Levitra enthält Vardenafil, das eine kürzere Wirkdauer als Tadalafil hat, dafür aber schnell einsetzt. Männer, die diskret bestellen möchten, suchen häufig nach levitra kaufen ohne rezept. Dabei spielt die rechtliche Lage in der Schweiz eine wichtige Rolle.
Kpa.or.jp
(英語)(English)
Regarding this translation, we did the best we could to match the
original Japanese text, however some parts were written in a suitable way to make it understandable to the English readers. Please refer to the Japanese instruction if necessary.
이 번역에 대해서는, 될수 있는한 일본어 원본과 가까이 해서 번역하고
있습니다만,100% 이 내용을 보증 할수는 없으므로 양해를 해주시기 바랍니다. 필요에 대해서는 일본어 첨부 설명서를 참고하여 주시기를 부탁드립니다.
Isinagawa ang pagsasalin na ito na sinunod sa abot ng makakaya ang
orihinal na teksto na nasa wikang Hapon, subalit may mga bahagi na isinulat upang madaling maintindihan ng mga mambabasa ng Tagalog. Mangyaring isangguni ang tagubilin na nasa wikang Hapon kung kinakailangan.
Apesar de todos os esforços não pode ser traduzido em cem por cento.
Esta tradução será anexado como um auxiliar de informação o mais próximo
possível do japonês para o português, apesar das limitações. Use a para melhor
entendimento das explicações em japonês.
A pesar de todos los esfuerzos tal vez no se ha logrado una traducción
al cien por ciento. Esta traduccion se adjunta como una información auxiliar lo mas cercana posible del japonés al espaňol, no obstante la limitaciónes. Usela para comprender mejor las explicaciónes en japonés que están a su disposición.
Product name: ACTIVIR Ointment (アクチビア軟膏)
※Please read the following directions carefully before using this product.
Product summary
For the treatment of recurring oral herpes. (Only those patients who have been diagnosed by a doctor
and have received medical treatment in the past can use this medicine.)
[Ingredient and Action]
The following ingredient is included in Activir 1g:
Active Ingredient
This medicine helps fight the herpes virus.
[Dosage and Usage]
Apply an appropriate amount of this medicine to the affected area 3 to 5 times a day. (Apply as soon
as you feel a tingle or prickle on or around your lips.) Description
• Tingles or prickles are signs of cold sore recurrence. Apply this product from the early stages of the
• In general, apply this product after every meal and before going to bed.
• Don't give this medicine to a family member who seems to have a cold sore for the first time.
〈Cautions on Dosage and Usage〉
• Please follow exactly the defined dosage and usage directions.
• Apply this medicine to a child only under parental guidance and supervision.
• Avoid contact with the eyes. If this medicine gets into your eyes, please rinse them immediately
with cold or lukewarm water. Please seek treatment from an eye doctor if your eyes still hurt after rinsing them.
• For external use only.
• Don't eat or lick this medicine, even though this medicine possesses no health threat.
Precautions for Use
Please read the following precautions carefully before using this product.
What not to do
(Failure to observe these warnings may lead to aggravation of the symptoms and increase the risk of
adverse reactions.) 1. The following persons should not use this medicine:
(1) Those who seem to have cold sore symptoms for the first time or have a large affected area.
(Consult a physician if you have the symptoms for the first time because it could worsen, or if you have a large affected area it could be a severe form of cold sore.)
(2) Those who have had an allergic reaction to this medicine or medicines containing valacyclovir
hydrochloride in the past. (This product may cause an allergic reaction again.)
(3) Children under the age of 6.
(There is a high possibility of a first time infection for babies and infants.)
2. Don't apply this medicine to the following areas of the body:
(1) In and around your eyes. (This medicine may irritate your eyes.) (2) Any body part other than your lips and the area around them. (Cold sores develop only on and
around your lips).
1. Consult a physician or pharmacist before using this medicine if any of the following apply to you:
(1) Those who are under treatment by two different physicians. (There may be the chance that
both physicians prescribe the same or similar medications.)
(2) Those who are or may be pregnant. (A pregnant woman must be careful about taking medicine.
lf you are or may be pregnant, consult your doctor and follow her instructions).
(3) Women who are breast-feeding. (Studies have shown that mothers who have taken this
medicine have it transfer to their breast milk.)
(4) Patients or their family members who have a tendency for allergies. (A person with an allergic
constitution may develop allergic symptoms by using this product.)
(5) Those who have allergic reactions to medicines. (lf you have had an allergic reaction to a
medicine in the past, this product could cause an allergic reaction as well.)
(6) Those who have developed severely moist skin or skin sores. (Consult a physician if your
symptoms are severe.)
(7) Those who have atopic dermatitis. (This product could worsen the atopic dermatitis. If you have
atopic dermatitis, consult a medical specialist.)
2. If any of the following applies to you, stop using this medicine immediately and consult a physician
or pharmacist with this insert: (1) When the following symptoms occur after using this medicine:
rash, redness, swelling, itching, skin eruption, irritation, pain, dry skin,
burning sensation, or flaking of the skin similar to dandruff
(The above-mentioned symptoms could be a result of allergic reaction to, or overt expression of
the pharmacological quality of, this product. The symptoms may worsen if you continue using.)
(2) When the symptoms do not improve after using this medicine after 7 days. (When the symptoms
have not subsided after using this product after 7 days, you should consider the symptoms are either severe or caused by another disease. Consult a physician or pharmacist as soon as possible).
Storage and Pharmaceutical Precautions:
(1) Make sure the cap is always on tight and store the medicine in a dry and cool place away from
direct sunlight at temperatures below 30° Celsius (86 degrees Fahrenheit).
(2) Keep out of the reach of children. (3) Wash your hands thoroughly before and after use. (4) Don't put this product into another container. (It can become ineffective or change in its quality.) (5) Don't use this product after the expiration date. Don't use product more than 6 months after it
has been opened, even if it is before the expiration date (to ensure product quality).
制作 (社)神奈川県薬剤師会
Product name: HERPECIA Ointment (ヘルペシア軟膏)
※Please read the following directions carefully before using this product.
Product summary
For the treatment of recurring oral herpes. (Only those patients who have been diagnosed by a doctor
and have received medical treatment in the past can use this medicine.)
[Ingredient and Action]
The following ingredient is included in Herpecia 1g:
Active Ingredient
This medicine helps fight the herpes virus.
[Dosage and Usage]
Apply an appropriate amount of this medicine to the affected area 3 to 5 times a day. (Apply as soon
as you feel a tingle or prickle on or around your lips.) Description
• Tingles or prickles are signs of cold sore recurrence. Apply this product from the early stages of the
• In general, apply this product after every meal and before going to bed.
• Don't give this medicine to a family member who seems to have a cold sore for the first time.
〈Cautions on Dosage and Usage〉
• Please follow exactly the defined dosage and usage directions.
• Apply this medicine to a child only under parental guidance and supervision.
• Avoid contact with the eyes. If this medicine gets into your eyes, please rinse them immediately
with cold or lukewarm water. Please seek treatment from an eye doctor if your eyes still hurt after rinsing them.
• For external use only.
• Don't eat or lick this medicine, even though this medicine possesses no health threat.
Precautions for Use
Please read the following precautions carefully before using this product.
What not to do
(Failure to observe these warnings may lead to aggravation of the symptoms and increase the risk of
adverse reactions.) 1. The following persons should not use this medicine:
(1) Those who seem to have cold sore symptoms for the first time or have a large affected area.
(Consult a physician if you have the symptoms for the first time because it could worsen, or if you have a large affected area it could be a severe form of cold sore.)
(2) Those who have had an allergic reaction to this medicine or medicines containing valacyclovir
hydrochloride in the past. (This product may cause an allergic reaction again.)
(3) Children under the age of 6.
(There is a high possibility of a first time infection for babies and infants.)
2. Don't apply this medicine to the following areas of the body:
(1) In and around your eyes. (This medicine may irritate your eyes.) (2) Any body part other than your lips and the area around them. (Cold sores develop only on and around your lips).
1. Consult a physician or pharmacist before using this medicine if any of the following apply to you:
(1) Those who are under treatment by two different physicians. (There may be the chance that
both physicians prescribe the same or similar medications.)
(2) Those who are or may be pregnant. (A pregnant woman must be careful about taking medicine.
lf you are or may be pregnant, consult your doctor and follow her instructions).
(3) A person who is breast-feeding. (Studies have shown that mothers who have taken this
medicine have it transfer to their breast milk.)
(4) Patients or their family members who have a tendency for allergies. (A person with an allergic
constitution may develop allergic symptoms by using this product.)
(5) Those who have allergic reactions to medicines. (lf you have had an allergic reaction to a
medicine in the past, this product could cause an allergic reaction as well.)
(6) Those who have developed severely moist skin or skin sores. (Consult a physician if your
symptoms are severe.)
(7) Those who have atopic dermatitis. (This product could worsen the atopic dermatitis. If you have
atopic dermatitis, consult a medical specialist.)
2. If any of the following applies to you, stop using this medicine immediately and consult a physician
or pharmacist with this insert: (1) When the following symptoms occur after using this medicine:
rash, redness, swelling, itching, skin eruption, irritation, pain, dry skin,
burning sensation, or flaking of the skin similar to dandruff
(The above-mentioned symptoms could be a result of allergic reaction to, or overt expression of
the pharmacological quality of, this product. The symptoms may worsen if you continue using.)
(2) When the symptoms do not improve after using this medicine after 7 days. (When the symptoms
have not subsided after using this product after 7 days, you should consider the symptoms are either severe or caused by another disease. Consult a physician or pharmacist as soon as possible).
Storage and Pharmaceutical Precautions:
(1) Make sure the cap is always on tight and store the medicine in a dry and cool place away from
direct sunlight at temperatures below 30° Celsius (86 degrees Fahrenheit).
(2) Keep out of the reach of children. (3) Wash your hands thoroughly before and after use. (4) Don't put this product into another container. (It can become ineffective or change in its quality.) (5) Don't use this product after the expiration date. Don't use this product more than 6 months
after it has been opened, even if it is before the expiration date (to ensure product quality).
制作 (社)神奈川県薬剤師会
Product name: S.TAC EVE FINE<
tablet> (エスタックイブファイン< 錠 剤>)
※Please read the following directions carefully before using this product.
Product summary
Relief of many symptoms of a cold: fever, chills, a cough, sputum, a sore throat, mucus, nasal
congestion, sneezing, a headache, joint pain, and muscle pain.
[Ingredients and Actions]
The following ingredients are included in 9 tablets (the one-day dosage for an adult):
Active Ingredient
Reduces fever, and helps relieve: headaches, sore
throats, and pain.
Ambroxol Hydrochloride
Helps fight the cause of a persistent cough.
Helps relieve a runny nose.
Iodide Dihydrocodeine Phosphate
Controls the cough, acting on its root cause.
dl-Methylephedrine
Widens bronchi (easing respiration), and helps suppress
Chlorpheniramine Maleate
Relieves sneezing, a runny nose, and congestion.
Anhydrous caffeine
Helps relieve headaches.
Ascorbic acid (vitamin C)
For the replenishment of this vitamin, which is easily
Thiamine nitrate
lacking when you have a cold.
(vitamin B1 nitrates)
[Dosage and Usage]
Take the tablets 3 times a day within 30 minutes after meals.
Adults (15 years old or older)
Under 15 years old
Don't take this medicine.
〈Cautions on Dosage and Usage〉
• Please follow exactly the defined dosage and usage directions.
Precautions for Use
Please read the following precautions carefully before using this product.
What not to do
(Failure to observe these warnings may lead to aggravation of the symptoms and increase the risk of
adverse reactions). 1. The following persons should not take this medicine:
(1) Those who have had an allergic reaction to this medicine in the past. (2) Those who have had an asthma attack after taking this medicine or other cold medicines such
as fever-reducers or painkillers.
(3) Those who are under 15 years old must not take this medicine.
2. When taking this medicine, be sure not to take any of the following medicines:
Other cold medicines, fever reducers, sedatives, cough suppressants, or any oral medicines that
contain an antihistamine, e.g. oral medicines for a runny nose, motion sickness pills, or allergy medications.
3. Please don't operate a vehicle or machinery after taking this medicine.
(This medicine may make you sleepy or affect your vision.)
4. Women who are breast-feeding should not take this medicine. If you take this medicine, please
stop breast-feeding your child.
5. Please refrain from drinking alcoholic beverages when taking this medicine. 6. Please don't take this medicine for more than 5 days.
1. Consult a physician or pharmacist before using this medicine if any of the following apply to you:
(1) You are currently under treatment by a physician or dentist (2) You are or may be pregnant. (3) You are an elderly person. (4) Those who are prone to allergies or have a family history of allergies. (5) You are someone who has had allergic reactions to medicines in the past. (6) People with the following symptoms: high fever, or have difficulty urinating. (7) Those who have been diagnosed with any of the following: hypertension, cardiac disease,
diabetes, glaucoma, liver disease, kidney diseases, inflammation of the thyroid gland, lupus, or Sharp syndrome.
(8) Those who have had the following diseases:
A gastroduodenal ulcer, idiopathic ulcerative colitis, or Crohn's disease.
2. lf you experience any of the following, stop using this medicine immediately and consult a physician
or pharmacist with this insert: (1) When the following symptoms appear after taking this product.
Rash, redness, or itching
Digestive system
Nausea, vomiting, poor appetite, stomachache, upset stomach, gas, heartburn, or abdominal pain
Blurred vision, ringing in the ears, difficulty in urinating,
edema, or numbness in your body
The following serious symptoms rarely may occur. If you have these symptoms, consult a physician immediately.
Symptom manifestation(s)
Face becomes pale, hands and feet become cold, you break
Shock (anaphylaxis)
out in a cold sweat, you break out in a rash, experience
edema, or breathing difficulty right after taking this medicine.
Stevens-Johnson syndrome
Intense manifestations include: rash, redness of the skin,
Toxic epidermal necrolysis
the appearance of burn-like blisters on the skin, mouth, or
(Lyell's syndrome)
around the eyes.
Chronic tiredness or jaundice (yellowing of the skin or eyes).
A decrease in urinary volume, swe1ling of the body, general
Kidney dysfunction
malaise, vomiting, blood in the urine, or albumin in the
urine. Include: an intense headache, fever, nausea / vomiting appear. ( As for these symptoms, they have often been
Aseptic meningitis
reported in patients treated for lupus or mixed connective tissue disease). Include: loss of breath, breathing difficulty, or a dry cough.
(If these symptoms, similar to those found in a cold, worsen
Interstitial pneumonia
please stop taking this medicine and consult your doctor as
soon as possible.)
(2) When your symptoms have not improved even if you have taken the medicine 5-6 times.
(In particular, if your fever continues for more than three days.)
3. The following symptoms may occasionally occur. If these symptoms continue or increase,
discontinue this medicine and consult a physician or pharmacist:
Constipation, loose stool, or dry mouth.
Storage and Pharmaceutical Precautions:
(1) Make sure the cap is always on tight and store the medicine in a dry and cool place away from
(2) Keep out of the reach of children. (3) Don't put this product into another container. (It can become ineffective or its quality may
(4) Don't touch this medicine with wet hands. The sugar coating may become uneven and its color
(5) The stuffing in the bottle is intended to prevent tablet damage during transport. Please remove
it after opening.
(6) Don't use this product after the expiration date. Don't use this product more than 6 months
after it has been opened, even if it is before the expiration date.
発行 (社)神奈川県薬剤師会
Product name: S.TAC EVE FINE<
granule> (エスタックイブファイン<顆粒>)
※Please read the following directions carefully before using this product.
Product summary
Relief of many symptoms of a cold: fever, chills, a cough, sputum, a sore throat, mucus, nasal congestion, sneezing, headache, joint pain, and muscle pain.
[Ingredients and Actions]
The following ingredients are included in one package (1.3g):
Active Ingredient
Reduces fever, and helps relieve: headaches, sore
throats, and pain.
Ambroxol Hydrochloride
Helps fight the cause of a persistent cough.
Isopropamide Iodide
Helps relieve a runny nose.
Dihydrocodeine Phosphate
Controls the cough, acting on its root cause.
dl-Methylephedrine
Widens bronchi (easing respiration) and helps
suppress a cough.
Chlorpheniramine Maleate
Relieves sneezing, a runny nose, and congestion.
Anhydrous caffeine
Helps relieve headaches.
Ascorbic acid (vitamin C)
For the replenishment of this vitamin, which is easily
Thiamine nitrate
lacking when you have a cold.
(vitamin B1 nitrates)
[Dosage and Usage]
Take a package 3 times a day within 30 minutes after meals.
Adults (15 years old or older)
Under 15 years old
Don't take this medicine.
〈Cautions on Dosage and Usage〉
• Please follow exactly the defined dosage and usage directions.
Precautions for Use
Please read the following precautions carefully before using this product.
What not to do
(Failure to observe these warnings may lead to aggravation of the symptoms and increase the risk of
adverse reactions). 1. The following persons should not take this medicine:
(1) Those who have had an allergic reaction to this medicine in the past. (2) Those who have had an asthma attack after taking this medicine or other cold medicines such
as fever-reducers or painkillers.
(3) Those who are under 15 years old must not take this medicine.
2. When taking this medicine, be sure not to take any of the following medicines:
Other cold medicines, fever reducers, sedatives, cough suppressants, or any oral medicines that
contain an antihistamine, e.g. oral medicines for a runny nose, motion sickness pills, or allergy medications.
3. Please don't operate a vehicle or machinery after taking this medicine.
(This medicine may make you sleepy or affect your vision.)
4. Women who are breast-feeding should not take this medicine. If you take this medicine, please
stop breast-feeding your child.
5. Please refrain from drinking alcoholic beverages when taking this medicine. 6. Please don't take this medicine for more than 5 days.
1. Consult a physician or pharmacist before using this medicine if any of the following apply to you:
(1) You are currently under treatment by a physician or dentist (2) You are or may be pregnant. (3) You are an elderly person. (4) You are prone to allergies or have a family history of allergies. (5) You are someone who has had allergic reactions to medicines in the past. (6) People with the following symptoms: high fever, or have difficulty urinating. (7) Those who have been diagnosed with any of the following: hypertension, cardiac disease,
diabetes, glaucoma, liver disease, kidney diseases, inflammation of the thyroid gland, lupus, or Sharp syndrome.
(8) Those who have had the following diseases:
A gastroduodenal ulcer, idiopathic ulcerative colitis, or Crohn's disease.
2. lf you experience any of the following, stop using this medicine immediately and consult a physician
or pharmacist with this insert: (1) When the following symptoms appear after taking this product.
Rash, redness, or itching
Digestive system
Nausea, vomiting, poor appetite, stomachache, upset stomach, gas, heartburn, or abdominal pain
Blurred vision, ringing in the ears, difficulty in urinating,
edema, or numbness in your body
The following serious symptoms rarely may occur. If you have these symptoms, consult a physician immediately.
Symptom manifestation(s)
Face becomes pale, hands and feet become cold, you break
out in a cold sweat, you break out in a rash, experience
edema, or breathing difficulty right after taking this medicine.
Stevens-Johnson syndrome
Intense manifestations include: rash, redness of the skin,
Toxic epidermal necrolysis
the appearance of burn-like blisters on the skin, mouth, or
(Lyell's syndrome)
around the eyes.
Chronic tiredness or jaundice (yellowing of the skin or eyes).
A decrease in urinary volume, swe1ling of the body, general
Kidney dysfunction
malaise, vomiting, blood in the urine, or albumin in the
urine. Include: an intense headache, fever, nausea / vomiting appear. ( As for these symptoms, they have often been
Aseptic meningitis
reported in patients treated for lupus or mixed connective tissue disease). Include: loss of breath, breathing difficulty, or a dry cough.
(If these symptoms, similar to those found in a cold, worsen
Interstitial pneumonia
please stop taking this medicine and consult your doctor as
soon as possible.)
(2) When your symptoms have not improved even if you have taken the medicine 5-6 times.
(In particular, if your fever continues for more than three days.)
3. The following symptoms may occasionally occur. If these symptoms continue or increase,
discontinue this medicine and consult a physician or pharmacist:
Constipation, loose stool, or dry mouth.
Storage and Pharmaceutical Precautions:
(1) Make sure the cap is always on tight and store the medicine in a dry and cool place away from
direct sunlight.
(2) Keep out of the reach of children. (3) Don't put this product into another container. (It can become ineffective or its quality may
(4) Don't use this product after the expiration date.
発行 (社)神奈川県薬剤師会
じょう ざ い
Product name: PABRON ACE AX< Tablet >(パブロンエースAX 錠
<錠剤
>)
※Please read the following directions carefully before using this product.
Product summary
Relief of many symptoms of a cold: fever, chills, a cough, sputum, a sore throat, mucus, nasal congestion, sneezing, a headache, joint pain, and muscle pain.
[Ingredients and Actions]
The following ingredients are included in 3 tablets (a single dose).
Active Ingredient
Reduces fever, and helps relieve: headaches, sore
throats, and pain.
Ambroxol Hydrochloride
Helps fight the cause of a persistent cough.
Dihydrocodeine Phosphate
Controls the cough, acting on its root cause.
dl-Methylephedrine
Widens bronchi (easing respiration) and helps suppress
Chlorpheniramine Maleate
Relieves sneezing, a runny nose, and congestion.
Anhydrous Caffeine
Helps relieve headaches.
Ascorbic Acid (vitamin C)
Thiamine Nitrate
For the replenishment of this vitamin, which is easily
(vitamin B1 Nitrate)
lacking when you have a cold.
Riboflavin (vitamin B2)
[Dosage and Usage]
Take the tablets 3 times a day within 30 minutes after meals.
Adults (15 years old or older)
Under 15 years old
Don't take this medicine.
〈Cautions on Dosage and Usage〉
• Please follow exactly the defined dosage and usage directions.
• How to remove a tablet from the packaging:
To remove a tablet from the PTP sheet, push the tablet gently through the aluminum foil. (Be
careful not to ingest any part of the PTP sheet, as doing so could lead to unexpected accidents, such as a piece of aluminum getting stuck in your throat.)
Precautions for Use
Please read the following precautions carefully before using this product.
What not to do
(Failure to observe these warnings may lead to aggravation of the symptoms and increase the risk of
adverse reactions.) 1. The following persons should not take this medicine:
(1) Those who have had an allergic reaction to this medicine. (2) Those who have had an asthma attack after taking this medicine or other cold medicines such
as: fever-reducers or painkillers.
(3) Those who are under 15 years old must not use this medicine.
2. When taking this medicine, be sure not to take any of the following medicines:
Other cold medicines, fever reducers, sedatives, cough suppressants, or any oral medicines that
contain an antihistamine, e.g. oral medicines for a runny nose, motion sickness pills, or allergy medications.
3. Please don't operate a vehicle or machinery after taking this medicine.
(This medicine may make you sleepy or affect your vision.)
4. Women who are breast-feeding should not take this medicine. If you take this medicine, please
stop breast-feeding your child.
5. Please refrain from drinking alcoholic beverages when taking this medicine. 6. Please don't take this medicine for more than 5 days.
1. Consult a physician or pharmacist before using this medicine if any of the following apply to you:
(1) You are currently under treatment by a physician or dentist (2) You are or may be pregnant. (3) You are an elderly person. (4) Those who are prone to allergies or have a family history of allergies. (5) You are someone who has had allergic reactions to medicines in the past. (6) People with the following symptoms: high fever, or have difficulty urinating. (7) Those who have been diagnosed with any of the following: hypertension, cardiac disease,
diabetes, glaucoma, liver disease, kidney diseases, inflammation of the thyroid gland, lupus, or Sharp syndrome.
(8) Those who have had the following diseases:
A gastroduodenal ulcer, idiopathic ulcerative colitis, or Crohn's disease.
2. lf you experience any of the following, stop using this medicine immediately and consult a physician
or pharmacist with this insert: (1) When the following symptoms appear after taking this product.
Rash, redness, or itching
Digestive system
Nausea, vomiting, poor appetite, stomachache, upset stomach, gas, heartburn, or abdominal pain
Dizziness, or numbness in your body
Blurred vision, ringing in the ears, difficulty in urinating,
The following serious symptoms rarely may occur. If you have these symptoms, consult a physician immediately.
Symptom manifestation(s)
Face becomes pale, hands and feet become cold, you break
out in a cold sweat, you break out in a rash, experience
edema, or breathing difficulty right after taking this medicine.
Stevens-Johnson syndrome
Intense manifestations include: rash, redness of the skin,
Toxic epidermal necrolysis
the appearance of burn-like blisters on the skin, mouth, or
(Lyell's syndrome)
around the eyes.
Chronic tiredness or jaundice (yellowing of the skin or eyes).
A decrease in urinary volume, swe1ling of the body, general
Kidney dysfunction
malaise, vomiting, blood in the urine, or albumin in the
urine. Include: an intense headache, fever, nausea / vomiting appear. ( As for these symptoms, they have often been
Aseptic meningitis
reported in patients treated for lupus or mixed connective tissue disease). Include: loss of breath, breathing difficulty, or a dry cough.
(If these symptoms, similar to those found in a cold, worsen
Interstitial pneumonia
please stop taking this medicine and consult your doctor as
soon as possible.)
(2) When your symptoms have not improved even if you have taken the medicine 5-6 times. (In
particular, if your fever continues for more than three days.)
3. The following symptoms may occasionally occur. If these symptoms continue or increase,
discontinue this medicine and consult a physician or pharmacist:
Constipation, loose stool, or dry mouth.
4. When taking this medicine, your urine may turn yellowish in color. This is due to the vitamin B2
content of this medicine.
Storage and Pharmaceutical Precautions:
(1) Make sure the cap is always on tight and store the medicine in a dry and cool place away from
(2) Keep out of the reach of children. (3) Don't put this product into another container. (It can become ineffective or change in its
(4) Don't use this product after the expiration date. Don't use this product more than 6 months
after it has been opened, even if it is before the expiration date (to ensure product quality).
発行 (社)神奈川県薬剤師会
Product name: PABRON ACE AX< Particle > (パブロンエースAX
<微粒
>)
※Please read the following directions carefully before using this product.
Product summary
Relief of many symptoms of a cold: fever, chills, a cough, sputum, a sore throat, mucus, nasal
congestion, sneezing, a headache, joint pain, and muscle pain.
[Ingredients and Actions]
The following ingredients are included in 1 package(1.3g).
Active Ingredient
Reduces fever, and helps relieve: headaches, sore
throats, and pain.
Ambroxol Hydrochloride
Helps fight the cause of a persistent cough.
Dihydrocodeine Phosphate
Controls the cough, acting on its root cause.
dl-Methylephedrine
Widens bronchi (easing respiration) and helps suppress
Chlorpheniramine Maleate
Relieves sneezing, a runny nose, and congestion.
Anhydrous Caffeine
Helps relieve headaches.
Ascorbic Acid (vitamin C)
Thiamine Nitrate
For the replenishment of this vitamin, which is easily
(vitamin B1 Nitrate)
lacking when you have a cold.
Riboflavin (vitamin B2)
[Dosage and Usage]
Take the 1 package 3 times a day within 30 minutes after meals.
Adults (15 years old or older)
Under 15 years old
Don't take this medicine.
〈Cautions on Dosage and Usage〉
• Please follow exactly the defined dosage and usage directions.
Precautions for Use
Please read the following precautions carefully before using this product.
What not to do
(Failure to observe these warnings may lead to aggravation of the symptoms and increase the risk of
adverse reactions.) 1. The following persons should not take this medicine:
(1) Those who have had an allergic reaction to this medicine. (2) Those who have had an asthma attack after taking this medicine or other cold medicines such
as: fever-reducers or painkillers.
(3) Those who are under 15 years old must not use this medicine.
2. When taking this medicine, be sure not to take any of the following medicines:
Other cold medicines, fever reducers, sedatives, cough suppressants, or any oral medicines that
contain an antihistamine, e.g. oral medicines for a runny nose, motion sickness pills, or allergy medications.
3. Please don't operate a vehicle or machinery after taking this medicine.
(This medicine may make you sleepy or affect your vision.)
4. Women who are breast-feeding should not take this medicine. If you take this medicine, please
stop breast-feeding your child.
5. Please refrain from drinking alcoholic beverages when taking this medicine. 6. Please don't take this medicine for more than 5 days.
1. Consult a physician or pharmacist before using this medicine if any of the following apply to you:
(1) You are currently under treatment by a physician or dentist (2) You are or may be pregnant. (3) You are an elderly person. (4) Those who are prone to allergies or have a family history of allergies. (5) You are someone who has had allergic reactions to medicines in the past. (6) People with the following symptoms: high fever, or have difficulty urinating. (7) Those who have been diagnosed with any of the following: hypertension, cardiac disease,
diabetes, glaucoma, liver disease, kidney diseases, inflammation of the thyroid gland, lupus, or Sharp syndrome.
(8) Those who have had the following diseases:
A gastroduodenal ulcer, idiopathic ulcerative colitis, or Crohn's disease.
2. lf you experience any of the following, stop using this medicine immediately and consult a physician
or pharmacist with this insert: (1) When the following symptoms appear after using this medicine, stop taking the medicine and
consult a physician or pharmacist:
Rash, redness, or itching
Digestive system
Nausea, vomiting, poor appetite, stomachache, upset stomach, gas, heartburn, or abdominal pain
Dizziness, or numbness in your body
Blurred vision, ringing in the ears, difficulty in urinating,
The following serious symptoms rarely may occur. If you have these symptoms, consult a physician immediately.
Symptom manifestation(s)
Face becomes pale, hands and feet become cold, you break
out in a cold sweat, you break out in a rash, experience
edema, or breathing difficulty right after taking this medicine.
Stevens-Johnson syndrome
Intense manifestations include: rash, redness of the skin,
Toxic epidermal necrolysis
the appearance of burn-like blisters on the skin, mouth, or
(Lyell's syndrome)
around the eyes.
Chronic tiredness or jaundice (yellowing of the skin or eyes).
A decrease in urinary volume, swe1ling of the body, general
Kidney dysfunction
malaise, vomiting, blood in the urine, or albumin in the
urine. Include: an intense headache, fever, nausea / vomiting appear. ( As for these symptoms, they have often been
Aseptic meningitis
reported in patients treated for lupus or mixed connective tissue disease). Include: loss of breath, breathing difficulty, or a dry cough.
(If these symptoms, similar to those found in a cold, worsen
Interstitial pneumonia
please stop taking this medicine and consult your doctor as
soon as possible.)
(2) When your symptoms have not improved even if you have taken the medicine 5-6 times.
(In particular, if your fever continues for more than three days.)
3. The following symptoms may occasionally occur. If these symptoms continue or increase,
discontinue this medicine and consult a physician or pharmacist:
Constipation, loose stool, or dry mouth.
4. When taking this medicine, your urine may turn yellowish in color.
This is due to the vitamin B2 content of this medicine.
Storage and Pharmaceutical Precautions:
(1) Make sure the cap is always on tight and store the medicine in a dry and cool place away from
direct sunlight.
(2) Keep out of the reach of children. (3) Don't put this product into another container. (It can become ineffective or change in its
(4) Don't use this product after the expiration date.
発行 (社)神奈川県薬剤師会
Product name: GASTER10<Powder> (ガスター
10 <散>)
※Please read the following directions carefully before using this product.
Product summary
Relief of symptoms such as stomachache, heartburn, and nausea. 〈Cautions on Usage〉
Don't use this medicine for symptoms other than those indicated above.
[Ingredient and Action]
The following ingredient is included in one package ( 0.5g.)
Ingredient Amount
This medicine helps control gastric acid.
[Dosage and Usage]
• Please take this medicine with cold or lukewarm water as follows:
Number of doses in a day
Adults (15 years old or older and less than 80 years old)
Children (under 15 years old)
Please don't take this medicine.
Elderly people (age 80 and over )
This medicine should not be taken on a fixed schedule, but only when symptoms appear.
You can take this medicine regardless of meal time (i.e. both before and after or meals is OK). When taking this medicine 2 times a day, you should wait 8 hours before taking the medicine a
second time as its effect lasts 8 hours.
• Please follow exactly the defined dosage and usage.
• If your symptoms have not improved after 8 hours, take l more package.
• If your symptoms have improved, stop taking this medicine.
• If your symptoms have not improved after taking this medicine for 3 days, stop taking it and
consult a physician or a pharmacist.
• Please avoid continuous use for over 2 weeks.
〈Cautions on Dosage and Usage〉
(1) Please refrain from drinking alcohol when taking this medicine.
(In general, never take medicine with any alcoholic beverages).
Precautions for Use
Please read the following precautions carefully before using this product.
What not to do
(Failure to observe these warnings may lead to aggravation of the symptoms and increase the risk of
adverse reactions.) 1. The following persons should not use this medicine:
(1) Those who have had an allergic reaction to this medicine (e.g. rash, redness, itching, swelling of
the throat, eyelids, or lips) or medicines containing H2 blockers such as Famotidine in the past.
(2) Those who received treatment for the following diseases:
Blood disease, kidney or liver disease, heart disease, gastro-duodenal disease, and immunological diseases such as asthma, rheumatism, or have been prescribed steroids,
antibiotics, anticancer agents, or azole-based antifungals. (Decreases in leukocytes, WBC, or
blood platelets may occur). In those patients with kidney or liver disease, excretion of the medicine is late and they might
have a strong reaction to it.
In those patients with heart disease such as myocardial infarction, heart valve disease, or myocardial disease (cardiomyopathy), pulse disorder with ECG (electrocardiographic)
abnormalities may occur. For those who are receiving treatment for gastro-duodenal disease, Famotidine, or similar
medicines, may probably be prescribed. However, avoid concomitant use of similar medicines.
The absorption of azole-based antifungals will decrease the effectiveness of this medicine.
(3) Those who have been notified by a physician about having abnormal blood (dysemia); few red
blood corpuscles (anemia), few platelets, or a low number of leukocytes.(This medicine may cause the recurrence of the blood abnormality).
(4) Children (under 15 years of age) and those who are elderly (80 years and over). (5) Those who are or may be pregnant and women who are breast-feeding.
2. Don't take the following medicines while taking this medicine:
other gastrointestinal agents.
1. Consult a physician or pharmacist before using this medicine if any of the following apply to you:
(1) You are currently under treatment by a physician or dentist (2) Those who are prone to allergies or have a family history of allergies. (3) You are someone who has had allergic reactions to medicines in the past. (4) Those who are elderly (65 years and over).
(Elderly persons often have reduced physiological functions).
(5) Those who have the following symptoms: sore throat, coughing, or a high fever.
(In those patients who have such disorders, a serious infection is possible and blood
abnormality such as decreased hemocytes may be observed. If the person had the disorder
before taking the medicine, it may worsen the disorder, leading to delayed detection of adverse reactions to the medication).
Weight loss of unknown cause, persistent abdominal pain.
(The symptoms may be caused by another disease).
2. When the following symptoms occur after using this medicine, stop taking the medicine and consult
a physician or pharmacist with this insert: (1) When the following symptoms occur after using this medicine:
Area of the body
rash, redness, itching, or swelling
Circulatory system
pulse irregularity
loss of consciousness or convulsions
you may feel bad, or a loss of energy, break out in a fever, or your body's condition may worsen in general.
The following serious symptoms rarely may occur. If you have these symptoms, consult a
physician immediately.
Symptom manifestations
Face becomes pale, hands and feet become cold, you break
out in a cold sweat, you break out in a rash, experience
edema, or breathing difficulty right after taking this
Stevens-Johnson syndrome
Intense manifestations include: rash, redness of the skin,
the appearance of burn-like blisters on the skin, mouth, or
Toxic epidermal necrolysis
(Lyell's syndrome)
around the eyes.
Rhabdomyolysis(A disease in Constitutional skeletal muscle aches and become stiff. which skeletal muscle melts.)
Urinary color becomes reddish brown.
Chronic tiredness or jaundice (yellowing of the skin or eyes).
A decrease in urinary volume, swe1ling of the body, general
Kidney dysfunction
malaise, vomiting, blood in the urine, or albumin in the
urine. Manifestations such as sore throat, fever, general malaise,
whitening of complexion and the backs of the eyelids
bleeding tendency (bleeding from the gums, nose, etc,) or
blue bruises appear.
(2) When taking the medicine more than in the directed dosage.
3. The following symptoms may occasionally occur. Be sure to stop taking this medicine and consult a
physician or pharmacist.
Constipation, loose stool, diarrhea, or oral dehydration.
Storage and Pharmaceutical Precautions:
(1) Make sure the cap is always on tight and store the medicine in a dry and cool place away from
direct sunlight.
(2) Keep out of the reach of children. (3) Don't put this product into another container. (It can become ineffective or change in its
(4) Don't use this product after the expiration date.
発行 (社)神奈川県薬剤師会
Product name: GASTER10 <Tablet> (ガスター
10 < 錠 >)
※Please read the following directions carefully before using this product.
Product summary
Relief of symptoms such as stomachache, heartburn, and nausea. 〈Cautions on Usage〉
Don't use this medicine for symptoms other than those indicated above.
[Ingredient and Action]
The following ingredient is included in one tablet.
Ingredient Amount
This medicine helps control gastric acid.
[Dosage and Usage]
• Please take this medicine with cold or lukewarm water as follows:
Number of doses in a day
Adults (15 years old or older and less than 80 years old)
Children (under 15 years old)
Please don't take this medicine.
Elderly people (age 80 and over )
This medicine should not be taken on a fixed schedule, but only when symptoms appear.
You can take this medicine regardless of meal time (i.e. both before and after or meals is OK).
When taking this medicine 2 times a day, you should wait 8 hours before taking the medicine a second time as its effect lasts 8 hours.
• Please follow exactly the defined dosage and usage.
• If your symptoms have not improved after 8 hours, take l more tablet.
• If your symptoms have improved, stop taking this medicine.
• If your symptoms have not improved after taking this medicine for 3 days, stop taking it and
consult a physician or a pharmacist.
• Please avoid continuous use for over 2 weeks.
〈Cautions on Dosage and Usage〉
(1) Please refrain from drinking alcohol when taking this medicine.
(In general, never take medicine with any alcoholic beverages).
(2) How to remove the tablets from the packaging
To remove a tablet from the PTP sheet, push the tablet gently through the aluminum foil. (Be
careful not to ingest any part of the PTP sheet, as doing so could lead to unexpected accidents,
such as a piece of aluminum getting stuck in your throat.)
Precautions for Use
Please read the following precautions carefully before using this product.
What not to do
(Failure to observe these warnings may lead to aggravation of the symptoms and increase the risk of
adverse reactions.) 1. The following persons should not use this medicine:
(1) Those who have had an allergic reaction to this medicine (e.g. rash, redness, itching, swelling of
the throat, eyelids, or lips) or medicines containing H2 blockers such as Famotidine in the past.
(2) Those who received treatment for the following diseases:
Blood disease, kidney or liver disease, heart disease, gastro-duodenal disease, and immunological diseases such as asthma, rheumatism, or have been prescribed steroids,
antibiotics, anticancer agents, or azole-based antifungals. (Decreases in leukocytes, WBC, or
blood platelets may occur). In those patients with kidney or liver disease, excretion of the medicine is late and they might
have a strong reaction to it.
In those patients with heart disease such as myocardial infarction, heart valve disease, or myocardial disease (cardiomyopathy), pulse disorder with ECG (electrocardiographic)
abnormalities may occur. For those who are receiving treatment for gastro-duodenal disease, Famotidine, or similar
medicines, may probably be prescribed. However, avoid concomitant use of similar medicines.
The absorption of azole-based antifungals will decrease the effectiveness of this medicine.
(3) Those who have been notified by a physician about having abnormal blood (dysemia); few red
blood corpuscles (anemia), few platelets, or a low number of leukocytes.(This medicine may cause the recurrence of the blood abnormality).
(4) Children (under 15 years of age) and those who are elderly (80 years and over). (5) Those who are or may be pregnant and women who are breast-feeding.
2. Don't take the following medicines while taking this medicine:
Other gastrointestinal agents.
1. Consult a physician or pharmacist before using this medicine if any of the following apply to you:
(1) You are currently under treatment by a physician or dentist (2) Those who are prone to allergies or have a family history of allergies. (3) You are someone who has had allergic reactions to medicines in the past. (4) Those who are elderly (65 years and over).
(Elderly persons often have reduced physiological functions).
(5) Those who have the following symptoms: sore throat, coughing, or a high fever.
(In those patients who have such disorders, a serious infection is possible and blood
abnormality such as decreased hemocytes may be observed. If the person had the disorder
before taking the medicine, it may worsen the disorder, leading to delayed detection of adverse reactions to the medication).
Weight loss of unknown cause, persistent abdominal pain.
(The symptoms may be caused by another disease).
2. When the following symptoms occur after using this medicine, stop taking the medicine and consult
a physician or pharmacist with this insert: (1) When the following symptoms occur after using this medicine:
Area of the body
rash, redness, itching, or swelling
Circulatory system
pulse irregularity
loss of consciousness or convulsions
you may feel bad, or a loss of energy, break out in a fever, or your body's condition may worsen in general.
The following serious symptoms rarely may occur. If you have these symptoms, consult a
physician immediately.
Symptom manifestations
Face becomes pale, hands and feet become cold, you break
out in a cold sweat, you break out in a rash, experience
edema, or breathing difficulty right after taking this
Stevens-Johnson syndrome
Intense manifestations include: rash, redness of the skin,
the appearance of burn-like blisters on the skin, mouth, or
Toxic epidermal necrolysis
(Lyell's syndrome)
around the eyes.
Rhabdomyolysis(A disease in Constitutional skeletal muscle aches and become stiff. which skeletal muscle melts.)
Urinary color becomes reddish brown.
Chronic tiredness or jaundice (yellowing of the skin or eyes).
A decrease in urinary volume, swe1ling of the body, general
Kidney dysfunction
malaise, vomiting, blood in the urine, or albumin in the
urine. Manifestations such as sore throat, fever, general malaise,
whitening of complexion and the backs of the eyelids
bleeding tendency (bleeding from the gums, nose, etc,) or
blue bruises appear.
(2) When taking the medicine more than in the directed dosage.
3. The following symptoms may occasionally occur. Be sure to stop taking this medicine and consult a
physician or pharmacist.
Constipation, loose stool, diarrhea, or oral dehydration.
Storage and Pharmaceutical Precautions:
(1) Make sure the cap is always on tight and store the medicine in a dry and cool place away from
direct sunlight.
(2) Keep out of the reach of children. (3) Don't put this product into another container. (It can become ineffective or change in its
(4) Don't use this product after the expiration date.
発行 (社)神奈川県薬剤師会
Product name: GASTER10 S Tablet
(ガスター
10S錠 )
※Please read the following directions carefully before using this product.
Product summary
Relief of symptoms such as stomachache, heartburn, and nausea. 〈Cautions on Usage〉
• Don't use this medicine for symptoms other than those indicated above.
[Ingredient and Action]
The following ingredient is included in one tablet.
This medicine helps control gastric acid.
[Dosage and Usage]
Please take this medicine with cold or lukewarm water as follows:
Number of doses in a day
Adults (15 years old or older and less than 80 years old)
Children (under 15 years old)
Please don't take this medicine.
Elderly people (age 80 and over )
This medicine should not be taken on a fixed schedule, but only when symptoms appear.
You can take this medicine regardless of meal time (i.e. both before and after or meals is OK).
When taking this medicine 2 times a day, you should wait 8 hours before taking the medicine a second time as its effect lasts 8 hours.
• Please follow exactly the defined dosage and usage.
• If your symptoms have not improved after 8 hours, take l more tablet.
• If your symptoms have improved, stop taking this medicine.
• If your symptoms have not improved after taking this medicine for 3 days, stop taking it and
consult a physician or a pharmacist.
• Please avoid continuous use for over 2 weeks.
〈Cautions on Dosage and Usage〉
(1) As this medicine easily melts in the mouth, but is not absorbed by the mucosa of the stomach,
please take it with water. As it is a normal tablet, you can take it with either hot or cold water. The effect will not change.
(2) Please refrain from drinking alcohol when taking this medicine.
(In general, never take medicine with any alcoholic beverages).
(3) How to remove the tablets from the packaging
As shown, to remove a tablet from the PTP sheet, push the tablet gently through the aluminum foil. (Be careful not to ingest any part of the PTP sheet, as doing so could lead to unexpected accidents, such as a piece of aluminum getting stuck in your throat.)
Precautions for Use
Please read the following precautions carefully before using this product.
What not to do
(Failure to observe these warnings may lead to aggravation of the symptoms and increase the risk of
adverse reactions.) 1. The following persons should not use this medicine:
(1) Those who have had an allergic reaction to this medicine (e.g. rash, redness, itching, swelling of
the throat, eyelids, or lips) or medicines containing H2 blockers such as Famotidine in the past.
(2) Those who received treatment for the following diseases:
Blood disease, kidney or liver disease, heart disease, gastro-duodenal disease, and immunological diseases such as asthma, rheumatism, or have been prescribed steroids,
antibiotics, anticancer agents, or azole-based antifungals. (Decreases in leukocytes, WBC, or
blood platelets may occur). In those patients with kidney or liver disease, excretion of the medicine is late and they might
have a strong reaction to it.
In those patients with heart disease such as myocardial infarction, heart valve disease, or myocardial disease (cardiomyopathy), pulse disorder with ECG (electrocardiographic)
abnormalities may occur. For those who are receiving treatment for gastro-duodenal disease, Famotidine, or similar
medicines, may probably be prescribed. However, avoid concomitant use of similar medicines.
The absorption of azole-based antifungals will decrease the effectiveness of this medicine.
(3) Those who have been notified by a physician about having abnormal blood (dysemia); few red
blood corpuscles (anemia), few platelets, or a low number of leukocytes.(This medicine may cause the recurrence of the blood abnormality).
(4) Those who have phenylketonuria. (This medicine includes Aspartame (an L-phenylalanine
(5) Children (under 15 years of age) and those who are elderly (80 years and over). (6) Those who are or may be pregnant and women who are breast-feeding.
2. Don't take the following medicines while taking this medicine:
Other gastrointestinal agents.
1. Consult a physician or pharmacist before using this medicine if any of the following apply to you:
(1) You are currently under treatment by a physician or dentist (2) Those who are prone to allergies or have a family history of allergies. (3) You are someone who has had allergic reactions to medicines in the past. (4) Those who are elderly (65 years and over).
(Elderly persons often have reduced physiological functions).
(5) Those who have the following symptoms: sore throat, coughing, or a high fever.
(In those patients who have such disorders, a serious infection is possible and blood
abnormality such as decreased hemocytes may be observed. If the person had the disorder
before taking the medicine, it may worsen the disorder, leading to delayed detection of adverse reactions to the medication).
Weight loss of unknown cause, persistent abdominal pain.
(The symptoms may be caused by another disease).
2. When the following symptoms occur after using this medicine, stop taking the medicine and consult
a physician or pharmacist: (1) When the following symptoms occur after using this medicine.
Area of the body
Rash, redness, itching, or swelling
Circulatory system
Pulse irregularity
Loss of consciousness or convulsions
You may feel bad, or a loss of energy, break out in a fever, or your body's condition may worsen in general.
The following serious symptoms rarely may occur. If you have these symptoms, consult a
physician immediately.
Symptom manifestations
Face becomes pale, hands and feet become cold, you break
out in a cold sweat, you break out in a rash, experience
edema, or breathing difficulty right after taking this
Stevens-Johnson syndrome
Intense manifestations include: rash, redness of the skin,
the appearance of burn-like blisters on the skin, mouth, or
Toxic epidermal necrolysis
(Lyell's syndrome)
around the eyes.
Rhabdomyolysis(A disease in Constitutional skeletal muscle aches and become stiff. which skeletal muscle melts.) Urinary color becomes reddish brown. Liver disorder
Chronic tiredness or jaundice (yellowing of the skin or eyes).
A decrease in urinary volume, swe1ling of the body, general
Kidney dysfunction
malaise, vomiting, blood in the urine, or albumin in the
urine. Manifestations such as sore throat, fever, general malaise,
whitening of complexion and the backs of the eyelids
bleeding tendency (bleeding from the gums, nose, etc,) or
blue bruises appear.
(2) When taking the medicine more than in the directed dosage.
3. The following symptoms may occasionally occur. Be sure to stop taking this medicine and consult a
physician or pharmacist.
Constipation, loose stool, diarrhea, or oral dehydration.
Storage and Pharmaceutical Precautions:
(1) Make sure the cap is always on tight and store the medicine in a dry and cool place away from
direct sunlight.
(2) Keep out of the reach of children. (3) Don't put this product into another container. (It can become ineffective or change in its
(4) Don't use this product after the expiration date.
発行 (社)神奈川県薬剤師会
Product name: ZADITEN AL Rhinitis Capsule
(ザジテン
AL 鼻炎カプセル )
※Please read the following directions carefully before using this product.
Product summary
For the relief of allergic symptoms from pollen or house dust (indoor dust): sneezing, runny nose (excessive nasal drip), or nasal congestion.
[Ingredient and Action]
The following ingredient is included in one capsule.
Active Ingredient
Ketotifen fumarate (as ketotifen)
[Dosage and Usage]
Please take a single dose once after breakfast and again before bed.
Adults (15 years old or older)
Children (under 15 years old)
Do not take this medicine.
〈Cautions on Dosage and Usage〉
• Please follow exactly the defined dosage and usage directions.
• If the symptoms are still not relieved after taking the medicine for about one week, please consult
a doctor or a pharmacist. (The medicinal effect varies from person to person. Depending on the person, it may take two weeks for effects to show.)
• How to remove the capsules from the packaging
To remove a capsule from the PTP sheet, push the capsule gently through the aluminum foil. (Be careful not to ingest any part of the PTP sheet, as doing so could lead to unexpected
accidents, such as a piece of aluminum getting stuck in your throat.)
Precautions for Use
Please read the following precautions carefully before using this product.
What not to do
(Failure to observe these warnings may lead to aggravation of the symptoms and increase the risk of
adverse reactions.) 1. The following persons should not use this medicine:
(1) Those who have had an allergic reaction to this medicine in the past. (2) Children under the age of 15.
2. Don't take the following medicines while taking this medicine:
Medicine for other allergies (including medicines for skin disorders or oral medicines for rhinitis),including antihistamines (cold remedies, antitussives,motion sickness pills,or sedatives).
3. Please don't operate a vehicle or machinery after taking this medicine. (This medicine may make
you sleepy or affect your vision.)
4. A person who is breast-feeding. (Studies have shown that mothers who have taken this medicine
have it transfer to their breast milk.)
5. Please refrain from drinking alcoholic beverages when taking this medicine.
1. Consult a physician or pharmacist before using this medicine if any of the following apply to you:
(1) You are currently under treatment by a physician or dentist. (2) You are currently receiving treatment for allergies such as reductive sensitizing therapy. (3) You are or may be pregnant. (4) You are an elderly person. (5) You are prone to allergies or have a family history of allergies. (6) You don't not know clearly whether your symptoms are a manifestation due to an allergy or due
to some other cause.
(7) You have been diagnosed with any of the following: bronchitic asthma, atopic dermatitis, or
(8) You have had the following symptom:
Difficulty in urinating (dysuria).
2. When the following symptoms occur after using this medicine, stop taking the medicine and consult
a physician or pharmacist with this insert: (1) When the following symptoms appear after using this medicine.
rash/ redness, itching, edema
dizziness, malaise, a headache, distortion in your sense of taste
(dysgeusia), or numbness. nausea, vomiting, poor appetite, stomachache, gastric region
Digestive system
unpleasantness、abdominal pain, or stomatitis. palpitations, hot flashes, frequent urination, pain when urinating, bloody urine(hematuria), a feeling of residual urine,
abnormalities in menses(emmeniopathy), or an increase in weight.
(2) If the symptoms have not improved after using this medicine for about one week, consult a
doctor or a pharmacist.
3. The following serious symptoms rarely may occur. If you have these symptoms, consult a physician
Symptom manifestation(s)
Central-nervous symptoms
transient unconsciousness, convulsions, or excitement appear. fever, rash, general malaise, jaundice (yellowing of the skin and
Impaired liver function
the whites of your eyes), or high fever appear.
4. The following symptoms may occasionally occur. If these symptoms continue or increase,
discontinue this medicine and consult a physician or pharmacist.
Constipation, loose stool, diarrhea, oral dehydration, or drowsiness
5. In the case of the following, please consult a physician or a pharmacist.
If the symptoms persist after taking the medicine for more than 2 weeks.
Storage and Pharmaceutical Precautions:
(1) Make sure the cap is always on tight and store the medicine in a dry and cool place away from
direct sunlight.
(2) Keep out of the reach of children. (3) Don't put this product into another container. (It can become ineffective or change in its
(4) Don't use this product after the expiration date.
発行 (社)神奈川県薬剤師会
Product name: PABRON Rhinitis Capsule Z (パブロン鼻炎カプセル
Z)
※Please read the following directions carefully before using this product.
Product summary
For the relief of allergic symptoms from pollen or house dust (indoor dust): sneezing, runny nose (excessive nasal drip), or nasal congestion.
[Ingredients and Actions]
The following ingredient is included in one capsule.
Ketotifen Fumarate (as ketotifen)
1.38mg(1.0mg)
[Dosage and Usage]
Please take a single dose after breakfast and again before going to bed.
Adults (15 years old or older)
Under 15 years old
Don't take this medicine.
〈Cautions on Dosage and Usage〉
• Please follow exactly the defined dosage and usage directions.
• If the symptoms are still not relieved after taking the medicine for about one week, please consult
a doctor or a pharmacist. (The medicinal effect varies from person to person. Depending on the person, it may take two weeks for effects to show.)
• How to remove the capsules from the packaging
To remove a capsule from the PTP sheet, push the capsule gently through the aluminum foil.
(Be careful not to ingest any part of the PTP sheet, as doing so could lead to unexpected
accidents, such as a piece of aluminum getting stuck in your throat.)
Precautions for Use
Please read the following precautions carefully before using this product.
What not to do
(Failure to observe these warnings may lead to aggravation of the symptoms and increase the risk of
adverse reactions.) 1. The following persons should not take this medicine:
(1) Those who have had an allergic reaction to this medicine. (2) Those who are under 15 years old must not use this medicine.
2. Don't take the following medicines while taking this medicine:
Medicine for other allergies (including medicines for skin disorders or oral medicines for rhinitis),including antihistamines (cold remedies, antitussives,motion sickness pills,or sedatives).
3. Please don't operate a vehicle or machinery after taking this medicine.
(This medicine may make you sleepy or affect your vision.)
4. Women who are breast-feeding should not take this medicine. If you take this medicine, please
avoid breast-feeding.
(Studies have shown that mothers who have taken this medicine have it transfer to their
5. Please refrain from drinking alcoholic beverages when taking this medicine.
1. Consult a physician or pharmacist before using this medicine if any of the following apply to you:
(1) You are currently under treatment by a physician or dentist. (2) Those who receive treatment for allergies such as reductive sensitizing therapy. (3) You are or may be pregnant. (4) You are an elderly person. (5) Those who are prone to allergies or have a family history of allergies. (Patients susceptible to
allergies may develop allergic symptoms by using this product).
(6) You don't not know clearly whether your symptoms are a manifestation due to an allergy or due
to some other cause.
(7) Those who have been diagnosed with any of the following: asthma, atopic dermatitis, or
(8) Those who have had the following symptoms: difficulty urinating (dysuria).
2. lf you experience any of the following, stop using this medicine immediately and consult a physician
or pharmacist with this insert: (1) When the following symptoms appear after using this medicine.
rash/ redness, itching, or edema
dizziness, malaise, a headache, distortion in your sense of taste
(dysgeusia), or numbness. nausea, vomiting, poor appetite, stomachache, gastric region
Digestive system
unpleasantness、abdominal pain, or stomatitis. palpitations, hot flashes, frequent urination, pain when urinating, bloody urine(hematuria), a feeling of residual urine,
abnormalities in menses(emmeniopathy), or an increase in weight.
(2) If the symptoms have not improved after using this medicine for about one week, consult a
doctor or a pharmacist.
3. The following serious symptoms rarely may occur. If you have these symptoms, consult a physician
Symptom manifestation(s)
Central-nervous symptoms
transient unconsciousness, convulsions, or excitement appear.
Impaired liver function
fever, rash, general malaise, jaundice (yellowing of the skin and the whites of your eyes), or high fever appear.
4. The following symptoms may occasionally occur. Discontinue this medicine and consult a physician
Oral dehydration, constipation, diarrhea, sleepiness.
5. In the case of the following, please consult a physician or a pharmacist.
If the symptoms persist after taking the medicine for more than 2 weeks.
Storage and Pharmaceutical Precautions:
(1) Make sure the cap is always on tight and store the medicine in a dry and cool place away from
(2) Keep out of the reach of children. (3) Don't put this product into another container. (It can become ineffective or change in its
(4) Don't use this product after the expiration date.
発行 (社)神奈川県薬剤師会
Product name: ZADITEN AL Rhinitis Spray
(ザジテン
AL 鼻炎スプレー )
※Please read the following directions carefully before using this product.
Product summary
For the relief of allergic symptoms from pollen or house dust (indoor dust): sneezing, runny nose
(excessive nasal drip), or nasal congestion.
[Ingredient and Action]
The following ingredient is included in 100 ml of this medicine (Include:8 ml per bottle).
Active Ingredient
Ketotifen fumarate (as ketotifen)
[Dosage and Usage]
Spray the following medicine inside the nasal cavities 4 times a day at morning, noon, evening, and
before bedtime. (Ketotifen 0.05 mg is included in 1 spray).
Adults (15 years old or older) and children Spray once in both nostrils
(ages 7 or older) Less than 7 years old
Do not take this medicine.
〈Cautions on Dosage and Usage〉
• Please follow exactly the defined dosage and usage directions.
• Apply this medicine to a child only under parental guidance and supervision.
• Please use for treating runny noses only.
• Avoid contact with the eyes. (If this medicine gets into your eyes, please rinse them immediately
with cold or lukewarm water and seek treatment from an eye doctor.)
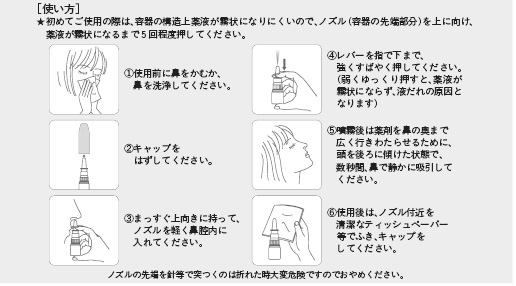
When using this for the first time, please push the nozzle about five times so the spray comes out in the form of a mist. Until the fifth time, the spray won't come out in a fine mist.
1. Before use, blow your nose. 2. Take off the cap of the container. 3. Straighten the container upward, and put the nozzle into a nasal cavity gently. 4. Strongly and quickly push the lever with a finger. (Otherwise a fine mist will not be produced.) 5. After spraying, keep your head tilted back in order to spread the medicine widely to the back of the
nose and breathe it in gently through the nose for several seconds.
6. After use, wipe around the nozzle with clean tissues and close it.
*Don't poke the tip of the nozzle with a needle because it is very dangerous if it breaks.
Precautions for Use
Please read the following precautions carefully before using this product.
What not to do
(Failure to observe these warnings may lead to aggravation of the symptoms and increase the risk of
adverse reactions.) 1. The following persons should not use this medicine: Children under the age of 7. 2. Please don't operate a vehicle or machinery after taking this medicine. (This medicine may make
you sleepy or affect your vision.)
3. Women who are breast-feeding should not take this medicine. If you take this medicine, please
avoid breast-feeding. (Studies have shown that mothers who have taken this medicine have it transfer to their breast milk.)
1. Consult a physician or pharmacist before using this medicine if any of the following apply to you:
(1) You are currently under treatment by a physician or dentist. (2) You are currently receiving treatment for allergies such as reductive sensitizing therapy. (lf you
have allergic tendencies, this product may produce an allergic reaction.)
(3) You are or may be pregnant. (4) You are someone who has had allergic reactions to medicines in the past. (5) You don't not know clearly whether your symptoms are a manifestation due to an allergy or due
to some other cause.
2. When the following symptoms occur after using this medicine, stop taking the medicine and consult
a physician or pharmacist this insert (1) When the following symptoms appear after using this medicine.
a dry or painful feeling
fatigue or headache
(2) If the symptoms have not improved after using this medicine for about one week, consult a
doctor or a pharmacist.
3. The following symptoms may occasionally occur. If these symptoms continue or increase,
discontinue this medicine and consult a physician or pharmacist: Drowsiness.
4. In the case of the following, please consult a physician or a pharmacist: If the symptoms persist
after taking the medicine for more than 2 weeks.
Storage and Pharmaceutical Precautions:
(1) Make sure the cap is always on tight and store the medicine in a dry and cool place away from
direct sunlight.
(2) Keep out of the reach of children. (3) Don't put this product into another container. (It can become ineffective or change in its
(4) Don't share this product with other people. (5) Don't use this product after the expiration date.After opening please use as soon as possible,
even if it is before the expiration date.
発行 (社)神奈川県薬剤師会
Product name: PABRON Nasal Spray Z < Rhinitis Spray >(パブロン点鼻
Z〈鼻炎スプレー〉)
※Please read the following directions carefully before using this product.
Product summary
For the relief of allergic symptoms from pollen or house dust (indoor dust): sneezing, runny nose
(excessive nasal drip), or nasal congestion.
[Ingredient and Action]
The following ingredient is included in 100 ml of this medicine (Include:8 ml per bottle).
Active Ingredient
Ketotifen fumarate (as ketotifen)
[Dosage and Usage]
Spray the following medicine inside the nasal cavities 4 times a day at morning, noon, evening, and
before bedtime. (Ketotifen 0.05 mg is included in 1 spray).
Adults (15 years old or older) and children
(ages 7 or older)
Spray once in both nostrils.
Less than 7 years old
Do not take this medicine.
〈Cautions on Dosage and Usage〉
• Please follow exactly the defined dosage and usage directions.
• Apply this medicine to a child only under parental guidance and supervision.
• Please use for treating runny noses only.
• Avoid contact with the eyes. (If this medicine gets into your eyes, please rinse them immediately
with cold or lukewarm water and seek treatment from an eye doctor.)
When using this for the first time, please push the nozzle about five times so the spray comes
out in the form of a mist. Until the fifth time, the spray won't come out in a fine mist.
1. Before use, blow your nose. 2. Take off the cap of the container. 3. Straighten the container upward, and put the nozzle into a nasal cavity gently. 4. Strongly and quickly push the lever with a finger. (Otherwise a fine mist will not be produced.) 5. After spraying, keep your head tilted back in order to spread the medicine widely to the back of the
nose and breathe it in gently through the nose for several seconds.
6. After use, wipe around the nozzle with clean tissues and close it.
*Don't poke the tip of the nozzle with a needle because it is very dangerous if it breaks.
Precautions for Use
Please read the following precautions carefully before using this product.
What not to do
(Failure to observe these warnings may lead to aggravation of the symptoms and increase the risk of
adverse reactions.) 1. The following persons should not use this medicine: Children under the age of 7. 2. Please don't operate a vehicle or machinery after taking this medicine. (This medicine may make
you sleepy or affect your vision.)
3. Women who are breast-feeding should not take this medicine. If you take this medicine, please
avoid breast-feeding. (Studies have shown that mothers who have taken this medicine have it transfer to their breast milk.)
1. Consult a physician or pharmacist before using this medicine if any of the following apply to you:
(1) You are currently under treatment by a physician or dentist. (2) You are currently receiving treatment for allergies such as reductive sensitizing therapy. (3) You are or may be pregnant. (4) You are someone who has had allergic reactions to medicines in the past. (5) You don't not know clearly whether your symptoms are a manifestation due to an allergy or due
to some other cause.
2. When the following symptoms occur after using this medicine, stop taking the medicine and consult
a physician or pharmacist: (1) When the following symptoms appear after using this medicine.
a dry or painful feeling
fatigue or headache
(2) If the symptoms have not improved after using this medicine for about one week, consult a
doctor or a pharmacist.
3. The following symptoms may occasionally occur. If these symptoms continue or increase,
discontinue this medicine and consult a physician or pharmacist: Drowsiness.
4. In the case of the following, please consult a physician or a pharmacist: If the symptoms persist
after taking the medicine for more than 2 weeks.
Storage and Pharmaceutical Precautions:
(1) Make sure the cap is always on tight and store the medicine in a dry and cool place away from
direct sunlight.
(2) Keep out of the reach of children. (3) Don't put this product into another container. (It can become ineffective or change in its
(4) Don't share this product with other people. (5) Don't use this product after the expiration date. After opening please use as soon as possible,
even if it is before the expiration date (to ensure product quality).
発行 (社)神奈川県薬剤師会
て ん が ん や く
て ん が ん え き
Product name: ZADITEN AL Eye Drop
(ザジテン AL点眼薬 <点眼液>)
※Please read the following directions carefully before using this product.
Product summary
For the relief of the following allergies caused by pollen or house dust (indoor dust): red eyes,
itchiness of the eyes, watery eyes, or the feeling of a foreign substance (feeling that a foreign substance rolls in the eyes.)
[Ingredient and Action]
The following ingredient is included in 100 ml of this medicine:
Ketotifen fumarate (as ketotifen)
[Dosage and Usage]
Put the following medicine inside the effected eye(s) 4 times a day in the morning, noon, evening, and
before going to bed.
Adults (15 years old or older)
and children ( 1 year old or older). Less than 1 year old
〈Cautions on Dosage and Usage〉
• Please follow exactly the defined dosage and usage.
• Apply this medicine to a child only under parental guidance and supervision.
• Be sure to wipe away any excess fluid after applying it to the eye.
• If the tip of the applicator touches the eyelid or eyelashes, the fluid may become infected with
germs, turning the eyes muddy with mucus.
• Don't use the eyewash as a solution for contact lens.
Refrain from using these drops when wearing contact lenses.
• Use only as an eyewash.
1. Wash your hands thoroughly before use. 2. Pull down your lower eyelid lightly and apply 1-2 drops
from directly above. Be sure to handle the bottle carefully so that the point of the container does not directly touch the eyelids or eyelashes. In addition, clean the tip of the cap after each use.
3. After the fluid enters the eye, please close the eye. Be
sure to tighten the cap after use.
4. Be sure to wipe away any excess fluid after applying it
Precautions
Please read the following cautions carefully before using this product.
What not to do
(Failure to observe these warnings may lead to aggravation of the symptoms and increase the risk of
adverse reactions). 1. The following persons should not use this medicine:
• Those who have had an allergic reaction to this medicine in the past.
• Children under 1 year old.
2. If used in combination with a nasal spray, be sure not to operate a vehicle or machinery.
(This medicine may make you sleepy or affect your vision.)
1. Consult a physician or pharmacist before using this medicine if any of the following apply to you:
(1) You are currently under treatment by a physician or dentist. (2) You are currently receiving treatment for allergies such as reductive sensitizing therapy. (3) You are or may be pregnant. (4) You are someone who has had allergic reactions to medicines in the past. (5) The person with the following symptoms: intense pain in the eye. (6) You don't not know clearly whether your symptoms are a manifestation due to an allergy or due
to some other cause.
lf any of the following correspond to you, consult a physician or pharmacist before use:
• When the symptoms are only in one eye.
• When the symptoms are found only in the eyes, and not in the nose.
• When eyesight is influenced.
• When there is discharge from the eyes.
2. lf any of the following apply to you, stop using these drops immediately and consult a physician or
pharmacist with this insert: (1) When the following symptoms occur after using this medicine.
itchiness bloodshot eyes, feeling of sharp pain*, pain*
swelling*, itchiness**, or the surrounding area of the eyes is also affected.
Other drowsiness
**For some persons, symptoms such as: severe itchiness, swollen eyes, and pain around the eyelids and eyes and cheeks, appear in the early stages when first starting use of the drops
(one or two days).
(2) When the haze of eyes is not improved. (3) If the symptoms have not improve after using this medicine about one week.
3. In the following case, consult a physician or a pharmacist.
If the symptoms are gone after taking the medicine for more than 2 weeks.
Storage and Pharmaceutical Precautions:
(1) Make sure the cap is always on tight and store the medicine in a dry and cool place away from
direct sunlight.
(2) Keep out of the reach of children. (3) Don't put this product into another container. (It can become ineffective or change in its
(4) Don't share this product with other people. (5) Don't use this product after the expiration date. After opening please use as soon as possible,
even if it is before the expiration date (to ensure product quality).
発行 (社)神奈川県薬剤師会
て ん が ん え き
Product name: IRIS ARREST<Eye Drops> (アイリスアレスト<点眼液>)
※Please read the following directions carefully before using this product.
Product summary
For the relief of the following allergies caused by pollen or house dust (indoor dust): red eyes,
itchiness of the eyes, watery eyes, or the feeling of a foreign substance (feeling that a foreign substance rolls in the eyes.)
[Ingredient and Action]
The following ingredient is included in 100 ml of this medicine: (Includes:10 ml per bottle)
Ketotifen fumarate (as ketotifen)
[Dosage and Usage]
Put the following medicine inside effected eye(s) 4 times a day in the morning, noon, evening, and
before going to bed.
Adults (15 years old or older)
and children ( 1 year old or older). Less than 1 year old
〈Cautions on Dosage and Usage〉
• Please follow exactly the defined dosage and usage.
• Apply this medicine to a child only under parental guidance and supervision.
• Be sure to wipe away any excess fluid after applying it to the eye.
• If the tip of the applicator touches the eyelid or eyelashes, the fluid may become infected with
germs, turning the eyes muddy with mucus.
• Don't use the eyewash as a solution for contact lens.
Refrain from using these drops when wearing contact lenses.
• Use only as an eyewash.
1. Wash your hands thoroughly before use. 2. Pull down your lower eyelid lightly and apply 1-2 drops from directly above.
Be sure to handle the bottle carefully so that the point of the container does not directly touch the eyelids or eyelashes. In addition, clean the tip of the cap after each use.
3. After the fluid enters the eye, please close the eye. Be sure to tighten the cap after use. 4. Be sure to wipe away any excess fluid after applying it to the eye.
Precautions
Please read the following cautions carefully before using this product.
What not to do
(Failure to observe these warnings may lead to aggravation of the symptoms and increase the risk of
adverse reactions). 1. The following persons should not use this medicine:
• Those who have had an allergic reaction to this medicine in the past. (This medicine may cause an
allergic reaction again).
• Children under 1 year old.
2. If used in combination with a nasal spray, be sure not to operate a vehicle or machinery.
(This medicine may make you sleepy or affect your vision.)
1. Consult a physician or pharmacist before using this medicine if any of the following apply to you:
(1) You are currently under treatment by a physician or dentist. (2) You are currently receiving treatment for allergies such as reductive sensitizing therapy. (3) You are or may be pregnant. (4) You are someone who has had allergic reactions to medicines in the past. (5) The person with the following symptoms: intense pain in the eye. (6) You don't not know clearly whether your symptoms are a manifestation due to an allergy or due
to some other cause.
lf any of the following correspond to you, consult a physician or pharmacist before use:
• When the symptoms are only in one eye.
• When the symptoms are found only in the eyes, and not in the nose.
• When eyesight is influenced.
• When there is discharge from the eyes.
2. lf any of the following apply to you, stop using these drops immediately and consult a physician or
pharmacist with this insert: (1) When the following symptoms occur after using this medicine:
itchiness bloodshot eyes, feeling of sharp pain*, pain*
swelling*, itchiness**, or the surrounding area of the eyes is also affected.
Other drowsiness
**For some persons, symptoms such as: severe itchiness, swollen eyes, and pain around the eyelids and eyes and cheeks, appear in the early stages when first starting use of the drops
(one or two days).
(2) When the haze of eyes is not improved. (3) If the symptoms have not improved after using this medicine about one week.
3. In the following case, consult a physician or a pharmacist.
If the symptoms are gone after taking the medicine for more than 2 weeks.
Storage and Pharmaceutical Precautions:
(1) Make sure the cap is always on tight and store the medicine in a dry and cool place away from
direct sunlight.
(2) Keep out of the reach of children. (3) Don't put this product into another container. (It can become ineffective or change in its
(4) Don't share this product with other people. (5) Avoid extended exposure to high temperatures, such as leaving it under the dashboard of a car.
(Modification of the container and the quality of the fluid may deteriorate)
(6) Don't use this product after the expiration date. After opening please use as soon as possible,
even if it is before the expiration date (to ensure product quality).
発行 (社)神奈川県薬剤師会
Source: http://www.kpa.or.jp/wp/wp-content/uploads/2013/05/2ea035a025b38f31131569e33fb596c1.pdf
Information about östradiolvalerat och dienogest This leaflet provides information about your new oral contraceptive, Qlaira®. It is important that you read this leaflet before starting to take the tablets. More information about Qlaira® can be found in the package insert that is included in the package. Oral contraceptives – a wise choice You have chosen to use oral contraceptives ("the pill") as your method of contraception. Oral contraceptives are one of the safest methods to protect against unwanted pregnancy, provided that they are used correctly. We have compiled this leaflet to help you: it provides the most important information you should know about Qlaira and oral contraceptives, and how they affect your body. Consult your midwife or doctor if you have any questions.
Antipsychotic Guidelines GUIDELINES TREATMENT OF SCHIZOPHRENIA AND PSYCHOSIS MI = Medicines Information service at Prospect Park Hospital Tel: 0118-960-5075, Monday – Friday 9am – 5pm Email: [email protected] Berkshire Healthcare NHS Foundation Trust Edition 6.0 March 2010 Antipsychotic Guidelines Authors: Ms Diane Booth, Chief Pharmacist, Berkshire Healthcare NHS Foundation Trust. Mrs Kiran Hewitt, Lead Clinical Pharmacist, Berkshire Healthcare NHS Foundation Trust. Mrs Katie Sims, Clinical Pharmacist, Berkshire Healthcare NHS Foundation Trust. Acknowledgements: The authors would like to thank the members of the pharmacy department, Prospect Park Hospital and the Drugs & Therapeutics Committee representatives of Berkshire Healthcare NHS Foundation Trust who provided help, advice and constructive feed back during the compilation of these guidelines. Any enquiries regarding these guidelines or other medication related queries should be forwarded to the MIS (Medicines Information Service), pharmacy department, Prospect Park Hospital, on 0118 960 5075/5059, or your ward/locality pharmacist.

