Levitra enthält Vardenafil, das eine kürzere Wirkdauer als Tadalafil hat, dafür aber schnell einsetzt. Männer, die diskret bestellen möchten, suchen häufig nach levitra kaufen ohne rezept. Dabei spielt die rechtliche Lage in der Schweiz eine wichtige Rolle.
Doi:10.1016/j.nbd.2006.09.010

Neurobiology of Disease 25 (2007) 274 – 283
The CB1 cannabinoid receptor antagonist rimonabant chronicallyprevents the nicotine-induced relapse to alcohol
José Antonio López-Moreno,⁎ Gustavo González-Cuevas, and Miguel Navarro⁎
Department of Psychobiology, Faculty of Psychology, Campus de Somosaguas, Complutense University of Madrid, 28223 Madrid, Spain
Received 7 July 2006; revised 12 September 2006; accepted 16 September 2006Available online 24 October 2006
Preclinical and clinical research shows that the cannabinoid brain
Some research has focused on the CB1 receptors and the
receptor type 1 (CB1) modulates alcohol- and nicotine-related
consumption, motivation and preference for alcohol. Generally,
behaviors. Throughout the nicotine-induced relapse to alcohol, the
these studies can be classified either by the use of:
rats were pre-treated for 10 days with the CB1 cannabinoid receptorantagonist rimonabant (0, 0.03, 0.3 and 3.0 mg/kg i.p.). In this
(1) Cannabinoid receptor agonists: treatment with the CB
condition, a long-lasting nicotine-induced relapse to alcohol was
cannabinoid receptor agonists WIN 55,212 and CP-55,940
observed, and this effect was reversed in a dose-dependent mannerwith rimonabant. Surprisingly, rats that were not exposed to nicotine
has been shown to increase alcohol consumption in Wistar
developed tolerance to the effects of rimonabant from the sixth day.
and Sardinian alcohol-preferring (sP) rats
Also, 3.0 mg/kg of rimonabant reduced the responses for sucrose.
Evaluation in the Elevated Plus-Maze after nicotine treatment did not
reveal anxiogenic effects. Finally, at the conclusion of rimonabant
(2) Cannabinoid receptor antagonists: treatment with the CB1
treatment, a rapid reinstatement of alcohol consumption was detected.
cannabinoid receptor antagonist rimonabant (SR 141716 or
These results suggest that rimonabant can prevent the relapse to
ACOMPLIA™) has been demonstrated to reduce operant
alcohol, even when an interaction with nicotine exists—the most
alcohol self-administration and alcohol intake
frequent situation in human alcohol abuse.
2006 Elsevier Inc. All rights reserved.
to reduce motivation toconsume alcohol ); to block the alcohol
Keywords: Nicotine; Relapse to alcohol; Rimonabant; Operant self-administration; Cannabinoid system; Sucrose
deprivation effect (ADE) to prevent theacquisition of drinking behavior ); and tosuppress extinction of the response for alcohol in sP rats). Furthermore, the new selective
antagonist of the CB1 cannabinoid receptor, SR147778, isable to reduce alcohol consumption and the motivational
In the last 9 years, numerous studies have been published that
properties of alcohol
demonstrate the interaction between the cannabinoid system and
alcohol. Neurochemical research has revealed that chronic alcohol
(3) Mice lacking the CB1 receptor: these mice show less
treatment elicits the release of endogenous cannabinoid brain
preference for alcohol and higher concentrations of ethanol
receptor type 1 (CB
in blood ); reduced ethanol-
1) agonists, causing a down-regulation of this
receptor and its signal transduction (
induced Conditioned Place Preference
). In addition, lower
); decreased alcohol self-administration
cannabinoid function is related to greater vulnerability to alcohol
and increased alcohol sensitivity
consumption (), and to the existence of
and a lack of alcohol-induced dopamine
cannabinoid-altered gene expression after intermittent exposure
release in the nucleus accumbens ().
to alcohol ).
Moreover, it seems that the CB1 receptor is also implicated in
nicotine addiction. For a review, see
⁎ Corresponding authors. Fax: +34 91 394 30 69.
. For instance, rimonabant causes decreased operant self-
E-mail addresses: (J.A. López-Moreno),
(M. Navarro).
administration of nicotine in rats and reverses nicotine seeking
Available online on ScienceDirect (www.sciencedirect.com).
after withdrawal ). In addition, mice
0969-9961/$ - see front matter 2006 Elsevier Inc. All rights reserved.
J.A. López-Moreno et al. / Neurobiology of Disease 25 (2007) 274–283
lacking the CB1 receptor do not show nicotine-induced Condi-
tioned Place Preference As of 2004,rimonabant was in phase III clinical trials (
99% (GC), liquid (Sigma Chemical Co., Madrid, Spain); 0.8 mg/
Despite all this evidence, the role of rimonabant in the
kg was dissolved in sterile physiological saline and administered
interaction between alcohol self-administration and nicotine has
subcutaneously (s.c.) between the shoulder blades in a volume of
not yet been studied. This is probably due, in part, to the complex
1 ml/kg. Nicotine was prepared daily before injection and ad-
interaction between alcohol and nicotine
justed to a pH of 7–7.2 with dilute NaOH. Alcohol solution was
Several authors have shown that nicotine can either increase
prepared daily as a 10% alcohol w/v. Rimonabant, [N-piperidino-
or decrease alcohol intake
carboxamide], was a kind gift from Sanofi-Aventis (Paris,
have demonstrated previously that when nicotine is administered
France). Doses of 0, 0.03, 0.3 and 3.0 mg/kg were first mixed
during the stage of alcohol deprivation, there is a long-term dose-
with 0.1% Tween 80, and then physiological saline was slowly
dependent increase in the relapse to alcohol, with the highest effect
added. Rimonabant was administered intraperitoneally (i.p.) in a
at the dose of 0.8 mg/kg of nicotine
volume of 1 ml/kg.
Furthermore, other studies noted that this dose induced an increasein alcohol self-administration in rats ). In the present
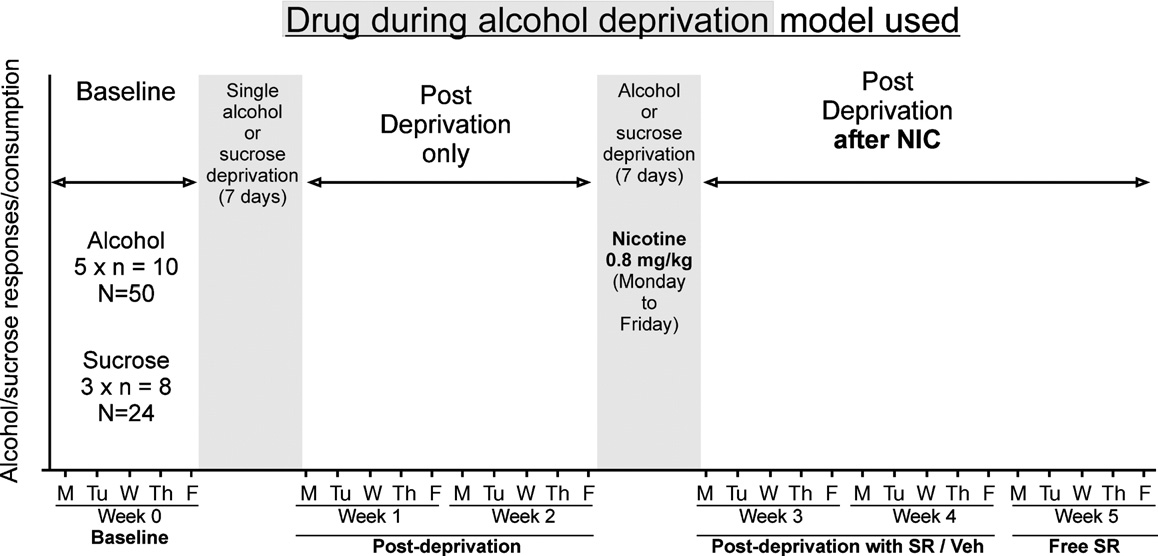
General protocol used—drug during alcohol deprivation
study, a protocol of Elevated Plus-Maze (EPM) was carried out inorder to evaluate a possible role of anxiety produced by nicotine.
Previous research in our laboratory has shown that exposure to
Finally, three additional groups responding for a natural reinforcer
drugs (i.e. nicotine and WIN 55,212-2) in the stage of alcohol
(sucrose) were added in order to evaluate whether the reduction of
deprivation is a useful method for evaluating long-lasting drug-
the response for alcohol with rimonabant treatment was exclusively
induced changes (). Here, we have
for alcohol and could be extended to a non-drug reinforcer
used this model as depicted in Briefly, the animals received
intermittent (Monday to Friday) and limited (30-min sessions)access to alcohol/sucrose per week. The experiments started once
Materials and methods
baseline had been reached following at least a 6-week period ofaccess to alcohol (10% w/v) or sucrose (0.25%). The exposure to
rimonabant was made 30 min before alcohol access.
Once this experiment was completed, two extra groups were
Adult male Wistar rats (Harlan, Barcelona, Spain) weighing
added; these groups were used to assess the anxiogenic/anxiolytic
200–225 g at the start of the experiments were housed two per cage
effects of nicotine by means of the EPM test, 24 h after the last
in a room with a controlled reversed light/dark photoperiod (lights
injection of nicotine.
on at 20:00) and controlled temperature/humidity environment (23 ±1°C). Food and water were available ad libitum in the home cage. All
Training procedure for operant alcohol/sucrose self-administration
experiments were conducted under dim red light, between 9:00 and21:00. All procedures described in the present study were in
Training was achieved using a modification of the method
accordance with the Guide for the Care and Use of Laboratory
described by that is described extensively in
Animals of the National Institutes of Health.
In brief, rats were placed on a water
Fig. 1. Schematic representation of the "Drug During Alcohol Deprivation Model" used. Note that the animals were treated with nicotine for 5 days duringabstinence from alcohol. Each standard week is composed of 5 days: Monday through Friday.
J.A. López-Moreno et al. / Neurobiology of Disease 25 (2007) 274–283
restriction schedule for 2–4 days to facilitate training of leverpressing. During the first 3 days of training, the animals received10% sucrose solution in the dipper. Thereafter, the followingsequence on a fixed ratio 1 schedule was used: 10% sucrose forfour sessions, 10% sucrose and 2% ethanol (EtOH) for twosessions, 8% sucrose and 4% EtOH for two sessions, 6% sucroseand 6% EtOH for four sessions, 4% sucrose and 8% EtOH for foursessions, 2% sucrose and 10% EtOH for four sessions, and 10%EtOH for 10–20 sessions. The chambers were equipped with tworetractable levers located on either side of a drinking reservoir(0.1 ml) positioned in the center of the front panel of the chamber.
The levers were counterbalanced to respond as either the active orinactive lever. Once animals had acquired stable responses toEtOH, the inactive lever was presented. A similar procedure wasused in the response for sucrose. Initially the animals had access toa 10% sucrose solution and the sucrose level was reducedprogressively similar to the EtOH schedule (but without EtOH)until a 0.25% sucrose solution was reached. This concentration waschosen because the animal response was similar to the alcoholsolution.
Elevated Plus-Maze
The EPM apparatus consisted of four arms (50 cm long × 10 cm
wide). The two enclosed arms had 40 cm-high dark walls, whereasthe two open arms had 0.5 cm high ledges. Lighting on the centerof the open arms was 50 lx. The maze was elevated to a height of50 cm. Rats were placed individually onto the center of theapparatus and faced toward an open arm. The 5-min experimentalsessions were recorded by video camera and viewed by a trainedexperimenter who was blind to the group assignment.
Data from weekly operant responses were analyzed by one way
ANOVA (rimonabant treatment), whereas daily operant responseswere performed by two-way repeated-measures ANOVA: numberof days (within-subjects factor) and different rimonabant treatment(between-groups factor). Data from the EPM were compared bythe t-test for unpaired variables (nicotine/saline treatment). Onlysignificant effects (p values < 0.05) in ANOVA analysis weresubjected to Tukey's honestly significant difference test (between-groups factor), and the post hoc analysis for repeated measuressubprogram of the SPSS statistical (Chicago, IL) software package(version 13.0) for Windows.
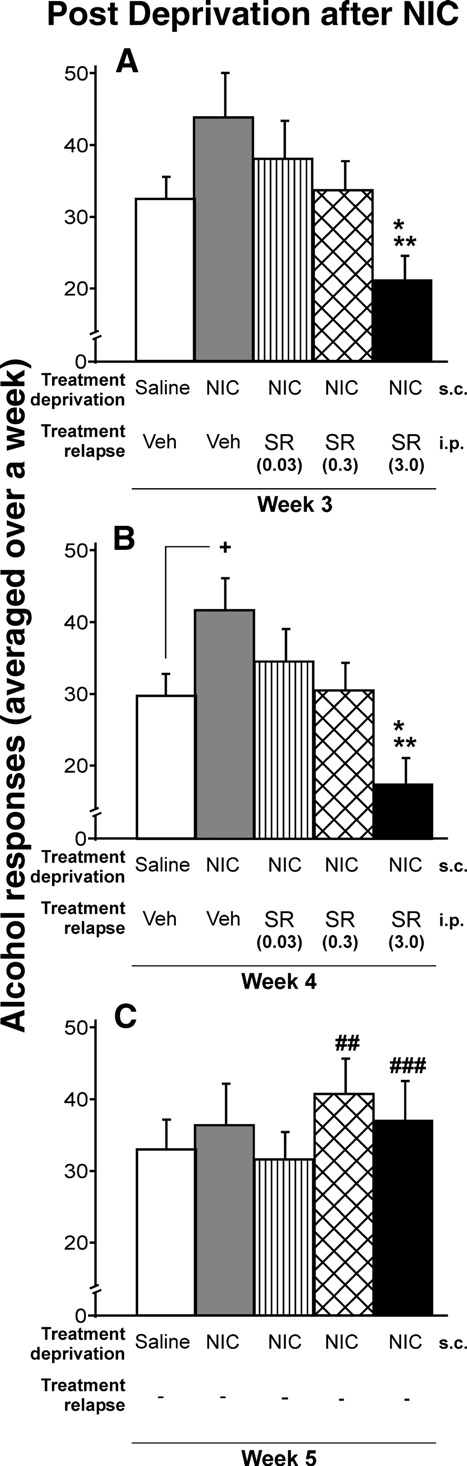
Rimonabant avoids the long-lasting nicotine-induced relapse toalcohol
Fig. 2. Effects of chronic administration of saline, vehicle, or rimonabant
and B show that the nicotine-induced relapse to
during the relapse to alcohol after nicotine exposure in the alcohol
alcohol was suppressed in a dose-dependent manner by rimonabant
deprivation period. The response for alcohol decreased in a dose-dependent
(SR) (ANOVA Week-3: F
manner. The highest dose of rimonabant showed significant differences
4,49 = 3.22, p < 0.05; ANOVA Week-4:
compared with the rest of the groups (A and B) (Tukey post hoc analysis;
F4,49 = 4.39, p < 0.01; ANOVA Week-5, rimonabant withdrawal,
*p < 0.05 and **p < 0.01). Only during the second week of nicotine-induced
F4,49 = 0.48, NS). The highest dose of rimonabant (3.0 mg/kg) fully
relapse to alcohol, significant increase in alcohol response in the vehicle-
reversed the increase in the nicotine-induced response for alcohol,
group when compared with the control group, was observed (B) (+p < 0.05).
as well as the alcohol intake in animals that were not treated with
After rimonabant treatment, a significant rebound increase in the response
nicotine; (Tukey post hoc analysis p < 0.05 and p < 0.01). This last
for alcohol was shown with doses of 0.3 and 3.0 mg/kg (C) (t-paired test;
finding is consistent with previous studies (i.e.
##p < 0.01 and ###p < 0.001). Data are represented as mean total alcohol
responses averaged over a week + SEM.
J.A. López-Moreno et al. / Neurobiology of Disease 25 (2007) 274–283
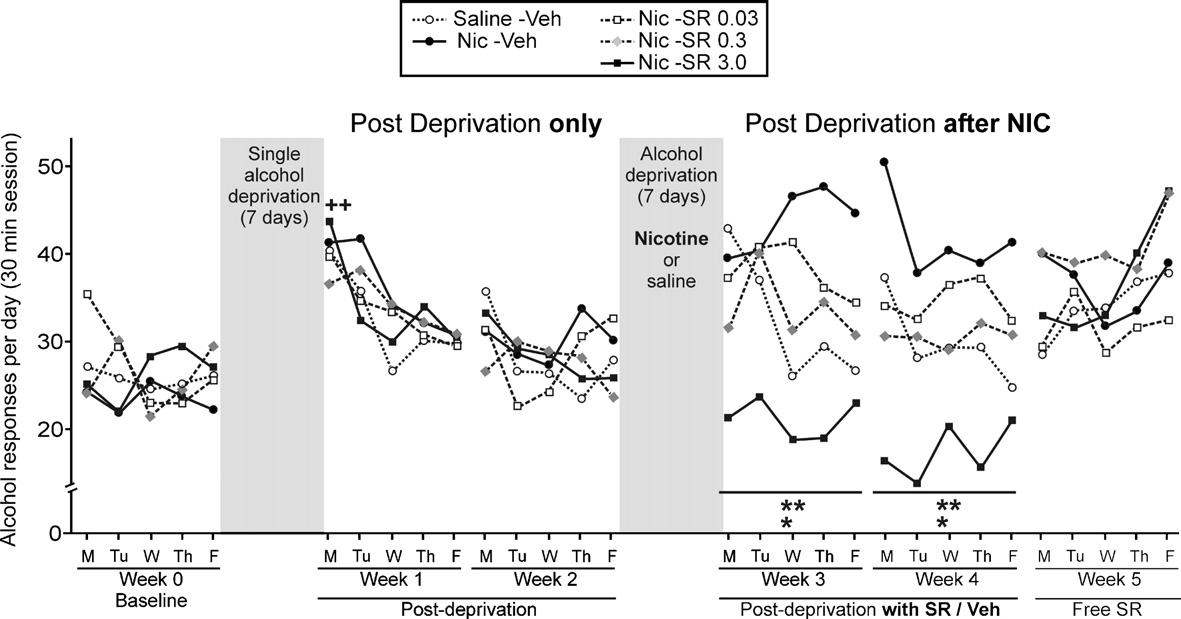
exposure to nicotine induced long-lasting relapse to alcohol when
alcohol showed a slight increase in alcohol intake over time (
compared with the group that was not exposed to nicotine
In contrast, the groups treated with rimonabant showed a
(p < 0.05). However, after rimonabant withdrawal, a significant
dose-dependent decrease in the number of alcohol reinforcers, and
rebound increase in alcohol consumption was observed (panel C).
the slope of the cumulative alcohol intake changed nearly to a
Interestingly, this rebound also occurred with a mild dose of
plane line C–E). Generally, the number of cumulative
rimonabant (0.3 mg/kg).
reinforcers reached 50–60% in the first 5 min, 75–85% at 10 min
The reversion of nicotine-induced relapse to alcohol after
and 92–94% at 20 min.
rimonabant treatment did not show tolerance throughout the 10days (see ). Also, highlights the time course of alcohol
Development of tolerance to the relapse-preventing effects of
response during the two consecutive cycles of alcohol deprivation.
rimonabant in animals not exposed to nicotine
As can be seen, the first day after alcohol abstinence alone therewas the characteristic ADE in all groups when compared with the
The two groups shown in were added to evaluate whether
baseline (t-paired test; p < 0.01). This pattern is strongly supported
or not the relapse-preventing effects of rimonabant on animals that
in the scientific literature However, the
had not been exposed to nicotine were specific. These animals
exposure to nicotine during the abstinence from alcohol noticeably
were deprived of alcohol for 7 days and both groups were treated
changed this pattern of relapse for the next 2 weeks. The group
only with saline. The highest dose of rimonabant (3.0 mg/kg) was
with the highest response for alcohol was the Nic-Veh group, and
chosen because it was the only one that totally reversed the
the response was even higher than the group Saline-Veh, that was
nicotine-induced relapse to alcohol. Intriguingly, the results
exposed neither to nicotine nor to rimonabant (Week-3: ANOVA
showed that the chronic ability of rimonabant to prevent the
between treatments F4,45 = 3.22, p < 0.05; interaction between days
relapse to alcohol seems to be specific for animals exposed to
and treatment F16,180 = 1.47, p = 0.12 NS; within days F4,180 = 1.11,
nicotine. Chronic rimonabant pre-treatment prevented relapse to
p = 0.35 NS/Week-4: ANOVA between treatments F4,45 = 4.39,
alcohol only the first 5 days when compared with the vehicle-group
p < 0.01; interaction between days and treatment F16,180 = 1.16,
(panel A) (t-independent test p < 0.001). Despite the presence of
p = 0.20 NS; within days F4,180 = 1.15, p = 0.29 NS). There were no
rimonabant pre-treatment, the next 5 days were followed by the
significant differences among any of the groups for the inactive
reinstatement of the response for alcohol and there were significant
lever (data not shown).
differences when compared with the previous alcohol response
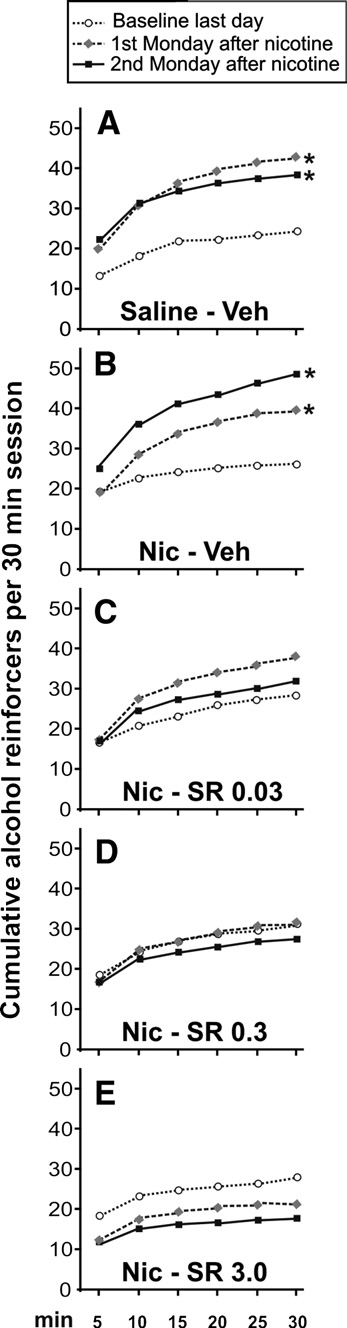
Alternatively, shows the cumulative alcohol reinforcers
(panel B) (t-independent test p < 0.001) that reached similar levels
obtained by the animals in three representative points: the last day
of alcohol consumption to that of the control-group. Non-
of baseline, and the first and second Monday after nicotine
significant differences were found after rimonabant withdrawal
exposure. It can be observed that two cycles of alcohol deprivation
(panel C). The two-way ANOVA analysis for the data shown in the
lead to a similar increase in the number of alcohol reinforcers
revealed statistically significant differences during the first
obtained A). However, the nicotine-induced relapse to
five days (ANOVA between treatments F1,14 = 59.51, p < 0.0001;
Fig. 3. Day-by-day time course of alcohol responses during baseline, during a single alcohol relapse, during a nicotine-induced relapse with chronic rimonabantpre-treatment and during the next days after rimonabant treatment. The response for alcohol increased the first day after a single alcohol abstinence whencompared with the last day of baseline (t-paired test; ++p < 0.01) (the alcohol deprivation effect). However, after the exposure to nicotine without rimonabant pre-treatment, a significant long-lasting increase in the response for alcohol was observed. The highest dose of rimonabant showed significant differences comparedwith the rest of the groups (Tukey post hoc analysis; *p < 0.05 and **p < 0.01). After rimonabant treatment, significant differences between groups disappeared(note that SEMs are not present in order to clarify the figure).
J.A. López-Moreno et al. / Neurobiology of Disease 25 (2007) 274–283
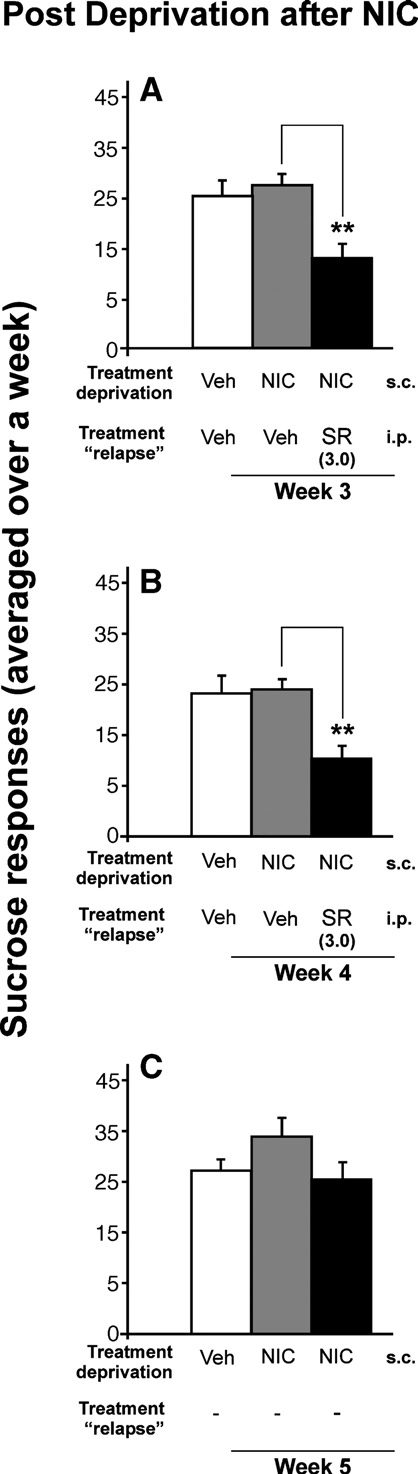
Nicotine had no effect on the relapse to sucrose, but rimonabantdecreased the response for sucrose
On the one hand, we found that after a period of abstinence
from nicotine, the relapse to sucrose was not modified for asolution of 0.25%. On the other hand, we observed that rimonabantdecreased the response for sucrose at 0.25% w/v concentration.
Panels a and b of show that the dose of 3.0 mg/kg ofrimonabant significantly reduced the sucrose intake (p < 0.01). Thisdose of rimonabant was used because it had proven previously thatthis dose produced the greatest reduction in the nicotine-inducedresponse for alcohol. Similarly, the same as the response foralcohol, there were no significant differences for the inactive lever(data not shown), and there was a rebound increase in sucroseconsumption after rimonabant withdrawal (panel C) (t-paired test;p < 0.01).
Nicotine had no effect on the anxiety-like behavior in the ElevatedPlus-Maze 24 h later
Twenty-four hours after the last nicotine injection, no sig-
nificant differences in anxiety-like behavior were found (percent open arm, t-test; p < 0.43, NS/percent open in the center,t-test; p < 0.75, NS/number of entries to the closed arms, t-test;p < 0.73 NS).
Body weight changes during operant alcohol/sucroseself-administration
shows the day-by-day time course of body weight during
two relapses after two forced deprivations: with or without nicotine.
During the nicotine-induced relapse to alcohol, the animals werepre-treated with rimonabant. Here, the data are presented as mean +SEM (ANOVA within days: F24,1176 = 60.16, p < 0.0001; interactionbetween days and treatments: F96,1176 = 2.99, p < 0.0001; betweentreatments: F4,49 = 4.29, p = 0.005). The animal's weight (mean 384 ±3.95) from the week of baseline was used as 100% weight. Onlysignificant differences between the sucrose group and all alcoholgroups are represented (*p < 0.05; **p < 0.01). Those receiving thehighest dose of rimonabant (3 mg/kg) showed a more significantreduction in body weight as compared with the sucrose group fromthe second day with rimonabant treatment (##p < 0.01), but therewere no differences between alcohol groups. However, this slightdifference in body weight with the highest dose of rimonabantdisappeared after rimonabant withdrawal.
Fig. 4. Cumulative alcohol reinforcers obtained by the animals during the30 min session in three representative days: the last baseline day, the first and
second Monday after nicotine treatment. Only saline and vehicle groupsshowed significant differences when compared with the last baseline day(A, B) (note the different response patterns), in contrast with the groups
The main findings of this study are as follows: (1) Rimonabant
treated with rimonabant (C–E) (Tukey post hoc analysis; *p < 0.05). Each
pre-treatment at 3.0 mg/kg totally abolished the relapse to alcohol
point represents the cumulative reinforcers obtained in 5-min intervals (note
during the first 5 days in the animals that were not exposed to
that SEMs are not present in order to clarify the figure).
nicotine during the phase of alcohol deprivation. (2) Exposure tonicotine during the stage of alcohol deprivation produced a long-term increase in the relapse to alcohol; however, this effect was
interaction between days and treatment F4,56 = 1.65, p = 0.17 NS;
reversed in a dose-dependent manner when the rats were
within days F4,56 = 6.08, p < 0.001), whereas the next days did not
chronically pre-treated with the cannabinoid receptor antagonist
show any significant differences, excepting the 8 day pre-treatment
rimonabant before the alcohol trial. The nicotine-induced relapse to
with rimonabant, where a transient increase in response for alcohol
alcohol seems to be specific to alcohol because nicotine treatment
was observed (p < 0.05). As can be seen in there was no
did not increase the intake of a natural reward (sucrose). (3) The
rebound in alcohol consumption after rimonabant withdrawal,
animals showed a rebound increase in alcohol consumption when
contrary to animals that were exposed to nicotine.
chronic treatment with 0.3 and 3.0 mg/kg rimonabant was
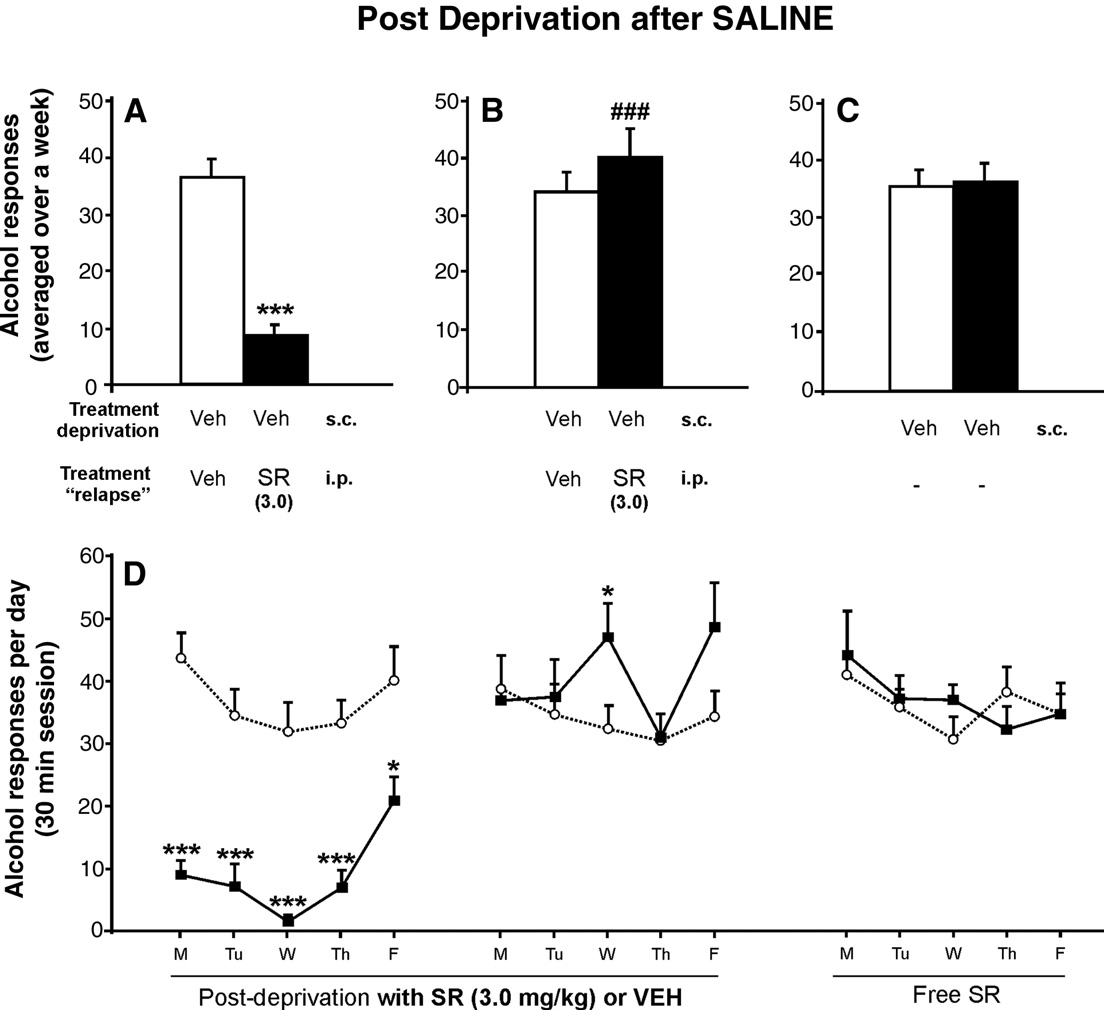
J.A. López-Moreno et al. / Neurobiology of Disease 25 (2007) 274–283
Fig. 5. Effects of chronic administration of rimonabant (SR) (3.0 mg/kg) during the relapse to alcohol after saline treatment in the phase of alcohol deprivation.
Panel A shows the prevention of alcohol relapse during the relapse to alcohol and the near abolishment of alcohol response, as compared with the control group(***p < 0.001). However, during the following week (B), these animals showed the development of tolerance to the relapse-preventing effects of rimonabant ascompared with the previous week (###p < 0.001). After withdrawal of rimonabant treatment (C), no significant changes were observed. Panel D depicts the day-by-day time course of the response for alcohol corresponding to the weeks depicted above (A–C) (***p < 0.001; *p < 0.05).
removed. (4) The group of rats that was not exposed to nicotine
including the GABA, glutamate and dopamine systems, as well as
developed tolerance to the reducing effect of rimonabant in alcohol
others ). Successive relapses to alcohol
responses from the sixth day. (5) The anti-motivational effects of
cause an imbalance between the two main excitatory and inhibitory
3.0 mg/kg of rimonabant seemed to be not specific for alcohol,
neurotransmitters: GABA and glutamate The
since there was also a significantly reduced response for 0.25%
interactions between alcohol and nicotine occur in the mesolimbic/
sucrose. (6) Nicotine treatment had no anxiogenic effects 24 h after
mesocortical reward system, as well as other regions (
the last nicotine injection in the EPM.
). Therefore, in this study, the proposed final common
The finding that rimonabant is a molecule that is able to
molecular pathway for the reinforcing effects of the abused drugs
modulate the relapse to alcohol and alcohol-related behaviors is not
() could be compro-
mised in some way.
Thus, the greater increase in the response for alcohol after
However, here we report the first evidence that
nicotine treatment compared with the increase in alcohol intake
rimonabant is able to suppress the relapse to alcohol when an
after two consecutive relapses could suggest a specific phenom-
interaction with nicotine exists, which is by far the most common
enon of cross-tolerance or/and cross-sensitization to the effects of
situation with a prevalence of 91.5% (Nicotine
alcohol, because sucrose responding was not altered. Importantly,
acts on the nicotinic acetylcholine receptors (nACHRs), and
this increase would be usually masked when two drugs are
usually activation of these receptors leads to an increase in the
administered at approximately the same time (either left-shifting
presynaptic release of neurotransmitters (e.g. GABA, glutamate,
the dose–response curve for alcohol or decreasing the total alcohol
acetylcholine and dopamine) On the other
intake). In addition, we have avoided any conditioned nicotine
hand, alcohol interacts with several neurotransmitter systems,
responses (i.e. nicotine-sensitization related to an environment),
J.A. López-Moreno et al. / Neurobiology of Disease 25 (2007) 274–283
Fig. 7. Twenty-four hours after the last nicotine injection, significantanxiogenic-like responses in the Elevated Plus-Maze were not detected. Thesaline/nicotine groups spent similar percentages of time in the open arms andin the center of apparatus. Similarly, there were no significant differences inthe number of entries into the closed arms. Data are represented as mean +SEM.
syndrome. For this reason, trials were carried out in the EPM, aparadigm widely used to evaluate anxioselective effects of drugs,as well as withdrawal ). A recentstudy has demonstrated that repeated alcohol abstinences preventwithdrawal-induced elevations of corticosterone and withdrawal-induced anxiety evaluated in the EPM ).
Despite previous studies showing the anxiogenic effects of 0.8 mg/kg of nicotine in mice () evaluated with
Fig. 6. Effects of chronic administration of rimonabant (SR) during sucrosereinstatement after a phase of sucrose deprivation with nicotine. Chronicpre-treatment with 3.0 mg/kg rimonabant significantly decreased theresponse for 0.25% sucrose (A, B) (**p < 0.01), without any development
Fig. 8. Change in animals' weight during the 2 weeks of alcohol/sucrose
of tolerance. However, this significant effect disappeared after rimonabant
relapse after a single alcohol deprivation (A), during the 2 weeks of alcohol/
treatment (C). Data are represented as mean total sucrose responses averaged
sucrose relapse after an alcohol deprivation with nicotine (0.8 mg/kg) and
over a week + SEM.
rimonabant (SR) pre-treatment (B), and the next days after rimonabantwithdrawal (C) (0, 0.03, 0.3 and 3.0 mg/kg). The animals' weight (mean 384g ± SEM) from the week of the establishment of baseline was used as 100% of
because it was administered in a context that was different from the
weight. *p < 0.05 and **p < 0.01 indicate significant increase in body weight
alcohol operating boxes, and was not contingent on alcohol access.
between the sucrose-group and all the other alcohol groups. ##p < 0.01
We are aware that nicotine-induced relapse to alcohol could be
indicates significant differences between the sucrose group and the alcohol
mediated by other factors, such as stress or a nicotine abstinence
group with the highest dose of rimonabant (3.0 mg/kg).
J.A. López-Moreno et al. / Neurobiology of Disease 25 (2007) 274–283
the EPM, anxiogenic effects were not found here. This could be
rimonabant could be used in the comorbid treatment of alcohol
due to the fact that the test trial was 24 h after the last injection of
and nicotine dependence (as well as other drugs of abuse), and on
nicotine (in order to investigate "post-effects" of nicotine in a
the other hand, its use in combination with other medications
similar way to what would occur in the operant alcohol boxes,
could lead to a reduction in the doses for the treatment of alcohol
instead of acute nicotine effects). Therefore, if the rats had access
dependence, and in consequence, a reduction in side effects.
to alcohol 72 h after the last injection of nicotine (Monday), and in
According with previous studies, we have data (not shown) that
a very familiar context (the operating boxes), it would be less likely
the highest dose of rimonabant used (3.0 mg/kg) neither caused
that stress or the nicotine withdrawal syndrome was the key
motor impairment nor reward/aversive effects evaluated in the
element to explain the greater relapse to alcohol following nicotine
Conditioned Place Preference paradigm
exposure. Interestingly, through internal data (and published
Furthermore, the rimonabant-mediated
results, we have observed that the
prevention of the relapse to alcohol was persistent throughout
nicotine-induced aversion in the Conditioned Place Preference
the 10 days and did not show any effect of tolerance or
paradigm after acute treatment (0.8 mg/kg) may be dissociated
sensitization. This lack of tolerance/sensitization was only
from posterior anxiogenic effects in the EPM. However, it has been
observed in the animals that were exposed to nicotine during
demonstrated that the effect of rimonabant could be modulated by
the alcohol deprivation period. Intriguingly, this is in contrast
the time following nicotine exposure.
with results when the animals were not exposed to nicotine;
have shown that rimonabant blocked the nicotine-induced Condi-
following 5 days of administration of rimonabant, tolerance to the
tioned Place Preference while administered immediately after the
protective effect in alcohol relapse was developed. Furthermore, a
conditioning phase, but not after prolonged nicotine withdrawal
similar pattern has been demonstrated, but with food reward: the
These evidences, together with our results,
tolerance to the anorectic effects of rimonabant was developed
strongly suggest complex interactions between nicotinic-acetylcho-
line and CB1 receptors.
A rebound increase in alcohol consumption was found with 0.3
CB1 receptors are widely distributed throughout the brain
and 3.0 mg/kg of rimonabant. It would be possible to misunder-
(including the mesocorticolimbic system) and they are the most
stand the ability of low doses to inhibit the greater nicotine-induced
abundant G protein-coupled receptors The
responding for alcohol; i.e. the dose of 0.3 mg/kg did not suppress
cannabinoid system modulates neurotransmitter release via pre-
alcohol intake, but was effective in reducing the extra alcohol
synaptic cannabinoid receptors (
consumption induced by nicotine. Interestingly, this reducing effect
Contrary to the main role of the nACHRs (the increase in
is evidenced after rimonabant withdrawal, when alcohol response
neurotransmitter release), the activation of the CB1 receptor
inhibits the neurotransmitter release (GABA, glutamate, dopamine,
In conclusion, the regulation of the endocannabinoid system
noradrenalin, serotonin, among others.) Logically, this wide range
could be an important therapeutic target for alcoholism, even when
of effects decreases the potential for elucidating the particular
an interaction with nicotine exists, the most frequent situation
mechanism of rimonabant in the prevention of the nicotine-induced
(). However, it seems that supple-
relapse to alcohol.
mentary strategies would be needed to avoid the reinstatement of
There are several studies that describe the reduction of alcohol
alcohol consumption after the withdrawal of rimonabant treatment.
consumption after rimonabant treatment (
). However, it seems that this effect is notspecific for alcohol, as supported here. Moreover, it reduced the
This work was supported by The European Fifth Framework
response for natural reinforces, such as 0.25% and 5% sucrose,
Programme QLRT-2000-01691, MEC SAF2005-04926, Fondo de
chocolate, food and NaCl in sodium-depleted rats (
Investigación Sanitaria (Red de Trastornos Adictivos G03/05),
Comunidad Autónoma de Madrid GR/SAL/0541/2004, and Plan
as well as other drugs of abuse: nicotine, cocaine and heroin
Nacional Sobre Drogas (Ministerio de Sanidad). We thank Luis
Franco and Ana I. de Tena for the help in the support and
Therefore, in a more general way, the blockade of the cannabinoid
maintenance of the chambers of operant self-administration, and
system may be affecting motivated behaviors.
José Mauricio Flórez de Uría and Santiago Climent for the
This raises the question: could the blockage of the cannabinoid
development of the computer software; and Miriam Philips for
system be a general approach to the treatment of addiction? If so,
English assistance.
this would be useful in the case of drug abuse: (1) when severaldrugs are co-abused, as is the most frequent situation in addiction(the aim of this work), or (2) using a sub-threshold dose of
rimonabant in combination with other sub-threshold doses ofAcamprosate, Disulfiram or Naltrexone, the current treatments for
Annual report 2004 from Sanofi-Aventis.
alcoholism approved by the U.S. Food and Drug Administration
Balerio, G.N., Aso, E., Maldonado, R., 2005. Involvement of the opioid
In fact, it has been demonstrated that low doses
system in the effects induced by nicotine on anxiety-like behaviour inmice. Psychopharmacology 181, 260–269.
of either naltrexone or naloxone (which did not have effects on
Balerio, G.N., Aso, E., Maldonado, R., 2006. Role of the cannabinoid
alcohol intake per se), in combination with a sub-threshold dose of
system in the effects induced by nicotine on anxiety-like behaviour in
rimonabant significantly decreased alcohol consumption
mice. Psychopharmacology 184, 504–513.
Basavarajappa, B.S., Hungund, B.L., 2002. Neuromodulatory role of the
These two points would have important clinical implications
endocannabinoid signaling system in alcoholism: an overview. Pros-
in the psychopharmacology of alcoholism. On the one hand,
taglandins Leukot. Essent. Fat. Acids 66, 287–299.
J.A. López-Moreno et al. / Neurobiology of Disease 25 (2007) 274–283
Borlikova, G.G., Le Merrer, J., Stephens, D.N., 2006. Previous experience
Freedland, C.S., Sharpe, A.L., Samson, H.H., Porrino, L.J., 2001. Effects of
of ethanol withdrawal increases withdrawal-induced c-fos expression in
SR141716A on ethanol and sucrose self-administration. Alcohol., Clin.
limbic areas, but not withdrawal-induced anxiety and prevents with-
Exp. 25, 277–282.
drawal-induced elevations in plasma corticosterone. Psychopharmacol-
Gallate, J.E., McGregor, I.S., 1999. The motivation for beer in rats: effects of
ogy 10, 1–13.
ritanserin, naloxone and SR 141716. Psychopharmacology 142,
Carobrez, A.P., Bertoglio, L.J., 2005. Ethological and temporal analyses of
anxiety-like behavior: the elevated plus-maze model 20 years on.
Gallate, J.E., Saharov, T., Mallet, P.E., McGregor, I.S., 1999. Increased
Neurosci. Biobehav. Rev. 29, 1193–1205.
motivation for beer in rats following administration of a cannabinoid
Castane, A., Valjent, E., Ledent, C., Parmentier, M., Maldonado, R.,
CB1 receptor agonist. Eur. J. Pharmacol. 370, 233–240.
Valverde, O., 2002. Lack of CB1 cannabinoid receptors modifies
Gallate, J.E., Mallet, P.E., McGregor, I.S., 2004. Combined low dose
nicotine behavioural responses, but not nicotine abstinence. Neurophar-
treatment with opioid and cannabinoid receptor antagonists synergisti-
macology 43, 857–867.
cally reduces the motivation to consume alcohol in rats. Psychopharma-
Cippitelli, A., Bilbao, A., Hansson, A.C., del Arco, I., Sommer, W., Heilig,
cology (Berlin) 173, 210–216.
M., Massi, M., Bermudez-Silva, F.J., Navarro, M., Ciccocioppo, R., de
Gessa, G.L., Serra, S., Vacca, G., Carai, M.A., Colombo, G., 2005.
Fonseca, F.R., The European TARGALC Consortium, 2005. Cannabi-
Suppressing effect of the cannabinoid CB1 receptor antagonist,
noid CB1 receptor antagonism reduces conditioned reinstatement of
SR147778, on alcohol intake and motivational properties of alcohol in
ethanol-seeking behavior in rats. Eur. J. Neurosci. 21, 2243–2251.
alcohol-preferring sP rats. Alcohol Alcohol. 40, 46–53.
Cohen, C., Perrault, G., Voltz, C., Steinberg, R., Soubrie, P., 2002.
Gessa, G.L., Orru, A., Lai, P., Maccioni, P., Lecca, R., Lobina, C., Carai,
SR141716, a central cannabinoid (CB(1)) receptor antagonist, blocks the
M.A., Colombo, G., 2006. Lack of tolerance to the suppressing effect
motivational and dopamine-releasing effects of nicotine in rats. Behav.
of rimonabant on chocolate intake in rats. Psychopharmacology 21
Pharmacol. Exp. Ther. 13, 451–463.
(electronic publication ahead of print).
Cohen, C., Perrault, G., Griebel, G., Soubrie, P., 2005. Nicotine-associated
Houchi, H., Babovic, D., Pierrefiche, O., Ledent, C., Daoust, M., Naassila,
cues maintain nicotine-seeking behavior in rats several weeks after
M., 2005. CB1 receptor knockout mice display reduced ethanol-induced
nicotine withdrawal: reversal by the cannabinoid (CB1) receptor
conditioned place preference and increased striatal dopamine D2
antagonist, rimonabant (SR141716). Neuropsychopharmacology 30,
receptors. Neuropsychopharmacology 30, 339–349.
Hughes, J., 1995. Clinical implications of the association between smoking
Colombo, G., Agabio, R., Diaz, G., Lobina, C., Reali, R., Gessa, G.L., 1998.
and alcoholism. In: Fertig, J.B., Allen, J.P. (Eds.), Alcohol and tobacco:
Appetite suppression and weight loss after the cannabinoid antagonist
from basic science to clinical practice NIAAA Research Monograph
SR 141716. Life Sci. 63, 113–117.
No. 3 NIH Publ. No 95-39-31. National Institute on Alcohol Abuse and
Colombo, G., Serra, S., Brunetti, G., Gomez, R., Melis, S., Vacca, G., Carai,
Alcoholism, Washington, DC, pp. 171–185.
M.M., Gessa, L., 2002. Stimulation of voluntary ethanol intake by
Hungund, B.L., Basavarajappa, B.S., 2004. Role of endocannabinoids and
cannabinoid receptor agonists in ethanol-preferring sP rats. Psycho-
cannabinoid CB1 receptors in alcohol-related behaviors. Ann. N. Y.
pharmacology (Berlin) 159, 181–187.
Acad. Sci. 25, 515–527.
Colombo, G., Vacca, G., Serra, S., Carai, M.A., Gessa, G.L., 2004.
Hungund, B.L., Szakall, I., Adam, A., Basavarajappa, B.S., Vadasz, C.,
Suppressing effect of the cannabinoid CB1 receptor antagonist, SR
2003. Cannabinoid CB1 receptor knockout mice exhibit markedly
141716, on alcohol's motivational properties in alcohol-prefering rats.
reduced voluntary alcohol consumption and lack alcohol-induced
Eur. J. Pharmacol. 498, 119–123.
dopamine release in the nucleus accumbens. J. Neurochem. 84,
Colombo, G., Serra, S., Vacca, G., Carai, M.A., Gessa, G.L., 2005.
Endocannabinoid system and alcohol addiction: pharmacological
Johnson, B.A., 2004. Topiramate-induced neuromodulation of cortico-
studies. Pharmacol. Biochem. Behav. 81, 369–380.
mesolimbic dopamine function: a new vista for the treatment of
Dani, J.A., Harris, R.A., 2005. Nicotine addiction and comorbidity with
comorbid alcohol and nicotine dependence? Addict. Behav. 29,
alcohol abuse and mental illness. Nat. Neurosci. 8, 1465–1470.
De Vries, T.J., Schoffelmeer, A.N., 2005. Cannabinoid CB1 receptors
Lallemand, F., de Witte, P., 2004. Ethanol induces higher BEC in CB1
control conditioned drug seeking. Trends Pharmacol. Sci. 26, 420–426.
cannabinoid receptor knockout mice while decreasing ethanol pre-
De Vries, T.J., de Vries, W., Janssen, M.C., Schoffelmeer, A.N., 2005.
ference. Alcohol Alcohol. 40, 54–62.
Suppression of conditioned nicotine and sucrose seeking by the
Larsson, A., Engel, J.A., 2004. Neurochemical and behavioral studies on
cannabinoid-1 receptor antagonist SR141716A. Behav. Brain Res.
ethanol and nicotine interactions. Neurosci. Biobehav. Rev. 27,
161, 164–168.
De Witte, P., 2004. Imbalance between neuroexcitatory and neuroinhibitory
Lê, A.D., Wang, A., Harding, S., Juzytsch, W., Shaham, Y., 2003. Nicotine
amino acids causes craving for ethanol. Addict. Behav. 29, 1325–1339.
increases alcohol self-administration and reinstates alcohol seeking in
Duarte, C., Alonso, R., Bichet, N., Cohen, C., Soubrie, P., Thiebot, M.H.,
rats. Psychopharmacology (Berlin) 168, 216–221.
2004. Blockade by the cannabinoid CB1 receptor antagonist, rimonabant
Le Foll, B., Goldberg, S.R., 2004. Rimonabant, a CB1 antagonist, blocks
(SR141716) of the potentiation by quinelorane of food-primed
nicotine-induced conditioned place preferences. NeuroReport 15,
reinstatement of food-seeking behavior. Neuropsychopharmacology
29, 911–920.
Le Foll, B., Goldberg, S.R., 2005. Cannabinoid CB1 receptor antagonists as
Economidou, D., Mattioli, L., Cifani, C., Perfumi, M., Massi, M., Cuomo,
promising new medications for drug dependence. J. Pharmacol. 312,
V., Trabace, L., Ciccocioppo, R., 2006. Effect of the cannabinoid CB1
receptor antagonist SR-141716A on ethanol self-administration and
Lopez-Moreno, J.A., Gonzalez-Cuevas, G., Rodriguez de Fonseca, F.,
ethanol-seeking behaviour in rats. Psychopharmacology 183, 394–403.
Navarro, M., 2004a. Long-lasting increase of alcohol relapse by the
Ericson, M., Engel, J.A., Soderpalm, B., 2000. Peripheral involvement in
cannabinoid receptor agonist WIN 55,212-2 during alcohol deprivation.
nicotine-induced enhancement of ethanol intake. Alcohol 21, 37–47.
J. Neurosci. 24, 8245–8252.
Fagerstrom, K., Balfour, D.J., 2006. Neuropharmacology and potential
Lopez-Moreno, J.A., Trigo-Diaz, J.M., Rodriguez de Fonseca, F.,
efficacy of new treatments for tobacco dependence. Expert Opin.
Gonzalez-Cuevas, G., Gomez de Heras, R., Crespo-Galan, I., Navarro,
Investig. Drugs 15, 107–116.
M., 2004b. Nicotine in alcohol deprivation increases alcohol operant
Forget, B., Hammon, M., Thiebot, M.H., 2005. Cannabinoid CB1 receptors
self-administration during reinstatement. Neuropharmacology 47,
are involved in motivational effects of nicotine in rats. Psychopharma-
cology (Berlin) 181, 722–734.
Naassila, M., Pierrefiche, O., Ledent, C., Daoust, M., 2004. Decreased
J.A. López-Moreno et al. / Neurobiology of Disease 25 (2007) 274–283
alcohol self-administration and increased alcohol sensitivity and with-
Cippitelli, A., Navarro, M., 2005. The endocannabinoid system:
drawal in CB1 receptor knockout mice. Neuropharmacology 46,
physiology and pharmacology. Alcohol Alcohol. 40, 2–14.
Role, L.W., Berg, D.K., 1996. Nicotinic receptors in the development and
Nadal, R., Samson, H.H., 1999. Operant ethanol self-administration after
modulation of CNS synapses. Neuron 16, 1077–1085.
nicotine treatment and withdrawal. Alcohol 17, 139–147.
Samson, H.H., Sharpe, A.L., Denning, C., 1999. Initiation of ethanol self-
Nestler, E.J., 2005. Is there a common molecular pathway for addiction?
administration in the rat using sucrose substitution in a sipper-tube
Nat. Neurosci. 8, 1445–1449.
procedure. Psychopharmacology (Berlin) 147, 274–279.
Ortiz, S., Oliva, J.M., Perez-Rial, S., Palomo, T., Manzanares, J., 2004.
Schlicker, E., Kathmann, M., 2001. Modulation of transmitter release via
Differences in basal cannabinoid CB1 receptor function in selective
presynaptic cannabinoid receptors. Trends Pharmacol. Sci. 22, 565–572.
brain areas and vulnerability to voluntary alcohol consumption in Fawn
Serra, S., Carai, M.A., Brunetti, G., Gomez, R., Melis, S., Vacca, G.,
Hooded and Wistar rats. Alcohol Alcohol. 39, 297–302.
Colombo, G., Gessa, G.L., 2001. The cannabinoid receptor antagonist
Pagotto, U., Marsicano, G., Cota, D., Lutz, B., Pasquali, R., 2006. The
SR 141716 prevents acquisition of drinking behavior in alcohol-
emerging role of the endocannabinoid system in endocrine regulation
preferring rats. Eur. J. Pharmacol. 430, 369–371.
and energy balance. Endocr. Rev. 27, 73–100.
Serra, S., Brunetti, G., Pani, M., Vacca, G., Carai, M.A., Gessa, G.L.,
Pierce, R.C., Kumaresan, V., 2005. The mesolimbic dopamine system: the
Colombo, G., 2002. Blockade by the cannabinoid CB(1) receptor
final common pathway for the reinforcing effect of drugs of abuse?
antagonist, SR 141716, of alcohol deprivation effect in alcohol-
Neurosci. Biobehav. Rev. 30, 215–238.
preferring rats. Eur. J. Pharmacol. 443, 95–97.
Rimondini, R., Arlinde, C., Sommer, W., Heilig, M., 2002. Long-lasting
Sharpe, A.L., Samson, H.H., 2002. Repeated nicotine injections decrease
increase in voluntary ethanol consumption and transcriptional regulation
operant ethanol self-administration. Alcohol 28, 1–7.
in the rat brain after intermittent exposure to alcohol. FASEB J. 16,
Singh, M.E., Verty, A.N., McGregor, I.S., Mallet, P.E., 2004. A cannabinoid
receptor antagonist attenuates conditioned place preference but not
Rinaldi-Carmona, M., Barth, F., Congy, C., Martinez, S., Oustric, D., Perio,
behavioural sensitization to morphine. Brain Res. 1026, 244–253.
A., Poncelet, M., Maruani, J., Arnone, M., Finance, O., Soubrie, P., Le
Thanos, P.K., Dimitrakakis, E.S., Rice, O., Gifford, A., Volkow, N.D., 2005.
Fur, G., 2004. SR147778 [5-(4-bromophenyl)-1-(2,4-dichlorophenyl)-4-
Ethanol self-administration and ethanol conditioned place preference are
ethyl-N-(1-piperidinyl)-1H-pyrazole-3-carboxamide], a new potent and
reduced in mice lacking cannabinoid CB1 receptors. Behav. Brain Res.
selective antagonist of the CB1 cannabinoid receptor: biochemical and
164, 206–213.
pharmacological characterization. J. Pharmacol. 310, 905–914.
Vengeliene, V., Bachteler, D., Danysz, W., Spanagel, R., 2005. The role of
Rodriguez de Fonseca, F., Roberts, A.J., Bilbao, A., Koob, G.F., Navarro,
the NMDA receptor in alcohol relapse: a pharmacological mapping
M., 1999. Cannabinoid receptor antagonist SR141716A decreases
study using the alcohol deprivation effect. Neuropharmacology 48,
operant ethanol self administration in rats exposed to ethanol-vapor
chambers. Zhongguo Yaoli Xuebao 20, 1109–1114.
Williams, S.H., 2005. Medications for treating alcohol dependence. Am.
Rodriguez de Fonseca, F., Del Arco, I., Bermudez-Silva, F.J., Bilbao, A.,
Fam. Phys. 72, 1775–1780.
Source: http://www.pbglab.com/pdf/publications/0004.pdf
Cl i ni cal Coref erence Annot at i o n G ui del i ne s ( w i t h excerpt s f rom O DIE gui del i nes an d m odi f i ed f or SH ARP n/ TH Y ME) Last updated November 2, 2013 The following is a summary of medical coreference annotation guidelines designed for the medical annotation projects. We acknowledge the ODIE project and OntoNotes coreference annotation guidelines in the creation of these guidelines. Our goals are:
MEDICINES MANAGEMENT GUIDE TO PRESCRIBING Medicines Management Team APPROVED BY: The Clinical Executive Team DATE OF ISSUE: July 2014 VERSION: Amendments for East Surrey – Jay Voralia Page 1 of 45 MEDICINES MANAGEMENT GUIDE TO PRESCRIBING Foreword This document aims to support the delivery of consistent prescribing advice to practitioners prescribing on behalf of the CCG with a purpose of:







