Levitra enthält Vardenafil, das eine kürzere Wirkdauer als Tadalafil hat, dafür aber schnell einsetzt. Männer, die diskret bestellen möchten, suchen häufig nach levitra kaufen ohne rezept. Dabei spielt die rechtliche Lage in der Schweiz eine wichtige Rolle.
Layout
Visit http://tinyurl.com/DMEandDR for online testing and instant CME certificate
Current Management of
Diabetic Macular Edema
and Diabetic Retinopathy
Clinical Cases
Original Release: January 2, 2015
Last Review: December 9, 2014
Expiration: January 31, 2016
This continuing medical education activity is jointly provided
by the University of Nebraska Medical Center, Center for
Continuing Education and MedEdicus LLC.
This continuing medical education activity is supported
through an unrestricted educational grant from
Regeneron Pharmaceuticals, Inc.
This activity intends to educate retina
This continuing medical education activity is
specialists, retina fellows, and comprehensive
supported through an unrestricted educational
ophthalmologists caring for patients with DR/DME.
grant from Regeneron Pharmaceuticals, Inc.
Learning Objectives
Upon completion of this activity, participants will
Diana V. Do, MD, had a financial relationship
be better able to:
during the past year with the following
• Recognize the importance of individualized
commercial interests that produce health care-
glycemic control in optimizing outcomes for
related products and/or services in the form of
patients with DR/DME
Consultant: Allergan, Inc; Genentech, Inc; and
Quan D. Nguyen, MD, MSc
• Discuss the utility of different diagnostic
Regeneron Pharmaceuticals, Inc.
imaging techniques in guiding the
Professor and Chair
Jeffrey S. Heier, MD, had a financial relationship
management of patients with DR/DME
during the past year with the following
• Describe the efficacy, dosing, and safety profiles
McGaw Memorial Endowed Chair
commercial interests that produce health care-
of current and emerging treatment options
related products and/or services in the form of
Grants/Research Support Recipient: Aerpio
Director of the Stanley M. Truhlsen
• Confidently tailor diagnostic and treatment
Therapeutics; Genentech, Inc; and Regeneron
strategies for various patients with DR/DME
Pharmaceuticals, Inc;
Consultant: Aerpio
University of Nebraska
• Communicate effectively with referring
Therapeutics; Allegro Ophthalmics, LLC;
physicians regarding the relevant systemic and
Genentech, Inc; Kala Pharmaceuticals Inc;
ophthalmic health issues of their mutual
Regeneron Pharmaceuticals, Inc; and Stealth
patients with DR/DME
Diana V. Do, MD
Joint Providership Credit Statement
Quan D. Nguyen, MD, MSc, had a financial
This activity has been planned and implemented
relationship during the past year with the
Associate Professor of Ophthalmology
in accordance with the accreditation
following commercial interests that produce
Vice Chair for Education
requirements and policies of the Accreditation
health care-related products and/or services in
Director of the Carl Camras Center
Council for Continuing Medical Education
the form of
Grants/Research Support Recipient
for Innovative Clinical Trials
through the joint providership of the University of
and Scientific Advisory Board: Bausch + Lomb
Nebraska Medical Center, Center for Continuing
Incorporated; Genentech, Inc; Regeneron
Director of the Ophthalmology
Education and MedEdicus LLC.
Pharmaceuticals, Inc; and Santen
Residency Training Program
Pharmaceutical Co, Ltd.
University of Nebraska
The University of Nebraska Medical Center,
Anne Peters, MD, had a financial relationship
Center for Continuing Education is accredited by
during the past year with the following
the Accreditation Council for Continuing Medical
commercial interests that produce health care-
Education to provide continuing medical
related products and/or services in the form of
Jeffrey S. Heier, MD
education for physicians.
Grants/Research Support Recipient: Medtronic
Director, Vitreoretinal Service
MiniMed, Inc;
Consultant: Abbott Diabetes Care
The University of Nebraska Medical Center,
Ophthalmic Consultants of Boston
Inc; Becton, Dickinson and Company; Bristol-
Center for Continuing Education designates this
Boston, Massachusetts
Myers Squibb/AstraZeneca; Eli Lilly and
enduring material for a maximum of 1.5
AMA
Company; Janssen Pharmaceuticals, Inc;
PRA Category 1 Credits™. Physicians should
Anne Peters, MD
Medtronic MiniMed, Inc; Novo Nordisk; and
claim only the credit commensurate with the
Professor of Medicine
Sanofi;
Speakers Bureau: Bristol-Myers
extent of their participation in the activity.
Director, University of Southern
Squibb/AstraZeneca; and Novo Nordisk.
California Westside Center
To Obtain AMA PRA Category 1 Credit™
It is the policy of the University of Nebraska
To obtain
AMA PRA Category 1 Credit™ for this
Medical Center to ensure balance,
Keck Medicine of University
activity, read the material in its entirety and
independence, objectivity, and scientific rigor in
of Southern California
consult referenced sources as necessary.
all its sponsored educational activities. All faculty,
Los Angeles, California
Complete the evaluation form along with the post
activity planners, and staff involved in the
test answer box within this supplement. Remove
development of this activity have disclosed any
the Activity Evaluation/Credit Request page from
significant financial interest or other relationship
the printed supplement or print the Activity
with manufacturer(s) of any commercial
Evaluation/Credit Request page from the Digital
product(s)/device(s) and/or provider(s) of
Edition. Return via fax to 1-203-286-1899. Your
commercial services included in this educational
certificate will be mailed to the address you
activity. The intent of this disclosure is not to
provide on the Activity Evaluation/Credit Request
prevent a faculty or staff member with a relevant
form. Please allow 4 weeks for Activity Evaluation/
financial or other relationship from participating
Credit Request forms to be processed. There are
in the activity, but rather to provide participants
no fees for participating in and receiving CME
with information on which they can base their
credit for this activity.
own judgments. The University of Nebraska
Medical Center has identified and resolved any
Alternatively, we offer instant certificate
and all faculty conflicts of interest prior to the
processing and support Green CME. Please take
release of this activity.
this post test and evaluation online by going to
you will receive your certificate immediately. You
The University of Nebraska Medical Center,
must score 70% or higher to receive credit for this
Center for Continuing Education presents this
activity, and may take the test up to 2 times.
information for educational purposes only. The
Upon registering and successfully completing the
content is provided solely by faculty who have
post test, your certificate will be made available
been selected because of recognized expertise
online and you can print it or file it.
in their field. Participants have the professional
responsibility to ensure that products are
The views and opinions expressed in this
prescribed and used appropriately on the basis
educational activity are those of the faculty and
of their own clinical judgment and accepted
do not necessarily represent the views of the
standards of care. The University of Nebraska
University of Nebraska Medical Center, Center for
Medical Center, Center for Continuing Education
Continuing Education; MedEdicus LLC;
assumes no liability for the information herein.
Regeneron Pharmaceuticals, Inc; or
Retina.
then remained.13 The patients who were tightly controlled
during the trial found it too difficult to maintain their
HbA1c levels at 7%, even with the tools and resources
Optimal management strategies for patients with diabetic
made available to them. Without the active conditions of
retinopathy (DR) and diabetic macular edema (DME)
the trial, the median HbA1c levels of the intensively treated
continue to evolve at a rapid pace. Careful consideration
patients went from 7.2% to 7.9%.13
of numerous patient factors and treatment options is
essential to the generation of positive visual outcomes. To
From these results, the phenomenon of metabolic memory
that end, we convened a multidisciplinary panel to discuss
was noted. If a patient's HbA1c is 9% for 10 years and is
current approaches to successful management of patients
subsequently lowered to 7% for the next 10 years, the risk for
with DR or DME. We have selected several challenging
microvascular and macrovascular complications is much
case scenarios that will highlight management options
worse than if the HbA1c starts out at 7% for the first 10 years
such as laser photocoagulation, anti-vascular endothelial
and then increases to 9% for the second 10 years. There is
growth factor (VEGF) therapies, intravitreal steroids, and
something about that first phase of diabetes during which if
glycemic control.
tight control is achieved, long-term outcomes are improved.
—Quan Dong Nguyen, MD, MSc
This is what was observed in the sustained follow-up to the
DCCT and the Epidemiology of Diabetes Interventions and
Complications (EDIC)—the intensive therapy group
Glycemic Control Strategies
continues to do better for many years. In a recent study, the
risk for further progression of retinopathy, progression to
Dr Peters: The recently published position statement of the
proliferative diabetic retinopathy (PDR), clinically significant
American Diabetes Association (ADA) for the treatment of
macular edema, and the need for intervention
type 1 diabetes mellitus addresses this condition across the
(photocoagulation or anti-VEGF) over 18 years of follow-up in
life span,1 and although we think of type 1 diabetes as a
the DCCT/EDIC were described.14 Although the cumulative
predominantly pediatric disease, it can develop at any age.
incidence of these outcomes continues to be lower in the
In the United States, there are as many as 3 million patients
group that initially received intensive treatment, the annual
with type 1 diabetes,2 with approximately 167,000 of them
incidence of these outcomes is now comparable between
being children or youths.3 Historically, HbA1c targets for
groups, largely because of a reduction in risk in the group
children were higher than those for adults because of the
that initially received conventional treatment.14
premise that severe, recurrent hypoglycemia in children
was associated with neurocognitive compromise,4 and that
There are other instances of metabolic memory found in
childhood was protective with respect to hyperglycemia.5,6
large studies looking at patients with type 2 diabetes.15,16
The concerns pertaining to hypoglycemia and
The UK Prospective Diabetes Study (UKPDS) also showed
neurocognitive problems have been allayed,1,7,8 and early
the benefit of early tight glycemic control. These patients
hyperglycemia and glucose variability may pose risk to the
had been recently diagnosed with type 2 diabetes and
central nervous system.9
randomized to 1 of 2 arms: an intensive treatment arm
(with either a sulfonylurea or insulin) or a conventional
On the other hand, people with type 1 diabetes used to
diet-controlled arm.15 The intensively treated patients had
die before they reached advanced age because of
a 12% reduction in all diabetes-related end points over
hypoglycemia and other complications. Now, patients with
10 years (
P=.029) and a 25% reduction in the risk for
type 1 diabetes are living longer.10,11 We have lowered
microvascular end points, largely because of the reduced
pediatric targets and raised targets for older adults.1 Our
need for laser photocoagulation.15 As in DCCT, the patients'
knowledge about type 1 diabetes is ever increasing, and
HbA1c values tended to drift up over time in the follow-up
we are doing more type 1-focused research.
study, but the benefits of early tight control were
demonstrated with a persistent 24% relative reduction in
Clinical evidence has supported the benefits of glycemic
risk for microvascular disease (
P=.001).17 Later tight control
control for patients with type 1 diabetes, with studies such
may not be as beneficial.
as the Diabetes Control and Complications Trial (DCCT),
which showed unequivocally that for the pathognomonic
How well are we doing? The Helmsley Charitable Trust has
complication for type 1 diabetes, DR,12 there is tremendous
established a registry of more than 26,000 patients from
benefit associated with intensive therapy. In the primary-
approximately 60 type 1 diabetes clinical centers in the
prevention cohort of the DCCT, there was a 76% reduction
United States, and it has shown that even the best centers
in the adjusted mean risk for retinopathy development for
are not able to get the average HbA1c of their patients to
those patients who received intensive therapy.12 With
less than 7%.18 Adolescence is a particularly difficult time
respect to the secondary-intervention cohort, the
for glycemic control,3,19 whereas older patients tend to do
progression of retinopathy was slowed by 54%, and the
better. Approximately 27% to 34% of adults are at target.20
development of proliferative or severe nonproliferative
The frequency of severe hypoglycemia increases with age,
retinopathy was reduced by 47%.12 Benefits were greater
and this is why the HbA1c targets for older patients with
in those patients who started intensive therapy earlier.
type 1 diabetes are not more aggressive.1 If a patient is
aged 65 years or older and has comorbidities and/or a
It is extremely difficult to achieve the same level of
short life expectancy, the HbA1c target becomes greater
glycemic control in patients with type 1 diabetes in the
than 7.5%.1 If the patient is particularly complex or in poor
world outside of clinical trials, because patients are trying
health, it becomes extremely difficult to establish a target
to balance high and low blood sugars often without
without increasing risk to the patient.1,21
the assistance of expert diabetes clinicians. When the
individuals in the control arm of the DCCT were made
When considering a strategy for glycemic control for
aware of the data from the trial, they lowered their HbA1c
patients with type 2 diabetes, the ADA/European
levels from a median value of 9.1% to 8.2%, where they
Association for the Study of Diabetes (EASD) position
statement advocates a patient-centered approach. The
receptor agonist, sodium-glucose co-transporter 2
other members of the Writing Group and I thoroughly
inhibitors, and metformin. Treatment of type 2 diabetes
reviewed the available evidence when we put the
has become much easier to manage given the new (along
statement together, and our recommendations are less
with some of the old) medications we have available.
algorithmic than previous approaches. Comparative
efficacy studies are limited; with respect to
pharmacotherapy, metformin should generally be
regarded as the optimal first-line drug, unless it is
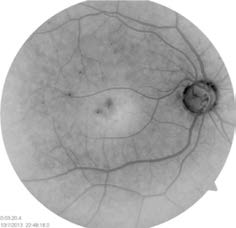
Dr Do: A 38-year-old gentleman with a history of type 1
contraindicated.22 After that, the picture is less clear. The
diabetes came in for his annual eye examination a few
Group spent hours looking at this and we were not able to
months ago. At the time of presentation, he had been
establish a definitive second-line step because of several
bothered by occasional blurred vision for several months.
variables such as practice setting, individual patient
His HbA1c was 7.0% three months prior to presentation.
characteristics, financial considerations, and the role of
His visual acuity was 20/30 in the right eye and 20/20 in
the left eye. A dilated examination showed some hard
exudates and some mild macular edema
(Figure 1).
Looking at the clinical trials, in addition to UKPDS and DCCT,
Dr Nguyen, when you assess your patients for suspected
there also are data from ACCORD,23,24 ADVANCE,25 and
DME, what imaging test(s) do you routinely obtain?
VADT.26 These trials were conducted in older patients who
had complications, many of whom had had macrovascular
events. It was thought that tightening glycemic control
would result in improved macrovascular outcomes. In the
latter 3 studies, some microvascular end points (pertaining
to retinopathy, nephropathy, and neuropathy) showed a
degree of improvement with tight control.
In ACCORD, the HbA1c target was below 6%.23 Trying toreach this target actually increased mortality, and the
Figure 1: Case 1 dilated
examination (OD).
study was stopped after a mean of 3.5 years of follow-up.23
Were these deaths due to the development of
Photo Courtesy of
hypoglycemia among patients? This turned out not to be
Diana V. Do, MD
the case.27,28 If a person with diabetes develops severe
hypoglycemia, whether on intensive therapy or not, it has
been shown that the risk for death increases 2- to 4-fold.27
For patients with long-standing diabetes, pushing
Dr Nguyen: In patients with new onset DME, I will obtain
their HbA1c values down with drugs that can cause
fluorescein angiography,29 as well as spectral domain
hypoglycemia is potentially dangerous. However, in
optical coherence tomography (OCT).30 If possible, the
addition to the finding of the risk for severe hypoglycemia
fluorescein angiogram could be done in a wide-angle
(noted in all studies), in ACCORD the treatment approach
system in order to assess the vasculature in the peripheral
designed to lower the HbA1c to less than 6% seemed to
increase mortality. It is doubtful an explanation for this will
be forthcoming, because all analyses done to date have
Dr Do: Dr Heier, what are your thoughts on the necessity of
been negative, but this study has changed current
angiography, given the sensitivity of OCT and the fact that
practice approaches and made individualization of A1C
many of our randomized clinical trials have not really
targets mandatory.
mandated the use of angiography?
In the aforementioned ADA/EASD position statement on
Dr Heier: I absolutely think that angiography is necessary.
type 2 diabetes, we focused on several domains when
In a straightforward patient like this, the OCT might show
trying to individualize a patient's target HbA1c.22 These
edema, and it might be fine for managing this particular
domains are all-encompassing, addressing the risk for
patient. There are patients, however, who may have what
complications, patient life expectancy, disease duration,
appears to be relatively subtle disease, and if they have
cardiovascular disease, and other factors. The goal is to
had diabetes for years, you can see gross nonperfusion
balance the patient with respect to all these domains, and
and unexpected neovascularization.32 I always obtain a
to arrive at an individualized target.
baseline fluorescein angiogram in patients with diabetes
and unexplained loss of visual acuity; I may not get
In the real world, patients exhibit a huge amount of
another one for years if the patient's disease is easily
variability with respect to these individual domains. Some
managed after initial assessment.
patients may be very worried about retinopathy, but
severe underlying cardiovascular disease may limit how
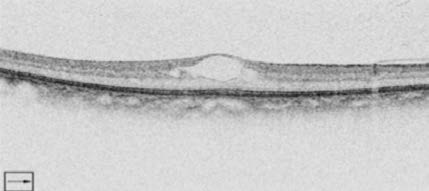
Dr Do: With this particular patient, we obtained both a
aggressive clinicians can be with glycemic control. Each
fluorescein angiogram and an OCT
(Figure 2). On the
patient should have his or her own target. The goal is to get
angiogram, there is evidence of leakage in the parafoveal
as close to normal blood sugar levels as possible without
region, and the OCT shows center-involved DME.
causing hypoglycemia or other adverse side effects.
He had not had any previous treatment. Dr Heier, for this
In order to minimize the risks of pharmacotherapy, my
patient who has a visual acuity of 20/30 and complains of
preference is to use drugs that do not cause hypoglycemia
occasional blurriness, what treatment option would you
and weight gain, and we have a lot of options to that end.
If patients are willing to work with me, I can usually get
even those with advanced type 2 diabetes to target using
Dr Heier: I am a little hesitant to start this patient on anti-
a combination of basal insulin, a glucagon-like peptide-1
VEGF therapy at this early stage. His HbA1c of 7.0% is fair,
Glycemic Control Questions on the Minds
of Practicing Retina Specialists
Dr Heier: Dr Peters, is there a general target for
early glycemic control? If a patient presents to an
ophthalmologist with an HbA1c of 9%, but no
Figure 2: Case 1 fluorescein
retinopathy, how aggressive should we be in initiating
angiogram and OCT.
a referral to an endocrinologist?
Photos Courtesy of
Dr Peters: Certainly an HbA
Diana V. Do, MD
1c of 9% is always
concerning, and that patient should be seen by an
endocrinologist. That being said, it is still important to
establish what the individual patient's target is. It is
also important for the patient and for all the medical
providers involved with the patient's care to establish
which provider is setting the patient's target.
Dr Nguyen: Do you believe that all patients with
diabetes should be managed by an endocrinologist?
Dr Peters: I think that all patients with type 1 diabetes,
if possible, should be monitored by an endocrinologist
because of the technical complexity of ongoing
but I would want to know if it was lower in the previous
management. I think that the vast majority of patients
assessments. I have had patients who averaged 6.0%,
with type 2 diabetes have to be managed in a primary
lost that level of control for a little bit, and subsequently
care setting. There are relatively few endocrinologists
developed fluid. Unless patients are very symptomatic, I
who focus primarily on the management of diabetes.
might try a short period of attempting to restore glycemic
If a patient with type 2 diabetes is complicated or
control (3-6 months often allows an adequate period for
having difficulty getting into a target range, then that
improvement) and managing other factors such as poorly
patient should be seen by an endocrinologist. Other
controlled hypertension, rather than essentially committing
providers, including Certified Diabetes Educators and
them to a series of injections. If they are very symptomatic,
dietitians, also have an important role. If you can
if their control has been excellent, and if their blood
connect your patients with the diabetes community,
pressure is under good control, I will discuss anti-VEGF
that can be very empowering for the patient.
therapy with them; and then if I am going to treat, anti-
Dr Nguyen: Do you have a morning glucose target in
VEGF would be my treatment of choice.
mind for most patients?
Dr Do: Dr Nguyen, if you were going to choose an anti-
Dr Peters: The ADA target is between 70 and 130, but if I
VEGF agent, which one would you choose for this patient?
have a patient who has difficulties with hypoglycemia, I
Let us assume patient insurance coverage and finances
would increase the fasting target to 100 to 130. I generally
are not factors.
aim for between 90 and 130 before meals, but it might be
Dr Nguyen: In a case such as this, if finances are not
lower or higher, depending on the individual patient.
a factor, I would choose either ranibizumab or
Dr Nguyen: How does the rate of glycemic reduction
aflibercept because both have been US Food and Drug
potentially worsen retinopathy?
Administration (FDA) approved for the indication of DME.
I am comfortable using either drug, but I may prefer
Dr Peters: In the DCCT, 13.1% of patients randomized to
ranibizumab because it has a longer record of safety
the intensive control arm had worsening of retinopathy
since it was approved several years before aflibercept.33,34
within the first year of treatment, compared with 7.6% of
the patients assigned to conventional treatment.1 Some
Dr Heier: I think most ophthalmologists would choose
of the risk factors for early worsening that were identified
bevacizumab as first-line therapy because cost cannot be
included higher HbA1c levels at screening and
ignored. I have gone on record a number of times stating
reduction of these levels within the first 6 months of
that I always use bevacizumab as my first-line therapy.
treatment. The DCCT authors did not find any evidence
I think that patients do well with it, and almost 90% of my
supporting the concept that more gradual glycemic
patients get bevacizumab. That being said, if cost was not
control might be associated with a lower risk for early
an issue, I would never use it because of the availability
worsening. That being said, they did recommend
of FDA-approved drugs that may be more efficacious in
ophthalmologic monitoring before initiation of intensive
some patients.35 Some information has recently been
treatment and at 3-month intervals for the first 6 to 12
released regarding the Diabetic Retinopathy Clinical
months of treatment.1 They also recommended delaying
Research Network (DRCR.net) Protocol T study, which
the initiation of intensive glycemic treatment until the
compared the safety and efficacy of 2.0-mg aflibercept,
retinopathy was treated, particularly for patients with
1.25-mg bevacizumab, and 0.5-mg ranibizumab in the
poorly controlled diabetes.1 The outcomes for those
treatment of patients with DME. These data have not
patients who were intensively controlled who had early
been peer reviewed. They indicate that there may be
worsening of retinopathy were the same or better than
differences among aflibercept, bevacizumab, and
for those in the conventional group who did not have
ranibizumab with respect to gains in visual acuity and
early worsening.
rates of cardiovascular events.36
1. Early worsening of diabetic retinopathy in the Diabetes Control and
Complications Trial. Arch Ophthalmol. 1998;116(7):874-886.
Dr Do: Many ophthalmologists are aware of the clinical
trial data showing that center-involved DME is best treated
20 RIDE RISE Pooled
with an intravitreal anti-VEGF agent. One of our first
landmark studies was from DRCR.net, which looked at
ranibizumab, given with either prompt or deferred laser,
and it showed that either dosing regimen of ranibizumab
was superior to preservative-free triamcinolone with laser
and also superior to focal/grid laser.37
A change, ETDRS letters
Regarding bevacizumab, which is the most popular choice
among the American Society of Retina Specialists
membership, the BOLT clinical trial that was conducted
4 6 8 10 12 14 16 18 20 22 24 26 28 30 32 34 36
in the United Kingdom additionally provides us some
prospective clinical trial data to suggest that bevacizumab
Ranibizumab 0.3 mg
Ranibizumab 0.5 mg
is also an effective option for center-involved DME.38
ETDRS=Early Treatment Diabetic Retinopathy Study.
Aflibercept was recently approved by the FDA for the
Figure 4: Mean change in best corrected visual acuity over time,
treatment of DME, based on the 1-year data from the
RISE and RIDE 3-year pooled data.33
phase 3 VISTA and VIVID studies.39 Aflibercept treatment,
whether dosed every 8 weeks or every 4 weeks, was
Returning to our case, the patient's visual acuity remained
superior to focal/grid laser, and eyes gained an average of
stable after a 1-month period of observation, but his edema
10.5 to 12.5 letters of visual acuity (Figure 3).39 Both dosing
increased on OCT, so I elected to treat him with the only
regimens of aflibercept had similar efficacy. We also have
on-label anti-VEGF treatment available at the time,
some of the 2-year data from VISTA, and both dosing
ranibizumab. I gave him 1 dose, but his visual acuity did
regimens resulted in sustained visual acuity with similar
not improve significantly, and his edema persisted.
Anti-Platelet Trialists' Collaboration-defined arterial
I administered a second ranibizumab injection, and his
thromboembolic events across all groups.40
visual acuity improved to 20/25 with some decrease in
central retinal thickness. After a third
ranibizumab injection, his visual acuity
improved to 20/20 and the center-involved
Another FDA-approved treatment option
that exists for patients with DME is the
A (letters) 15
dexamethasone delivery device.* The
MEAD study looked at the safety and
efficacy of this option, and in a recent
subanalysis of the study, dexamethasone
was found to be more effective than sham
therapy in all subgroups, regardless of
duration of DME, type of DME, duration of
Mean change from Baseline BCV
diabetes, patient age, or perfusion status.41
12 16 20 24 28 32 36 40 44 48 52
12 16 20 24 28 32 36 40 44 48 52
Patients who were pseudophakic at
Time (weeks)
Time (weeks)
baseline showed benefit from
dexamethasone at each chronological
BCVA=best corrected visual acuity; IAI=intravitreal aflibercept injection.
point that was evaluated, while patients
who were phakic at baseline did not show
Figure 3: Mean standard deviation change in best corrected
continued benefit from dexamethasone
visual acuity from baseline through week 52 with censoring of
after the first year of treatment because of the emergence
values after additional treatment was given (last observation
of cataracts. However, when these patients had their
carried forward).39
cataracts removed, their visual acuity results were
comparable to those patients who were pseudophakic
at baseline. We do not know the optimal dosing strategy
When we further probe our armamentarium, we see that
for this implant; in recent phase 3 clinical trials,
the RISE and RIDE studies demonstrated the superiority of
dexamethasone was given every 6 months.42 Most
ranibizumab to sham treatment.33 Looking at the extension
of us would say that it needs to be dosed every 3 to 4
study, patients who were initially randomized to sham
months, according to our clinical experience with the
treatment and crossed over to treatment with ranibizumab
dexamethasone implant for retinal vein occlusion.
2 years later never matched the gains in visual acuity
Dr Nguyen, when would you recommend the
seen in patients who were initially treated with
dexamethasone implant for DME?
ranibizumab (Figure 4).33
Dr Nguyen: I would tend to select anti-VEGF therapy as an
This suggests that a long delay in beginning anti-VEGF
initial treatment based on the clinical outcomes data. If
therapy for DME causes some level of irreversible damage
you compare the overall gains in vision and percentage
to the retina, and those eyes will not catch up to eyes that
of patients who gained more than 3 lines of vision in
began anti-VEGF therapy much earlier. Maybe you could
RISE/RIDE and VISTA/VIVID against the gains in the recent
delay for a few months, but certainly do not delay for a
dexamethasone study, the anti-VEGF therapies had an
period of years.
* A fluocinolone delivery device also has been approved by the FDA recently for the treatment of DME in patients who have been previously treated with a course of
corticosteroids and did not have a clinically significant rise in intraocular pressure. It is expected to be available early 2015.
Dr Do: Dr Heier, if we are concerned about the treatment
Metabolic Parameters and the Response
burden to the patient and the patient's family, then the
to Pharmacotherapy in DME
dexamethasone delivery device may be an attractive
treatment option because it can be given every 3 to 4
Given the prominent role of anti-VEGF therapy and
months. For some patients, this interval might even be
steroid therapy in the armamentarium for the treatment
stretched out further. What is your perspective on the
of DME, it is important to assess parameters that may
influence their efficacy. Although no double-masked
prospective studies have been conducted to assess
Dr Heier: I am happy that the dexamethasone implant
the relationship between glycemic control and
was approved, and I do think that it will help some of our
responsiveness to pharmacotherapy for patients with
patients. But I still think that anti-VEGF therapy is the best
DME, the question has been addressed with other
first line of therapy, largely for its safety profile. Although
investigations. The limitations of investigation designs
cataracts would not be an issue for the pseudophakic
and variation in results have hindered the ability to
patient, the problem of treatment-induced glaucoma
draw any definitive conclusions.
remains,42 and patients with diabetes are already more
likely to have elevations in intraocular pressure than
A recent subanalysis of the MEAD data, which looked at
the role of intravitreal dexamethasone implant therapy
patients without diabetes.43 As you mentioned, I believe
in the treatment of DME, found that there was a trend
that the number of patients who will be able to get to
toward greater influence of dexamethasone in patients
6 months with 1 implant will be relatively low. Three to
who had better control of their diabetes.1
4 months seems a more likely interval.
A retrospective study conducted by Ozturk and
Dr Nguyen: Dr Peters, if a patient's HbA1c is between 7%
colleagues was designed to assess the effects of glucose
and 7.5%, and he or she continues to have problems with
regulation on visual outcomes for patients with DME
recurrent macular edema, is there any utility to lowering
who were treated with ranibizumab. In this study, the
the patient's HbA1c further?
patients' HbA1c values negatively correlated with
Dr Peters: I think that there is a benefit regarding the
the change in central subfield macular thickness
retinopathy issue. I am not sure there is always a benefit in
(coefficient = –0.50, P<.001).2
terms of the entire person, and that is where we providers
Another recent retrospective case analysis conducted
have to collaborate. Some patients are quite fragile, and
by Matsuda and colleagues enrolled 124 consecutive
the risks for hypoglycemia are too great.
patients with DME to determine the role of systemic
factors on functional and anatomic outcomes of anti-
VEGF therapy (bevacizumab).3 Patients with a serum
Dr Heier: This case features a 36-year-old woman with a 25-
HbA1c of ≤7.0% had a more robust response with respect to best corrected visual acuity and central subfield
year history of type 1 diabetes who presented with a 5-day
macular thickness than those whose HbA
history of "black blobs" in the central vision of her right eye.
>7.0%. Patients whose glycemic control improved during
Her most recent HbA1c was 8%. She received panretinal
the study had lower retinal thickness than patients
photocoagulation in her right eye and focal treatment in
her left eye in 2011 (the laser was performed prior to our
1c was stable or had deteriorated.3
care of her). Her visual acuity at the time of presentation
1. Loewenstein A. MEAD: Diabetic Macular Edema Trial Subanalysis. Presented
was 20/25 in her right eye and 20/20 in the left.
at: Retina Subspecialty Day, American Academy of Ophthalmology. October
17-18, 2014; Chicago, IL.
The patient's imaging shows some preretinal hemorrhage
2. Ozturk BT, Kerimoglu H, Adam M, Gunduz K, Okudan S. Glucose regulation
influences treatment outcome in ranibizumab treatment for diabetic macular
inferiorly in the right eye; there is evidence of previous
edema. J Diabetes Complications. 2011;25(5):298-302.
laser. The left eye looks good. Dr Do, how would you
3. Matsuda S, Tam T, Singh RP, et al. The impact of metabolic parameters on
suggest this patient be managed?
clinical response to VEGF inhibitors for diabetic macular edema. J Diabetes
Dr Do: I would recommend obtaining a fluorescein
angiogram to evaluate the retinal vasculature.29 I suspect
that there will be significant capillary nonperfusion and
multiple areas of neovascularization in her right eye. She
may have more retinopathy problems with her left eye as
well. If this patient has poor glycemic control, retinopathy
is likely to be fairly symmetric in both eyes.
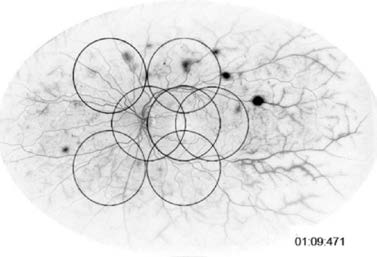
Dr Heier: You are correct. There are some areas of
neovascularization and perhaps some capillary
nonperfusion. Her widefield angiogram shows that there
are a number of areas of neovascularization and extensive
capillary nonperfusion (Figure 5). Dr Nguyen, how would
you approach this patient?
Dr Nguyen: This patient has PDR that seems to be laser
deficient at the time of this imaging. I would perform
additional panretinal photocoagulation, because there
is evidence to support its efficacy in controlling the
progression of the PDR.29,44 Because there is no macular
Figure 5: Case 2 widefield angiogram (OD).
edema, I would delay pharmacologic therapy at this time.
Photo Courtesy of Jeffrey S. Heier, MD
Dr Heier: Dr Do, if there was macular edema, would you
a much greater extent of disease pathology than does
approach this patient differently?
7 standard field imaging, and may, in fact, alter the
classification of DR in as many as 10% of eyes evaluated
Dr Do: I would recommend anti-VEGF injection to treat the
by the 7 standard field imaging technique.32,48
macular edema, and panretinal photocoagulation laser to
control the proliferative aspect.
I get baseline widefield imaging on every patient with
diabetes, as well as on patients with retinal vein occlusion.
Dr Heier: Would you administer these 2 treatment
For this particular patient, we were amazed by the extent
modalities at the same time or would you do the anti-VEGF
of disease in her left eye.
first and then the laser?
Dr Do, when treating a patient with DR with anti-VEGF
Dr Do: I tend to do both procedures at the same visit to
therapy, do you follow the patient with angiography?
avoid the need for the patient to come back multiple
times. I also try to do all the panretinal photocoagulation
Dr Do: I think that when treating DME, an angiogram at
baseline is helpful. For routine follow-up and ongoing
management decisions, OCT is more practical. In my
Dr Peters: This young woman is the perfect example of a
opinion, you need to repeat the angiogram only if
patient who should be referred to an endocrinologist, if she
something changes or if the patient does not respond as
is not already under the care of one. Given the fact that
you would expect.
she is of reproductive age, any attempts to treat her
ophthalmic problems would be significantly complicated
Dr Heier: We have recently conducted a study looking at
by a pregnancy.45 Contraception should be discussed. You
just such an issue, and we are currently evaluating the
do not want a patient with poor glycemic control or
results.49 We treated patients who had PDR with either 12
unstable vision becoming pregnant.
monthly injections of aflibercept or 6 monthly injections
followed by a period of 6 months during which the
Dr Do: Yes, I agree completely. We do not know the effects
injections were given every other month. We then followed
of anti-VEGF therapy on pregnant women, so we certainly
the patients with widefield angiography, with the intent of
do not advocate using it in patients who are pregnant. We
examining the degree of nonperfusion and how the anti-
always counsel our young female patients to use a reliable
VEGF therapy affected it.49 I expect to have those results
birth control method, as you have advised.
If this patient with progressive eye disease was to become
pregnant, I would attempt focal/grid laser first for DME,
because that is the safest option.46 If the edema does not
Dr Do: We next have a case of a 62-year-old woman with
respond, and her vision is being further compromised,
a 5-year history of type 2 diabetes who presented with a
then an intravitreal steroid injection may be the next
complaint of decreased vision in her left eye. Her diabetes
best option. The safety of intravitreal anti-VEGF agents
was initially treated with oral antiglycemic agents, but she
in pregnancy is unclear, and we do not recommend
subsequently required insulin. Her most recent HbA1c was
anti-VEGF injections in this population.47 In my opinion,
8.5%. At the time of presentation, she was noted to have
anti-VEGF would be a first-line agent for women of
center-involved DME in her left eye with a visual acuity of
reproductive age who have diabetes and DME, if they are
20/80 (Figure 7).
able to be reliable with contraception. If not, then laser or
intravitreal steroids might be other options to consider.
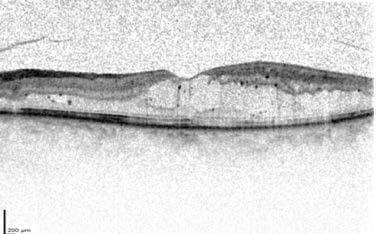
Dr Heier: Let us move on to the patient's left eye; her OCT
shows a few cysts but a nice contour.
There is some evidence of neovascularization on her
7 standard field imaging, and on her widefield imaging
(Figure 6) gross nonperfusion is evident.
Figure 7: Case 3 baseline OCT OS (20/80–1).
Photo Courtesy of Retina Consultants of Houston
Her retina specialist elected to treat her with ranibizumab,
and after 1 injection, her visual acuity improved to 20/60.
Her edema was still persistent, and her ophthalmologist
administered a second injection. Her vision then was
20/60+2, and her foveal contour returned. Dr Heier, in this
patient, would you continue treatment or begin a period of
observation at this point?
Figure 6: Case 2 widefield angiogram (OS).
Photo Courtesy of Jeffrey S. Heier, MD
Dr Heier: I would continue treatment here. As in RISE and
RIDE, we often see slow recovery of vision in patients with
Recent studies, such as those conducted at the Joslin
diabetes.33 There are still some exudates and fluid
Diabetes Center and Weill Cornell Medical Center, have
temporarily, so I would continue until I was absolutely
shown that ultra-widefield angiography potentially reveals
convinced that she had maximized visual gain.
Dr Do: Dr Nguyen, do you ever consider combining anti-
Dr Do: That is what her specialist did. He administered
VEGF with laser, and if so, when do you add the laser?
another ranibizumab injection. Her macula looked great,
with no edema. Her visual acuity improved to 20/40.
Dr Nguyen: I usually start with anti-VEGF injections alone.
If the eye has a suboptimal response to the intravitreal
Subsequently, her provider decided to administer yet
VEGF blockers, I may switch anti-VEGF agents or add
another injection, and her vision improved by 1 line to
focal/grid laser to the injections.
20/30. Dr Nguyen, what would you do now? Do you think
that the eye will go to 20/20 if you give 1 more injection?
Dr Heier: While I am not yet convinced that subthreshold
Should we continue?
micropulse diode laser50 will work, if the problem is
recurrent, as it is in this scenario, I would be interested
Dr Nguyen: She continues to improve, so I would say to
to see if such an approach would help.
continue monthly therapy.
Dr Do: I know many of our colleagues like to combine
Dr Do: When you look at the visual acuity response curves
the effects of anti-VEGF therapy with focal/grid laser.
from the randomized clinical trials pertaining to the
Interestingly, the DRCR.net Protocol I demonstrated that
treatment of wet macular degeneration and DME, you can
in year 3, eyes randomized to ranibizumab with deferred
see that visual gains rise quickly in age-related macular
laser (laser given at month 6 or later) had gained almost
degeneration (AMD) and may also plateau more quickly
3 letters more compared with eyes randomized to
in AMD than they do in DME (Figure 8).33,52
ranibizumab with prompt laser. These data suggested that
anti-VEGF treatment with deferred laser may be more
We do not know why this slight difference occurs. One
beneficial than when laser is used at the beginning.51
study looking at bevacizumab for the treatment of
DME found that although anti-VEGF therapy did lower
In this case, the patient's retina specialist provided another
intraocular VEGF levels dramatically, the effect on other
anti-VEGF treatment, and her vision improved to 20/40.
cytokines involved in disease progression was not as great
Dr Nguyen, what would you do at this time? Would you
as it is in AMD.53,54
observe, or continue the anti-VEGF therapy? When would
your end point be?
Dr Nguyen: The patient has continued to show
Dr Nguyen: I think that there are several key messages to
improvement in vision, so I would like to make sure that
highlight. First, we need to be patient with our treatment
we have maximized her potential gain in visual acuity.
choices with DME, because it appears that the time to
I would continue to treat her at this point, because there
maximal effect of anti-VEGF therapy may be longer for DME
may yet be some level of edema that we could eliminate.
than it is for some other retinal vascular diseases. Anti-VEGF
therapy does appear to be a therapeutic cornerstone for
DME, particularly for those patients with central involvement.
Second, an individualized approach to glycemic control may
benefit patients with diabetes more than trying to treat to a
specific HbA1c goal. Third, DR and DME are quite complex
and variable in their presentations, and it may be
worthwhile to consider widefield angiography as a means of
detecting and assessing the true scope of these diseases.
My appreciation to our panelists for a lively discussion of
some essential management strategies for our complex
ean Change in Visual Acuity (no. of letters)
patients with DR and DME. An individualized approach
can provide great improvements in glycemic control as
well as in visual outcomes.
Trial Abbreviations Used
Action to Control Cardiovascular Risk in Diabetes
Action in Diabetes and Vascular Disease: Preterax
and Diamicron MR Controlled Evaluation
A prospective randomized trial of intravitreal
bevacizumab or laser therapy in the management
of diabetic macular edema
Macular Edema: Assessment of Implantable
Dexamethasone in Diabetes
ean BCVA Change, ETDRS letters
A study of ranibizumab injection in subjects with
clinically significant macular edema with center
involvement secondary to diabetes mellitus
Ranibizumab 0.3 mg
Ranibizumab 0.5 mg
Veterans Affairs Diabetes Trial
Figure 8: Visual acuity response curves from ANCHOR (AMD)52
A study of intravitreal administration of aflibercept
and RISE/RIDE (DME).33
in patients with diabetic macular edema
1. Chiang JL, Kirkman MS, Laffel LM, Peters AL; Type 1 Diabetes Sourcebook
25. ADVANCE Collaborative Group, Patel A, MacMahon S, Chalmers J, et al.
Authors. Type 1 diabetes through the life span: a position statement of the
Intensive blood glucose control and vascular outcomes in patients with type 2
American Diabetes Association. Diabetes Care. 2014;37(7):2034-2054.
diabetes. N Engl J Med. 2008;358(24):2560-2572.
2. Type 1 Diabetes, 2010; Prime Group for JDRF, Mar 2011.
26. Duckworth W, Abraira C, Moritz T, et al; VADT Investigators. Glucose control
3. Pettitt DJ, Talton J, Dabelea D, et al; SEARCH for Diabetes in Youth Study Group.
and vascular complications in veterans with type 2 diabetes. N Engl J Med.
Prevalence of diabetes in U.S. youth in 2009: the SEARCH for Diabetes in Youth
Study. Diabetes Care. 2014;37(2);402-408.
27. Bonds DE, Miller ME, Bergenstal RM, et al. The association between
4. Rovet JF, Ehrlich RM. The effect of hypoglycemic seizures on cognitive function
symptomatic, severe hypoclycaemia and mortality in type 2 diabetes:
retrospective epidemiological analysis of the ACCORD study. BMJ. 2010;
in children with diabetes: a 7-year prospective study. J Pediatr. 1999;134(4):
28. Boyko EJ. ACCORD glycemia results continue to puzzle. Diabetes Care. 2010;
5. Krolewski AS, Warram JH, Christlieb AR, Busick EJ, Kahn CR. The changing
natural history of nephropathy in type 1 diabetes. Am J Med. 1985;78(5):
29. AAO Retina/Vitreous PPP Panel, Hoskins Center for Quality Eye Care. Diabetic
Retinopathy Summary Benchmark – 2014. http://one.aao.org/summary-
6. Kostraba JN, Dorman JS, Orchard TJ, et al. Contribution of diabetes duration
before puberty to development of microvascular complications in IDDM
Accessed September 12, 2014.
subjects. Diabetes Care. 1989;12(10):686-693.
30. Al-Iatayfeh MM, Sun JK, Aiello LP. Ocular coherence tomography and diabetic
7. Cato MA, Mauras N, Ambrosino J, et al; Diabetes Research in Children Network
eye disease. Semin Ophthalmol. 2010;25(5-6):192-197.
(DirecNet). Cognitive functioning in young children with type 1 diabetes. J Int
Neuropsychol Soc. 2014;20(2):238-247.
31. Wessel MM, Nair N, Aaker GD, Ehrlich JR, D'Amico DJ, Kiss S. Peripheral retinal
ischaemia, as evaluated by ultra-widefield fluorescein angiography, is
8. Marzelli MJ. Mazaika PK, Barnea-Goraly N, et al; Diabetes Research in Children
associated with diabetic macular oedema. Br J Ophthalmol. 2012;96(5):694-698.
Network (DirecNet). Neuroanatomical correlates of dysglycemia in young
children with type 1 diabetes. Diabetes. 2014;63(1):343-353.
32. Wessel MM, Aaker GD, Parlitsis G, Cho M, D'Amico DJ, Kiss S. Ultra-widefield
angiography improves the detection and classification of diabetic retinopathy.
9. Barnea-Goraly N, Raman M, Mazaika P, et al; Diabetes Research in Children
Network (DirecNet). Alterations in white matter structure in young children with
33. Brown DM, Nguyen QD, Marcus DM, et al; RIDE and RISE Research Group.
type 1 diabetes. Diabetes Care. 2014;37(2):332-340.
Long-term outcomes of ranibizumab therapy for diabetic macular edema:
10. Miller RG, Secrest AM, Sharma RK, Songer TJ, Orchard TJ. Improvements in the
the 36-month results from two phase III trials: RISE and RIDE. Ophthalmology.
life expectancy of type 1 diabetes: the Pittsburgh Epidemiology of Diabetes
Complications study cohort. Diabetes. 2012;61(11):2987-2992.
34. Lucentis [package insert]. South San Francisco, CA: Genentech, Inc; 2014.
11. Livingstone SJ; Scottish Diabetes Research Network epidemiology group;
35. Nepomuceno AB, Takaki E, Paes de Almeida FP, et al. A prospective
Diabetes Epidemiology Unit, University of Dundee. Life expectancy in Type 1
randomized trial of intravitreal bevacizumab versus ranibizumab for the
diabetes: a Scottish Registry Linkage study. Presented at: European Association
management of diabetic macular edema. Am J Ophthalmol. 2013;156(3):
for the Study of Diabetes Annual Meeting; September 23-27, 2013; Barcelona,
Spain. Abstract No. 301. http://www.abstractsonline.com/Plan/ViewAbstract.
36. EyewireTV. Breaking industry news from the AAO meeting in Chicago.
Accessed November 24, 2014.
the-aao-meeting-in-chicago/. Accessed October 22, 2014.
12. The effect of intensive treatment of diabetes on the development and
37. Diabetic Retinopathy Clinical Research Network, Elman MJ, Aiello LP, Beck RW,
progression of long-term complications in insulin-dependent diabetes mellitus.
et al. Randomized trial evaluating ranibizumab plus prompt or deferred laser or
triamcinolone plus prompt laser for diabetic macular edema. Ophthalmology.
The Diabetes Control and Complications Trial Research Group. N Engl J Med.
38. Rajendram R, Fraser-Bell S, Kaines A, et al. A 2-year prospective randomized
13. Retinopathy and nephropathy in patients with type 1 diabetes four years
controlled trial of intravitreal bevacizumab or laser therapy (BOLT) in the
after a trial of intensive therapy. The Diabetes Control and Complications
management of diabetic macular edema: 24-month data: report 3. Arch
Trial/Epidemiology of Diabetes Interventions and Complications Research
Group. N Engl J Med. 2000;342(6):381-389.
39. Korobelnik JF, Do DV, Schmidt-Erfurth U, et al. Intravitreal aflibercept for
14. The Diabetes Control and Complications Trial (DCCT)/Epidemiology of Diabetes
diabetic macular edema. Ophthalmology. 2014 Jul 8. [Epub ahead of print]
Interventions and Complications (EDIC) Research Group. Effect of intensive
diabetes therapy on the progression of diabetic retinopathy in patients with
40. American Society of Retina Specialists. Two-year results of phase 3 VISTA trial
type 1 diabetes: 18 years of follow-up in the DCCT/EDIC. Diabetes. 2014 Sep 9.
of aflibercept for DME treatment show sustained vision improvement.
[Epub ahead of print]
15. Intensive blood-glucose control with sulphonylureas or insulin compared with
improvement. Accessed September 12, 2014.
conventional treatment and risk of complications in patients with type 2
diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet.
41. Loewenstein A. MEAD: Diabetic Macular Edema Trial Subanalysis. Presented at:
Retina Subspecialty Day, American Academy of Ophthalmology. October 17-
18, 2014; Chicago, IL.
16. Shichiri M, Kishikawa H, Ohkubo Y, Wake N. Long-term results of the Kumamoto
42. Boyer DS, Yoon YH, Belfort R Jr, et al. Three-year, randomized, sham-controlled
Study on optimal diabetes control in type 2 diabetic patients. Diabetes Care.
trial of dexamethasone intravitreal implant in patients with diabetic macular
2000;23 suppl 2:B21-B29.
edema. Ophthalmology. 2014;121(10):1904-1914.
17. Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of
43. Newman-Casey PA, Talwar N, Nan B, Musch DC, Stein JD. The relationship
intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359(15):
between components of metabolic syndrome and open-angle glaucoma.
18. Schiller M, Hochberg D, Garner K. Established T1D Clinical Research Roadmap:
44. Bressler NM, Beck RW, Ferris FL 3rd. Panretinal photocoagulation for proliferative
Paving a New Path. Health Advances. June 2012. http://cdn.jdrf.org/wp-
diabetic retinopathy. N Engl J Med. 2011;365(16):1520-1526.
September 12, 2014.
45. Pescosolido N, Campagna O, Barbato A. Diabetic retinopathy and pregnancy.
Int Ophthalmol. 2014;34(4):989-997.
19. Peters A, Laffel L; American Diabetes Association Transitions Working Group.
Diabetes care for emerging adults: recommendations for transition from
46. Errera MH, Kohly RP, da Cruz L. Pregnancy-associated retinal diseases and their
pediatric to adult diabetes care systems: a position statement of the American
management. Surv Ophthalmol. 2013;58(2):127-142.
Diabetes Association, with representation by the American College of
47. Georgalas I, Petrou P, Koutsandrea C. Safety of intravitreal anti-VEGFs during
Osteopathic Family Physicians, the American Academy of Pediatrics, the
pregnancy is unclear. BMJ. 2012;345:e4526.
American Association of Clinical Endocrinologists, the American Osteopathic
48. Silva PS, Cavallerano JD, Sun JK, Soliman AZ, Aiello LM, Aiello LP. Peripheral
Association, the Centers for Disease Control and Prevention, Children with
lesions identified by mydriatic ultrawide field imaging: distribution and
Diabetes, The Endocrine Society, the International Society for Pediatric and
potential impact on diabetic retinopathy severity. Ophthalmology.
Adolescent Diabetes, Juvenile Diabetes Research Foundation International,
the National Diabetes Education Program, and the Pediatric Endocrine Society
49. ClinicalTrials.gov. Impact of intravitreal aflibercept injections on capillary
(formerly Lawson Wilkins Pediatric Endocrine Society). Diabetes Care. 2011;
non-perfusion (ANDROID). NCT01724554. https://clinicaltrials.gov/ct2/show/
NCT01724554. Accessed September 4, 2014.
20. T1D Exchange Clinic Registry; Jaeb Center for Health Research. The Leona M.
50. Othman IS, Eissa SA, Kotb MS, Sadek SH. Subthreshold diode-laser micropulse
and Harry B. Helmsley Charitable Trust (2012).
photocoagulation as a primary and secondary line of treatment in
21. Kirkman MS, Briscoe VJ, Clark N, et al. Diabetes in older adults. Diabetes Care.
management of diabetic macular edema. Clin Ophthalmol. 2014;8:653-659.
51. Diabetic Retinopathy Clinical Research Network, Elman MJ, Qin H, Aiello LP,
22. Inzucchi SE, Bergenstal RM, Buse JB, et al; American Diabetes Association
et al. Intravitreal ranibizumab for diabetic macular edema with prompt versus
(ADA); European Association for the Study of Diabetes (EASD). Management
deferred laser treatment: three-year randomized trial results. Ophthalmology.
of hyperglycemia in type 2 diabetes: a patient-centered approach: position
statement of the American Diabetes Association (ADA) and the European
52. Brown DM, Kaiser PK, Michels M, et al. Ranibizumab versus verteporfin for
Association for the Study of Diabetes (EASD). Diabetes Care. 2012;35(6):1364-1379.
neovascular age-related macular degeneration. N Engl J Med. 2006;355(14):
23. Action to Control Cardiovascular Risk in Diabetes Study Group, Gerstein HC,
Miller ME, Byington RP, et al. Effects of intensive glucose lowering in type 2
53. Funk M, Schmidinger G, Maar N, et al. Angiogenic and inflammatory markers
diabetes. N Engl J Med. 2008;358(24):2545-2559.
in the intraocular fluid of eyes with diabetic macular edema and influence of
24. ACCORD Study Group; ACCORD Eye Study Group, Chew EY, Ambrosius WT,
therapy with bevacizumab. Retina. 2010;30(9):1412-1419.
Davis MD, et al. Effects of medical therapies on retinopathy progression in
54. Schmidt-Erfurth U. Current concepts in the management of diabetic macular
type 2 diabetes. N Engl J Med. 2010;363(3):233-244.
edema. Adv Stud Ophthalmol. 2010;7(2):52-59.
CME Post Test Questions
To obtain AMA PRA Category 1 Credit ™, please see detailed instructions on page 2.
1. Which of the following factors would tend to favor more
6. Which of the following statements regarding the
stringent management of hyperglycemia for a patient
relationship between type 1 diabetes and patient age
with type 2 diabetes?
a. High level of risk potentially associated with
a. Older patients are less successful with glycemic
control than adolescent patients
b. Low patient motivation
b. Approximately 30% of adult patients are at
c. Long-standing duration of disease
d. Lack of established vascular complications
c. The frequency of severe hypoglycemia decreases
2. Which of the following statements regarding the
d. Pediatric glycemic goals have been made less
management of hyperglycemia in type 1 diabetes
stringent because of validated concerns regarding
a. Adult glycemic targets are more stringent now
than they have ever been
7. A patient with evidence of clinically significant diabetic
b. Early problems with hyperglycemia do not
macular edema should have an HbA1c target value:
predispose children to complications as adults
c. Ophthalmologic monitoring should take place
before and during the first year of increased
glycemic control efforts
d. That takes into account multiple individual patient
d. Patients with long-standing diabetes should
factors, including risk for hypoglycemia
always aim for an HbA1c value of ≤6%
8. All the following statements regarding the use of OCT in
3. The use of ultra-widefield angiography for patients with
the management of DME are true, except:
diabetic macular edema:
a. OCT imagery has a high level of correlation with
a. Has been mandated as a means of following
anatomic outcomes in clinical trials
b. OCT is a highly reproducible method of measuring
b. Has revealed a correlation between the degree of
pathological features of DME
retinal ischemia and macular thickness
c. OCT can monitor response to therapies such as
c. Has the potential to change the classification of a
surgical intervention and intravitreal
patient's ophthalmic disease
d. Has been shown to be less efficacious than
d. OCT may be performed in conjunction with
7 standard field imaging as a means of detecting
fluorescein angiography
diabetic pathology
9. All the following factors may adversely influence visual
4. Dexamethasone implant use for the treatment of
health for patients with diabetes, except:
diabetic macular edema:
a. Is currently FDA approved for pseudophakic adult
c. Poor glycemic control
b. Provides up to 2 years of medication per implant
d. Low serum triglyceride levels
c. Carries no appreciable risk for elevations in
intraocular pressure
10. When assessing the response of patients with DME to
d. Is a Pregnancy Category X treatment
anti-VEGF therapy, it is important to consider that:
a. Visual gains plateau more quickly in DME than
5. Anti-VEGF therapy for clinically significant diabetic
b. Glycemic control influences the efficacy of all
a. Is regarded as a first-line choice for this condition
when it involves the foveal center
c. Prolonged delays in anti-VEGF therapy may limit
b. Has worse functional and visual outcomes than
the magnitude of visual gains for patients who are
laser photocoagulation
candidates for it
c. Has only 1 FDA-approved option
d. Anti-VEGF therapy should be combined with laser
d. Typically achieves maximum functional gains
therapy within the first month of pharmacologic
by 2 months of treatment
Activity Evaluation/Credit Request
Original Release: January 2, 2015 • Last Review: December 9, 2014 • Expiration: January 31, 2016
Current Management of Diabetic Macular Edema and Diabetic Retinopathy:
A Multidisciplinary Discussion of Clinical Cases
PARTICIPANT INFORMATION (Please Print)
❏ Home ❏ Office
Last Name _ First Name Birth Month/Day (mm/dd)
Specialty Degree ❏ MD ❏ DO ❏ OD ❏ PharmD ❏ RPh ❏ NP ❏ RN ❏ PA ❏ Other
City State _ ZIP Code Country
E-mail Phone Fax _
The objectives were achieved.
Upon completion of this activity, participants will be better able to:
Recognize the importance of individualized glycemic control in optimizing outcomes for patients with DR/DME
Discuss the utility of different diagnostic imaging techniques in guiding the management of patients with DR/DME
Describe the efficacy, dosing, and safety profiles of current and emerging treatment options for DME
Confidently tailor diagnostic and treatment strategies for various patients with DR/DME
Communicate effectively with referring physicians regarding the relevant systemic and ophthalmic health issues of their mutual patients with DR/DME
FINANCIAL INTEREST AND BIAS
Disclosure of relevant financial interests of presenters and planners was stated. ❏ Yes ❏ No
This educational activity was free of commercial bias. ❏ Yes ❏ No
If no, please explain.
IMPLEMENTING INTO PRACTICE
Do you intend to make changes or to apply new knowledge as a result of this educational activity?
I intend to make changes to improve my effectiveness. ❏ Yes ❏ No
This experience will not change my practice, as my current behavior is already consistent with the information provided. ❏ Yes ❏ No
If no, please explain.
What strategies for improvement or changes do you plan to implement following this educational activity?
Please indicate all barriers you perceive in implementing these changes. (check all that apply)
❏ Lack of professional guidelines or consensus
❏ Patient compliance issues
❏ Opportunity to practice
❏ Lack of resources
❏ Lack of health system support
❏ Further training is needed
❏ Cost/Reimbursement/Insurance issues
❏ Other, please specify _
How do you think your changes will affect patient outcomes?
POST TEST ANSWER BOX
Source: http://www.oftalmo.epm.br/no/diabetic.pdf
INSTALLATION AND MAINTENANCE MANUAL Original instructions MAN3300010 rel. 03 dated 27.09.2011 www.rheavendors.com Caffè Europa by this manual is intended to describe the Caffè Europa vending machine in its three versions: basic, multimedia and multitouch; by using the same basic components, the three machines differ from each other in components and in the
Private experience and observational learning in pharmaceutical demand Tanja Saxell∗† February 13, 2014 I quantify the roles of the physician's own experience and the past choices of other doctorsin pharmaceutical demand. I develop a model of medical decision-making under uncer-tainty about the quality of the match between the patient and drug treatment. Unlikeprevious demand models, I take into account both private and social learning, and allowheterogeneity in product quality across individuals. I test whether information on thepast choices of other doctors improves drug choices. Using rich data from the market forcholesterol drugs, I show that treatment patterns relying heavily on the past choices ofother doctors can lead to over-prescribing in terms of e�ciency. My results suggest thatcontinuity of care, where a patient is repeatedly consulting the same doctor, is an e�cientpolicy to limit such behavior.




