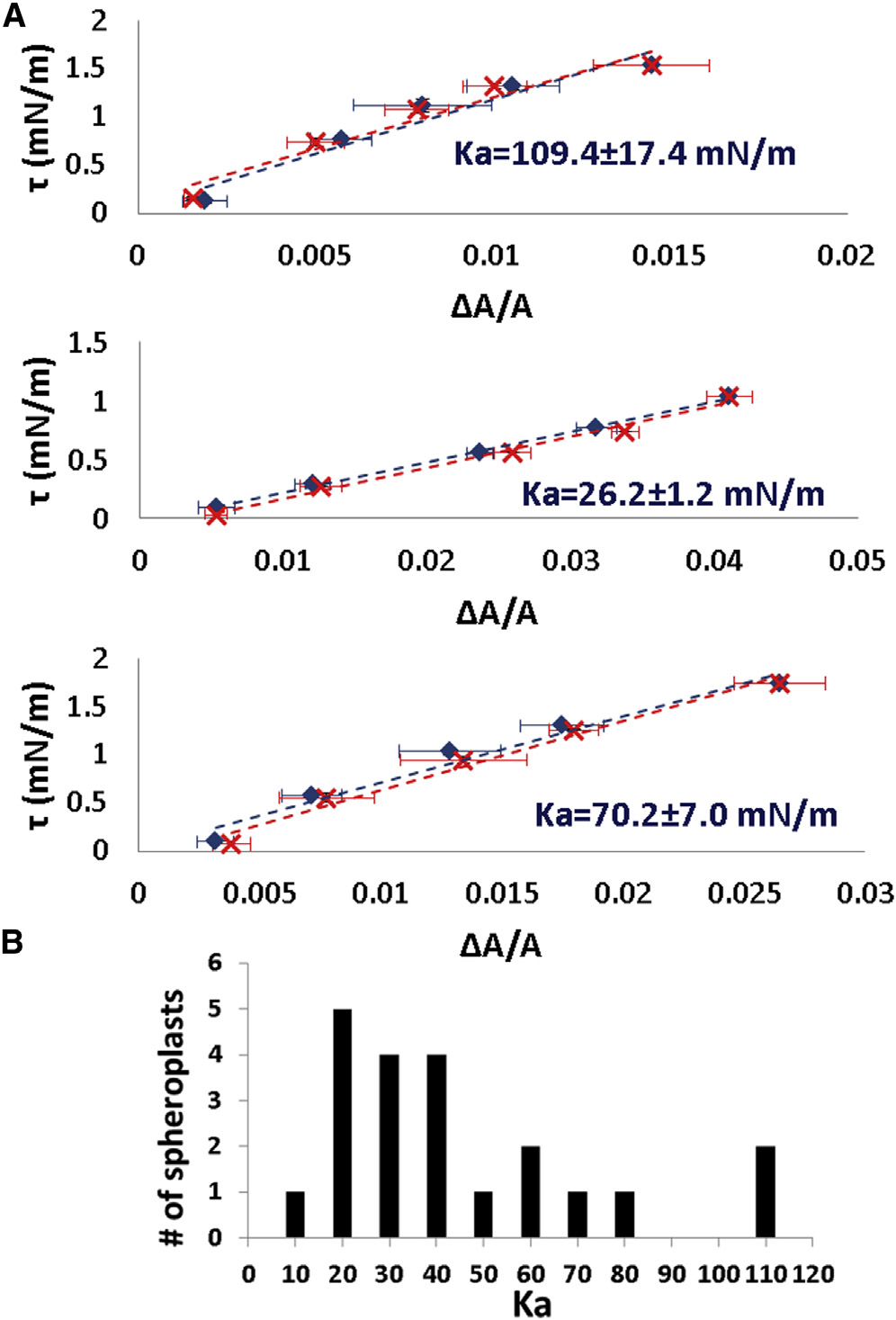
(A) Three examples of reversible tension t versus fractional
Materials and Methods). Importantly we found that the
area change DA=A of spheroplast membranes. In each example blue data
stress-strain relation is reversible and repeatable
are for increasing tension and red for decreasing tension. The data points
and ) within errors. From the linear relation, we define
are equilibrium values. The error bars represent the uncertainties in calcu-
the apparent stretching modulus K
lating the area from the microscope images. The uncertainties in tension
a ¼ t=ðDA=AÞ. We
calculation are smaller than the data symbols. K
a, the apparent stretching
a of the spheroplasts taken from a population
modulus, was measured from the slope of each curve. (B) Ka of a sphero-
equilibrated in external osmolality 0.237 Osm (B).
plast population in 28% STOP solution. To see this figure in color, go online.
The largest values of Ka from the population are 100 mN/m. In comparison, the typical values of Ka for lipidbilayers are 200 mN/m ). Only very swollen sphero-
plasts. The requirement of a 100� microscope objective
plasts exhibit Ka values in the same range (see below).
and its short working distance made it very difficult to trans-fer an aspirated spheroplast from one chamber to another, as
Temperature dependence
we did with GUVs ). Also, an aspirated spheroplast was
Bacterial membranes may have a gel-fluid phase transition
easily detached from the micropipette by a flow in the cham-
below the growth temperature (). Thus it is important to
ber, making it difficult to change the solution in the sample
test the possible temperature dependence of their elastic prop-
chamber. We could only change the solution osmolality
erties. A number of randomly chosen spheroplasts were
qualitatively. This was done by introducing an open tube
measured by t versus DA=A, first in room temperature 25�C
containing a 100% STOP solution that included 1 mM car-
then at 37�C, the growth temperature (In all cases,
boxyfluorescein (CF) into the chamber. We let the diffusion
we detected no significant temperature dependence. Thus
from the tube slowly increased the osmolality inside the
we performed the rest of experiments in room temperature.
chamber until the fluorescence intensity of CF in the cham-ber appeared to be uniform. We measured K
Stretching modulus K
a changes with osmolality
plast before and after the introduction of the open tube.
Measurements of the same spheroplast in different osmolal-
We found that Ka invariably decreased if the external osmo-
ities were difficult because of the small size of the sphero-
lality was increased
Biophysical Journal 107(9) 2082–2090
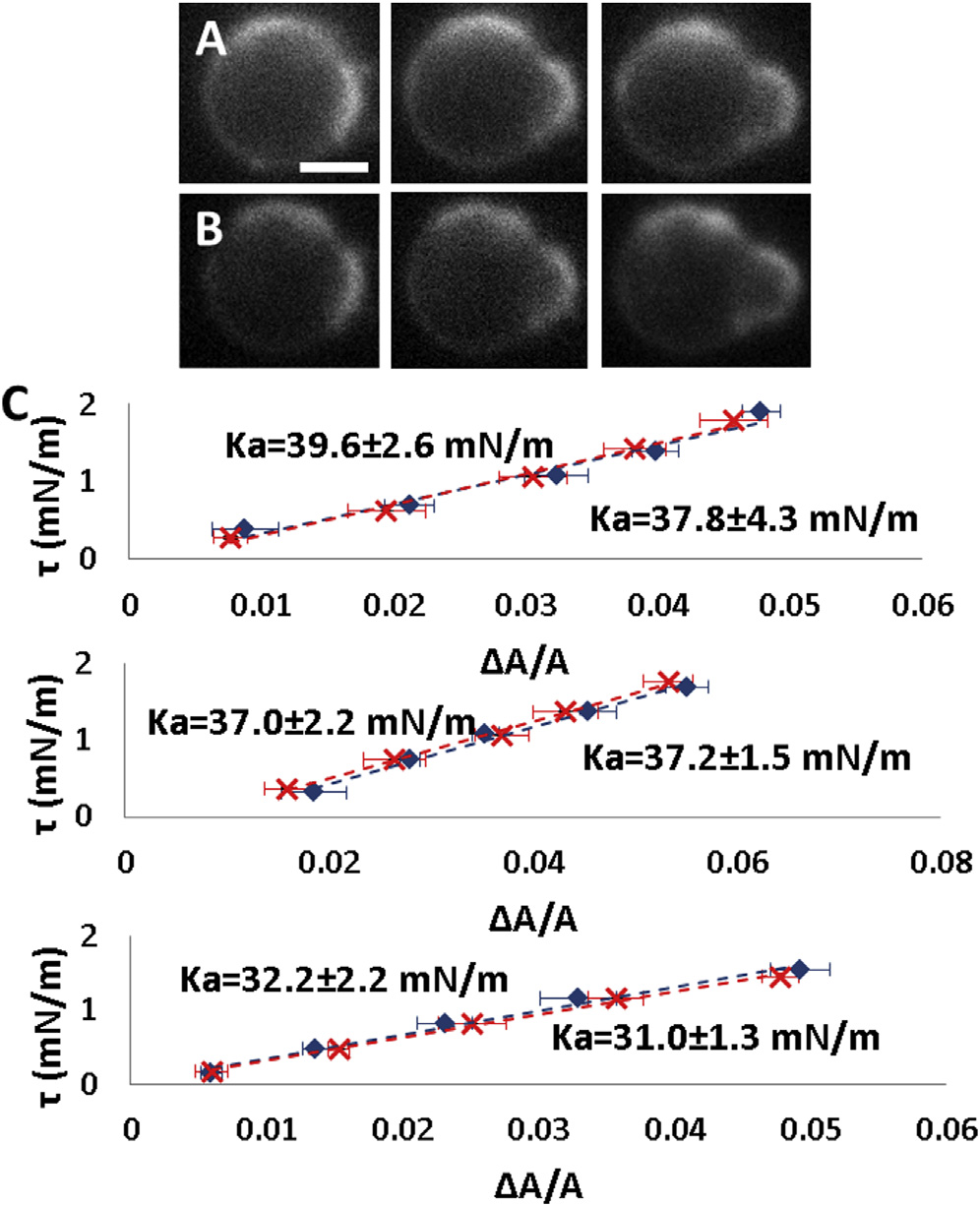
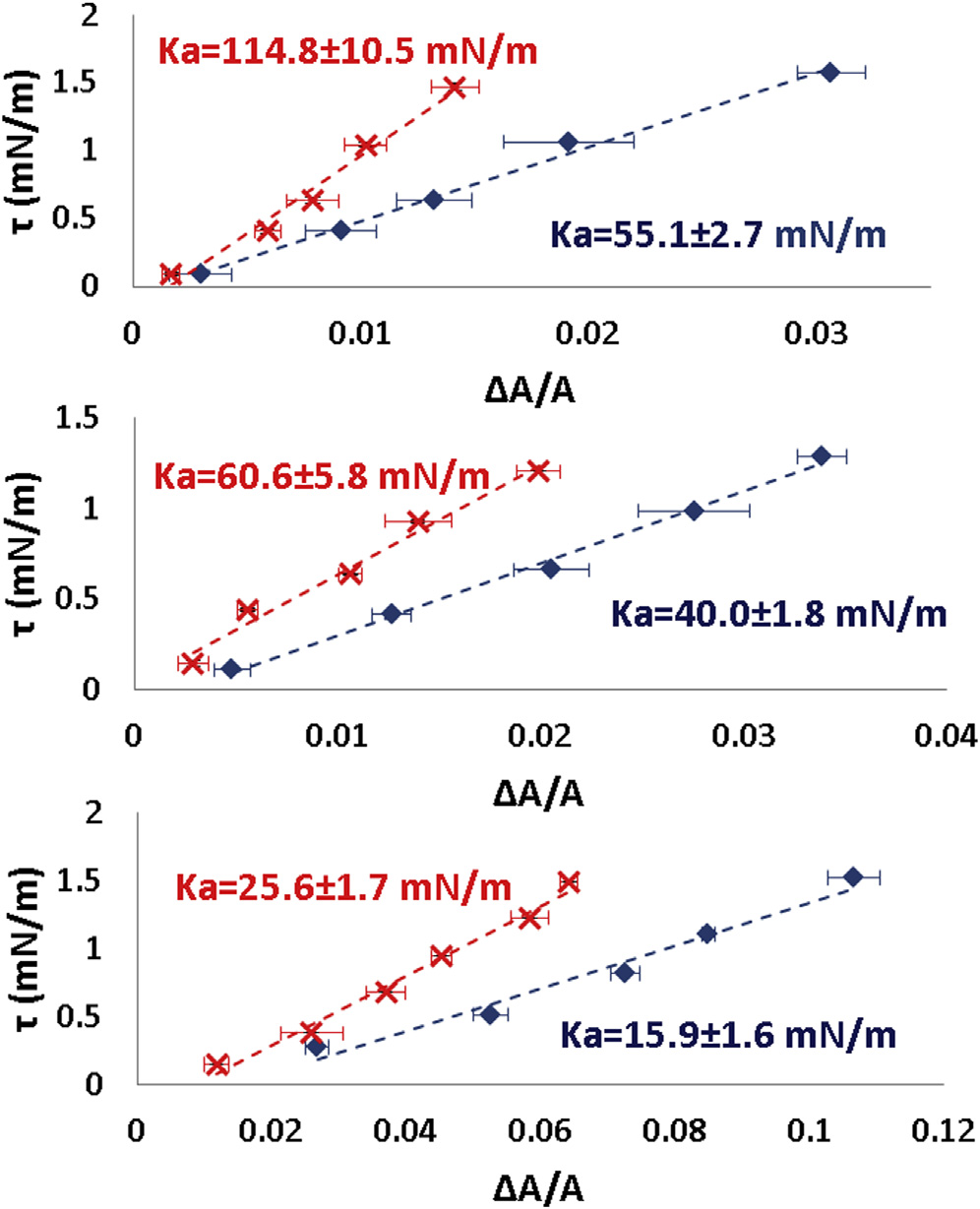
Tension t versus DA=A measurement at 25�C and at 37�C.
Ka decreased if the external osmolality was increased. Ka
(A) A spheroplast was first measured at 25�C. The image from left to right
was first measured in a 28% STOP solution (red points). Then an open
at tension t ¼ 0.34, 1.21, and 2.14 mN/m. (B) The same measurement was
tube containing a 100% STOP solution and carboxyfluorescein (CF) was
repeated at 37�C. Scale bar ¼ 2.5 mm. (C) Data from three spheroplasts:
introduced into the chamber. The diffusion from the tube slowly increased
solid line (blue diamonds) at 25�C; dotted line (red crosses) at 37�C. No
the osmolality inside the chamber until the intensity of CF in the chamber
significant temperature dependence was detected. To see this figure in color,
became uniform. Then Ka was measured again (blue points). The value of
Ka always decreased. Three measurements are shown. To see this figure incolor, go online.
Very swollen spheroplast
In all of the previous examples to ), we detected
membrane surface area was lost, i.e., when the tension was
no initial membrane tension in the aspirated spheroplasts.
reduced to smaller values, the membrane did not recover its
In contrast, very swollen spheroplasts (as judged by small
original smaller area ).
interior phase contrast) were under a surface tension. For
Spheroplasts in the presence of the metabolic inhibitor CCCP
such spheroplasts, a larger suction pressure was requiredfor aspiration and the initial tension was T 1 mN/m
To test if the stretching reversibility is related to bacterial
The values of Ka for very swollen spheroplasts
metabolism, we added 100 mM metabolic inhibitor CCCP
are 200 mN/m, close to the values of Ka measured from
() into a spheroplast suspension in 100% STOP solu-
GUVs of E. coli total lipid extract (
tion. After 1 h, 30 ml of the suspension was injected into anobservation chamber containing 28% STOP solution and
Spheroplasts that lost the interior phase contrast
100 mM CCCP. The spheroplast population in the observa-
In a normal spheroplast population, there were always some
tion chamber looked normal initially. But after each addi-
that appeared to have lost the interior phase contrast (but
tional hour, more and more spheroplasts lost the interior
still clearly visible by their boundaries). If the solution con-
phase contrast. The aspiration experiments were performed
tained the nucleic acid-staining, bacterial viability indicator
soon after the spheroplasts were introduced into the ob-
Sytox Green (the indicator appeared to first enter such
servation chamber. Several spheroplasts with intact phase
spheroplasts and then leaked out (). This implies that
contrast as well as some without interior phase contract
the no-contrast spheroplasts have lost their nucleic acids
were randomly chosen for measurement. The results
). The response of no-contrast spheroplasts to aspiration
(were the same as shown in . The spheroplasts
was different from normal spheroplasts. If the membranes of
poisoned by CCCP but still had intact phase contrast
no-contrast spheroplasts were stretched, the reversibility of
behaved exactly the same as those spheroplasts that had
Biophysical Journal 107(9) 2082–2090
E. coli Spheroplast Membrane
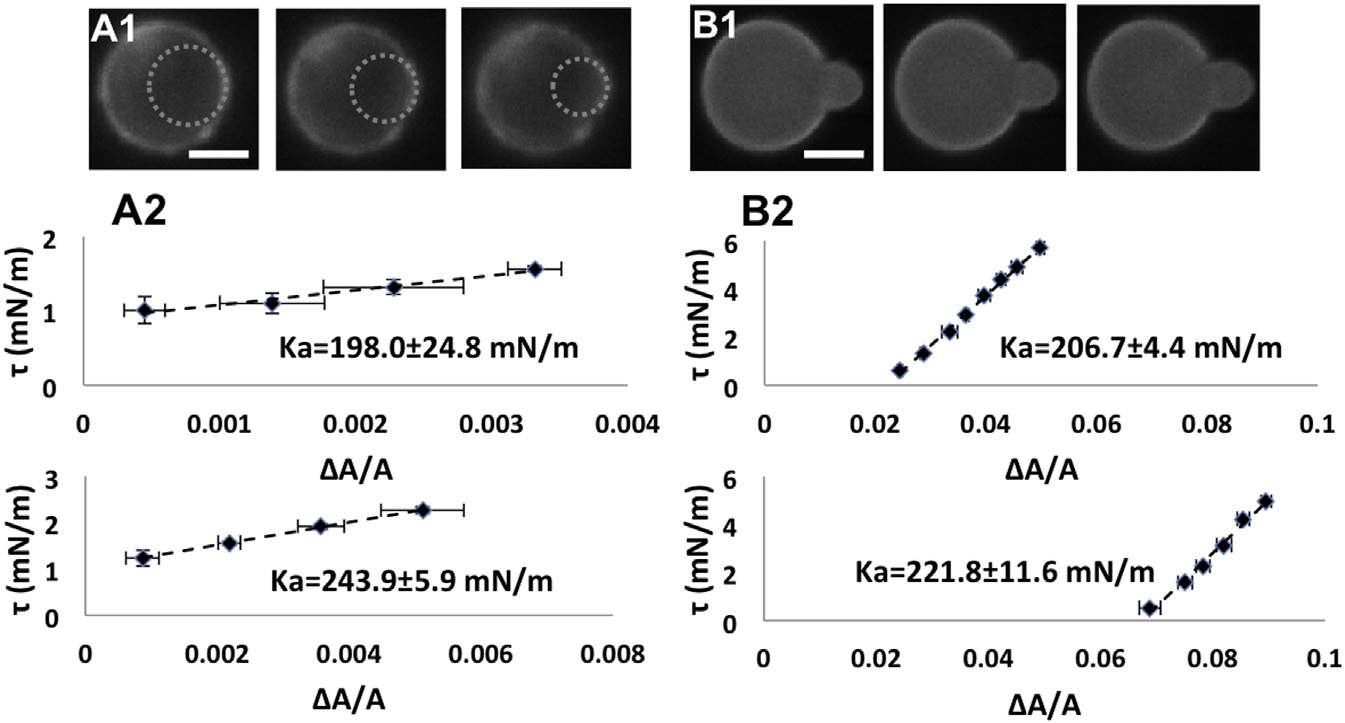
Very swollen E. coli spheroplasts
were compared with GUVs of total E. coli lipidextract. (A1) A very swollen spheroplast aspiratedby a micropipette in a series of increasing tension:left to right, t ¼ 1.00, 1.11, and 1.56 mN/m. Scalebar ¼ 2.5 mm. The dotted circles are the fits to theprotrusions into the micropipette that are used tocalculate the area changes (see ). (A2)Two t versus DA/A measurements of very swollenspheroplasts. (B1) Aspiration measurement of aGUV of E. coli total lipid extract at, left to right,t ¼ 0.3, 1.02, and 1.92 mN/m. Scale bar ¼10 mm. (B2) Two t versus DA/A measurementsof GUVs of E. coli total lipid extract. To see thisfigure in color, go online.
lost the interior phase contrast, without or with CCCP. Thus
hered membranes (). Only in a very swollen condition,
it appeared that the refolding capability of the membrane
the membrane of a red blood cell behaves like a lipid bilayer
reservoir was metabolically maintained.
with a Ka value comparable with a bilayer with comparablecholesterol content In irregularly shaped cells with arigid cytoskeleton, the plasma membrane superstructures
are complicated. Many forms of membrane reservoir were
Comparison with eukryotic cells
visible in electron micrographs Membrane tension,measurable by the tether technique is dominated
In eukaryotic cells, the plasma membrane adheres to the un-
by membrane-cytoskeleton adhesion and is intimately
derlying cytoskeleton, mediated by phospholipid-binding
coupled to cell functions ().
proteins (). Their membrane properties are very different
E. coli. spheroplasts are spherical in shape. Thus the cyto-
from what were measured here. Micropipette aspiration
plasmic membrane is most likely not adhered to an underly-
studies on blood cells essentially characterized the mechan-
ing cytoskeleton, if any. The requirement to survive over a
ical properties of the whole cells, rather than that of unad-
wide range of external osmolality appears to produce aunique design for the cytoplasmic membrane of E. coli. Wefound that the spheroplast membrane has its own character-istic properties not found in eukaryotic cells. The cytoplasmicmembrane appears to have a total area larger than the spher-ical surface area of the cell. The excessive area is kept in amembrane reservoir that behaves like membrane folds. Asthe cell volume expands or contracts by osmolality downshiftor upshift, the spheroplast maintains a minimum sphericalsurface area without tension by adjusting the amount ofmembrane folds. Membrane tension appeared, only when
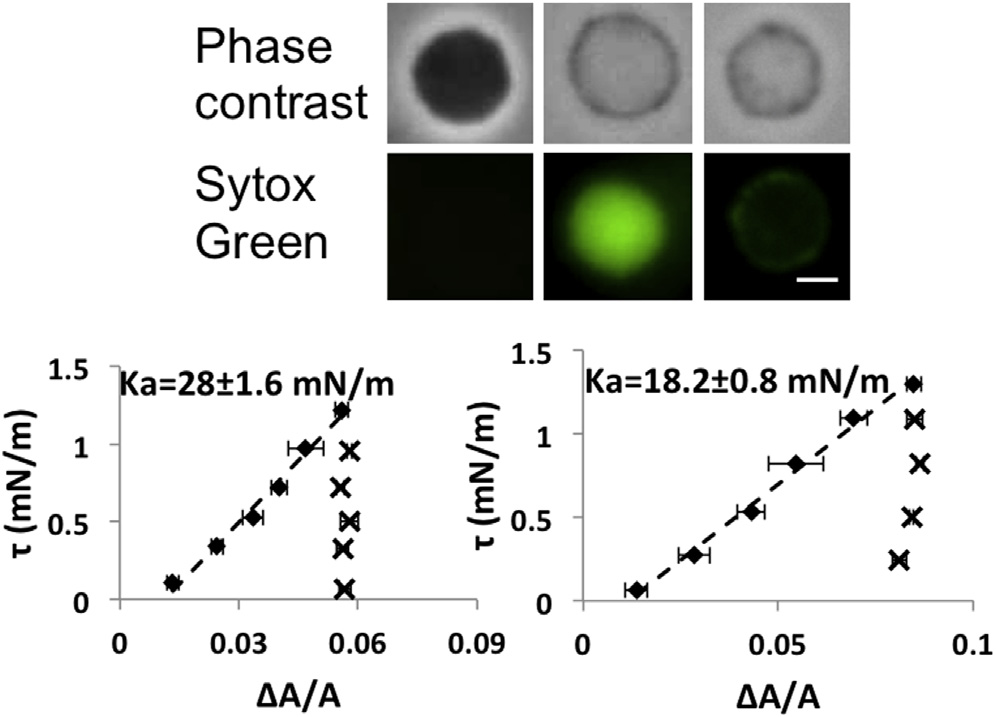
(Top) Paired images of spheroplasts in phase contrast and in
the green channel to detect the nucleic acid stain of Sytox Green (at 1 mM):1st column, the spheroplast with a strong phase contrast showed little pene-tration by Sytox Green; 2nd column, a spheroplast without interior phasecontrast showing very bright Sytox Green in the interior; 3rd column, aspheroplast without interior phase contrast had Sytox Green only around
Aspiration experiments on spheroplasts poisoned by CCCP.
the periphery indicating that the Sytox Green had leaked out. (Bottom)
The spheroplast membrane was stretched by increasing tension (diamonds).
Aspiration experiments on spheroplasts without interior phase contrast
Then the tension was reduced to the initial near zero value (crosses). Unlike
(showing two examples). Once the membranes were stretched (diamonds),
normal spheroplasts, the membrane area did not recover the initial values.
the membrane area could not recover the low tension value when the tension
Two examples were shown with the phase contrast images of the poisoned
was reduced (crosses). To see this figure in color, go online.
Biophysical Journal 107(9) 2082–2090
the volume expansion exhausted the membrane reservoir.
imum surface area for the cell at zero tension over a wide
Perhaps the mechanosensitive channels and osmoregulators
range of external osmolality; 2), it increases or decreases
are operative only under such extreme conditions (
membrane area reversibly in response to an applied tension;3), it changes the membrane area by a relaxation process; 4),the membrane stretching elastic constant changes with
Physical properties of spheroplast membranes
external osmolality; 5), when the reservoir is exhausted by
We have used two different ways of expanding the surface
swelling, the spheroplast membrane has the stretching elas-
area of a spheroplast. The surface area of a spheroplast
ticity of a lipid bilayer; and 6), the refolding capability of the
could expand 2.4 times in average (the average radius
reservoir is maintained metabolically.
increased from 1.3 to 2 mm) by osmolality downshift
We do not know the underlying mechanisms that give rise
This expansion or swelling was because of water
to these properties. However, they are understandable, if we
influx into the cell. It appeared that during the expansion
assume that the membrane reservoir is metabolically main-
the internal osmolality was adjusted to the external value
tained by reversible chemical bonds (In the following
and there was no pressure difference across the membrane,
we describe the characteristics of a simple generic model
until the swelling reached its limit. Thus we found the cyto-
for membrane stretching elasticity based on a membrane
plasmic membrane of E. coli tensionless, unless the bacteria
reservoir maintained by reversible chemical bonds. We as-
were subject a very low external osmolality.
sume that each bond is responsible for an area dA of mem-
On the other hand, at a constant external osmolality, the
brane, so that when N bonds were broken the surface area
aspiration-induced surface area expansion created tension
will increase by dA,N.
to counter the force of aspiration. The volume of cytoplasmwas constant during this operation. Very importantly we
Membrane area changes as a relaxation process
found that, unlike the stretching of a lipid bilayer that
We assume that, at zero tension, the bound state (B) and
follows the rules of elasticity of material deformation
the unbound state (U) of a chemical bond are in kinetic equi-
the area increase of spheroplast membrane by ten-
librium. This is expressed as a reversible reaction B%U,
sion was a relaxation process with a time-constant in the or-
with an unbonding rate constant ko� and a bonding rate con-
der of tens of second (). When the applied tension was
stant koþ. At time zero, a tension is applied to the bonds, that
reduced to the initial smaller values, the membrane area
changes the rate constants to k� and kþ. As a function of
recovered the original values also by a relaxation process.
time, under the constant tension, the number of unbound
The equilibrium area-tension relation was strictly reversible,
and linear over a small range of tension. Thus phenomeno-
u ¼ Nfu þ ðNiu � NfuÞe�lt, where l ¼ kþ þ k�, Niu
logically the area-tension relation of spheroplast membranes
and Nfu are the initial and final values. For area expansion,
can be described by an apparent area stretching elastic con-
we set the initial DA ¼ 0, so as a function of time
a ¼ t=ðDA=AÞ. Our water-column manometer was
dAðNu � NiuÞ ¼ dAðNfu � NiuÞf1 � e�ltg. After the
not long enough to measure the entire t versus DA=A curve
area expansion reached equilibrium, the tension was
starting from the least swollen condition to the very swollen
reduced to zero. Then the area decrease is described
condition. Instead we measured Ka by aspiration at different
DA ¼ dAðNu � NfuÞ ¼ dAðNiu � NfuÞe�lot,
values of external osmolality. The values of Ka were the
lo ¼ koþ þ ko�. These are the expressions used to fit the
same at 25�C and at 37�C We found that the value
data for area increase and decrease in . 1=l and 1=lo
of Ka increases with the degree of swelling ), up to
are the time constants of the relaxation processes.
about Ka � 100 mN/m (No higher Ka values werefound except in very swollen spheroplasts that behave like
The effect of tension
a GUV made of E. coli total lipid extract. We found that
Assume a binding energy �eo for each bond. Initially, in the
both the very swollen spheroplasts and the GUVs of
absence of tension, there are Ni
E. coli lipid extract had values of K
u broken (or unbound) bonds
Interestingly, the reversibility of membrane area
b intact bonds among a total of No : Niu=Nib ¼ 1=ebeo ,
stretching was lost when spheroplasts were poisoned by
BT, kB is the Boltzmann constant and
T is the temperature. From the equilibrium condition
the metabolic inhibitor CCCP, suggesting that the mem-
brane reservoir is maintained metabolically.
þNiu ¼ ko�Nib, one obtains the relation for the rate constants
It is clear that the elasticity of spheroplast membranes
ko�=koþ ¼ 1=ebeo.
with Ka(100 mN=m is not that of stretching a lipid bilayer,
If a tension t is applied to a chemical bond, its effect can
because the values of Ka for lipid bilayers are typically
be expressed by a decrease of the binding energy by ta,
200 mN/m without cholesterol ) and higher with choles-
where a is a parameter such that ta is the amount of work
terol (). Rather the spheroplast membrane area is con-
done by tension per bond (Then the rate constants are
trolled by a membrane reservoir. This membrane reservoir
modified to k�=kþ ¼ 1=ebðeo�taÞ. The increase of k�=kþ
exhibits six characteristic properties: 1), it maintains a min-
relative to ko�=koþ, by a factor of ebta, increases Nu=Nb to
Biophysical Journal 107(9) 2082–2090
E. coli Spheroplast Membrane
1=ebðeo�taÞ, from which one can derive the tension versus
2. Needham, D., and R. M. Hochmuth. 1992. A sensitive measure of sur-
face stress in the resting neutrophil. Biophys. J. 61:1664–1670.
area relation.
3. Tsai, M. A., R. S. Frank, and R. E. Waugh. 1994. Passive mechanical
We can make a rough estimate for the value of the bond
behavior of human neutrophils: effect of cytochalasin B. Biophys. J.
energy. Take the example of Ka � 70 mN/m in
an applied tension tz2 mN=m ¼ 4:8 � 105kBT=ðmmÞ2
4. Evans, E., and A. Yeung. 1989. Apparent viscosity and cortical tension
increased the surface area of a spheroplast by 2.5%; the
of blood granulocytes determined by micropipet aspiration. Biophys. J.
diameter of the spheroplast 5 mm; thus DA � 2:0 ðmmÞ2;
the work done is t,DAz9:6 � 105 k
5. Hochmuth, R. M. 2000. Micropipette aspiration of living cells.
BT. Because the mem-
J. Biomech. 33:15–22.
brane thickness is 50 �
A, we assume the minimum area size
6. Sheetz, M. P., and J. Dai. 1996. Modulation of membrane dynamics and
per chemical bond dA � ð100Þ2�
A . Thus the stretching has
cell motility by membrane tension. Trends Cell Biol. 6:85–89.
broken � 2 � 104 bonds. Therefore the binding energy per
7. Sheetz, M. P., J. E. Sable, and H. G. Do¨bereiner. 2006. Continuous
bond is eo � 48 kBT (when Ka � 70 mN/m). The value of
membrane-cytoskeleton adhesion requires continuous accommodation
the apparent area-stretching modulus varies from near
to lipid and cytoskeleton dynamics. Annu. Rev. Biophys. Biomol.
Struct. 35:417–434.
zero to 100 mN/m, as the membrane progressively un-
8. Sheetz, M. P. 2001. Cell control by membrane-cytoskeleton adhesion.
folds. This implies that the strength of the chemical bonds
Nat. Rev. Mol. Cell Biol. 2:392–396.
for the membrane reservoir covers a range of value, and
9. Gauthier, N. C., T. A. Masters, and M. P. Sheetz. 2012. Mechanical
the bonds are progressively broken from the weaker to the
feedback between membrane tension and dynamics. Trends Cell
stronger ones as the membranes are unfolded.
Biol. 22:527–535.
10. Dai, J., and M. P. Sheetz. 1999. Membrane tether formation from bleb-
bing cells. Biophys. J. 77:3363–3370.
11. Jones, W. R., H. P. Ting-Beall, ., F. Guilak. 1999. Alterations in the
Young's modulus and volumetric properties of chondrocytes isolated
E. coli is the most studied microorganism, but the physical
from normal and osteoarthritic human cartilage. J. Biomech. 32:
property of its cytoplasmic membrane has been unknown,
because it is normally shielded by an outer membrane. Our
12. Martinac, B., M. Buechner, ., C. Kung. 1987. Pressure-sensitive ion
main results are the discovery of the tensionless condition
channel in Escherichia coli. Proc. Natl. Acad. Sci. USA. 84:2297–2301.
of cytoplasmic membranes of E. coli and their unusual
13. Ruthe, H. J., and J. Adler. 1985. Fusion of bacterial spheroplasts by
stretching properties. A membrane reservoir appears to serve
electric fields. Biochim. Biophys. Acta. 819:105–113.
the function of adjusting the cytoplasmic volume so as to
14. Renner, L. D., and D. B. Weibel. 2011. Cardiolipin microdomains
localize to negatively curved regions of Escherichia coli membranes.
maintain equal osmolality on both sides of the membrane.
Proc. Natl. Acad. Sci. USA. 108:6264–6269.
At the least swollen state of spheroplast, less than one-half
15. Randall, C. P., K. R. Mariner, ., A. J. O'Neill. 2013. The target of dap-
of membrane forms the surface area. More than one-half
tomycin is absent from Escherichia coli and other gram-negative path-
of the membrane is in reservoir. When a tension is applied
ogens. Antimicrob. Agents Chemother. 57:637–639.
to the spheroplast membrane, its area increases by a relaxa-
16. White, D., J. Drummond, and C. Fuqua. 2012. The Physiology and
Biochemistry of Prokaryoties. Oxford University Press, Oxford, UK.
tion process but the area would decrease reversibly when the
17. Wood, J. M. 1999. Osmosensing by bacteria: signals and membrane-
tension is removed. Most interestingly this reversibility is
based sensors. Microbiol. Mol. Biol. Rev. 63:230–262.
lost in the presence of metabolic inhibitor CCCP. The cyto-
18. Chang, G., R. H. Spencer, ., D. C. Rees. 1998. Structure of the MscL
plasmic membrane is under a tension only when the mem-
homolog from Mycobacterium tuberculosis: a gated mechanosensitive
brane reservoir is completely unfolded by swelling.
ion channel. Science. 282:2220–2226.
These physical properties are important for experiments
19. Jensen, M. O., and O. G. Mouritsen. 2004. Lipids do influence protein
using spheroplast membranes, particularly for the investiga-
function—the hydrophobic matching hypothesis revisited. Biochim.
Biophys. Acta. 1666:205–226.
tions of how peptides and proteins interact with bacterial
20. Berchtold, D., M. Piccolis, ., R. Loewith. 2012. Plasma membrane
stress induces relocalization of Slm proteins and activation ofTORC2 to promote sphingolipid synthesis. Nat. Cell Biol. 14:542–547.
21. Sochacki, K. A., K. J. Barns, ., J. C. Weisshaar. 2011. Real-time
SUPPORTING MATERIAL
attack on single Escherichia coli cells by the human antimicrobial pep-tide LL-37. Proc. Natl. Acad. Sci. USA. 108:E77–E81.
Four figures are available at
22. Barns, K. J., and J. C. Weisshaar. 2013. Real-time attack of LL-37 on
single Bacillus subtilis cells. Biochim. Biophys. Acta. 1828:1511–1520.
This work was supported by NIH Grant GM55203 and the Robert A. Welch
23. Rangarajan, N., S. Bakshi, and J. C. Weisshaar. 2013. Localized perme-
Foundation Grant C-0991.
abilization of E. coli membranes by the antimicrobial peptide CecropinA. Biochemistry. 52:6584–6594.
24. Pogliano, J., N. Pogliano, and J. A. Silverman. 2012. Daptomycin-
mediated reorganization of membrane architecture causes mislocaliza-tion of essential cell division proteins. J. Bacteriol. 194:4494–4504.
1. Mohandas, N., and E. Evans. 1994. Mechanical properties of the red
25. Huang, H. W. 2009. Free energies of molecular bound states in lipid
cell membrane in relation to molecular structure and genetic defects.
bilayers: lethal concentrations of antimicrobial peptides. Biophys. J.
Annu. Rev. Biophys. Biomol. Struct. 23:787–818.
Biophysical Journal 107(9) 2082–2090
26. Galinski, E. A. 1995. Osmoadaptation in bacteria. Adv. Microb. Phys-
41. Lee, M. T., W. C. Hung, ., H. W. Huang. 2008. Mechanism and ki-
iol. 37:272–328.
netics of pore formation in membranes by water-soluble amphipathic
27. Lippert, K., and E. A. Galinski. 1992. Enzyme stabilization by ectoine-
peptides. Proc. Natl. Acad. Sci. USA. 105:5087–5092.
type compatible solutes: protection against heating, freezing and dry-
42. Lee, M. T., T. L. Sun, ., H. W. Huang. 2013. Process of inducing pores
ing. Appl. Microbiol. Biotechnol. 37:61–65.
in membranes by melittin. Proc. Natl. Acad. Sci. USA. 110:14243–
28. Kennedy, E. P., and M. K. Rumley. 1988. Osmotic regulation of biosyn-
thesis of membrane-derived oligosaccharides in Escherichia coli.
43. Landau, L. D., and E. M. Lifshitz. 1970. Theory of Elasticity. Perga-
J. Bacteriol. 170:2457–2461.
mon Press, Oxford, UK, pp. 101–122.
29. Record, Jr., M. T., E. S. Courtenay, ., H. J. Guttman. 1998. Responses
44. Bell, G. I. 1978. Models for the specific adhesion of cells to cells. Sci-
of E. coli to osmotic stress: large changes in amounts of cytoplasmic
solutes and water. Trends Biochem. Sci. 23:143–148.
45. Kappel, C., N. Do¨lker, ., H. Grubmu
30. Cayley, D. S., H. J. Guttman, and M. T. Record, Jr. 2000. Biophysical
¨ller. 2012. Universal relaxation
governs the nonequilibrium elasticity of biomolecules. Phys. Rev.
characterization of changes in amounts and activity of Escherichia coli
Lett. 109:118304.
cell and compartment water and turgor pressure in response to osmoticstress. Biophys. J. 78:1748–1764.
46. Moy, V. T., E. L. Florin, and H. E. Gaub. 1994. Intermolecular forces
31. Stock, J. B., B. Rauch, and S. Roseman. 1977. Periplasmic space in
and energies between ligands and receptors. Science. 266:257–259.
Salmonella typhimurium and Escherichia coli. J. Biol. Chem.
47. Kellermayer, M. S., S. B. Smith, ., C. Bustamante. 1997. Folding-un-
folding transitions in single titin molecules characterized with laser
32. Sen, K., J. Hellman, and H. Nikaido. 1988. Porin channels in intact
tweezers. Science. 276:1112–1116.
cells of Escherichia coli are not affected by Donnan potentials across
48. Koga, Y. 2012. Thermal adaptation of the archaeal and bacterial lipid
the outer membrane. J. Biol. Chem. 263:1182–1187.
membranes. Archaea. 2012:789652.
33. Kwok, R., and E. Evans. 1981. Thermoelasticity of large lecithin
49. Roth, B. L., M. Poot, ., P. J. Millard. 1997. Bacterial viability and
bilayer vesicles. Biophys. J. 35:637–652.
antibiotic susceptibility testing with SYTOX green nucleic acid stain.
34. Sun, Y., W. C. Hung, ., H. W. Huang. 2009. Interaction of tea catechin
Appl. Environ. Microbiol. 63:2421–2431.
(-)-epigallocatechin gallate with lipid bilayers. Biophys. J. 96:1026–1035.
50. Diez-Gonzalez, F., and J. B. Russell. 1997. Effects of carbonylcyanide-
m-chlorophenylhydrazone (CCCP) and acetate on Escherichia coli
35. Sun, Y., C. C. Lee, and H. W. Huang. 2011. Adhesion and merging of
O157:H7 and K-12: uncoupling versus anion accumulation. FEMS Mi-
lipid bilayers: a method for measuring the free energy of adhesion and
crobiol. Lett. 151:71–76.
hemifusion. Biophys. J. 100:987–995.
51. Chapple, D. S., D. J. Mason, ., R. W. Evans. 1998. Structure-function
36. Onitsuka, M. O., Y. Rikihisa, and H. B. Maruyama. 1979. Biochem-
relationship of antibacterial synthetic peptides homologous to a helical
ical and topographical studies on Escherichia coli cell surface. IV. Gi-
surface region on human lactoferrin against Escherichia coli serotype
ant spheroplast formation from a filamentous cell. J. Bacteriol.
O111. Infect. Immun. 66:2434–2440.
52. Piddock, L. J., Y. F. Jin, ., A. E. Asuquo. 1999. Quinolone accumula-
37. Ishihara, A., J. E. Segall, ., H. C. Berg. 1983. Coordination of flagella
tion by Pseudomonas aeruginosa, Staphylococcus aureus and Escheri-
on filamentous cells of Escherichia coli. J. Bacteriol. 155:228–237.
chia coli. J. Antimicrob. Chemother. 43:61–70.
38. Kubalski, A. 1995. Generation of giant protoplasts of Escherichia coli
and an inner-membrane anion selective conductance. Biochim. Bio-
53. Fazal, F. M., and S. M. Block. 2011. Optical tweezers study life under
phys. Acta. 1238:177–182.
tension. Nat. Photonics. 5:318–321.
39. Buechner, M., A. H. Delcour, ., C. Kung. 1990. Ion channel activities
54. Belyy, V., K. Kamaraju, ., S. Sukharev. 2010. Adaptive behavior of
in the Escherichia coli outer membrane. Biochim. Biophys. Acta.
bacterial mechanosensitive channels is coupled to membrane me-
chanics. J. Gen. Physiol. 135:641–652.
40. Rawicz, W., K. C. Olbrich, ., E. Evans. 2000. Effect of chain length
55. Helfrich, W. 1973. Elastic properties of lipid bilayers: theory and
and unsaturation on elasticity of lipid bilayers. Biophys. J. 79:328–339.
possible experiments. Z. Naturforsch. C. 28:693–703.
Biophysical Journal 107(9) 2082–2090
Supplemental Information
Physical Properties of Escherichia coli Spheroplast Membranes
Yen Sun, Tzu-Lin Sun and Huey W. Huang
Department of Physics & Astronomy, Rice University, Houston, Texas
Fig. S1 Geometries of an aspirated GUV and an aspirated spheroplast are the same except in size. Two different situations are shown. A. Aspiration produced a cylindrical protrusion
(shown with a GUV image; scale bar = 10 µm): definitions for 𝑅𝑣, 𝑅𝑝, 𝐿𝑝. B. Aspiration produced a spherical protrusion (shown with a spheroplast image; scale bar = 2.5 µm): definitions for 𝑟, ℎ, and 𝐻. C. Formulas used for the situation B : the volume on the right side
of the red plane = 𝜋ℎ2 �𝑟 − ℎ�; the area of the partial sphere on the right side of the red plane =
Fig. S2 (Left image) A spheroplast was held by a micropipette with a small suction pressure initially in 30% STOP solution. (Middle image) Then the solution was changed to 85% STOP solution. (Right image) Finally the solution was changed back to 30% STOP solution. Both the swelling and the phase contrast changes were reversible. In sequence, the diameter of the
spheroplast was 5.8, 4.7, 5.8 µm; the phase contrast (𝐼0 − 𝐼)/𝐼0 was 0.15, 0.25, 0.17 (see Fig. 1). Scale bar = 2 µm.
Fig. S3 The images from left to right show the response of a spheroplast in 100% STOP
solution to a sucking pressure by micropipette aspiration. Note that the applied suction pressure was very small 50 Pa, but the spheroplast was already entirely sucked into the pipette. Scale
bar = 5 µm. The images were taken, from left to right, at t = 0, 79, 110, 169 s. This could be due to the size of the micropipette being too close to the size of the spheroplast, or it could be what was described as a liquid drop model in the micropipette-aspiration experiments of human blood cells . Since our interests are solely on the membrane property of spheroplasts, we will not discuss spheroplasts from 100% STOP solution.
Fig. S4 The tension vs. area change measurement of a spheroplast is reversible and repeatable. In this example, the spheroplast membrane was stretched and released twice (four different colors). The error bars are explained in Fig. 3. 1.
Sun, Y., C. C. Lee, and H. W. Huang. 2011. Adhesion and merging of lipid bilayers: a method for
measuring the free energy of adhesion and hemifusion. Biophys J 100:987-995.
Evans, E., and A. Yeung. 1989. Apparent viscosity and cortical tension of blood granulocytes
determined by micropipet aspiration. Biophys J 56:151-160.
Jones, W. R., H. P. Ting-Beal , G. M. Lee, S. S. Kel ey, R. M. Hochmuth, and F. Guilak. 1999.
Alterations in the Young's modulus and volumetric properties of chondrocytes isolated from
normal and osteoarthritic human cartilage. J Biomech 32:119-127.
Hochmuth, R. M. 2000. Micropipette aspiration of living cells. J Biomech 33:15-22.
Source: http://hwhuang.rice.edu/pdfs/Sun%20E.%20coli%202014.comb.pdf
THE CENTRE FOR HEALTH IN THE HEART OF RODNEY Breast reconstruction following a mastectomy using Brava and autologous fat transfer (AFT) Rodney Surgical Centre is leading You adjust the pressure, starting at a comfortable level and gradually the way with a scientifically-proven increasing it, depending on your tolerance. Most patients ramp it up
INTEGRATED PEST MANAGEMENT PACKAGE M Srinivas Prasad National Centre for Integrated Pest Management LBS Building, IARI Campus, New Delhi – 110 012 Directorate of Plant Protection, National Institute of Plant Health Quarantine & Storage (DPPQ&S) CGO Complex, NH IV, Faridabad DAC, Min of Agri., Rajendranagar, Hyderabad- 500030








