Levitra enthält Vardenafil, das eine kürzere Wirkdauer als Tadalafil hat, dafür aber schnell einsetzt. Männer, die diskret bestellen möchten, suchen häufig nach levitra kaufen ohne rezept. Dabei spielt die rechtliche Lage in der Schweiz eine wichtige Rolle.
Microsoft word - nanogen hair growth factor serum study report
Evaluation of the hair growth and retention activity
of two solutions on human hair explants
Study Directed by Dr E. Lati of Laboratoire Bio-EC, Centre de Recherches Biologiques
et d'Experimentations Cutanees, on behalf of Pangaea Laboratories Ltd.

Evaluation of the hair growth and retention activity of two solutions on human hair explants Independent Laboratory Evaluation and Experimental Report
Study Promoter:
Pangaea Laboratories Ltd.
Tel +44 (0) 208 4582500
Fax: +44 (0)870 460 1690
www.pangaea.co.uk
Study Realised by:
Laboratoire Bio-EC
1 Chemin de Saulxier
91165 Longjumeau
Tel: +33 0169 414221
Technical Manager:
Study Director:
Quality Manager:
M. A. Shahiri MSc
2012 Copyright Nanogen Hair Research. Al rights reserved.
This independent study report is for internal and professional use only and should not be shared with or acted upon by consumers.

Evaluation of the hair growth and retention activity of two solutions on human hair explants Independent Laboratory Evaluation and Experimental Report
Study Schedule Commence:
Technical Manager
2012 Copyright Nanogen Hair Research. Al rights reserved.
This independent study report is for internal and professional use only and should not be shared with or acted upon by consumers.

Evaluation of the hair growth and retention activity of two solutions on human hair explants Independent Laboratory Evaluation and Experimental Report
Hair loss is often caused by abnormal hair fol icle cycling and changes in hair fol icle morphology. The molecules and biochemical pathways that control normal hair fol icle formation, hair growth, and hair loss are beginning to be understood. Despite advances in hair research, current treatments on the market such as minoxidil and finasteride have limitations. Both solutions work very slowly, and finasteride particularly has some negative side effects therefore patient compliance for these treatments is low. An alternative or complementary treatment to topical minoxidil solution is highly desirable. Vascular Endothelial Growth Factor (VEGF), a cytokine natural y produced in the body has been characterised and identified as a key mediator of hair growth. With the aid of recombinant DNA technology, safe, identical copies of VEGF protein can be biosynthesised and used in treatments.
The objective of this study was to assess and evaluate the efficacy of Nanogen VEGF, or "Hair Growth Factor" and 5% minoxidil solution alone and in combination with each other. An ex vivo experimental model utilising human hair was designed to compare the effects of these two treatments.
Nanogen Hair Growth Factor outperformed minoxidil by as much as 237% and showed a complementary effect on minoxidil treatment when used in combination. Al results were statistical y significant.
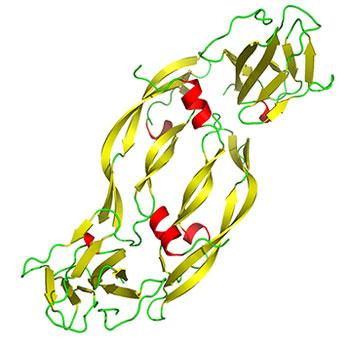
In the field of hair research, VEGF (Vascular Endothelial Growth Factor) has been identified as a key mediator of
Throughout the history of medicine, a tremendous
hair growth (Figure 1). It can influence al stages of hair
amount of time has been dedicated to discovering
growth, from cel proliferation and migration through to
external chemical molecules or elements, and applying
angiogenesis and preventing apoptosis. It was
them to the body to induce a physiological response.
hypothesised that cytokines and particularly VEGF can enhance hair growth and increase survival, reducing hair
It is only very recently that advances in molecular
loss. If this were the case, VEGF could be used as an
biology, and particularly proteomics, have described the
alternative to wel known treatments such as minoxidil. It
mechanism of intercel ular signals in great depth. We
was also hypothesised that VEGF can improve response
now know that many cel ular functions, from mitosis and
to minoxidil treatment if used in combination.
differentiation right through to apoptosis, are control ed by an array of biological molecules (proteins) known as
cytokines. They can control al aspects of cel ular proliferation and differentiation. Tissues in the body communicate constantly through a flurry of these signals.
Their existence is not a surprise to the scientific community, however the intensity and breadth of their effects was not ful y understood and appreciated until now. The effect of cytokines or "growth factors" as they are commonly known is so significant that they would have great utility if they could be characterised, isolated
Figure 1 Tertiary structure (3D) of Vascular Endothelial
and applied correctly as therapeutic agents.
2012 Copyright Nanogen Hair Research. Al rights reserved.
This independent study report is for internal and professional use only and should not be shared with or acted upon by consumers.




Evaluation of the hair growth and retention activity of two solutions on human hair explants Independent Laboratory Evaluation and Experimental Report
Experimental Design & Methods
To test these hypotheses, an experiment was designed
A scalp plasty from a human donor was micro-dissected
to compare the effects of Nanogen VEGF with 5%
to isolated 80 individual hairs and their bulbs. Each hair
minoxidil, via an ex vivo experimental model utilising
was cut approximately 1mm above the infundibulum.
human hair. The objectives of this study were to
(Figure 2.1, 2.2).
measure and compare:
Hair growth rates after topical application of Nanogen Hair Growth Factors and minoxidil.
Hair fol icle survival after topical application of Nanogen Hair Growth Factors and minoxidil.
The effects of combination therapy with Nanogen Hair Growth Factors and minoxidil.
Bio-EC - an independent clinical testing laboratory based in France, was instructed to conduct a study investigating Nanogen Hair Growth Factors, using
minoxidil as a positive control. The study utilised
Figure 2.2 Micro-dissection of a hair plasty.
impartial measuring techniques, and was blind as the
containers of growth factor and minoxidil were label ed anonymously. Products tested
Figure 2.1 Anatomy of human hair
2012 Copyright Nanogen Hair Research. Al rights reserved.
This independent study report is for internal and professional use only and should not be shared with or acted upon by consumers.
Evaluation of the hair growth and retention activity of two solutions on human hair explants Independent Laboratory Evaluation and Experimental Report
The hairs were then placed in 48-wel plates and kept in classical survival cel culture conditions (Philpott medium, at 37°C in a 5% CO2 atmosphere). The hairs were re-measured on day -4 (4 days post micro-dissection). Those demonstrating a healthy total growth of 54 to 1641 µm were selected, randomised and distributed into different batches of 7 hairs for product testing.
Treatment Hair Batch
Mean growth from D-4 to D0
Figure 2.3 Individual hair plasty in culture medium.
Treatment & Sampling
After screening, the culture medium was renewed on
The length of each individual hair was measured from
day 0, 2, 4, 7 and 8. New treatment solution was also
the bulb to the hair tip with the use of Olympus Cel D
added to every wel at each time point.
Treatment solution
Volume added to culture
Hair samples were taken and measured at day 0, 4, 8 and 11. The growth of each hair was fol owed individual y.
Figure 2.4 Micro-dissection and micro-photography
2012 Copyright Nanogen Hair Research. Al rights reserved.
This independent study report is for internal and professional use only and should not be shared with or acted upon by consumers.
Evaluation of the hair growth and retention activity of two solutions on human hair explants Independent Laboratory Evaluation and Experimental Report
treatment (p-value = 0.007). The complementary effect of Nanogen Hair Growth Factors on minoxidil treatment
When compared to minoxidil, Nanogen Hair Growth
is clearly evident. However, the combination did not
Factors are significantly more effective at stimulating hair
increase survival as expected. There is no theoretical
growth. Up to 237% faster growth rate was shown
basis for the two treatments to interact; therefore this
in the growth factor group compared to the minoxidil
may be due to the smal er sample number in the second
positive control (Figure 3.1). A Student's t-test
test, or perhaps the increased dilution of Philpott
found a statistical y significant difference between the
medium caused by adding both treatment solutions.
At the end of the hair cycle, as before hair fal s out, necrosis (death) of some cel s in the bulb region can occur. This is responsible for the smal reduction in hair length. Treatment with Nanogen Hair Growth Factors al owed the hairs to continue growing at a healthy rate. In contrast, with minoxidil monotherapy, hair length begins to decline from day 9, indicating necrosis prior to the onset of hair loss. This shows that Nanogen Hair Growth Factors prolonged hair survival more effectively than minoxidil.
Figure 3.2 Graph to compare average hair growth rate
over time in minoxidil against a combination of minoxidil
and Nanogen Hair Growth Factor.
It can be concluded that:
Nanogen Hair Growth Factor treatment can significantly increase hair growth rate, up to 237% more than minoxidil monotherapy.
Figure 3.1 Graph to compare average hair growth rate
Nanogen Hair Growth Factor treatment
over time in minoxidil against Nanogen Hair Growth
prolongs hair survival, and therefore can
prevent or reduce hair loss.
After these results, the test was repeated to investigate the combination of Nanogen Hair Growth Factors with
Nanogen Hair Growth Factors in combination with minoxidil can significantly increase hair
minoxidil, in comparison to minoxidil alone. 133% higher
growth rate by up to 133% more than minoxidil
hair growth rate was observed with the combination
treatment than with minoxidil treatment alone (Figure
3.2). A Student's t-test found a statistical y significant
difference in hair growth rate between the two groups of
2012 Copyright Nanogen Hair Research. Al rights reserved.
This independent study report is for internal and professional use only and should not be shared with or acted upon by consumers.
Evaluation of the hair growth and retention activity of two solutions on human hair explants Independent Laboratory Evaluation and Experimental Report
QUALITY CONTROL STATEMENT
This study was conducted in compliance with Good Laboratory Practices (Arrêté du 10 Août 2004), as wel as in compliance with the validated procedures and SOP of Laboratoire BIO-EC. The audits performed ensure that al the steps of the study are control ed. The dates and steps inspected during the various audits are presented in the table below: Audit type
Dates of report to Study
Dates of report to the
Laboratory histology
Laboratory histology
Laboratory histology
This report was reviewed by the Quality Manager and confirms that the methods, standard operating procedures and observations are faithful y and completely described, and that the results reported reflect with accuracy the raw data of the study.
2012 Copyright Nanogen Hair Research. Al rights reserved.
This independent study report is for internal and professional use only and should not be shared with or acted upon by consumers.
Evaluation of the hair growth and retention activity of two solutions on human hair explants Independent Laboratory Evaluation and Experimental Report
Appendix: Selected microscope images of hair biopsies in wells at various stages.
2012 Copyright Nanogen Hair Research. Al rights reserved.
This independent study report is for internal and professional use only and should not be shared with or acted upon by consumers.
Evaluation of the hair growth and retention activity of two solutions on human hair explants Independent Laboratory Evaluation and Experimental Report
2012 Copyright Nanogen Hair Research. Al rights reserved.
This independent study report is for internal and professional use only and should not be shared with or acted upon by consumers.
Evaluation of the hair growth and retention activity of two solutions on human hair explants Independent Laboratory Evaluation and Experimental Report
2012 Copyright Nanogen Hair Research. Al rights reserved.
This independent study report is for internal and professional use only and should not be shared with or acted upon by consumers.
Source: http://www.nanogen.nl/producten/Serum-VEGF-studie.pdf
Making sense of Making sense of antipsychotics This booklet is for anyone who has been prescribed antipsychotic drugs, or who thinks they may be offered them, and also for their friends and family. It explains why these drugs may be prescribed, what their effects are, and when to avoid them. Note:Antipsychotics are sometimes called ‘neuroleptics' or ‘major tranquillisers'.
UNITED STATES COURT OF APPEALS FOR THE SECOND CIRCUIT ARKANSAS CARPENTERS HEALTH AND WELFARE FUND, MARIA LOCURTO, PAPER, ALLIED-INDUS, UNITED FOOD AND COMMERCIAL WORKERS UNION-EMPLOYER, LOUISIANA WHOLESALE DRUG CO., INC., CVS PHARMACY, INC., RITE AID CORPORATION, ARTHUR'S DRUG STORE, INC. SOL LUBIN, ANN STUART, LINDA K. MCINTYRE, PLAINTIFFS, -AGAINST-








