Levitra enthält Vardenafil, das eine kürzere Wirkdauer als Tadalafil hat, dafür aber schnell einsetzt. Männer, die diskret bestellen möchten, suchen häufig nach levitra kaufen ohne rezept. Dabei spielt die rechtliche Lage in der Schweiz eine wichtige Rolle.
Microsoft word - elf mf alter cancer cells.docx
ISSN: 1536·8378 (print), 1536-8386 (electronic)
Electromagn Bioi Med, Early Online: 1-12
AND MEDICINE
2013 lnforma Healthcare USA, Inc. DOl: 10.3109/15368378.2013.817334·
ORIGINAL ARTICLE
Extra-low-frequency magnetic fields alter cancer cells through
metabolic restriction
Ying Li1 2 and PauI Heroux 2
'lnVitroPJus Laboratol'f, Department of Surgel'f, Royal Victoria Hospital,Montreal, QC,Canada and 2Department of Epidemiology, Biostatistics and
Occupational Health, McGiJJ University, Montreal, QC, Canada
Background: Biological effects of extra-low-frequency {ELF) magnetic fields {MFs) have lacked a AMP-activated protein kinase,
credible mechanism of interaction between MFs and living material. Obiectives: To examine the
ATP synthase, chromosome instability,
effect of ELF·MFs on cancer cells. Methods: Five cancer cell lines were exposed to ELF·MFs
extra-low-frequency, magnetic field
within the range of0.025-Sf.1T, and the cells were examined for karyotype changes after 6d.
Results: All cancer cells lines lost chromosomes from MF exposure, with a mostly flat dose· History
response. Constant MF exposures for three weeks allow a rising return to the baseline, Received 8 January 2013
unperturbed karyotypes. From this point, small MF increases or decreases are again capable Revised 3 May 201 3
of inducing karyotype contractions {KCs). Our data suggest that the KCs are caused by Accepted 26 May 2013
MF interference with mitochondria's adenosine triphosphate synthase {ATPS), compensated Published online 31 July 2013
by the action of adenosine monophosphate-activated protein kinase {AMPK). The effects of MFs are similar to those of the ATPS inhibitor, oligomycin. They are amplified by metformin, an AMPK stimulator, and attenuated by resistin, an AMPK inhibitor. Over environmental MFs, KCs of various cancer cell lines show exceptionally wide and flat dose-responses, except for those of erythroleukemia cells, which display a progressive rise from 0.025 to 0.4 f.1T. Conclusions: The biological effects of MFs are connected to an alteration in the structure of water that impedes the flux of protons in ATPS channels. These results may be environmentally important, in view of the central roles played in human physiology by ATPS and AMPK, particularly in their links to diabetes, cancer and longevity.
(Phillips et al., 1986), inhibition of differentiation with
increased cell proliferation (Chen et al., 2000) as well
Since the Wertheimer and Leeper (1979) article l:inking wire
as DNA breaks with apoptosis and necrosis (Lai and Singh,
codes to childhood cancer, the relation between cancer and
power-frequency magnetic fields (MFs) has been under
In the early days of extra-low-frequency (ELF)-MF
investigation (Heroux, 1991). Population, in vivo and
research, Senllkhina et al. (Sem.ild:rina et al., 1988;
in vitro studies have falled to provide a clear link. The
Semikhina and Kiselev, 1981) documented by electrical
exception is childhood leukemia (Ahlbom et al., 2000),
dissipation factor (roRC, also known in electrical engineering
leading the International Agency for Research on Cancer to
as tg o) and optical measurements (the dimerization of dilute
attach the class 2B carcinogen designation to MFs in June
rhodamine 60 solutions) that alternating lviFs in the range
2001 (IARC, 2002).
25nT-879T disrupt the anangement of water molecules,
It has been argued that envirorunental 60-Hz lviFs, as
particularly under high concentrations of hydrogen bonds and
non-ionizing radiation and incapable of raising tissue tem-
protons. The effects were absent above 40-50°C, as water
peratures, could not have significant impacts on cells. But
structure changes. The maximum effect was detected at
effects on breast cancer cells, MCF-7, were confirmed by a
156.2Hz and 15.45T for 7oc pure water. NatTow reson-
number oflaboratories near 1.2T (Ishido et al, 2001). Many
ances were observed, easily broadened by the presence of
have also reported a diversity of effects above 2.5 T, higher
even small levels of impurities. The MF effects on water
than common environmental exposures. These include
progressed over 5h and dissipated over 2h after the field was
(Goodman et al., 1979), increased soft agar colony formation
Interestingly, when alternating lviFs were kept below
25nT, an influence of static MFs on water could also
be detected. Removing the static MF acted on water
Address correspondence to Dr Paul Heroux, Faculty of Medicine,
McGill University, Montreal, QC H3A 1A3, C:mada. Tel: 1-514-398-
variables (dissipation factor and optical measurements) in a
6988. E-mail: [email protected]
direction opposite to the application of ELF-lviFs larger

Y Li & P. Heroux
Electromagn Bioi Med, Early Online: 1-12
than 25 nT. Thus, it seemed that elimination of both ELF and
insulin 1mgll (Sigma15500), iron saturated bovine transferrin
static MFs allowed water to "opti.rnlze" its molecular
25mgll (Sigma T1408), sodium bicarbonate 2g/1 (SigmaS-
6014) and bovine serum albumin 4g/1 (Sigma A3311,
These observations created ground to attempt an inter-
Oakville, Canada). Vented T-25s (Sarstedt 83.1810.502,
pretation of ELF-MF health effects based on water structure
Ntirnbrecht, Germany) and T-12s (Falcon 353018, BD,
alterations brought about by the MF itself, as opposed
Franklin Lakes, NJ) were used for experiments, and cells
to magnetically induced currents. We investigated this possi-
are seeded at 5000/cm2 and kept in the same medium for 6 d.
bility by setting up baseline cancer cell lines maintained under
In longer tests (3 weeks), new medium is added weekly.
power-frequency MFs lower than 4 nT, and also under anoxia.
Oxygen was eliminated by enclosing T-25s and T-12s in large
As 82% of oxygen readings in solid tumors are less than
polycarbonate containers (1.61, Starfrit Lock & Lock,
0.33% (Kizaka-Kondoh et al., 2003), and stem cells are hosted
Longueuil, Canada) flushed with medical-grade nitrogen
in niches that are very low in oxygen (Hill et al., 2009), anoxia
(95%) and C02 (5%). pH readings were conducted under
is a better simulation of the tumor environment than routinely
isothennal conditions (water bath) for samples as well as
used 21% oxygen. Our cells are also hyperploid, displaying
calibration buffers, using Corning 445 meters (Corning, NY).
a range of chromosomes numbers larger than 46, as a result
of the enhanced metabolism typical of cancer cells. The
absence of oxygen reduces chromosome numbers to some Unexposed cells for experiments are kept in T-12 or T-25
extent, but not back to normal, and also narrows their range
culture flasks under anoxia and MFs below 4 nT.Three 6.3 mm
(Li et al., 2012).
thick layers of structural steel reduce ELF-MFs from incuba-
Metabolic restrictors, chemicals that impair oxygen tors and the environment. Culture vessels are centered in a
metabolism, adenosine triphosphate (ATP) synthesis or ATP
rectangular st.ructw:al steel pipe 5.1 x 7.6 em, itself contained
use, can bring back chromosome numbers in cancer cell lines
in a7.6 x 10.2mm pipe, both 20 em long.These two shields are
even closer to their original46 than anoxia alone, an effect we
placed in a 15.2 x 24.5 x 36 em long pipe. This reduces 60-
labeled karyotype contraction (KC). KC is a rapid and
HzMFs by a factor of 144, providing unexposed cells with a
reversible loss of clu·omosomes resulting from metabolic
MF environment of 3nT, slightly below the measurement floor
restriction (Li et al., 2012).
(5 nT at 60Hz) of our Narda EPA-300 instrument (Hauppauge,
A critical enzyme in ATP production is ATP synthase
NY).The incubator is a Forma 3310 (ThermoFisher, Waltham,
(ATPS).The structure of ATPS is documented in detail (Boyer;
MA), with low average MF (0.4 ).11).
2002; Sasada and Marcey, 2010) as a rotating motor-generator
MFs are applied by rectangular coils (19 x 25.6 em) with
structure activated by the trickle of high-density protons from
20-50 turns of #25 AWG varnished copper wire wound on
the inter-membrane space into the matrix of mitochondria.
13mm polycarbonate, providing8 ft The coil is under the
Proton diffusion along the 15nm thick inter-membrane space
two irmer shields and over an ac1ylic spacer at the bottom of
does not limit their transit time of 1-2 JlS (Procopio and Fornes,
the outer shield. 60-Hz fields above 0.4 JlT are from sector-
1997). Protons enter the Fo of ATPS along an entry half-
cormected variable transformers fitted with a passive low-pass
channel made of four hydrophilic ex-helices, to reach a rotating
capacitive filters, with all harmonics at less than 20 dB.
helix. After rotation, protons flow out through a similar exit
Smaller 60-Hz fields and other frequencies were generated
half-channel.The rotation is used by the F segment of ATPS to
with computer-based synthesizers with a background noise at
produce ATP (Procopio and Fornes, 1997).
less than 40dB. MFs are within 10% of nominal in the
These hydrophilic channels (Fillingame etal.,2003) provide
whole cell culture area.
a high density of hydrogen bonds, while the mitochondrial
The ''NIM'' shield cancelling both alternating and static
inter-membrane space feedsATPS a high-density of protons, as
MFs is an acrylic cylinder 5.7 em in internal diameter 'vith a
required for maximum MF effect on water by Semikhina et al.
0.38 em wall and 38 em in length, covered by six layers of
(Semikhina et al., 1988; Semikhina and Kiselev, 1981). The
0.4mm nickel-iron-molybdenum foil (ASTM A753 Type 4)
high-density protons (pH 1; Procopio and Fornes, 1997) are
wound in a spiral, together with a 1.6 mm neoprene
driven through the half-channels by a 180kV/cm electric field
membrane spacer (Futurplast, St-Laurent, Canada).
(Zorov et al., 2009) across the inner membrane (Mitchell,
60-Hz 5 JlT exposures produce no measurable temperature
1966). In this study, we assess the ability of 1-fFs at common
rises. K562 is a good the1mal sentinel, hype1ihermia being
environmental levels to induce KCs.
detectable from larger cell sizes at +0.5 K, while +1K
seriously impairs proliferation and +2K over a few days is
Cells and culture conditions
Chromosome, cell and adenosine monophosphate-
The cell lines, K562 and HEL 92.1.7 (erythroleukemias),
activated protein kinase assays
MCF7 (breast cancer), NCI-H460 (lung cancer) and
COL0320DM (colon cancer) were obtained from ATCC
Metaphase preparation and cytogenetic analysis were per-
(Manassas, VA). Cells are maintained under 5% carbon
formed according to the standard trypsin-Gien1sa banding
dioxide and 90% humidity, and grown in synthetic culture
technique. Karyotypes are obtained using x100 oil immer-
medilll11. because changes in serum can alter chromosome
sion, a Laborlux D (Leitz, Leica, Wetzlar, Germany) micro-
counts. The medium is RPMI-1640 with L-glutamine (Sigma
scope, and an Infinity X (21 Mpixels) CMOS camera
61-030-RM), sodium selenite, 20nM (Sigma S-5261), bovine
(Lumenera, Ottawa, Canada).

DOl: 10.31091153683782()13.817334
Extra-low-frequency magnetic fields alter cancer cells
Cell proliferation and cell size histograms of are fi:om a
the horizontal coil induces cments six times larger
Scepter automated cell counter (Millipore, Billerica, MA).
because the exposed culture dish area is 34 x 34 mm for
Metformin was obtained from Sigma (D150959) and resistin
the horizontal coil, compared to 5.8 x 34mm for the
from Prospec Protein Specialists, East Bnmswick, NJ.
vertical coil. As KCs after 6 d at 1J.LT come out similarly
for both orientations (Figure 1), we conclude that the effect
on chromosome numbers are dependent on the MF itself.
Ve assume direct MF, rather than induced cment action on
Because of the controlled MFs and of anoxia, our reference
the basis that variations of cunent density by a factor of 6 do
K562 cultures are karyotypically and otherwise exceptionally
not affect KC. But this would also occur if induced cments
stable. Seventy-five percent of the cells have just
had a flat dose-response, already saturated at the lower
t vo chromosome numbers, 62 and 61, compared to a wider
current. Furthemwre, direct MF action on KC does not
range under 21% oxygen (Li et al., 2012). The stability of
preclude that other effects of MFs may depend on induced
chromosome numbers in baseline anoxic K562 has been
pe1iodically confirmed in our laboratory over 5 years.
These cultures provide an extremely precise reference
point, as shown in the nanow baselines of Figures 1, 2
(top) and 4. This is of great advantage in obtaining statistical
Figure 2 (top) shows the chromosome number losses
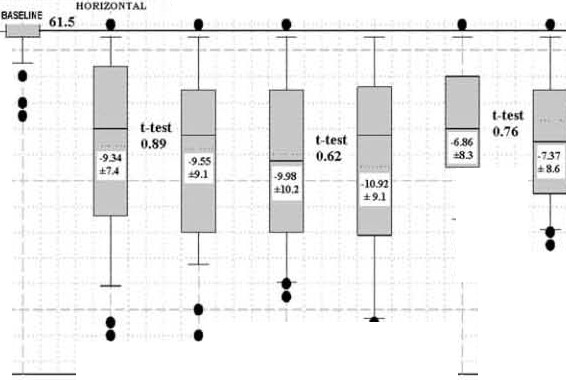
significance in our data. Figure 1 sho -vs little overlap between
experienced by previously shielded anoxic K562 cells after
baseline and exposed data, yielding small numbers in
6 d in various MFs. Under any exposure, the nanow baseline
Student's t-tests. In Figure 2 (top), the p value between
expands, and there are substantial KCs.
baseline and 0.025 J.LT is 0.00012. In Figure 3, even when
Three features are of importance. First, a no-effect-level
using 21% oxygen, the large number of karyotypes performed,
lower than 25nT. Second, a progression of KCs up to 0.4 J.LT.
and the strong shifts in average chromosome numbers Third, the relatively flat dose-response bet veen 0.1 and produced by MFs result in extremely small p values
(0.000006 for MCF7).
The graph spans time-averaged MFs representing domestic
(0-0.2J.LT), commercial (0.07-0.5 J. LT) and occupational (0.1-
1J.lT) environments (Heroux, 1987).
Induced currents
Later sections of this article will argue that MFs act most
'hether biological effects of power-frequency MFs relate to
directly on the structure of water, leading to an alteration in
the MF itself, or to the CUlTents induced in tissues by the
proton mobility. As proton mobility is tightly related to pH, a
fields, has been a perennial question. Many think that effects
measurement of hydrogen ion activity, some perturbation of
occur through potentials produced by magnetically induced
pH values might be expected. A lasting effect of MFs on
currents blocked by the thin membranes, within or bordering
aqueous fluids is actually observable from pH measurements
living cells. Such cments and membrane potentials are
in cell culture media, which turn slightly more acidic under
familiar to conventional electrophysiology.
short MF exposures. After 20h, there is a difference
In the results of Figure 1, one aliquot of an anoxic
of 0.09 pH units with a 95% confidence interval of
K562 cell culture is placed in a vertical and the second in
0.045 between unexposed versus 5 J.LT 60-Hz exposed
a horizontal MF exposure system. At the same MF,
media (Figure 2; bottom) for the widely used RPMI-1640
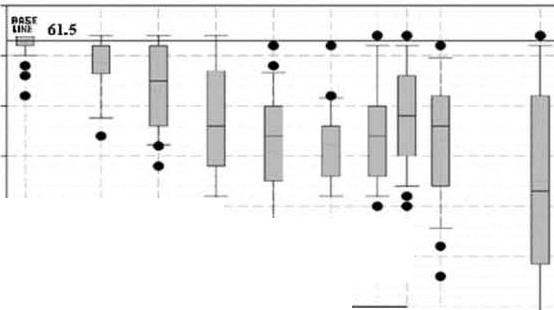
Figure 1. Baseline anoxic K562 cells at less
70-- ------------ ------ ------------ ------ -,
tl1an 4nT (60Hz) with an average of 61.5 chromosomes (horizontal line) and a very
narrow distribution (at left) are simultan-
eously transferred for 6 d to 11-1T MFs applied
IIOKIZOI<TAI.
eitl1er horizontally or vertically. Three inde-
pendent 6-d assays show the resulting
chromosome numbers. Box plots show
median (solid), average (dotted), 25 and 75%
(box), 10 and 90% limits (whiskers) and
outside values (dots). Fifty-si'< (assay 1), 50
(assay 2) and 51(assay 3) metaphases were
karyotyped in each orientation. Inside the box
plots are average chromosome losses. TI1e
Student's t-test results quantify the probabil-
ity that the horizontal and vertical results are
Y Li & P. Heroux
Electromagn Bioi Med, Early Online: 1- 12
Figure 2. Top: K562 chromosome numbers as
a function of 60Hz magnetic flux density.
Six-day assays with, in sequence, 65, 28, 50,
77, 46, 33, 65, 102, 56 and 50 metaphases.
1 vo to 6 experiments at each MF.
Approximate ranges for domestic, commer- cial and occupational exposures are
shown. Bottom: pH differences between
two cell medium aliquots, one exposed for
20h to <4nT at 60Hz and the second to the
MF density in the figure. Medium is RPMl-
1640 with 10% FBS. Isotherm measurements
using auto read were made with the same
probe, alternating between the two aliquots.
TI1ree measurements for each aliquot and
three repeats at each MF density.
ComtnetSlll
IA.SCUHI:
Magnetic Flux Density (1-JT)
with 10% serum. The pH shift was confirmed for a variety of
numbers is even less than what is observed in the long-term
cell culture media
baseline culture, as shown in the last measurement of Figure 4
(top) and in the central measurements of Figure 4 (middle and
Across cell lines
bottom). Chromosome numbers restore earlier than chromo-
some number dispersions.
Beyond K562, we investigated four more hyperploid cancer
After 3 weeks, if the MF is altered by a small percentage of
cell lines to determine the generality of KC by MFs.Over two
the original value, either positively or negatively, KCs are
orders ofMF magnitude, erythroleukemia (HEL 92.1.7), breast
again observed, as shown in Figure 4 (middle and bottom).
(l 1CF7) and lung (NCI-H460) cancer cells lose between 8 and
Starting from low (middle, 0.1 T) or high (bottom. 1 T)
13 chromosomes (Figure 3).BEL, our second erythroleukemia
baselines, symmetrical KCs are observed. This bilateral
cell line, shows fewer losses at lower fields, similar to K562.
sensitivity to changes is unforeseen by conventional toxico-
Three of the four results reported in Figure 3 were obtained
logical principles. KC is also observed when fields are reduced
under standard (21% oxygen) culture conditions.
from 50 to 4nT (not shown).
Classical toxicology and epidemiology, where smoothly
The KCs will be interpreted below as caused by magnet-
climbing dose-responses are justified by binding chemistry
ically induced perturbations in intra-cellular AlP levels.
and the central tendency theorem. do not expect the flat dose-
These results cast doubt on the stability of cancer cell models
responses observed in Figures 2 (top) and 3.The effects found
housed in incubators with MFs that are highly variable over
for different cell types are strikingly similar, with similar low-
space and time (l 1ild et al., 2009; Su and Heroux, 2012).
field deviations in the two erythroleukemia lines, suggesting
common, basic mechanisms.
Differential action
We measured in anoxic K562 6-d tests at 1 T, the average
KCs over frequency as follows:
K562 cells with magnetic KCs, such as in Figure 2 (top),
progressively recover their original chromosome numbers
3.6 ± 0.79 at 50-Hz,
9.36 ± 1.06 at 60-Hz,
after 3 weeks, even as the :MF is maintained at a constant level
12.71 ± 1.82 at 120-Hz and
9.8 ± 1.31 at 155-Hz.
(Figure 4; top). Surprisingly, in cells recovering over 3 weeks
from a MF disturbance, the deviation of chromosome
A polynomial fit predicts maximum KC at 113Hz for 1T .
DOl: 10.31091153683782()13.817334
Extra-low-frequency magnetic fields alter cancer cells
Figure 3. Average chromosome losses in erythroleukemia, breast, lung and colon cancer cells as a function of 60Hz magnetic flux density. The cllromosome number baseline(' '0") averages for <4nT cells at 60Hz, 80% range and metapllase number are: HEL: 66, 62-67 and 32; MCF7: 74,
61-75 and 30;NO-H460: 57,53-65and 30; and COLO 320DM: 54,49-61and 30. Six-day assayswitll, in sequence, 32, 22, 29, 32; 19, 22, 19, 21; 29,
22, 24; 22, 34 and 46 metapllases. Two to five experiments at each MF.HEL, NO-H460 and COL 320DM assays used 21% oxygen, ratller tl1an anoxic
conditions, as some anoxic karyotype modes are too close to 46 to allow easy statistical separation from MF-exposed samples.
(less than 5 n'I) and the static field to 3 T. Karyotyping
Static MF removed
revealed a very slow drift downward, but a strong effect on
The :influence of the static MF was investigated by observing
proliferation rate was observed. After 4 d, cell numbers in the
K562 cells transferred from a steel shield that eliminated ELF
NIM shield were increased by a factor of 2.05 ± 0.13 (SD)
MFs (to less than 5 n'I), but had a static field of 74 T, to
over cells kept in the steel shield, indicating enhanced
a second shield (NIM) that attenuated both the ELF-MF
metabolism. The effect is persistent over time.
6 Y Li & P. Heroux
Electromagn Bioi Med, Early Online: 1-12
Figure 4. Top: K562 chromosome numbers
return to baseline after 3 weeks of continuous
1f.lT lVIF exposure. Sixty -five, 102, 50 and
37 metaphases. Two experiments at each lVIF.
Middle: K562 chromosome numbers obtained after 6 d by altering baseline lVIFs of
0.1f.lT. Twenty, 31, 37 (baseline), 31 and
!l 10
35 metaphases. Three to six e.-xperiments at
each lVIF. Bottom: For 1f.lT, 28, 28, 37
8
(baseline), 28 and 28 metaphases. Three
experiments at each lv!F. Although the
symmetry of the chromosome numbers
is strong, there is more cell decay with
increased than with reduced fields.
Weeks of 1 T Exposure
Il l
""0""'
009 010 O lt
";2"":
< Field Oeorease : Faeld Increase >
lb'!fllnt'
Magnetic F1eld Density (IJT)
DOl: 10.31091153683782()13.817334
Extra-low-frequency magnetic fields alter cancer cells
MF and oligomycin
MF and adenosine monophosphate-activated protein
Previous experiments (Li et al., 2012) on the five cancer cell
lines used in this article show a link between metabolic
The similarity between 0.4 )lT and oligomycin suggests that
restriction and KC. Anoxia alone induces partial KCs of 6-8
the MF may be an inhibitor of ATPS, as oligomycin is a
chromosomes. Deeper contractions, almost to normalization
highly specific inhibitor of ATPS. If this were the case,
of the karyotypes to 46, are produced by IC50 doses (allov.ring
inhibition of mitochondrial ATPS by lYIFs would activate
50% of the nonnal cell division rate) of the metabolic
adenosine monophosphate-activated protein kinase (AMPK),
restrictors oligomycin and imatirrib. Similar KCs are
because healthy cells must maintain a high level of phos-
produced by physiological levels of melatonin and vitamin
phorylation capacity (ATP:ADP10) to function well
(Hardie and Hawley, 2001). AMPK is a sensitive ATP
We believed that comparison of metabolically restricted
regulator that switches on catabolic pathways and off many
cultures with MF-exposed cultures could provide clues on
ATP-consmning processes, both acutely and clu·orrically,
action mechanisms, as the different metabolic restrictors through gene expression. mentioned above have different sites of action.Figure 5 (top),
The MF>ATPS>AMPK pathway was investigated using
displays the similarity in cell size distribution after 6-d
metfonrrin and resistin. Metfonnin is a diabetes drug that
between two of seven anoxic K562 assays, one exposed to a
activates AMPK, leading to reduced glucose production in the
very effective MF, 0.4 )lT at 60-Hz, and the second to
liver and reduced insuJin resistance in muscle. It is an
oligomycin at IC50 (2.5 ng/mJ). TI1e two distributions
attractive anti-aging drug that usually causes weight and
stand apart, with smaller cell diameters and higher ratios of
cells-to-objects below 11J.llll, the decay particles and
Resistin, a product of the RSTN gene, is a 9.9 kDa protein
apobodies. This suggests that lYIFs and oligomycin share a
containing 93 alllino acid residues which, at 20ng/rn1 or more,
common mode of action. Despite the closeness between inhibits AMPK. It interferes vlth phosphorylation of Akt oligomycin and MF assays in Figure 5 (top), oligomycin is
(serine/threonine protein kinase), active in multiple cellular
faster-acting than 0.4 )lT: changes in cell size, revealing of
processes such as glucose metabolism, cell proliferation,
KC, are visible at 1d, em·lier than for the IVIF. But more
apoptosis, transcription and cell migration.
efficant MFs, such as 5 )lT at 60-Hz or 1)lT at 120-Hz, show
Metfonrrin (0.01mg/1) and resistin (40ngll) alone for 3d
effects earlier (not shown).
induce average KCs of 9 and 10, respectively, in K562.
Figure 5. Top: Object diameter histograms
for 6-d anoxic exposures of K562 cultures to
0.4 f!T MF at 60-Hz and oligomycin at ICso
(2.5 ngfml). The lower four ICso curves are:
imatinib (0.04 J!gfml), resistin (40ngtml),
metfonnin (0.01 mg/ml) and melatonin-vita- min C (0.3 J.lg!ml and 26 J.lg!ml). All cultures
are adjusted to a common small particle
count maximum. Bottom: Object diameter
histograms for 7-h 21% oxygen exposures of
three K562 cultures under typical incubator
MF. Aliquots of RPMl-1640 witl1 10% FBS
medium exposed for 15h to very small MF
(<4nT at 60Hz, 3 f!T static), incubator MF
(2-2.7 f!T at 60Hz) or Inhibitory MF
(0.62 f!T at 120Hz) were seeded with cells at
Object Diamete•·(l.tm)
time 0 and measured with a lvfillipore Scepter
at 7h. Average of three repeats for each condition. TI1e p value between average
levels (l2-16J!m) for very small MF and
inhibitory MF is 0.001 (n-4).
Object Diameter (Jtm)
8 Y Li & P. Heroux
Electromagn Bioi Med, Early Online: 1-12
'hen, in the 6-d trials routinely used for MF tests, 1T is
There is an increase (Figure 5; bottom) in the number of
combined with metfornrin, even larger KCs are observed (9
living cells observed nnder the very small MF condition,
becomes 11± 0.34). 'hen 1 Tis combined with resistin, the
compared to the inhibitory MF condition, with the incubator
KC of resistin reduces from 10 to 4 ± 0.46, aJso less than the
MF condition rating in between.
KC of 1T alone, at 7.5. The conclusion from summary of
When stressed cells from a culture with depleted medium
Table 1 is that l .1Fs enhance the action of metformin, but
(lower pH) were used, the inhibitory MF had the effect of
neutralize the effect of resistin, again suggesting a connection
increasing the level of decay products (object diameters less
between l .1Fs and ATPS.
than 11 )in the culture (not shown).
Experiments on medium alone
NCI-H460 and MCF7 proliferation
Cells grown for 7 h nnder identical incubator MF (2-2.7 Tat
Beyond strong effects on cancer cells karyotypes, MFs also
60-Hz) conditions fared differently according to whether the
impact proliferation rate, adhesion and cell shape, but in
culture medium added at Oh originated from closed flasks
specific cases, such as for lnng (NCI-H460) and breast cancer
exposed for 15 h to: very small MF (<4 nT at 60-Hz, 3 T (MCF7), effects are particularly striking. Figure 6(C) illus- static), incubator MF (2-2.7T at 60-Hz) or inhibitory MF
trates that the cell connts of lnng cancer cells after 4 d in our
(0.62T at 120Hz).
synthetic medium at 50nT, 400nT and 5 Tare 8, 9.2 and
After the sealed flasks v.>ith media (only) are exposed
14.8, respectively, times larger than those of nnexposed (4 n'I)
to their respective MFs, cell culture aliquots are introduced
cells. NCI-H460 and MCF7 cells grown at less than 4nT in
into each flask, and incubated for 7 h nnder incubator
our synthetic medium do not attach to the growth surface, but
iv!F conditions. Measurements of cells numbers of each
do so nnder any MF exposure. Figure 6 illustrates that the
S1Ze are acquired at 0h, as well as at 7 h for each flask.
appearance of MCF7 cells after 4 d.is completely different at
<4nT (A) than at 5T (B). These MF-related differences in
adhesion, appearance and proliferation last over two passages,
Table 1. K562 karyotype contractions under the action of AMPK
modulators and MFs.
but finally return to the (A) characteristic, the "non-
adherent'' style, which is coherent with the restoration of
Agent Concentration/intensity
chromosome numbers nnder constant l .1F exposures displayed
Metformin (activator)
in Figure 4 (top). The proliferation results for NCI-H460 are
Resistin (inhibitor)
also compatible with our metfornrin and resistin experiments,
which suggest that MFs stimulate Akt, which in turn activates
protein synthesis and apoptosis inhibition in NCI-H460
(Hovelmann et al., 2004).
'" I l l
"".tnooc Flo4d Oon•IIJ IIITI
------r------- I-----1
Figure 6. EffectofMFs on MCF7 adhesion and shape and NCI-H460 proliferation. MCF7 (breast cancer) a:nd NO-H460 (lwlg cancer) cells originally under shielded levels (4nT). (A) MCF7 exposed to 4nTfor 4d. (B) MCF7 exposed to 5 J.lT for4 d. (C) Proliferation ofNCI-H460 over4 d as a function of MF density. (D) Evolution of NO-H460 over 8d for 4nT and 5 J.lT exposures. Three experiments per determination.
limited number of binding sites, but still manage to elicit a strong
001: 10.'3109/153683782()13.817334
response (Vandenberg et aL, 2012). In our case, the "binding
Discussion
2008; Zorov et al., 2009). The transitions between para and
ortho forms are forbidden by quantum mechanics. They rarely
Possible site of action of Mf s
occur during random thermodynantic fluctuations as a proton
There are documented examples of hydrogen bonding
drifts away from its OH radical, releases Pauli exclusion
impacting biological processes. The hydrogen bond is 4%
principle constraints and allows spin flipping according to an
longer for heavy water than for light water, which results in
external MF. The ortho state is sensitive to the l .1F. This
lethal rnamrnalian toxicity at about 50% heavy water (Kushner
dependence of the transitions on thermodynantic instability is
et al., 1999). Small changes in hydrogen bond lengths and
convenient to explain the slow rate of action ofMFs on water.
angles are critical in determining how binding pockets react,
The detailed physics within the water twmel is complex
as demonstrated in the selective uptake of phosphorous over
because proton tunneling is probably coordinated with
arsenic in bacteria (Elias et al, 2012).
electron tunneling (Gray and Winkler, 1996; Moser et al.,
The involvement of water structure disruptions through
1992). Proton-coupled electron transfer underpins many bio-
alterations in hydrogen bonding was also anticipated by recent
logical reactions and may occur as unidirectional or bidirec-
views on EMF bioeffects (De Ninno and Castellano, 2011;
tional, and synchronous or asynchronous, transfer of protons
Novikov et al., 2010).
and electrons (Reece and Nocera. 2009). Most enzymatic
If the effect on water described by Semildrina and
reactions would involve a single such transfer, wbile ATPS is
Klselev is involved, it would be more prominent "the
exquisitely sensitive to MFs because its structure is dependent
higher the concentrations of hydrogen bonds and proton
on a serial layout of such transfers.
containing groups in aqueous systems'' (Semikhina et al.,
Finally, the metabolic ''restriction'' that we attribute to the
1988). The only known location in the biota where these
action of MFs on ATPS could be labeled as a metabolic
two conditions are met with such intensity are the entry
"disruption" and bas at least three characteristics in common
and exit water channels of ATPS. That protons can
with endocrine disruption.
proceed through water channels by tunneling bas been
First, dependence on very specific receptors, namely ATPS
confirmed in water-filled nanotube models where neutron
channels of mitochondria for the MF, and ligand-receptor
Compton scattering has detected as double wells the
systems in the cell membrane, cytosol or nucleus for
quantum delocalization of protons (Reiter et al, 2011).
endocrine disrupters. Second, the quickly saturating dose-
Proton movement is sensitive to the global configuration
response curves observed in Figures 2 (top) and 3 are similar,
of hydrogen bonds.
for example, to the effects of atrazine on the size of the larynx.
The intense electric field (Zorov et al., 2009) applied to
Atrazine does not shrink the larynx, but it inhibits the
ATPS Fo in mitochondria lowers the potential barriers
androgen stimulus (Hayes et al., 2002). Third, thresholds of
inhibiting proton movement. In low-barrier hydrogen bonds,
action at very low levels: 25nT for l .1Fs and pico-molar to
hydrogen is free to move between two oxygen atoms (Cleland,
nano-molar levels
endocrine-disrupting
2000). According to this mechanism, MFs would impede the
(Vandenberg et al, 2012).
quantum mechanical proton flow through the hydrophilic
The increased metabolism observed when alternating and
channels, and MF removal would improve proton flow,
static MFs are removed. and the ability of MF-conditioned
directly impacting ATPS efficiency. The dose-responses
culture media to influence cellular development are aD
of Figures 2 (top) and 3 would be determined by rising
compatible with Russia11 water data. The presence of a KC
proton impedance (decreased soliton tunneling) through
resonance wider tha11that observed for pure water by Russian
ATPS half-channels.
physicist adds support. Finally, the ratio between frequency
The involvement of protons relieves the area of EMF
and field intensity (fiB) for maximum biological effects is
bioeffects of the "kT problem", because EMF would act not
suggestive of a coupling with the gyromagnetic ratio of the
on molecules, but on particles (protons and electrons), wbich,
as ferrnions, do not follow Maxwell-Boltzmann statistics, but
In tbis context, similar fingerprints between the 0.4}-LT and
Fermi-Dirac statistics, and are governed by quantum
oligomycin (Figure 5; top), known to inhibit ATPS by binding to the o subunit of the Po segment of ATPS (also named
electrodynamics.
Numerous elements documented by Russian physicists in
oligomycin sensitivity conferral protein) comes as no surprise. Another intriguing link between MFs and ATPS is provided
their studies of :MF's on water (Semikhina et al, 1988;
by the fact that rhodamine 6 G, used by Semikbina to detect
Senrikbina and Kiselev, 1981) are compatible with our own
MF effects on water, also happens to inhibit the F
biological observations.
It is particularly notable that the KC threshold of 25nT in
Figure 2 (top) falls in line with the water effect threshold detected by Russian physicists (Semikhina et al., 1988). The extended, flat response is also compatible with their obser- vations, and there is an interesting biological aspect to this flat dose-response. Quickly saturating dose-responses are observed in endocrine disrupters that attach to a
Y Li & P. Heroux
Electromagn Bioi Med, Early Online: 1-12
KC,AMPK and diabetes
Mfs and cancer epidemiology
Perturbations of A1P concentrations trigger AMPK, which
For many cancer cell types, the dose-response of KC versus
activates p53 and reduces both ATP consumption and DNA
MFs is remarkably flat (Figure 3). The deviation from
synthesis (Jones et al., 2005; Motoshima et al., 2006).
flatness in erythroleukemia cells (Figures 2 (top) and 3; HEL)
The suppression ofDNA synthesis, part of AMPK's catabolic
is due, we suspect, to extra-mitochondrial A1P secretion in
control, leads to KCs through suppression of chromosome
the cell membrane (Arakaki et al., 2003) where pH is at a
endoreduplication, the mechanism probably responsible
physiological 7.3 rather than 1, a probable feature of this cell
for rapid chromosome number increases :in cancer cells
type (Das et al., 1994).
(Li et al., 2012).
If KC is indeed a marker of increased malignancy, there is
Two unusual aspects of MF action, adaptation to a stable
a possibility of carcinogenicity from MF exposures. fu such a
field over three weeks (Figure 4; top) and the unusual
case, the phenomenon would not be easy to document through
shorter-term sensitivity to small MF increases and decreases
epidemiology.First, the threshold for the effect (25n1)is very
(Figure 4; middle and bottom) are compatible with AMPK
low, which means that all the population is "exposed''.
physiology. As far as we know, this is the first example of an
Second, the dose-response is unusually flat (Figure 3), such
agent presenting this kind of symmetry, making it possible to
that useful low and high exposure groups vlth otherwise
sustain KCs indefinitely by judicious selection of MF
similar characteristics would be difficult to assemble. Third,
sequences. AlYIPK is easily triggered by small changes in
the differential action of MFs may confuse conventional
A1P levels (Hardie and Hawley, 2001), and also controls
exposure analysis.
long-term dynamic adaptation in muscle (Winder et al.,
Occupational studies are often at the forefront of
2000). The connection between metabolic restrictors, includ-
epidemiological discovery because of their higher and better
ing MFs, and KC can be explained by AMPK physiology.
docwnented exposures. According to Figure 2 (top), occupa-
The MF >A1PS > AlYIPK pathway is easily detectable in
tional populations of low (0.1JlT) and high exposures (1)l1)
cancer cells because of KC, but there is no reason to think that
have a KC difference of "1 chromosome" between them.
the A1PS of normal cells is spared under MF exposure. A
Domestic MF epidemiology on leukemia may have been
major regulator of metabolism (Liu et al., 2006), AMPK
successful (Ahlborn et al., 2000; Svendsen et al., 2007)
modulates insulin secretion by pancreatic beta-cells (Winder
because it benefited from a KC of "10 chromosomes"
and Hardie, 1999) and is investigated for the treatment of
between 0 and 0.4 JlT (Figure 2; top).
diabetes (Viollet et al., 2009).AMPK is tied with body weight
The increased proliferation rates reported for lung cancer
(Kim et al., 2004) as well as with immWle cell behavior
cultures may also be important.Lung cancer was pointed in at
(Kanellis et al., 2006). Type 2 diabetes has been linked to
least three studies related to EMFs (Armstrong et al., 1994;
prolonged and persistent exposures to endocrine disruptors
l'vfiller et al., 1996; Vagero and Olin, 1983).
(Lee et al., 2010).
Conclusion
The following evidence swpmt the inhibition of A1PS
Cancer cells depend on glycolysis and significantly upregu-
late it when respiration is inhibited. The Warburg effect manifests as increased glycolysis and reduced mitochondrial
Mfs alter metabolism
respiration (Jezek et al., 2010; Wu et al., 2006). These
capabilities of cancer cells allow growth under meta-
(1) MFs induce KCs in five cancer cell lines, as do other
bolic restriction by concentration of their resources on
metabolic restrictors (Li et al., 2012).
bio-synthesis through the elimination of detoxification mech-
(2) MFs interact with metformin and resistin as would an
anisms associated with oxygen exposure, such as
glutathione-S-transferase and CYP3A4 expression (Nagai
(3) Elimination of alternating and static MFs produces a
et al., 2004). The smaller karyotypes maintained under
durable increase in cell proliferation.
metabolic restriction contribute to tumor core expansion, as
fewer chromosomes can be more rapidly duplicated.
The survival of twnors could thus be enhanced by certain
levels of chronic metabolic restrictions from hypoxia,
(4) The KC threshold (25 n1), as well as its extent over two
oligomycin or MFs.
orders of magnitude, is predicted by the work of Russian
It has been repeatedly confirmed that cancer cells
physicists on water (Semikhina and Kiselev, 1981).
become more malignant under metabolic restriction (Hill
Lack of sensitivity to MF intensity or to cell type
et al., 2009; Rockel and Vaupel, 2001; Jogi et al., 2003)
suggest tl1e knockout of a biological enzyme by physics.
in vitro (Anderson et al., 1989) and in the clinic (Brizel et al.,
(5) MF-exposed culture meditun. without cells, is a vector
1996; Nordsmark et al., 1996), to the point where it has
of MF action (proliferation and cell decay).
become a central issue in twnor physiology and treatment
(6) Measured changes in the pH of cell culture media from
(Rockel and Vaupel, 2001). From our data, it is logical to
conclude that KC observed under metabolic restriction is a
(7) A wide KC resonance (113Hz at 1)l1) is compatible
possible indicator of meta-genetic promotion in cancer cells
with the work of Russian physicists on water (Semikhina
(Li et al., 2012).
and Kiselev, 1981).
Y Li & P. Heroux
Electromagn Bioi Med, Early Online: 1-12
laboratory support and Michel Bourdages,
DOl: 10.31091153683782()13.817334
Institut de Recherche d'Hydro-Quebec, for
(8) KC is maximized at specific frequency-amplitude (fiB)
equipment contributions. We thank Louis Slesin for
combinations (Semikhina and Kiselev, 1981), suggest-
reviewing the document We also thank an anonymous
ing an interaction with water's protons.
reviewer for contributions to the article.
Mfs alter ATPS Fo
Declaration of interest
(9) ATPS Fo is the only site in the biota where conditions
We declare no competing financial interests.
for maximum sensitivity to MF action (Semik:hina et al.,
The work was suppmted by Royal Victoria Hospital
1988) happen together: high concentrations of protons
Research Institute Fund 65891.
and hydrophilic bonds in a narrow channel.
(10) Strongly acting MFs induce cell cultme characteristics
(Figure 5; top), closely matching those of a specific
References
ATPS Fo inhibitor, oligomycin.
(11) 1-IF activation of AlVIPK implies a perturbation to ATP
Ahlborn, A., Day, N,, Feychting, M., et al. (2000), A pooled analysis of
levels, thus a change in ATPS performance.
magnetic fields and childhood leukaemia. Br. J. Cane. 83:692-698.
Anderson, G. R., Stoler, D. L., Scarcello, L A. (1989). Normal
(12) Rhodamine 6 G, used by Russian physicists (Semik:hina
:fibroblasts responding to anoxia exllibit features of tlle malignant
and Kiselev, 1981) to detect MF effects, is also an
phenotype. J. Biol. Chem 264:14885-14892 (accessed 5 Jul2013).
inhibitor of ATPS Fo.
Andreev, S. N., Makarov, V. P., Tikhonov, V.I., Volkov, A. A. (2008).
Environmental :rv!Fs act on the core of human metabolism.
Ortho and para molecules of water in electric field. Available from:
llttp://arxiv.orgfabstphysicsf0703038v1 (accessed 5 Jul 2013).
Past evaluations of MF bio-effects were at a serious
Arakaki, N., Nagao, T., Niki, R., et al. (2003). Possible role of cell
disadvantage because of traditional toxicological and
surface H+-ATP synthase in the extracellular ATP syntl1esis and
epidemiological assumptions, that larger exposures induce
proliferation of human umbilical vein endotl1elial cells. MoL Cane.
larger responses. The controls of in vitro scientists were
Res. 1:931-939.
Armstrong, B., Theriault, G., Guenel, P., et al. (1994). Association
ah·eady randomly exposed by the :rv!Fs of their incubators.
between exposure to pulsed electromagnetic fields and cancer in
The flatness ofMFs' dose-response impaired epidemiological
electric utility workers in Quebec, Canada, and France. Am. J.
work, as most studies, except for domestic leukemia, used
Epidermiol. 140:805-820.
tainted controls (Milham, 2010). The interaction between
Boyer, P. D. (2002). A research journey witl1 ATP synt11ase. J. BioL
Chern. 277:39045-39061.
power-frequency :rv!Fs and living cells may have been
Brizel, D. M., Scully, S. P., Harrelson, J. M., et al. (1996). Thmour
underestimated for a long time, because of these unexpected
oxygenation predicts for tl1e likelihood of distant metastases in human
characteristics.
soft tissue sarcoma Cancer Res. 56:941-943.
Some diseases appear to have strengthened, with no
Bulterijs, S. (2011). Metfonnin as ageroprotector. Rejuvenation Res. 14:
clear causation, as more advanced technology, in great part
Chen, G., Upham, B. L., Sun, W., et al. (2000). Effect ofElVfF exposure
based on electricity, has expanded. Chronic diseases that
on chemically induced differentiation of friend erythroleukemia cells.
increased or decreased in the past century, and that are
Ercv. Health Persp. 108:967-972.
connected to ATP metabolism, should be examined for a link
Cleland, W. W. (2000). Low-barrier hydrogen bonds and enzymatic
catalysis. Arch. Biochem Biophys. 382:1-5.
with MFs. But our understanding of AMPK and metabolism
Das, B., Mondragon, M. 0. H., Sadeghian, M., et al. (1994). A novel
is incomplete (Jones and Thompson, 2009), making a link
ligand in lymphocyte-mediated cytotoxicity: E:<pression of the/3
between MFs and any specific disease, such as diabetes,
subunit of H+ transporting ATP synthase 011 the surface of tumor cell
lines. J. Exp. Med. 180:273-281.
De Ninno, A., Castellano, A. C. (2011). On tl1e effect of weak magnetic
According to our data, MFs are physiological agonists of
:field on solutions of glutamic acid: The function of water. J. Phys.
metformin, perhaps through inhibition of ATPS. But metfor-
Con(. 329:012025.
min is a knovm geroprotector (Bulterijs, 2011). Beyond the
Elias, M., Wellner, A., Goldin-Azulay, K., et al. (2012). The molecular
anti-oxidants we investigated and :rv!Fs, there are other agents
basis of phosphate discriminatiotl in arsenate-rich environments.
known to impair the use of oxygen. H
Nature 491:134-137.
2S, for example, inhibits
Fillingame, R. H., Angevine, C. M., Dmitriev, 0. Y. (2003). Mechanics
the cytochrome enzymes of mitochondria, reversibly slowing
of coupling proton movements to c-ring rotation in ATP syntl1ase.
the metabolism of mice, lowering their body temperatme,
FEBS Lett. 555:29-34.
breathing rate and use of oxygen. Ftuthermore,
Goodm Ul, E. M., Greenebaum, B., Marron, M. T. (1979). Bioeffects of
extremely low-frequency electromagnetic fields: variation with inten-
Caenorhabditis elegans, when grown in 50 ppm H2S, lives
sity, waveform, and individual or combined electric and magnetic
70% longer than in normal air (Miller and Roth, 2007). Such
:fields. Radiat. Res. 78:485-501.
models suggest that the ability of MFs to suppress ATPS
Gray, H. B., Winkler, J. R. (1996). Electron transfer in proteins. Annu.
(Hootkooper et al., 2013) may have played a role in the
Rev. Biochem 65:537-561.
Hardie, D. G., Hawley, S. A. (2001). AMP-activated protein kinase: The
increased lifespan observed in developed countries in the past
energy charge hypothesis revisited. BioEssays 23:1112-1119.
Hayes, T. B., Collins, A., Lee, M., et al. (2002). Hermaphroditic,
demasculinized frogs after exposure to t11e herbicide atrazine at low
ecologically relevant doses. ?roc. Natl. Acad. Sci. USA 99: 5476-5480.
Authors acknowledge the late Nancy Wertheimer and Edward
Herou:'<, P. (1987). 60Hz electric and magnetic fields generated by a
Leeper, who saw it first; Semikhina and Kise]ev, whose work
distribution network. Bioelectromagnetics 8:135-148.
Heroux, P. (1991). A dosimeter for assessment of exposures to ELF
made our analysis possible. We are grateful to Janet Moir
:fields. Bioelectronw.gnetics 12:241-257.
and Lome Beckman of the Royal Victoria Hospital for
Hill, R. P., Marie-Egyptienne, D. T., Hedley, D. W. (2009). Cancer stem
cells, hypoxia and metastasis. Semi.rr. Radiat. OncoL 19:106-111.
Moser, C. C., Keske, J. K., Warncke, K., et al. (1992). Nature of
biological electron transfer. Nacure 355:796-802.
, M., Vanpel, P. (2001). Tumour hypoxia: Definitions and current
Motoshima, H., Goldstein, B. J., lgata, M., Araki, E. (2006). AMPK and
clinical, biologic, and molecular aspects. J. Natl. Cancer lnstic. 93:
cell proliferation - AMPK as a therapeutic target for atherosclerosis
and cancer. J. Physiol. 574:63-71.
Houtkooper, R. H., Moucl1iroud, L., Ryu, D., et al. (2013). Mitonuclear
Nagai, F., Kato, E., Tamura, H. (2004). Oxidative stress induces GSTP1
protein imbalance as a conserved longevity mechanism. Nature 497:
and CYP3A4 expression in the hwnan erythroleukemia cell line,
K562. BioL Pharm. Bull. 27:492-495.
Hovelmann, S., Beckers, T. L., Schmidt, M. (2004). Molecular
Nordsmark, M., Hoyer, M., Keller, J., et a!. (1996). The relationship
alterations in apoptotic pathways after PKB/Akt mediated chemore-
between twnour oxygenation and cell proliferation in hwna11 soft
sistance in NCI H460 cells. Br. J. Cane. 90:2370-2377.
tissue sarcomas. Inc. J. Radiat Oncol. Biol. Phys. 35:701-708.
International Agency for Research on Cancer. (2002). IARC Monographs
Novikov V. V., Ponomarev, V. 0., Novikov, G. V., et al. (2010). Effects
on che Evaluacion of Carcinogenic Risks co Humans. Non-ion ing
and molecular mechanisms of tl1e biological action of weak and
Radiacion, Part 1: Scatic and Excremely Low-Frequency (ELF)
extremely weak magnetic fields. Biophysics 55:565-572.
Eleccric and Magnecic fields. Volume 80. Lyon, France: IARC Press.
Phillips, J. L., Winters, W. D., Rutledge, L. (1986). In vitro exposure to
lshido, M., Nitta, H., Kabuto, M. (2001). Magnetic fields of 50-Hz at
electromagnetic fields: Changes in tumour cell properties. Int J.
1.2 f!T as well as 100 f!T cause uncoupling of inhibitory pathways of
Radiac. Biol. 49:463-469.
adenylyl cyclase mediated by melatonin 1a receptor in MF-sensitive
Procopio, J., Fornes, J. A. (1997). Fluctuations of the proton-
MCF-7 cells. Carcinogenesis 22:1043-1048.
electromotive force across the inner mitochondrial membrane. Phys.
Jezek, P., Plecita-Hiavata, L., Smolkova, K., Rossignol, R. (2010).
Rev. 55:6285-6288.
Distinctions and similarities of cell bioenergetics and t11e role of
Reece, S. Y., Nocera, D. G. (2009). Proton-coupled electron transfer in
mitochondria in hypoxia, cancer, and embryonic development. Tnt J.
biology: Results from synergistic studies in natural and model
Biochem. Cell Biol. 42:604-622.
Jogi, A., 0ra, 1, Nilsson, H., et al. (2003). Hypoxia-induced dediffer-
systems. Ann. Rev. Biochem 78:673-699. http://aiXiv.org/abs/
entiation in neuroblastoma cells. Cancer Lect 197:145-150.
1101.4994v1 (accessed 5 Jul 2013).
Jones, R. G., TI10mpson, C. B. (2009). Tumor suppressors and cell
Reiter, G. F., Kolesnikov, A. 1,Paddison, S. J., eta!. (2011).Evidence of
metabolism: A recipe for cancer growt11. Genes Dev. 23:537-548.
a new qua11tum state of nano-confined water. Available from: http://
Jones, R. G., Plas, D. R., Kubek, S., et al. (2005). AMP-activated protein
arxiv.org/abs/ll01.4994v1(accessed 5 Jul 2013).
kinase induces a p53-dependent metabolic checkpoint MoL CelL 18:
Sasada, R., Marcey, D. (2010). ATP Syntl1ase. Available from: http://
Kapralov, P. 0., Artemov, V. G., Leskin, A. A., et al. (2008). On the
html#fig1 (accessed 5 Jul2013).
possibility of sorting ortho and para water molecules during diffusion
Semikhina, L. P., Kiselev, V. F., Levshin, L. V., Salets.kii, A.M. (1988).
in nanopores. BulL Lebedev Phys. Insc. 35:221-223.
Effect of weak magnetic fields on tl1e luminescence-spectral proper-
Kanellis, J., Kandane, R. K., Etemadmoghadam, D., et a!. (2006).
ties of a dye in an aqueous solution. J. Appl. Speccros. 48:556-559.
Activators of t11e energy sensing kinase AJvlPK inhibit random cell
Semiklrina, L. P., Kiselev, V. F. (1981). Effect of weak magnetic fields on
movement and chemotaxis in U937 cells. Immunol. Cell Biol. 84:
the properties of water and ice. Russ. Plrys. J. 31:5351-5354.
Su, D., Heroux, P. (2012). Survey of extra-low frequency and very-low
Kim, E. K., },ifiller, 1, Aja, S., et al. (2004). C75, a fatty acid synthase
frequency magnetic fields in cell culture incubators. Available from:
inhibitor, reduces food intake via hypot11alamic AMP-activated
llttp://arxiv.org/abs/1211.2458 (accessed 5 Jul 2013).
protein .kinase. J. Biol. Chem 279:1997 19976.
Svendsen, A.L., Weihkopf, T., Kaatsch, P., Schtiz, J. (2007). Exposure to
Kizaka-Kondoh, S., Inoue, M., Harada, H., Hlraoka, M. (2003). Tumor
magnetic fields a11d survival after diagnosis of clri!dllOod leukemia: A
hypoxia: A target for selective cancer t11erapy. Cancer Sci 94:
german cohort study. Cancer Epidemiol. Biomarkers Prev. 6:
Kus!Uler, D. J., Baker, A., Dunstall, T. G. (1999). Pharmacological uses
Tikhonov, V. I., Volkov, A. A. (2002). Separation of water into its ortl1o
and perspectives of heavy water and deuterated compow1ds. Can. J.
and para isomers. Science 296:2363.
PhysioL Plw.rmacol. 77:79-88.
Vagero, D., Olin, R. (1983). Incidence of ca11cer in tl1e electronics
Lai, H., Singh, N. P. (2004). Magnetic-field-induced DNA strand breaks
industry: Using the new Swedish Cancer Environment Registry as a
in brain cells of the rat. Environ. Healch Perspecc. 112:687-694.
screening instrument Br. J. Inti Med 40:188--192.
Lee, D., Steffes, M. W., Sjodin, A., et al. (2010). Low dose of some
Vandenberg, L. N., Colborn, T., Hayes, T. B., et a!. (2012). Hormones
persistent organic pollutants predicts type 2 diabetes: A nested case-
and endocrine-disrupting chemicals: Low-dose effects and nonmono-
control study. Environ. Healch Perspect 118:1235-1242.
tonic dose responses. Endocr. Rev. 33:378--455.
Li, Y., Heroux, P., Kyrychenko,1(2012).Metabolic restriction of cancer
Viollet, B., Lantier, L., Devin-Leclerc, J., et al. (2009). Targeting the
cells in vitro canses karyotype contraction - An mdicator of cancer
AMPK pathway for t11e treatment of Type 2 diabetes. Fronc. Biosci.
promotion? Tumor BioL 33:195-205.
Liu, L., Cash, T. P., Jones, R. G., et al. (2006). Hypoxia-induced energy
Wert11eimer, N., Leeper, E. (1979). Electrical wiring configurations and
stress regulates mRNA translation and cell growth. Mol. Cell 21:
childhood cancer. Am J. EpidemioL 109:273-284.
Winder, W. W., Holmes, B. F., Rubink, D. S., eta!. (2000). Activation of
},l{ild, K. H., Wilen, J., Mattsson, M. 0., Simko, M. (2009). Background
AMP-activated protein kinase increases mitochondrial enzymes in
ELF magnetic fields in incubators: A factor of importance in cell
skeletal muscle. J. Appl. Physiol. 88:2219-2226.
culture work. Cell Biol. Inc. 33:755-757.
Winder, W. W., Hardie, D. G. (1999). AMP-activated protein kinase, a
},l{i]Jer, A. B., Teresa, T., Agnew, D. A., et a!. (1996). Leukemia
metabolic master switch: possible roles in Type 2 diabetes. Am. J.
following occupational exposure to 60-Hz electric and magnetic fields
PhysioL Endocrinol. Mecab. 277:E1-E10.
among Ontario electric utility workers. Am. J. EpidemioL 144:
Wu, M., Neilson, A., Swift, A. L., et a!. (2006). Multiparameter
metabolic analysis reveals a close link betlveen attenuated mitochon-
}, {iller, D. L., Rotl1, M. B. (2007). Hydrogen sulfide increases
drial bioenergetic function and enhanced glycolysis dependency in
thermotolerulCe and lifespan in Caenorhabdicis elegans. Proc. Nacl.
human tumor cells. Am J. PlzysioL Cell. Physiol. 292:C125-C136.
Acad. Sci. USA. 104:20618-20622.
Zorov, D. B., Juhaszova, M., Yaniv, Y., et al. (2009). Regulation and
},l{ilham, S. (2010). Hlstorical evidence that electrification cansed the
pharmacology of tl1e mitochondrial permeability transition pore.
20th century epidemic of "diseases of civilization". Med. Hypocheses
Cardiovas. Res. 83:213-225.
Mitchell, P. (1966). Chemiosmotic coupling in oxidative and photosyn-
thetic phosphorylation. Biol. Rev. 41:445-501.
Source: http://www.lifeharmonizer.name/userfiles/file/ELF%20MF%20cancer%20cells.pdf
Available online at Anomalous self-experience and childhood trauma in Elisabeth Hauga,⁎, Merete Øiea,b, Ole A. Andreassen c, Unni Bratlien a, Barnaby Nelson d, Monica Aas c, Paul Møller e, Ingrid Melle c aDivision of Mental Health, Innlandet Hospital Trust, Ottestad, Norway bDepartment of Psychology, University of Oslo, Oslo, Norway cNORMENT, KG Jebsen Centre for Psychosis Research, Institute of Clinical Medicine, Division of Mental Health and Addiction, University of Oslo, and Oslo
Flexible Benefit Plan Enrollment Guide Ozarks Technical Community College 01/01/16 – 12/31/16 Instructions for Using This Guide: 1. Review the information and decide how this plan benefits you. 2. Estimate your out-of-pocket health care expenses using the worksheet. 3. Enroll or waive participation by completing the election process.