Levitra enthält Vardenafil, das eine kürzere Wirkdauer als Tadalafil hat, dafür aber schnell einsetzt. Männer, die diskret bestellen möchten, suchen häufig nach levitra kaufen ohne rezept. Dabei spielt die rechtliche Lage in der Schweiz eine wichtige Rolle.
Prema.or.th
Harmonization and Standardization of the
ASEAN Medical Industry
"Healthcare is a priority for ASEAN. Countries must adhere to international
standards and not create their own … We know it is important, we see efforts to
harmonize, but progress is slow."
-Martin Hutagalung
Regional Director, US-ASEAN Business Council
Mason School of Business
The College of William & Mary
Table of Contents
3. Intellectual Property Rights ……………………………………………….19
vi. Regulatory Data Protection ……………………………………………36
1. General Assessment of ASEAN Member Countries………………………….54
2. Top 10 Pharmaceutical Companies…………………………………………….54
5. Indonesia - Intellectual Property Rights……………………………………….56
6. Malaysia - Medical Device Products ………………………………………….57
7. Malaysia - Medical Device Products – Risk Level…………………………….57
8. Malaysia - Medical Device Product Framework………………………………58
9. Malaysia – Medical Device Placement…………………………………….58
10. Malaysia – Incentive Program…………………………………………………59
11. Singapore – Pharmaceutical Industry……………………………………….60
12. Singapore – Product License Approvals……………………………………….60
13. Singapore – Clinical Trial Certificate Issues………………………………….61
14. Singapore – Good Manufacturing Practice……………………………………62
15. Singapore – Post-Market Alert System……………………………………….63
17. ASEAN - ACTR Format………………………………………………………64
18. ASEAN - ACTD Organization……………………………………………….64
19. Thailand – Allowable Drugs in Hospitals/Clinics…………………………….65
20. Thailand – Intellectual Property Rights……………………………………….65
The Association of Southeast Asian Nations (ASEAN) was formed to provide its member countries with an avenue for discussing foreign affairs, growth, and peace in a cooperative environment. Lately, however, attention has shifted to focus on key areas of development, one of which is healthcare. Although there is some evidence of healthcare standardization occurring in the region, overall progress is slow given the infrastructure, institutional and economic disparities that exist between the ASEAN-6 and ASEAN-4 nations. Countries which have made the greatest advancements include Singapore and Malaysia, while Cambodia and Laos remain the least committed.1
Research from both primary and secondary sources reveals that there are five areas either where progress is occurring or are "hot topics" in the region. 2
Key areas of research include:
ASEAN Trade Agreement Good Practices Parallel Importation ASEAN Common Technical Dossier (ACTD) Mutual Recognition Agreement (MRA)
In addition to the harmonization areas uncovered, research also reveals several emerging trends in the region, which are of particular importance to multinational and local pharmaceutical companies. On one hand, foreign direct investment, public and private investments are making healthcare more accessible to rural populations in the remote regions of ASEAN. Such endeavors provide unique opportunities for pharmaceutical companies to expand their existing operations. Unfortunately, however, that opportunity comes at a price. With greater government intervention into the pricing of consumer medicines and limited to no Intellectual Property Right recognition in others, pharmaceutical companies must be both cognizant and wary when enlarging their presence in these areas. Admittedly, some of the biggest offenders such as Cambodia have made strides in battling against the unregistered and counterfeit drug markets, but such attempts have a limited impact in the overall scheme.
The hope is that with Indonesia receiving chairmanship of ASEAN from Vietnam, new perspectives will be articulated on ASEAN's road map to harmonization. But as recent events in the European Union have showcased, it is a tremendous endeavor – especially by 2015 – to bring together ten countries to benefit all as one.
1 For the purposes of this project, Myanmar and Brunei Darussalam were not examined.
2 Secondary sources include reputable databases and reports such as Business Monitor International, Factiva
EBSCOHost and ASEAN Economic Bulletin.
The Association of Southeast Asian Nations (ASEAN) was formed in 1967 to provide its member countries with an outlet for discussing foreign affairs, growth, and peace in a cooperative environment. Currently, the primary emphasis is on economic issues. The ASEAN member countries include: Thailand, Cambodia, Vietnam, Singapore, Philippines, Myanmar, Malaysia, Laos, Indonesia, and Brunei Darussalam (ASEAN Background). Exhibit 1 depicts ASEAN countries researched and a general assessment on entering the health care market in these countries based upon current political, economic and social conditions.
ASEAN and the United States
Progressive talks between the U.S. and ASEAN formally began in 1977. Initially, the focus was on markets, technology and energy resources, but beginning in 1990, the focus shifted towardtrade, investments and human resources. The U.S. currently participates in ASEAN consulting meetings such as the ASEAN Regional Forum (ARF) and the Post Ministerial Conferences (PMC). Through these meetings, the U.S. has been able to enhance its relationship with ASEAN, especially through trade. Total trade between ASEAN and the U.S. increased 10% from 2006 to 2008 and the total foreign direct investment flow to ASEAN nearly doubled. ASEAN has become the fastest growing export market and the U.S. is becoming its major trading partner.
U.S. and European Medical Products Industry
Business Monitor International has ranked the US number one in the pharmaceuticals market, with spending reaching US$306bn in 2009 (BMI, 2010). The Food and Drug Administration (FDA) is the regulatory regime in the United States. It was established in 1906, under the Department of Health and Human Services, and is considered the superior regulatory model worldwide. The healthcare arm of the FDA is responsible for protecting the public health by assuring the safety, efficacy, and security of human and veterinary drugs, biological products, and medical devices (U.S. Food, 2010). The FDA approval process is cumbersome and can cost millions of dollars for a company. On average, it takes 12 years and over US$350 million to get a new drug from laboratory onto the pharmacy shelf (Roth, 2010). The company, research institutions, and other organizations are responsible for all testing costs and providing supporting evidence to the FDA for their Investigational New Drug Application (IND). Based upon the evidence provided in the application, the FDA will decide if it is safe for the drug to be tested in humans. Only 1 in 1,000 drugs make it to human testing (Roth, 2010).
Due to healthcare reform initiatives in the US, many manufacturers may see pricing strategy changes in the near future due to expanding state insurance laws. Coverage for the poor and patients with pre-existing medical conditions will no longer be excluded by their insurers. Because drug manufacturers negotiate directly with insurers and healthcare providers for the
pricing of drugs, there are no formal regulations. Innovative drugs are priced at a premium until the patent expires, at which point competition from generic drug makers move into the arena.
Johnson & Johnson (J&J) is listed in the top ten for both medical devices and pharmaceuticals, but does not have a strong foothold in the vaccine area. That is about to change in late 2010 or early 2011. Dutch biotechnology vaccine group Crucell will have the remaining 82.5% of their company bought by Johnson & Johnson (Johnson & Johnson, 2010). Crucell has offices aroundthe world, but the two that are in the ASEAN countries are Malaysia and Viet Nam. With J&J having offices in these two countries, it may help not only with providing vaccines to thesecountries, but could also help influence the need for FDA approved drugs from US to fund local clinical trials in Viet Nam.
In April 2008, Switzerland and Singapore signed a memorandum of understanding (MoU) relating to therapeutic products and the collaboration of the two countries' pharmaceutical administrations. The agreement, which pertains to both pharmaceuticals and medical devices, will aim to encourage data and inspection exchange, in a bid to improve market surveillance, approvals processes and pharmacovigilance (Hollis, 2010). With Novartis the number three revenue generating pharmaceutical company in the world and having already had similar MoU'ssigned with US, Australia, and Canada there could be more MoU's signed with other ASEAN countries particularly Indonesia and Thailand.
The medical device industry has 4 major players (Medtronic, Boston Scientific, Haemonetics Corp, and Cyberonics, Inc.) that are all based out of the U.S. (BMI, 2010). Twelve major players dominant the pharmaceutical industry. Of those, 7 originate from the United States. A complete list is available in Exhibit 2. Each country regulates its pharmaceutical drugs in varying ways (Exhibit 3). For example, Germany allows its wholesalers and retailers to commit to any price over the manufacturers as well as allowing the product to be taxed afterwards. However, the UK's government handles the price points for their pharmaceuticals at certain benchmarks. On the manufacturing side, most countries adhere to a 20-year life span patent (Exhibit 4).
ASEAN proposed tariff regulations to encourage a free flow of capital to increase economic growth among the countries. The top four intra-exporters are Singapore, Malaysia, Thailand and Indonesia. The top three intra-importers are Singapore, Malaysia and Thailand. The average CEPT rate for ASEAN countries is 3.71%; however, Cambodia is the benchmark high with 9.3% and Singapore is the low with a nonexistent rate (Oktaviani, 2007). The ASEAN agreement was to lower the CEPT rates among the countries to encourage intra-ASEAN trade. On the other hand, these countries trade more outside of these countries than within because of the similarities of each country's products and services. Conflicting reports have been published about intellectual property rights (IPR) and foreign direct investments (FDI). Traditional research has shown that strong IPR increases FDI. Other research has shown that strong IPR inhibits
imitation, which brings in more FDI, but it is spread amongst competitors (Yang, 2008). Overall, FDI is expected to decrease. Countervailing has been present within ASEAN as a way to attract large company buyers to off-patent drugs (Ellison, 2010). Anti-dumping is also present in ASEAN as a measure to reduce surges of imports (Deardorff, 2005).
Cambodia suffers from high levels of corruption as evidenced by its 154th spot out of 178 countries on Transparency International's Corruption Perception Index (Transparency International, 2010). Corruption is particularly prevalent among civil servants, whose salaries remain lower than garment workers and are insufficient to cover basic needs. Moreover, in a recent report, the Cambodian economy ranked lowest out of 16 Asia Pacific markets studied (Research and Markets, 2010). Thus, the combination of a poor economy and low wages only encourages government officials and civil servants to resort to corruption.
Cambodia is also the dumping country of choice for foreign nations, especially since the EU placed dumping restrictions on China and Viet Nam. For example, back in 2006, it was well documented that a number of Taiwanese and Hong Kong shoemakers began building plants in Cambodia in order to sidestep EU regulations (Ho, 2006). Additionally, Cambodia has the highest tariffs in Southeast Asia for both imported and exported goods and does little trade with its neighboring countries. Cambodia has a CEPT rate of 9.3%, indicating that the country only exports 9.3% to other ASEAN countries. And like the Philippines, Cambodia has the lowest intra-ASEAN import intensity at 19% (Oktaviani, 2007).
While high levels of corruption, limited economic growth and prevalent anti-dumping puts Cambodia at a disadvantage compared to its ASEAN peers, other social and government elements further compound the problem. As a direct result of the Khmer Rouge atrocities committed in the 1970s, 60% of the population is under age 24 with the median age being 22.6 years old (Central Intelligence Agency, 2010). And because of the country's poor infrastructure that population is predominately rural. Led by Prime Minister Hun Sen and the Cambodia's People Party (CPP) Cambodia is politically stable, but the government is excessively bureaucratic, which slows down the decision making process and lacks transparency (Research and Markets, 2010).
On the plus side, Cambodia's pharmaceutical industry is predicted to grow by double digits –about 11.2% - over the next decade. Only in the past ten years has Cambodia experienced somewhat stabilized economic growth and political stability, which has allowed officials to put more effort into the country's healthcare program. But, the industry substantially lags behind more advanced nations such as Singapore (Research and Markets, 2010). In order to remedy that disparity, Cambodia is receiving high levels of foreign aid and foreign direct investment from South Korea and China. In 2008 Cambodia also received a $12.35 million grant from the World Bank for a trade development program. The aim of the grant is to develop trade policy, build performance monitoring systems and provide implementation support to the Ministry of Commerce in Cambodia (The World Bank, 2008).
Traditionally, Cambodia has not been viewed as an ideal country for vaccine trials or developments because the country's population is much lower compared to other ASEAN nations such as Indonesia or Thailand. Moreover, attempted vaccine trials have met with little success. Back in 2004 despite green lighting a trial for Gilead Sciences AIDS drug, Viread, the Cambodian government later halted the study. Even though Gilead Sciences was willing to sell the medication for $300 – a significant discount from the US price of $3,900 – the project was met with opposition from Cambodian sex workers who demanded additional counseling and free follow-up care if they became infected or suffered adverse side effects (Chase, 2004).
The Cambodian pharmaceutical market is currently quite small, with per capita expenditure on medicine and pharmaceutical products much lower compared to other countries. Cambodia, however, has a high growth potential over the next decade. IBM forecasts that the demand for drugs and drug consumption in Cambodia is expected to increase at a rate of 11.6% and willreach a level of $302 million by 2014. By 2019, the market will have expanded to $516 million (Research and Markets, 2010). As such Cambodia is increasingly becoming a target country for drug makers.
While the national health care budget in Cambodia is increasing, it is mostly an effect of higher foreign contribution levels. Such reliance on foreign money, however, leads to instability in the health care market because programs and agendas are pushed and are then abruptly curtailed when funds are no longer available. While policies have been constructed on paper to address this issue, there is no evidence of implementing these checks in order to prevent the situation from occurring (Park, 2010).
Even more troubling, however, is that the counterfeit drug market is an enormous problem in Cambodia. A number of reports have been published on the effect and damage that counterfeit drugs have had on the region. A Ministry of Health study in 2006 found 58% counterfeit and substandard anti-malaria drugs exist in licensed outlets and 75% in non-licensed outlets. A 2003 study performed by USP DQI discovered that 27% of 451 drug samples tested where counterfeit (USP revisiting medicare, 2006). A later survey conducted by C.T. Lon and associates found that 79% of the drugs were not registered ( Lon, et al. 2006) . Most troubling, however, is that the World Health Organization estimates that Cambodia has close to 2800 illegal medicines sellers and 1000 unregistered medical products (WHO, 2003).
Intellectual Property Rights
There is little to no respect for intellectual property rights in Cambodia as evidenced by number of counterfeit drugs in the region.
Because Cambodia has only been a relatively stable country for ten years, the infrastructure and income gap between Cambodia and ASEAN countries is much more pronounced. As such, Cambodia has opted not to sign several key ASEAN agreements and has made little progress on many of the health care agendas currently being propagated by the ASEAN council. While
leaders of ASEAN's four less developed member countries – Cambodia, Laos, Myanmar and Vietnam –met in November, 2010 in Phnom Penh, where they vowed to promote health care development projects any tangible results are at least five years away (Kyodo, 2010).
Despite only having a per capita income of $1,900, which ranks Cambodia 187th out of 227 countries tracked, there are some instances of health care advancements (Central Intelligence Agency, 2010). According to Ambassador at Large, Roland Eng, both public and private institutions are building hospitals and clinics in remote regions of the country to ensure health services are more accessible to rural populations. Such projects are meeting with success.
Additionally, there have been efforts made to crack down on the counterfeit drug trade. In 2008, over 200 raids were carried out across Southeast Asia, including Cambodia, which seized $6.6 million worth of counterfeit anti-HIV, anti-malaria, anti-tuberculosis drugs and other medications (The Telegraph, 2008). Also, Pharmelp, a company that aims to bring affordable equipment to detect counterfeit and substandard medicines to developing nations, is bringing its machines to Cambodia (Taylor, 2010). The equipment will be installed in February 2011 at the National Health Product Quality Control Center in Phnom Penh.
The market for pharmaceuticals in Indonesia has incredible growth potential. While Business Monitor International ranks the country 11th out of 17 for investment within the Southeast Asian market, the biggest growth sources come from its stable political system, year-over-year growth of the domestic pharmaceutical market, and the growth of an already large population. The main drawbacks are low spending on pharmaceuticals, higher levels of corruption, and a relatively small percentage of elderly in the country. With this in mind, there are several trends emerging within the Indonesian market ranging from increased collaboration with countries within ASEAN to the growth of the over-the-counter (OTC) and generic markets. (Indonesia Pharmaceuticals, 2010). Finally, one of the biggest trends taking place in Indonesia is the reliance on domestically produced and manufactured drugs. The biggest question arising from this movement centers on what specific steps Indonesia will take to continue to encourage domestic consumption of pharmaceuticals over foreign products.
The pharmaceutical industry in Indonesia is controlled by the Directorate General of Drug and Food Control (DG DFC) which falls under the Ministry of Health. This agency is in charge of everything from the control of production to providing licenses for drug imports. The DG DFC is responsible for monitoring and making changes to the National List of Essential Medicines (NLEM). The NLEM consists of medicines that the Ministry of Health decides are the most needed and most available. The Ministry of Health states, "the implementation of NLEM is meant to increase the efficacy, safety, rational use, and medicine management which altogether increase efficiency of available cost, which in turn extends coverage and increases the average quality of medicine prescriptions" (National List of Essential, 2008).
Within the Ministry of Health also lie the National Agency of Food and Drug Control (BTOM). This is similar to the Food and Drug Agency in the US. One of the biggest pushes by the government and BTOM has centered on the increased use of generic pharmaceuticals over brand names. The Indonesian government believes that due to the lower price and relatively equal effectiveness and safety of generics over name brands, more citizens will have access to medicines that they would not normally be able to afford. The push for generics has gone so far as to require all branded pharmaceutical products to print the generic name at least 80% as big as and directly below the trade name of the product. The hope is to spread the knowledge of generics to as many people as possible in the country. In order to guarantee the safety and equivalence of the generic drugs, the BPOM has required that any drug being submitted for generic approval must also submit bioequivalence and bioavailability data (Indonesia Pharmaceuticals, 2010).
Labeling
Labeling for pharmaceuticals in Indonesia requires that all labels be printed in the Indonesian
language. Some specific items that must be listed on all packaging for all pharmaceuticals
include the product name, the name and strength of active ingredients, the generic name (if
applicable, at 80% size of the brand name), the name of the manufacturer if it is an imported
drug, and the expiration date. While many of these requirements are in place, most are not being
enforced (ASEAN Labeling Requirements).
Quotas
The government of Indonesia does not have any quotas established to limit the imports of
pharmaceuticals. Currently, the government has the equivalent of $180 million allocated to
import pharmaceutical ingredients in case of an emergency. In such an event, the ingredients
would be used to produce generic pharmaceuticals that could then be sold to private or public
institutions (Indonesia: Supply and Demand, 2004). Without quotas in place, Indonesia has
instead controlled foreign drug companies through its somewhat difficult process of obtaining
import licenses (Indonesia: Supply and Demand, 2004).
Import Licenses
In 2008, the Ministry of Health set new guidelines for the registration and importation of
pharmaceuticals into Indonesia. Decree 1010/08 separates manufacturers from wholesalers in
order to protect consumers. Only Indonesian manufacturers are allowed to register
pharmaceutical products, and only wholesalers are allowed to distribute them within Indonesia.
Foreign companies can only register their products with Indonesian manufacturing companies
based in the country, and once they have done so, they are required to sell directly to
wholesalers. Regarding this policy, the Ministry of Health states, "This distinction is considered
to represent the least trade restrictive approach to achieve the legitimate goals set" (Global Trade
Alert, 2009). The "legitimate goals" refer to increasing consumer safety and quality of products
within Indonesia (Indonesia: Regulating Registration, 2009).
Harmonization
Many of the items touched on above can provide some proof to Indonesia's attempts at
harmonizing with ASEAN standards and collaborating with other countries in the region. Decree
1010/08 that established rules and procedures for import licenses was developed to be the least
imposing on trade in the region. One of ASEAN's main goals was to establish a region with free
trade. Indonesia can only be seen to be attempting to aid this goal through Decree 1010/08. The
government is trying to promote safe products and practices within its own country while
keeping trade in the region as unrestricted as possible. The lack of quotas for pharmaceuticals
can also be seen as an attempt at harmonization within ASEAN. Currently, Indonesia has a 10%
tariff on all imports of pharmaceuticals; however, by 2011, Indonesia will be required to cut this
to zero in accordance with the ASEAN free trade agreement (Indonesia Pharmaceuticals, 2010).
One of the biggest steps toward harmonization can be seen in labeling for pharmaceuticals. While many countries may not have exactly the same procedures and requirements, there are many trends seen throughout the region such as the distinction between what is required to be printed on the exterior carton verses a vial and the difference between requirements for domestically produced pharmaceutical products verses foreign products. If successful, labeling may be the first full step toward harmonization (ASEAN Labeling Requirements).
In the realm of intellectual property rights, Indonesia has taken a huge step in combating the country's serious counterfeiting problem. The National Anti-Counterfeiting Task Force was set up by the government in hopes of fighting a problem that results in almost 30% of all pharmaceuticals in the country being counterfeits. Indonesia still remains on the US trade association Pharmaceutical Research and Manufacturers of America's (PhRMA) Watch List, having been moved down from its Priority Watch List in 2007 (Indonesia Pharmaceuticals, 2010).
Finally, collaboration with other countries in ASEAN has begun as there has been a growing trend of foreign companies within ASEAN starting joint-ventures within Indonesia. One such example is the Singaporean Drug Company Innogene Kalbiotech and Malaysian research company Info Kinetics Sdn Bhd. The two companies have set up a research unit in a Jakarta hospital [names not released] to study bioavailability and bioequivalence within the Indonesian pharmaceutical space. The research will also be supported by Indonesian company PT Pharma Metric. This sort of collaboration can only lead to positive working relationships within ASEAN (Indonesia Pharmaceuticals, 2010).
Labeling
A regulation issue for ASEAN is whether or not medical device labels should all be in the
language of the country of where it is made or if labels need to be translated into all countries
where it is distributed.
Import/Export
Companies must obtain an import license from the Ministry of Industry and Trade. In order to
import products, a company also needs a permit from the Ministry of Health. There are hardly
any restrictions on imports into Indonesia. The registration of medical devices has been under the
National Agency of Drug and Food Control (known as BPOM in Indonesia) since 2001. The
general rules that Indonesia follows regarding imports are in keeping with World Trade
Organization standards. Any companies in Indonesia, whether it be a limited liability company, a
state-owned limited liability company or a cooperative company are able to obtain an import
license. The company must have adequate room and equipment to store serums and vaccines,
along with an arrangement with a laboratory appointed by the Ministry of Health. Recently
Indonesia has liberalized its trade regime in order to reduce protection. There are no quotas on
importing medical devices. Distributors have to register the medical device after which it usually takes 6 months to obtain a license (BMI, 2010).
Intellectual Property Rights
According to the National Agency of Drug and Food Control in Jakarta, Indonesia, patents last
for a period of 20 years. Agreement on Trade-Related Aspects of Intellectual Property Rights
(TRIP) will slow the introduction of generics, increase prices, and reduce access to various
medicines for many patients. In order to compensate the protection of the innovation of
medicines, testing and approval of generics are allowed before the patent expires (DGIPR,
Indonesia).
Indonesia's Intellectual Property Law creates significant barriers to promotion of foreign investment within the country. As of 2005, only drugs manufactured and produced within Indonesia are eligible for patent protection. Accordingly, drugs sold and registered with the Ministry of Health are able to be legally copied and distributed under a generic name by Indonesian countries. Additionally, applications for pharmaceutical product regulation can only be filed for by local companies, forcing foreign companies to invest in manufacturing centers within Indonesia, or pursuing joint-venture operations with Indonesian firms. Additionally, Indonesia – like other ASEAN countries – faces issues regarding law enforcement, resulting in a high number of counterfeit drugs in distribution (Ratanawijitrasin, 2005). Exhibit 5 details laws written to protect intellectual property.
Laos is a landlocked country in Southeast Asia and has little transparency regulations or the healthcare sector. Much of the data collected is outdated, but unfortunately, recent data is not available.
With the country's economic liberation in the 1980s, the private provisions of drugs increased dramatically. By 1997, 80% of pharmaceuticals available in Lao PDR were being provided by the private sector, mostly because of the development of private pharmacies.i Good Pharmacy Practices were developed and applied in a sample collection of Lao PDR pharmacies.ii There are three areas where quality seemed to be lower in districts than other districts further afield. The qualities and their percentages in each case are as follows:
Poor Dispensing Practices – 59%
Drugs not being labled – 47%
Different drugs being placed in the same package – 26%
Prices for drugs are not stable and can vary depending on districts. A research study on the price of four drugs revealed that prices varied very little in remote districts to as much as ten-fold in one district (Stenson, 2001).
The Ministry of Health is the lead regulator in Lao PDR. Unfortunately, they do not view pharmaceuticals, vaccines, or medical devices as a priority. Moreover, they do not see a high importance on Intellectual Property, parallel importation, counterfeits, or other issues of ASEAN harmonization. Instead, the three goals the Ministry of Health Lao PDR saw in 2003 were (Ministry of Health of Lao PDR):
By 2020-Free Health Care Services from state of underdevelopment, ensure the full HS coverage, justice and equity to increase quality of life of all Lao ethnic groups
By 2010-free the poverty of all Lao ethnic groups from the state of Laos
By 2005-the poverty of all Lao ethnic groups will be reduced in half of the current figure
According to Stenson, Syhakhang and Tomson, "the regulatory system seems to be focused on entry into the pharmaceutical retail market and dealing with basic issues of product quality and conditions of sale. An enforcement system including sanctions is being developed; other policy instruments such as information and economic means are hardly being used at all. The government presently faces a trade-off between quality of pharmaceutical services and geographical equity of access. The study shows that regulation is strongly influenced by the general socioeconomic context"(Stenson, 2001).
Intellectual Property Rights
Intellectual Property Rights are becoming more important in the global economic community. In January 1998, only a few countries, including Laos, did not have the full range of Intellectual Property laws on their statute books in accordance with the Paris and Berne Conventions (Endeshaw, 1998).
Malaysia score remains at 70 for market risk, which refers to a subjective assessment of the country's IP laws, policy and reimbursement regimes, as well as to the speed and efficiency of the approvals process. Despite the positive prospect of harmonization, however, the significant counterfeit drug industry, the difficulty in applying process patents, the lack of data exclusivity and generally poor regulatory enforcement will continue to pose major drawbacks to multinationals interested in Malaysia (BMI, 2010).
The manufacturing and marketing of pharmaceutical products in Malaysia are heavily regulated as in most developed countries. Medicines marketed in Malaysia are required to be registered by the Drug Control Authority (DCA) of the Ministry of Health. All manufacturers, importers andwholesalers are required to be licensed by the DCA. The registrations of prescription and OTC medicines require proof of efficacy, quality and safety, and are subjected to stringent screening and testing as well as regular and random post-marketing surveillance and testing. All manufacturers in Malaysia are subjected to regular and random inspection by DCA inspectors. Medicines are regulated under several acts such as: the Poisons Act, the Dangerous Drugs Act and the Drugs Act. Medicine advertisements require prior approval by the Medicines Advertisement Board. Malaysia is a member of the WTO and has acceded to the TRIPS agreement. Patents are registered and copyrights are protected (BMI, 2010).
Analytical method protocol for the testing of the raw materials (only the active pharmaceutical ingredients and preservatives if any) is used for injections. This should include the specifications and certificate of analysis. All analytical test procedures where possible should be in accordance with the official monograph of that ingredient in the latest edition of the official pharmacopoeia such as British Pharmacopoeia, United States Pharmacopoeia or World Health Organization(NPCB).
At present, Malaysian private clinics and pharmacies are not required to comply with standard labeling regulations. However, this means that most of the medicines, which are usually taken out of standard packs and repacked, do not come with appropriate usage and indication information. In October 2005, the Ministry of Health issued further guidance on the requirement that all registered pharmaceutical products be labeled with a Meditag, a hologram security patch. Labeling laws for dispensed medicines came under scrutiny in early 2006 for not providing clear information to patients, especially to those who receive more than one medication. Additionally, labels for generics medicines are also thought to be lacking, with professional groups lobbying
the government for appropriate changes in legislation, which would complement the recent efforts to make drug monitoring more effective (NPCB).
Packing/Storage
Containers used should afford protection to reference substances from moisture, light and
oxygen. From the point of stability, sealed glass ampoules are recommended. An important
matter to be considered when choosing suitable containers is their permeability to moisture.
Additional measures may be necessary to ensure long term integrity and stability. The filling of
reference substances into containers can be carried out in a glove box with facility for nitrogen
supply, where humidity is controlled.
Theoretically, the stability of the substances should be enhanced by keeping them at low temperatures but, for substances that contain water, storage below 00C may impair the stability. It should also be remembered that the relative humidity in normal refrigerators or cold-rooms may be high and, unless ampoules or other tightly closed containers are used, the improvement in stability may be more than offset by degradation due to the absorption of moisture. Storage at about +50C, with precautions to prevent such absorption, have proved satisfactory for most chemical reference substances.
The storage and maintenance of unopened containers of the chemical reference substance, in accordance with information provided, are integral to its suitability of use. To avoid any doubts concerning the integrity of opened containers, it is suggested that potential users obtain only the quantities of substances necessary for short-term need and obtain fresh stocks (held under controlled and known conditions) when needed. Long-term storage of substances in opened containers is to be avoided. Similarly, efforts should be made to avoid possible degradation, contamination and/or introduction of moisture during the repeated use of a substance (NPCB).
In May 2005, the Malaysian Medical Association implemented a guideline permitting doctors and hospitals to advertise their medical services. While the guideline have a number of restrictions, doctors and hospitals are able to advertise their medical specialties and any new or technologically advanced medical equipment. However, the advertisements are limited in their claims, prohibiting medical providers from exaggerating their abilities, asserting their achievements or ‘overselling' a product. Nevertheless, some hospitals have already taken advantage of this new advertising privilege through new product launches. The Ministry of Health intends to pass new regulations enabling legislation for these advertising rules. Under the new regulations, the Ministry must first clear claims that any pharmaceutical or medical device prevents or treats an illness or condition. Approval will also be necessary in order to sell a product commercially in Malaysia. The Ministry of Health hopes that this proposed regulation will allow for product testing in order to ensure the safety and effectiveness of products prior to their advertising within the country (BMI, 2010).
Intellectual Property Rights
Despite a major revision of patent law in 2001 and subsequent amendments in 2003, patent protection continues to be the cause of friction between the government and international drug manufacturers. While the government revised the period of protection for pharmaceuticals by increasing it to 20 years, it also implemented legal provisions that have come under heavy criticism from the industry. For example, the stipulation that limited the manufacturing, use and sale of a generic drug before the expiry of the original's patent are no longer considered patent infringement. Also, provisions allow the licensing and production of medicines by the government under certain conditions, without the patent holder's consent. The 2003 amendment attempted to make registering a patent easier and less expensive. Under this system, international patent applications may be made in any one of the countries of the Patent Co-operation Treaty, an initiative by the World Intellectual Property Organization (WIPO). Previously, the applicant had to make the application in each and every country where the patent was to be applicable. With procedures increasingly aligned with regional and international norms, it still did not address the issues at the center of the debate between government and industry.
In 2005, the United States Trade Representative (USTR) listed Malaysia as a Watch List country in its Special Report on Intellectual Property Protection.
The USTR and PhRMA criticized the Malaysian government on a number of points, including the level of counterfeiting taking place in the country (despite the introduction of holograms on pharmaceutical packaging), the difficulty in applying process patents, the lack of data exclusivity [which has not been aligned with the World Trade Organization (WTO)'s TRIPS agreement] and the overall poor standard of regulatory enforcement. Additionally, the association has criticized the lack of patent linkage as part of the registration process, which has led to instances of generic products being launched while original patents are still in effect.
In 2007, Malaysia remained on the Watch List, despite showing a solid commitment to strengthening IP protection and enforcement in 2006. However, the report welcomed the process of establishing a specialized IP court, which is designed to more effectively handle civil and criminal copyright cases. The US has indicated that it will continue to work with Malaysia to encourage full implementation of WIPO Internet Treaties. While international criticism of the current state of patent legislation is expected to continue, the government is unlikely to significantly amend the law in the short term, not wishing to further pressure the indigenous industry. In the meantime, financial gains from parallel trade, which is encouraged as a cheaper option for the state-funded healthcare, will continue to be made almost exclusively by the middle traders, thus not achieving its aim, but instead serving to further antagonize multinational pharmaceutical players. Consequently, Malaysia remained featured on the Watch List for 2008 as well as for 2009 (BMI, 2010). Only in 2010 was Malaysia removed from PhRMA's list (PhRMA, 2010).
More than 65% of Malaysia's pharmaceuticals and medical equipment are imported (mostly from the US, Japan and Germany), partly because most doctors are reluctant to switch to local brands because they perceive imported drugs as being higher quality, especially novel and specialist treatments. Most drugs produced by local companies are basic medicines, such as painkillers and antibiotics, since it is not economically feasible to target a specialized segment Increasing domestic demand, a significant proportion of which the local manufacturing industry will be unable to meet, will stimulate the growth of pharmaceutical imports over the next five years. The market will remain receptive to foreign products, particularly at the hi-tech end of the scale. The overall trade balance will increasingly shift in favor of imports throughout the forecast period, indicating sizeable business opportunities for foreign companies (MIDA).
In May 2007, as a sign of its strength in FTA negotiations with the US, Malaysia stated that it is seeking the right to issue compulsory licenses on patented drugs. While Malaysia is legally within its rights, as permitted by WTO rules, the country will be strongly discouraged to do so by the US, because the profits of multinational drug makers will be negatively impacted. Given that the average monthly wage in Malaysia is approximately US$1,000, compulsory licenses have made antiretroviral treatments affordable to the vast majority of the population. The USTR also kept Malaysia on the Watch List for 2010, pointing to the lack of resources and training that lead to a backlog at specialized IPR courts in the country. The report also highlighted the absence of effective protection against unauthorized disclosure or unfair commercial use of test data produced for product approval in the pharmaceutical market. The report also urged the development of an effective and prompt patent system for new pharmaceutical products.
Parallel imports have recently been legalized in a bid to cut costs in the public healthcare sector, undermining revenues on branded products. The practice is angering the multinational sector, with foreign players critical of the government's biased approach to regulatory and enforcement issues. Pharmaniaga Logistics Sdn Bhd (formerly known as Remedi Pharmaceuticals). This subsidiary of leading drug company Pharmaniaga is responsible for around 75% of medicines purchased by public healthcare institutions. The strict policy results in public prices being set below market prices, which is necessary, given that the government is responsible for around 60% of reimbursement amounts. Nevertheless, the country is considering price ceilings for selected essential drugs to improve access. Portending further reform, studies have found wide variability in the public and private sector prices of both patented drugs and generics. In the private sector, pricing is free in theory, although the government has increasingly been using the prices on the Ministry of Health drug list as a guideline when dealing with private companies. A full proposal for private-sector pricing regulation has yet to emerge. Out-of-pocket spending on drugs accounts for around 25% of the total, with private insurance covering some 15%. According to reports in the New Strait Times, around 15% of the population has private
insurance. Malaysia is committed to the ASEAN Common Effective Preferential Tariffs (CEPT) scheme under which all industrial goods traded within ASEAN are imposed import duties of 0% to 5%.
Increasing domestic demand, a significant proportion of which the local manufacturing industry will be unable to meet, will stimulate the growth of pharmaceutical imports over the next five years. The market will remain receptive to foreign products, particularly at the hi-tech end of the scale. The overall trade balance will increasingly shift further in favor of imports throughout the forecast period, indicating sizeable business opportunities for foreign companies (BMI, 2010).
Clinical Trial Exemption (CTX)
An approval by the DCA authorizing the applicant to manufacture any local product for the
purpose of clinical trial.
Clinical Trial Import License (CTIL)
A license in Form 4 in the schedule of The Control of Drugs and Cosmetics Regulations of 1984, authorizing the licensee to import any product for purposes of clinical trials, notwithstanding that the product is not a registered product. Prior to importation/manufacturing product locally, the investigator or sponsor is required to apply for CTIL/CTX from the Drug Control Authority (DCA). The following products will require a CTIL/CTX:
3.1 A product including placebo which are not registered with the DCA and are intended to be imported for clinical trial purpose.
3.2 A product with a marketing authorization when used or assembled (formulated or packaged) in a way different from the approved form and when used for unapproved indication / when use to gain further information about
an approved use for clinical trial purpose.
3.3 An unregistered product including placebo manufactured locally for the purpose of the clinical trial (Ismail, 2009).
Despite the introduction of holograms on pharmaceutical packaging, the level of counterfeit trade in Malaysia remains significant due to lax enforcement and other issues. A small – but not unimportant – proportion of drugs on the market are counterfeit (a 1997 study by the Ministry of Health found that 5.3% of sampled drugs fell into this category, although other estimates are at least double that amount), which has continued to represent a point of contention between the
government and the international industry. According to the Pharmaceutical Services Division, around 5.28% of all OTCs on sale in Malaysia were counterfeit in 2008, with slimming products accounting for around 10% of all illegal medicines seized in 2007. Enforcement work has become very challenging as these products use advanced technologies to avoid detection and exploit Malaysia's land and sea borders with Thailand and Indonesia.
In order to deter sales of imitation drugs, the government is looking to enforce stricter punishments for counterfeiters. Currently, most offences lead to prison sentences of no longer than five years, in addition to a fine of between MYR2,000 and MYR20,000 per infringement. After consulting with the Pharmaceutical Association of Malaysia, the Ministry of Domestic Trade and Consumer Affairs initiated calls for new legislation against the illegal trade.
The Malaysian International Chamber of Commerce recommended that the Trade Descriptions Act 1972, Sales of Drugs Act 1952 and the Poisons Act 1952 be amended so that there is a minimum fine for each counterfeit item and a mandatory jail sentence. A draft bill was expected in 2009, although no developments on the issue were reported by early 2010. Nevertheless – and despite the fact that the country has no legislation that specifically targets online counterfeiting –authorities (through a dedicated unit) have reportedly been successful with regard to reducing online sales of fake medicines (BMI, 2010).
All pharmaceutical products must be registered with the Drug Control Authority before it can be marketed in Malaysia. A foreign company wishing to bring pharmaceutical products into Malaysia would first have to appoint a local agent to be the holder of the registration certificate. The appointed agent would then be responsible for all matters pertaining to the registration of the products. There are specific forms to complete during the process of registration. Under the labeling requirements for products registered with the Drug Control Authority, the name and address of the actual manufacturer must be declared on the label.
At point of entry, imported products without holograms are a signal to the enforcement officers regarding its authenticity. Enforcement will not be carried out at the point of entry. Over the past decade, the DCA has cancelled or suspended 213 generic drug registrations for failing bioequivalence examinations. In 2008-2009, the authority rejected 66 new product applications because they did not include the required data (BMI, 2010).
Malaysia has played an important role in the global medical devices industry, as both an importer and exporter. While the country is actively seeking investment and shows promise as a growing market, it is important to understand the regulations and practices in place. Importers will find a growing market for high-end medical equipment while companies looking to position manufacturing facilities in Malaysia will find an encouraging and accommodating host.
Companies should be most concerned with Malaysia's vague policy on parallel importation of patented products.
Malaysia's primary focus within the medical device industry has traditionally been the manufacturing and exporting of medical gloves and catheters, accounting for 60% and 80% of the world market respectively (Industries in Malaysia, 2010). For a full list of products manufactured in Malaysia, please refer to Exhibit 6. The Ministry of Health Malaysia (MOH) oversees the Medical Device Bureau (MDB) with the following objectives, "One is to protect the public health and safety and, two, to ensure that new technology is made available for use for patients in a timely manner and at the same time facilitating trades and medical device industry ("Medical device bureau," 2010). Current growth in the market is expected to remain at 9.5% per year until 2015 at which the market is expected to reach US$1.7 billion ("Medical device bureau", 2010).
The medical device industry in Malaysia is largely self-regulated, although significant changes are expected soon. Medical devices are encouraged to conform to "internationally recognized quality standards such as the Food and Drug Administration (United States), Department of Health (United Kingdom) and Bundesgesundheitsamt (Germany)" (Industries in Malaysia,2010).
Intellectual Property Rights
Malaysia, a member of the World Intellectual Property Organization (WIPO) and a signatory to the Paris Convention and Berne Convention, is also a signatory to the Agreement on Trade Related Aspects of Intellectual Property Rights (TRIPS) under the World Trade Organization and therefore considered in conformance with international standards per periodic review (Invest in Malaysia: Intellectual, 2010).
While IPR are in place and have viable means of enforcement, it is important to note that foreign organizations/applicants wishing to apply must find a registered patent or trademark agent in Malaysia to act on their behalf. Products may be patented for the duration of 20 years. Trademarks are protected for ten years, and can be renewed every ten years, as specified by the Trade Marks Act of 1976 and Trade Marks Regulations of 1997. In the last decade, there seems to be no significant change in trademark policy, and it is important to note that patented products in other country markets can also be imported under the parallel import model. This can have significant implications for medical devices and other pharmaceuticals, which may rely on their patents to generate revenue for extensive R&D costs. While the government has officially stated that Malaysia will prohibit the registration of well-known trademarks by unauthorized parties to prevent counterfeits, the only official position on the issue of parallel imports is that the government can, "Prohibit exploitation of patents for reasons of public order or morality (Invest in Malaysia: Intellectual, 2010)."
The MDB runs the Voluntary Registration of Medical Devices Establishments (MeDVER), which applies to manufactures, importers, and distributors of medical devices. Companies should note that the Medical Devices Bill, expected to pass into law by the end of 2010, would make registration required and increase government oversight.
Although the current level of enforcement of the regulatory system is unknown, a comprehensive publication of all guidance notes is available with the Medical Device Bureau. The guidance publication contains "classification, essential principles of safety and performance, risk management, labeling and packaging, conformity assessment, nomenclature, quality management system, clinical evidence, recall, incident investigation and adverse event reporting, effective operation and maintenance management as well as disposal" (Medical device bureau2008).
Significant points within the guidance notes are the establishment of classification influencing regulatory importance, steps for placement of medical devices on the Malaysia market, and essential principles of safety and performance. The definition and examples of medical device classifications are available in Exhibit 7. A summary of the regulations relating to pre-market, placement on market and post-market are provided in Exhibit 8.
The placement of medical devices on the Malaysian market is dependent on a process of evaluation and approval known as a conformity assessment and includes 1) Conformity of the Quality Management System (QMS) and System for Post-Market Surveillance (PMS) 2) Conformity Assessment of Medical Device Safety and Performance through summary technical documentation and Declaration of Conformity (DoC) and 3) Registration of medical devices and establishments; there are three parties involved in the conformity assessment: the manufacturer of the medical device, Conformity Assessment Body (CAB), and the Regulatory Authority (RA) (Ministry of Health). For a figure summarizing the conformity assessment system, please see Exhibit 9.
The MDB is working with SIRM Berhad, formally the Standards and Industrial Research Institute of Malaysia (wholly owned by the Malaysian Government), to develop medical device standards (Medical device bureau, 2008). These standards and future regulatory guidelines are currently part of the Medical Devices Bill. In addition to the creation of the Medical Devices Bureau and Medical Devices Bill to develop a regulatory system, Malaysia also offers a variety of incentive programs for foreign corporations.
There are a variety of incentives for manufacturing companies doing business in Malaysia. Manufacturing companies will benefit from Pioneer Status and the Investment Tax Allowance, which are detailed in Exhibit 10. As of 2009, the corporate tax rate and maximum individual tax rate were reduced to 25% and 27% respectively (Why Malaysia: supportive, 2010).
Exports also receive a tax exemption "on statutory income equivalent to 10% of the value of increased exports, provided that the goods exported attain at least 30% value-added; or a tax exemption on statutory income equivalent to 15% of the value of increased exports, provided that the goods exported attain at least 50% value-added" (Invest in Malaysia: incentives, 2010).
Malaysia's current commitment to ASEAN harmonization remains verbal until the passage of the Medical Devices Bill and subsequent actions. In theory, Malaysia has agreed to align their regulations to those published by the ASEAN Harmonization Working Party (AHWP) and the Global Harmonization Work Force (Industries in Malaysia, 2010).
Malaysia has committed to harmonization by providing guidance notes for Medical Device regulation adapted from the Global Harmonization Task Force (GHTF). The GHTF is an international body representing Europe, Asia-Pacific, and North America with the goal to create "a global consultative partnership aimed at harmonizing medical device regulatory practices"(GHTF: overview and, 2007).
Malaysia also hopes to play a lead role in the ASEAN and other Asian regions by developing "the common submission dossier template for medical devices market approval and formalization of a post-market alert system for ASEAN and Asia" (Medical device bureau,2010).
Despite a major revision of patent law in 2001 and subsequent amendments in 2003, patent protection continues to be the cause of friction between the government and international drug manufacturers. While the government revised the period of protection for pharmaceuticals (increasing it to 20 years) following pressure from the international pharmaceutical community, it also implemented legal provisions that have come under heavy criticism from the industry (BMI, 2010).
Despite the introduction of holograms on pharmaceutical packaging, the level of counterfeit trade in Malaysia remains significant due to lax enforcement and other issues (NPCB).
By the end of 2006, Malaysia, Singapore, Thailand, Indonesia and Vietnam succeeded in harmonizing their pharmaceutical exports as part of the ASEAN Common Technical Dossiers (ACTD) program (BMI, 2010).
BMI's Pharmaceuticals & Healthcare Business Environment Ratings (BERs) reveal that Malaysia is a fairly attractive proposition to multinational drug makers. The country scores 55.7 out of 100, which is above both the regional (54.2) and global (51.9) averages. Indicating the developed nature of the DCA, Malaysia scores 7out of 10 for its approvals process, intellectual property laws and policy/reimbursement (BMI, 2010).
The expiry of patents on 47 drugs with high sales figures in the next five years is a chance for manufacturers to produce generic versions. Presently, generics are poorly promoted in Malaysia, with branded drugs generally viewed as superior in quality (BMI, 2010).
Other growth drivers are the rising quality of generics, cost-containment needs and implementation of the ASEAN Free Trade Area (AFTA) agreement, with products from signatory countries to be exempt from import barriers and tariffs. The flow of imports is expected to increase, tightening competition and pushing local manufacturers to create competitive advantages (BMI, 2010).
The Philippines is a middle-income country with imbalanced distribution of economic growth and productive resources. The Philippines consists of a large and growing population with currently low levels of drug consumption. In recent years, the government has been strongly committed to providing greater access to medicines. As economic growth strengths, total health spending is expected to increase reaching US$9.9 billion in 2013 (Philippines Healthcare Report, 2009). The country spent around US$2.58 billion on pharmaceuticals in 2009, up by over 200% compared to 2003 (Philippines Pharmaceuticals, 2010).
Primary healthcare services in rural areas are of a basic standard due to the lack of infrastructure and investment. The country is a major provider of healthcare workers and medical staff internationally. A significant proportion of overseas Filipino workers are doctors and nurses. ASEAN Mutual Recognition Agreement (MRA) allows health professionals to practice in other ASEAN-10 countries without having to obtain another license. The negative aspect of this is that healthcare provision in the Philippines is being undermined by the departure of so many medical professionals to work overseas (Philippines Healthcare Report, 2009). The Philippines Health Insurance Corporation (PhilHealth) had more than 80 million beneficiaries in 2009 which is equivalent to about eight out of ten Filipinos. Many poorer families still do not have PhilHealth cards entitling them to health services. Out-of-pocket spending has increased in the past few years, reflecting budgetary constraints on government expenditure (Philippines Healthcare Report, 2009).
Pharmaceuticals
Medicaments containing antibiotics or their derivates are #73 out of the top 93 imports into the
Philippines. However, pharmaceuticals cost more relative to drug prices in other Asian
countries. Furthermore, pharmaceutical consumption per head in the Philippines is still
relatively low.
In 2009, spending on generics has reached US$361 million and the market share
increased to about 14% from 12% in 2008. Key drivers of the generics sector are the increasing
need for low-cost drugs, budgetary increases, new legislation, patent expirations and the push to
increase compliance with public-sector generic prescribing and substitution (Philippines
Pharmaceuticals, 2010).
The 1988 Generics Act has had little positive impact on the generics market; in fact, generics drug sales only account for 10% of the medicine market. Patent protection is a major concern for multinational pharmaceutical companies with operations in the Philippines. The Philippines wasone of the top two IPR offenders according to PHrMA in 2009 because of the Universally Accessible Cheaper & Quality Medicines Act passed in June, 2008. The law aims to increase the use of affordable generic medicines while also reducing the country's reliance on foreign patented medicines by permitting health authorities to import patented drugs from other countries and to allow local drug producers to register their own versions of patented drugs once the
original patent has expired. Furthermore, the legislation makes it more difficult for patent holders to renew their patents (Philippines Healthcare Report, 2009).
According to a September 2008 statement by a senior lawmaker, corrupt practices in the drug procurement system are a cause of high-priced medicines. In November 2010, the National Bureau of Investigation (NBI) seized P25 million worth of fake medicines imported from India, Pakistan and Singapore (Araneta, 2010). A report from the Commission on Audit states that the Department of Health has a weak purchasing and monitoring system, which results in incorrect maintenance of medicine inventories and the frequent expiration of medicines. In August 2010, Samahan Laban sa Pekeng Gamot, a healthcare advocacy group, blamed the government and its policies on counterfeit drugs for worsening the problem of counterfeit drugs.
The Philippines relies heavily on foreign drugs, which account for about 75% of the market by value (Philippines Pharmaceuticals, 2010). The top manufacturers selling products in the Philippines include Wyeth, Bristol-Myers Squibb, Abbott Laboratories, Pfizer, Schering Plough, Eli Lilly and Merck Sharp & Dohme, all of the US, GlaxoSmithKline and AstraZeneca of the UK, Roche and Novartis of Switzerland, Boehringer Ingelheim and Bayer Pharama of Germany and sanofiaventis of France (Philippines Healthcare Report, 2009).
The Expanded Program on Immunization (EPI) in the Philippines began in 1979 and was launched in cooperation with the WHO. EPI has responded to the Universal Child Immunization goal with a set of standards for child immunization. Immunization is performed weekly, monthly, and quarterly in the Philippines depending on the area of the country (Philippines Healthcare Report, 2009).
Regulation of medical equipment is controlled by the Bureau of Health Devices and Technology (BHDT). The BHDT ensures the safety, efficacy and quality of medical devices and is the government contact for importers looking to sell their products in the Philippines.
The Philippines represents a modest but growing proposition for medical device manufacturers. Long-term drivers of the market include strong economic growth, a rapidly expanding and aging population, and development of the private hospital sector and the upgrade of government healthcare facilities. Medical device expenditure is projected to reach US$361 million by 2014. The public healthcare system is due for modernization but no extensive plans to upgrade facilities have been announced (Philippines Pharmaceuticals, 2010).
Local production of medical devices is almost non-existent and limited to low-end devices such as surgical gloves, syringes and needles. The Philippines mainly imports medical devices from the US due to its historical ties with the country. The next leading importers are Germany, Japan
and Singapore. However, India and China are expected to sell more products to the Philippines in the near future. The Philippines imposes a 3% tariff duty and a 10% VAT on imported medical equipment. Medical equipment in the Philippines must have a Good Manufacturing Practice (GMP) certificate.
Pharmaceuticals
Singapore's pharmaceutical industry remains one of the country's strongest components of its
biomedical sciences sector since 1997 (Exhibit 11). It is the fourth pillar of manufacturing in
Singapore, including electronics, chemicals and engineering (Yeo). The rapidly growing city-
state has developed into one of Asia's fastest growing bio-clusters to develop new medicines for
regional and global markets. Today, Singapore is leading the way in the global manufacturing of
innovative medicines and is now home to over 4,300 researchers, more than 50 companies and
30 public sector institutes with more than a billion dollars per year dedicated to research and
development (SEDB, 2010).
The Centre for Drug Administration (CDA), under the Ministry of Health and Health Sciences Authority, is the main regulatory committee which monitors the quality and safety of all pharmaceutical production and imports. CDA also evaluates the applications and decides on product license approvals (Exhibit 12). Despite its relatively small – in regional terms –pharmaceutical market of US$560 million, Singapore still remains an attraction for multinational companies due to its well-developed economy and high per capita spending (BMI, 2010). Pharmaceutical market forecasts indicate the pharmaceutical market will reach US$601 million and have a compound annual growth rate of 3.26% by 2014.
Clinical Trials
The Medicines Act of 1978 and the Medicines Regulation of 1998 are the statutory legislations
governing clinical trials in Singapore. The legislations demand that any investigator who is
partaking in a clinical trial must comply with Good Clinical Practice (GCP), and obtain both
ethical and regulatory approvals including a Clinical Trial Certificate (CTC) by the HSA. CTCs
are required by the National Pharmaceutical Administration (NPA) and are valid for up to two
years (HSA August 4, 2010). The Medical Clinical Research Committee (MCRC) is the
committee which advises on the licensing of the clinical trials. Along with stringent requirements
from HSA and NPA, clinical investigators are required to follow a set of ethical and scientific
standards for conducting clinical trials called Singapore Guideline for Good Clinical Practices
(SGGCP). Any person who fails to comply with the regulations as set forth by the Medicines Act
shall be liable on conviction to a fine of $5,000 or to imprisonment or both (1978).
According to Business Monitor International's report, "Singapore: Pharmaceutical & Healthcare, September 10, 2010," Singapore is leading the way in clinical trials. Over the next five years, Singapore plans to double the number of studies regarding Phase I trials. According to ClinicalTrials.gov, the city-state conducted 625 clinical trials as of February 2010. Exhibit 13shows the number of clinical trial certificates issued by the HSA within the past decade (HSA,2010).
The HSA forces all pharmaceutical manufacturers to adhere to the Good Manufacturing Practice (GMP) and Good Distribution Practice (GDP) guidelines as set forth by ASEAN to increase harmonization efforts. GMP for Medicinal Products are guidelines for manufacturers to ensure that all medicinal products are "consistently produced and controlled to the quality standards appropriate to their intended use and as required by the marketing authorization or product specification (ASEAN, 2006). Please refer to Exhibit 14 for excerpts from the official GMP guidelines as provided by HSA on their website. According to GMP, all packages must be traceable, provide consistent quality, provide product protection and offer child-resistant protection when applicable (Lithgow, 2002).
HSA also requires all pharmaceutical products to show the following on labels (1987):- name of the person to whom the product is to be administered.
- name of the registered pharmacy where the medicine is supplied.
- date upon which the product is dispensed.
- the direction for use.
- name of the medicinal product being either the appropriate non-proprietary name. - quantitative particulars of the active ingredients in the medicinal product.
In terms of packaging and labeling, Singapore is fully harmonized with ASEAN's GMP and GDP guidelines, according to an interview with Senior Regulatory Representative from the Health Sciences Authority, Evelyn Lee. Lee stated that Singapore is not looking to modify these guidelines anytime soon.
Marketing Requirements
There are four types of medicinal advertisements and sales promotion permits required by HSA:
still media, sound media, light & sound media and sales promotions (HSA, 2009). All permits
approved by the HSA are valid for one year and all advertisements are required to print the
permit number on advertisements and promotional items. All marketing requirements are
controlled under the Medicines Act of 1975 and its subsidiary Medicines Regulation Act of 1977
and the Singapore Code of Advertising Practices (SCAP), drawn up by the Advertising
Standards of Authority of Singapore. The objective of these legislations is to ensure that all sales,
promotions and advertisements are truthful and do not mislead or induce unnecessary use of the
medicinal product by the public (HSA, 2008).
As part of ASEAN harmonization scheme, Singapore has established Mutual Recognition Agreement (MRA) on the Post-Marketing Alert System (PMA). The objective of the PMA is to establish an efficient and effective system of alert on post-marketing issues which affect the pharmaceutical quality and safety. According to ASEAN, PMA is compulsory for all its member countries. This system also supports the International Medicinal Anti-Counterfeiting Taskforce
Program or IMPACT, as established by the World Health Organization, WHO (Laetzel, 2007). Exhibit 15 shows the Post-Market Alert System statistics for 2007 (Hui-Keng, 2009).
Intellectual Property Rights
Among the Southeast Asian countries, Singapore is one of the most advanced regarding
Intellectual Property laws. Singapore is a member of the World Intellectual Property
Organization (WIPO), THE World Trade Organization (WTO) and Trade-Related Intellectual
Property Rights (TRIPS). TRIPS requires all member countries to provide patent protection to
last for 20 years in all fields of technology, copyright to last for at least 50 years from the date of
publication and WTO members to allow patents in processes. Singapore also adheres to the
standards of protection as stated by the Berne and Paris Conventions (IDA, 2009).
BMI's 2010 report on Singapore's Pharmaceutical Industry suggests that there are still many concerns regarding Intellectual Property Rights and parallel trade which are pending the integration of the present healthcare and medicines acts. Singapore has passed several IP bills, including an amendment to the Medicines Act to protect pharmaceutical innovation which further tightens domestic regulations. BMI also suggests in their fourth quarter report that Singapore is not likely to exercise the TRIPS agreement, which enables the export of cheaper versions of patented medicines to address public health problems due to its close ties with surrounding countries.
However, a different message is conveyed by the Info-communications Development Authority of Singapore (IDA) and the Intellectual Property Office of Singapore (IPOS). These government offices indicate that they are working hard to "strike a balance between the property protection of owners of creative works and increased public access to intellectual property." Singapore'sintellectual property governing bodies have ensured that the intellectual property laws are harmonized globally. In 1999, The Copyright Act (Chapter 63) was amended to reinforce the city-state's commitment to provide a strong and conducive Intellectual Property Rights regime while promoting the growth of a knowledge-based economy (IDA, 2009).
Parallel Trade
All imported pharmaceuticals require the approval of the Health Sciences Authority of
Singapore. According to HSA, import license will only be issued to importers who have been
authorized by the product license holder to import licensed products on their behalf. Along with
a license, importers must also comply with HSA's Good Distribution Practice (GDP) guidelines
in order to be granted approval. HSA's regulatory Inspectors audit distributors in accordance
with the HSA Guidance Notes on Good Distribution Practice (HSA, 2010).
Despite such explicit requirements and guidelines, some sources reveal that parallel trade is still a concern in Singapore. One resource suggests that Singapore's legislations actually support parallel importation and that there are no legal and practical impediments against such trade. The
authors suggest that this is due to the fact that, "Singapore is a small domestic market and the costs of proving an alleged infringement of the consent of the copyright owner as against the benefits of a small domestic market." Sections 32 and 33 of the Singapore Copyrights Act (Chapter 63) allows for parallel importation as long as the importer can show that the product he/she is importing was made with the consent or license of the copyright owner (Partners, 2001). According to Maskus, Singapore prefers an open regime when it comes to parallel importation "because of their nature as centers of entrepot trade" (Maskus, 2001).
However, according to an interview with Senior Regulatory Specialist at HSA, Evelyn Lee, there are strict guidelines and criteria set forth by the HSA which require all new distributors of pharmaceuticals to apply for a distribution license. In order to eliminate parallel importation, licenses will only be given to wholesalers that are approved by the original licensed party. All guidelines and application for licenses for imported pharmaceuticals are available on HSA's website. It was also stated that IPRs also eliminate parallel trade (Zaman, 2010). According to research done by Frank Langer, IPR owners can take action against the parallel trading party for infringing on copyright or trademark issues. Furthermore, the license holder can include a restriction notice in licensing and purchasing agreements in order to prevent parallel trade. But Langer also suggests that this protection is subject to whether it is considered anticompetitive by prevailing competitive laws (Muller-Langer, 2007).
Despite parallel trade and intellectual property issues, Singapore's prescription Drug Market Forecast is forecasted to remain stable, with sales expecting reach US$ 579 million by 2014 (BMI, 2010).
Regulatory Data Protection
According to an interview with Senior Regulatory Specialist, Evelyn Lee, there is no
harmonization effort in place for RPD as far as ASEAN is concerned. However, Singapore does
have data protection for all pharmaceuticals. All new licenses are protected for 5 years and any
new application that infringes on the data protection is not accepted by the HSA. HSA has a
surveillance group in place to check and make sure all pharmaceutical data is protected.
Government Participation
The government of Singapore has taken an active role in setting legislations and guidelines for
the sale, advertising, clinical trials, manufacturing, and distribution of pharmaceutical products.
HSA adheres to the following legislations as set forth by the government of Singapore:
Medicines Act, Health Products Act, Poison Act, Medicine Act for Sales and Advertisements,
Sale of Drugs Act and Smoking Act. All legislations regarding medicinal products can be
obtained from the Singapore Statutes Online website.
In efforts to harmonize with ASEAN, Singapore is a member of ASEAN's Pharmaceutical Product Working Group, PPWG, formed in 1999. PPWG's objective is to harmonize
pharmaceutical regulations across ASEAN and to facilitate the ASEAN Free Trade Agreement (AFTA) while promoting product quality, efficacy and safety (Javroongrit, 2006). PPWG seeks to harmonize pharmaceutical regulations by trading information on existing regulations, conducting comparative studies, studying harmonization procedures and regulatory systems. In efforts to further harmonization among ASEAN member countries, PPWG has established ASEAN Common Technical Requirements (ACTR) and ASEAN Common Technical Dossier (ACTD) for all pharmaceutical products. Exhibit 16 shows ASEANs Harmonization Milestones from 1999 to 2009 (Hui-Keng, 2009).
ACTR is a set of written materials intended to guide the applicants to prepare application dossiers in a way that is consistent with ASEAN Drug Regulatory Authority. ACTD is a part of marketing authorization application dossier which requires all applicants to provide a letter of authorization, certification, labeling information, product information and proper language for the product (Haq). Exhibits 17 and 18 show the ACTR format and the ACTD structure ASEAN countries must adhere to for all new pharmaceutical products (Javroongrit, 2006).
Along with PPWG, ASEAN Consultative Committee of Standards and Quality (ACCSQ) initiated efforts to harmonize regulatory requirements among ASEAN member countries. Singapore and ASEAN have signed the AFTA to eliminate tariff on imported goods. The ASEAN member countries are to apply a tariff rate of 0 to 5%. This is known as the Common Effective Preferential Tariff, CEPT (Ratanawijitrasin, 2005). All ASEAN countries have agreed to enact a zero tariff on virtually all imported goods by 2010 (SECRETARIAT 2010).
An interview with Evelyn Lee, Senior Regulatory Specialist for HSA, indicated that Singapore has fully implemented the ACTR and ACTD dossier in efforts to increase harmonization with ASEAN and its member countries in April 2004. Lee stated that Singapore has no near-future plans to change or modify these policies.
Medical Devices in Singapore are regulated by the Centre for Medical Device Regulation (CMDR). Operating under the umbrella Health Sciences Authority (HSA), CMDR is the healthcare equipment supervisory body operating in Singapore. Historically, CMDR's regulations were limited. Few medical devices were actually covered under statutory control within the Medicines Act and Radiation Protection Act.
In 2007, the power of the CMDR to regulate medical devices increased with the Health Products Act of 2007. The finished act was a result of extensive research into best practices utilized by other foreign agencies, including US Food and Drug Administration (FDA) and the European Medicines Agency (EMEA). After years of evaluation, CMDR decided on a set of guidelines similar to the ones used by the Global Harmonization Task Force (BMI, 2010).
Testing Methods
As of the fourth quarter of 2010, nearly 80 of the 200 registered pharmaceutical firms in
Thailand are multinational companies (BMI Thailand, 2010). Accounting for 40% of the overall
industry, these multinational companies are increasingly concerned with the progression of the
generic drug market in the country. Currently, generic medicines accounts for approximately
50% of market share value, and this percentage is expected to increase over the next five years
with greater technology and development capabilities (BMI Thailand, 2010).
Multinationals have expressed concern about the Thai government's support of the generic
market through biased pricing towards local firms, reimbursement, and disadvantageous Intellectual Property environments. Despite these setbacks, there have been some positive signs of intergovernmental acceptance of testing methods with the resumption of free trade agreement (FTA) negotiations with the United States. These talks have the potential to decrease the number of generic drugs on the Thai market and keep the demand of prescription and patented drugs high, especially since physicians and hospitals will continue to prescribe specialized medicines and are the main gatekeepers of the healthcare system.
In addition to concerns regarding the generic market, multinationals are also dissatisfied with
the April 2009 amendments to the Thailand term of reference (TOR) regulations. Under the 2009 amendment, government officials no longer require a formalized preregistration for narcotic and psychotropic drugs to be purchased (BMI Thailand, 2010). The amendment leaves multinational companies at a significant disadvantage since neither laboratory validations nor Thai-based clinical trials data are required for drugs to go to market, allowing non-researched based competitors to have a lower barrier to entry and have easier access to the pharmaceutical market. Additionally, this regulation change removes vital safety and quality checks on potentially hazardous products that may have adverse effects on patients.
The Thai government has a clearly established set of labeling requirements for all modern drugs to be sold in the country. All drug labels must include the following information: Drug Name, Quantity, Active Ingredients, Lot/Batch Number, Manufacturer's Name and Province, Date of Production, and Expiration Date.
Additionally, according to the Drug Acts, the following words must be clearly written in Thai:Dangerous Drug, Specially Controlled Drug, For External Use, Common Household, Remedy, Topical Use, and Expiration Date.
The Ministerial Notification requires standard warning and precaution labels for specific
medicines. Additionally, certain drugs are only distributed through limited channels in clinics and hospitals and are not available for use outside these designations. Drugs that are to be used only in hospitals and clinics can be seen in Exhibit 19 (ASEAN Labeling Requirements).
The Thai Food and Drug Administration (FDA) requires high standards for medicinal advertisements and other promotional supplements to ensure that information is delivered to consumers and health-care professionals in a clear and truthful manner. (FDA Thailand) All advertisements must be pre-approved by government officials before they are allowed to be dispatched and promoted. Prescription drugs and other pharmacy-based medicines are only allowed to be distributed to health-care professionals and not to the general public. In contrast, household medicines may be directly advertised to consumers and specialized restrictions do not apply.
With the growing popularity of online browsing, the Thai government is now focusing
regulatory efforts on Internet advertisements. Currently over 85% of online drug advertisements are being run without FDA approval. The government is working to expand its monitoring capabilities in addition to creating more comprehensive guidelines and programs for handling online advertisement infractions (FDA Thailand).
Post-Marketing Surveillance
To ensure the safety and quality of all drugs advertised on the Thai market, the Medical Sciences
Department, a branch of the Ministry of Health and Welfare (MOHW), regularly tests samples of
medicines currently being retailed. This department is responsible for not only monitoring new
and established drugs on the market but also for responding to product complaints, informing
consumers of possible drug risks (including investigating the cause of the risk and potentially
removing the drug from market if needed), inspecting manufacturing plants for compliance
standards and monitoring changes in manufacturing processes (Gross and Weintraub, 2005).
Since October 2008, the Thai government under the Ministry of Public Health has been committed to maintaining compulsory patent licensing policies, which effectively limit multinationals investments in the pharmaceutical industry. As of March 2010, the government has suspended patent protection for expensive drugs that treat HIV/AIDS, cancer and heart
disease in an effort increase the availability of cheaper, generic versions of the drugs for patients. This decision is in line with the government's continued push for more generic drugs in the pharmaceutical market (BMI Thailand, 2010).
Anti-Dumping
The Thai government created The Act Countering Market Dumping and Subsidy of Goods from
Abroad to protect consumers, the domestic industry and public interest by taking actions against
foreign companies engaging in dumping practices (Thailand Legal Basics, 2009). The Act is
enforced for all foreign manufacturers, exporters and importers of specified goods. Additionally,
governments and trade associations that condemn dumping activities are subject to investigation.
Dumping is said to occur when goods are exported for commercial gains, lowering the export
price below the below the normal value of the goods. The export price is the price of the goods that should have been paid upon leaving the country of origin and the normal value is the price an independent buyer in the domestic country would pay for similar goods. The Thai government takes counteraction against dumping practices because it has a detrimental effect onThai industries. The Foreign Trade Department is responsible for evaluating alleged dumping cases and submits its findings to a specialized committee to make final decisions on the matter. If dumping is found to occur, then the Committee has the power to collect temporary security against the payment of the duty in question to cover damages incurred. Countermeasures against dumping practices may only be cancelled if the Committee and the exporter reach an agreement to change pricing or remove exports at dumping prices (Thailand Legal Basics, 2009).
Counterfeit Drugs
The United States government estimates that approximately 50% of all pharmaceuticals
purchased in Thailand are counterfeit or modified from their factory form. Not only does this
illegal activity serve as a serious health risk for Thai consumers, but it also decreases sales for
approved pharmaceutical firms. Despite shrinking sales to counterfeit drugs, many firms are
hesitant to publicize the issue for fear that bad press will only result in greater profit losses and
decreased consumer confidence in the industry.
The scope of the counterfeit drug problem is so large that the Thai government is ill-equipped
with sufficient resources to tackle the issue. Additionally, the little regulatory control that is in place is not enough to deter counterfeiters from abandoning current practices. Until criminal charges against counterfeiters are universally applied and regulations are harmonized, the government will continue to fight a losing battle in the counterfeit drug war (BMI Thailand, 2010).
Government Participation
Thailand continues to refrain from being a signatory to the WTO Agreement of Government
Procurement and the government continues to place restrictions related to its procurement
tenders on foreign bidders (Barriers to Trade). Although highly decentralized, the various governmental agencies – Public Procurement Management Office, Committee in Charge of Procurement – maintain control over which bids to accept or reject and have the right to alter technical requirements during the bidding process, permitting them to provide additional preferential treatment to domestic suppliers (they already automatically receive a 15% price advantage in the initial bidding round). Additionally, this leaves little room for foreign bidders to challenge government procedures.
Under the Counter Trade Act, foreign bidders are required to counter-purchase Thai
commodities at a minimum of 50% of the original value of the principal contract for any government procurement contract over 300 million Baht (Barriers to trade). The Thai government is also able to delineate specific markets in which foreign commodities are barred from being sold; these are typically markets where domestic products achieve clear competitive advantages. Thus, not only do these procedures leave foreign bidders at a significant disadvantage, but they also drastically increase operational costs.
The Act Countering Market Dumping and Subsidy of Goods from Abroad defines a subsidy "as the receipt of benefit from the originating government granting financial aid and income or price support in any form, directly and indirectly to increase exports or reduce imports of goods" (Thailand Legal Basics). In the event that a subsidy requires counteraction, The Thailand Foreign Trade Department contacts the subsidiary granting country in question and proposes that the dispute be settled according to WTO procedures. Additionally, the Foreign Trade Department may take any protective actions it sees necessary.
All foreign companies importing goods to Thailand are required to pay the following duties before goods are released from Customs:
· Customs Import Duties with an ad valorem rate· Excise Tax for Excise Department and Interior Tax· Value Added Tax (VAT) for the Revenue Department
Additionally the Thai government has several regulations regarding restricted and prohibited imports. In order for a foreign company to import pharmaceuticals, the company is required to provide a license issued by the government agency connected to Customs at the time of hosting a Customs goods declaration (Thailand, 2010).
Intellectual Property Rights
The Thai Food and Drug Administration (FDA) requires high standards for medicinal advertisements and other promotional supplements to ensure that information is delivered to
consumers and health-care professionals in a clear and truthful manner (FDA Thailand). All advertisements must be pre-approved by government officials before they are allowed to be dispatched and promoted. Prescription drug and other pharmacy-based medicines are only allowed to be distributed to health-care professionals not the general public. In contrast, household medicines may be directly advertised to consumers and specialized restrictions do not apply.
With the growing popularity of online browsing, the Thai government is now focusing regulatory efforts on internet advertisements. Currently, over 85% of online drug advertisements are being run without FDA approval. The government is working to expand its monitoring capabilities in addition to creating more comprehensive guidelines and programs for handling online advertisement infractions (FDA Thailand).
The Ministry of Public Health (MOPH) implemented the first Medical Device Act in 1988. This
act requires all products imported into Thailand to be approved by the Thai FDA. In order to control medical devices more effectively they are categorized into three main levels. The levels are general control (Level I), pre-marketing notification (Level II) and pre-marketing approval (Level III). The Medical Device Act prohibits the manufacture, import or sale of the following devices: fake devices, substandard devices, deteriorated devices and unsafe devices. (Kongkamee, 2005).
General control (Level I) devices require only a certificate of free sales to be commercialized. This document certifies that the devices are legal. Level I devices are classified as a condom, examination glove, surgical glove, hypodermic syringe, insulin syringe and HIV test kits (for diagnostic use). The Thai FDA does accept the opinion of other regulatory bodies such as the US FDA; all approval must be through their government. Medical devices must follow the quality and standard declared by The Ministry of Public Health. Producers, importers or distributors who produce, import, or sell Level I devices require them to submit the certificate of free sale (CFS), but they do not require a license. The certificate of free sale is issued by the Thai government or approved private organization. If the medical device is made abroad, the Thai FDA must give approval; this consists of a registration document, which is valid for 2 years (FDA, 2010).
Pre-market notification (Level II) devices require the same standards as Level I pre-market notification. Level II devices also require special labeling, mandatory performance standards, post marketing surveillance, operating service manuals and wiring diagrams (if applicable). The Ministry has the authority to declare what kinds of medical devices require FDA approval with regard to their effectiveness, quality and safety. Producers, importers or distributors of medical devices in pre-marketing notification are required to submit details on product descriptions, usage, specifications, labeling and name of producers and distributors to the FDA. A certificate
of free sale in the country of manufacture is also required for importing devices in this category(FDA, 2010).
Pre-marketing Approval (Level III) devices requires the same standards of Level II pre-market entry and also pre-market approval, which usually takes 3 months. Devices that fall under Level III are required to register with the FDA for approval before they can be manufactured, imported or sold. However, unlike Level I and II, the devices specified under category III must have a license for production, import, or sale (FDA, 2010).
Every medical device sold or possessed for sale must have a label on its container or package
written in Thai. The following information must appear on the label: name, category, and type of the medical device; name and location of producer or importer; in case of importer, the name of producer and place of origin of such device must also be shown; quantity contained therein; number or letters indicating a lot number of production; license number; purpose of use, instructions for usage and storage; a medical device for single use must display the words "disposable" or "for single use" in clearly visible red color; warnings and precautions; an expiry date, if any; and lastly any other messages as notified by the Minister in the Government. All claims imprinted on the labels of medical device products must have the support evidence kept in a ready-to-be-delivered manner when inquired about by any medical device control officer.
With regards to labeling standards, generic drug registration requires, "Labeling and packaging should consist of name of the drug, registration number, quantity of drug per packaging, formula which shows active ingredient(s) and quantity of strength, lot no. batch control number, name of manufacturer and address, manufacturing date, the words " dangerous drug"/ "specially controlled"/ "for external use"/ "for topical use" written in Thai and in red color if the drug is considered to be of them, the word "household remedy drug" written in Thai if the drug is considered to be, the word "for veterinary use" written in Thai if the drug is considered to be, and the expire date" (Thai Drug Control Authority, 2003).
The Medical Devices Act also prohibits advertisements with false or exaggerated statements
regarding the purpose of use, quality, quantity, standard or place of origin of any devices. The FDA must approve any type of advertisement, messages, audio, or videos for beneficial purposes (Medical device control in Thailand).
The purpose of post-marketing control is to ensure that medical devices distributed to consumers have quality that complies with the medical device standards. These standards are enforced by inspection of all medical device factories. These inspections are conducted regularly, together
with the sampling of devices so that they can be analyzed and ensure they are in compliance with legal requirements. Market surveillance includes the investigation of product labeling and the collection of marketed product samples at the inspecting place (Medical device control in Thailand).
Market authorization requests or vaccines are vetted by the Thai FDA, which in turn works closely with the Division of Biological, Department of Medical Sciences to coordinate testing protocol after an application for marketing authorization for a vaccine has been filed. The Thai FDA is subsequently allowed to determine course of actions with regards to GMP (GoodManufacturing Practices), Good Clinical Practice, or Good Laboratory Practice inspections, samples for analysis, as well as liaison with environmental agencies. After validation of the application, Market Authorization will be determined within 480 working days. Market Authorization decision is based on a "scientific opinion assessment report [which] shall contain the conclusions on the Quality, the Safety and Efficacy and will take into account appropriate benefit/risk scenarios on the populations and conditions of use as documented with clinical data by the applicant" (Thai FDA, 2008).
Tariffs
General import duty rates for selected medical device categories: Surgical and dental supplies
have a 15% tariff. Medical, surgical and instruments, patient monitors and surgical appliances
have 1 % tariff. Mechanotherapy, oxygen therapy and therapeutic respiratory apparatus have a
1% tariff free. Orthopedic appliance, implantable devices, pacemakers, cardiovascular devices,
and X-ray/irradiation apparatus for medical uses are free.
For the purposes of our research, a vaccine is defined by the Thailand Food and Drug Authority as "an immunogen, the administration of which is intended to stimulate immune system to result in the prevention, amelioration or therapy of any disease or infection. A vaccine may be a live attenuated preparation of bacteria, viruses or parasites, inactivated (killed) whole organisms, living irradiated cells, crude fractions or purified immunogens, including those derived from recombinant DNA in a host cell, conjugates formed by covalent linkage of components, synthetic antigens, polynucleotides (such as the plasmid DNA vaccines), living vectored cells expressing specific heterologous immunogens, or cells pulsed with immunogen. It may also be a combination of vaccines listed above." The Thai FDA follows guidelines established by the World Health Organization (Thai FDA 2008).
Licensing
Licenses are issued on the basis of the Drug Control Authority to any person who wishes to sell,
manufacture, or import drugs on the basis of 9 categories:
License to manufacture modern medicines License to import modern medicines License to sell modern medicines
License as a wholesaler of modern medicines License to sell modern medicines in sealed packages which are classified as neither
dangerous nor specially-controlled medicines
License to sell modern veterinary medicines in sealed packages License to manufacture traditional medicines License to sell traditional medicines License to import traditional medicines (Thai FDA, 2003)
The Thai government and Multinational Corporations are often in disagreement over various governmental practices, including compulsory licensing. Although accepted under WHO guidelines, many research-focused multinational companies criticize the government for their policies. Additional regulatory decisions on behalf of the government are decreasing the willingness of foreign companies to participate in the Thai economy and deterring the creation of new products. However, the market is primarily foreign company dominated (Red Orbit, 2008).
Intellectual Property Rights
IPR law for medical devices in Thailand is Exhibit 20.
The drug supply in Viet Nam is run by two different groups. The first group is the state-owned manufacturing industries that fall under the General Pharmaceutical Company, where the Ministry of Health is the supervisory body. The second group is the manufacturing plants that are managed by the People's Committee for each respective province/city (Wondemagegnehu, 1991).
Antibiotics being used to treat the common cold or runny nose is causing Vietnam citizens to become immune to certain viruses because the enzyme NDM-1 (New Delhi Metallo-beta lactamose) is now resistant to the most powerful antibiotics (Widespread Antibiotic Abuse in Vietnam Said Increasing Drug-Resistance, 2010). The cause of the resistance is mainly due to pharmacies willing to sell drugs without prescriptions for profit. Other reasons include: doctors prescribed the drug because their knowledge was limited or they were bribed by pharmaceutical companies. People with diseases preferred to use antibiotics directly because they could avoid paying examination fees. Most of all, pharmacies are willing to sell the drugs without prescription for profit (Widespread Antibiotic Abuse in Vietnam Said Increasing Drug-Resistance, 2010). To help with this problem and to enhance competitiveness, the Vietnamese pharmaceutical industry has decided to develop and maintain Good Pharmacy Practice (GPP) (Building GPP Pharmacies - Obstacles Still Exist, 2010
). The GPP construction plan had required all hospitals to be GPP by the end of 2008 and the all pharmacies by January 1, 2011. With the current report being completed in April 2010, it is highly unlikely that the country will achieve the goals of all hospitals and pharmacies being compliant with these aggressive goals. Currently, only 13% of pharmacies are GPP certified in Ho Chi Minh City and Hanoi has less than 20% (Building GPP Pharmacies - Obstacles Still Exist, 2010). The main reason for the lack of compliance is due to higher investment costs. In order to comply, the pharmacies must hire certified pharmacists and invest in infrastructure and equipment. This is very hard for the smaller pharmacies to do with little sales. And if pharmacies have to turn away patients with improper prescriptions, it could cost them their business. The first GPP certified pharmacy in Hanoi had to turn away 40% of their customers due to lack of proper prescriptions (Building GPP Pharmacies - Obstacles Still Exist, 2010). The government is giving incentives to the GPP certified pharmacies, but in the end it will be up to the pharmacies to know the purpose and reasoning behind the increased costs of doing business according to good practices.
Currently Viet Nam is requiring local clinical trials for any drugs sold in their country, even if they have been approved by the US FDA or the ICH. In April 2009, they also started to require clinical trials for first time medical-device imports. These imported devices do not need to be registered to be marketed, but they do need an import license (Clinical Trials in Vietnam
Required for First-Time Device Imports, 2009). The Department of Medical Equipment and Health Works (DMEHW) will give the approval on a manufacturer's application for clinical trials. Prior to the import permit application, the device must go through local clinical trials in three government-designated hospitals in Vietnam (Clinical Trials in Vietnam Required for First-Time Device Imports, 2009). All hope is not lost, as the DMEHW can waive the local clinical trials if the product has been deemed safe and approved for marketing in another country, but this is only on a case by case basis (Clinical Trials in Vietnam Required for First-Time Device Imports, 2009). To become more harmonized with the ASEAN community, Vietnam hopes to adopt the Common Submission Dossier Template. They also have a post-marketing surveillance system currently in development.
Vietnam Black/Counterfeit market is estimated to be $674.8M and is comprised of cigarette smuggling, counterfeit/piracy general market, part in global drug trade, piratedbooks/movies/software and wildlife (Vietnam Black Market Value, 2010). Vietnam outlines 5 "forbidden acts" in Article 9 of their Pharmaceutical "Black Market" Law. It is "forbidden" to be caught with (Vietnam Black Market Value, 2010):
Conducting drug trade without Certificate of SDTC
Conducting drug trade without Certificate of PP
Trading of drugs with unclear origins
Counterfeiting, renting, borrowing, leasing certificates of pharmaceutical practices
Selling drugs at places which are not lawful drug selling establishments
In recent developments, the Counterfeit Drug Challenges developed a 2009 report that showcases 6.6 pharmacy outlets with 1,000 people. This same report lists that only 20-30% of pharmacy users have a prescription. The black pharmaceutical market is driven by both shortages of qualified pharmacists and low awareness of intellectual property rights (Counterfeit Drugs Challenge, 2010). The Vietnamese Government actively supports the importation (parallel) of generic drugs (i.e. over half of the domestic demand is met by imports) and they are fully aware that similar names and packaging make distinguishing nearly impossible for the untrained consumer (Counterfeit Drugs Challenge, 2010).
Customs and regulatory officials are understaffed and lack regulatory backbone making counterfeit enforcement nearly nonexistent. Vietnamese officials all claim that they are incapable of stopping the growth of counterfeit trade. Currently, there are no specific penalties for the over 100,000 cases IP law breaking cases (Quang, 2010). Viet Nam is becoming a transit hub for fake goods due to the loophole for newly-created fake goods. "The customs official further claimed that his department is only empowered to investigate or refuse clearance for shipments of products that businesses suspect of violating intellectual properties" (Quang, 2010).
The main authority is the Ministry of Health (MoH) and the Drug Administration of Viet Nam (DAV), established in 1996. By 2005 more than 10,000 kinds of medicines were registered for sale in Viet Nam. Of these 10,000, more than 6,000 were produced locally and the remaining 4,000 were from foreign companies. To minimize the foreign companies from bringing in their medicines, the MoH and DAV requires local clinical trials of vaccines. The US manufacturers believe the products that have been approved by the US FDA or ICH should be exempt from this local testing in accordance with the PhRMA 2010 submission (BMI, 2010).
Vietnam entered the WTO in January 2007. They were able to meet the membership requirements of WTO based on improved transparency, uniformity of the tariffs system and reducing the tariffs of forty-seven pharmaceutical categories from 10-15% to less than 2.5% after 5 years (BMI, 2010).
Import Licenses/Parallel Trade
The Ministry of Health has reported that the rate of counterfeit drugs in the country was 0.09% for the 16,500 medicines examined in 2005, the highest level for five years. Among the examined products, 3.4% were ‘low quality', down from a figure of 3.74% in 2003. Vietnam's testing system has the capacity to analyse around 500 pharmaceutical ingredients or about 50% of the total licensed for sale. In the five years to September 2007, some 35 million doses of fake medicines circulated in the local market (BMI, 2010).
In fact, as of the start of 2009, local entities that are fully owned by foreign companies are no longer barred from importing pharmaceuticals into the country in an unrestricted fashion. Clarification is still reportedly needed from the MoH on requirements for importing entities, according to PhRMA's 2010 submission. Currently, foreign-owned distribution companies in Vietnam must be licensed by the MoH and prove that they comply with international standards(BMI, 2010).
Over 95% of the market is made up of foreign goods. The main sources are the US, Germany and Japan, with Taiwan, Italy, France and South Korea also accounting for significant shares. Local production is extremely limited in terms of value but volume levels suggest the foundation for ascent up the value chain. There are presently 50 domestic firms making approximately 600 products officially licensed by the Ministry of Health. The vast majority are low-end offerings such as dressings, plastic gloves and syringes. Importantly, however, quality is high since ISO 9001-2000 came in effect in November 2004. Nevertheless, the government has not achieved its goal of domestic medical equipment meeting 40% of demand by 2005 and 60% by 2010, as set out in its 2002-2010 national policy on medical devices (BMI, 2010).
In the meantime, as a greater proportion of Vietnam's vast population of around 88 millionbecome consumers of pharmaceuticals, various state agencies are looking for local producers or
Vietnamese subsidiaries of foreign firms to increase output rather than rely on imports. For example, the health ministry is facilitating this goal by improving the legal framework for setting up medicine manufacturing businesses. Indeed, by October 2007 Vietnam had licensed 370 foreign companies to make pharmaceuticals – a significant increase on 2006. Along these lines, in Q409, DAV announced that the country's pharmaceutical industry aims to meet 60% of the market's demand by 2010 (BMI, 2010).
Meanwhile, foreign direct investment (FDI) is playing an increasing, but still marginal, role in improving standards in Vietnam's domestic pharmaceutical manufacturing. By the end of 2008, the Ministry of Health had licensed a cumulative total of 37 FDI projects in the pharmaceutical sector worth US$282.6mn. Having said this, only two thirds of the projects have actually been initiated and the pace of investment appears to have slowed, with only one of these licenses being granted in 2008. The limited impact of FDI to date lends weight to the Ministry of Health's view that foreign companies would prefer to seek out local suppliers than construct their own pharmaceutical plant in the country, despite a relaxation of rules on foreign company activities in recent years (BMI, 2010).
With unmet pharmaceutical demand, the Vietnamese government actively supports the importation of generic drugs. Lack of significant new information since 2004 shows the lag between parallel importation regulations and IP law. An update on Vietnamese parallel importation guidelines on 2004 showed all approvals of goods are submitted through the Ministry of Health. Additional details describe that goods to be approved are imports of products that are not registered with Vietnamese government, but are identical to products that are registered from the same manufacturer. Additionally, parallel imported goods must be sold at a price less than the price that manufacturers sell at and must be labeled specifically to denote parallel import status (Minh, 2004).
NOTE: no new significant information since 2004
Customs and entry procedures
Vietnamese customs and entry procedures are controlled by a tiered organization that is led by the Ministry of Finance. The General Department of Vietnam Customs is then subdivided into Assistance Unit, Administrative Offices, and Local Customs Department. For 2010, the Vietnamese Customs office forecasts that trafficking of drugs, addictives, arms, and reactionary publications will only increase. This forecast also includes fraudulent tricks of violation of copyright material. As a strategy to combat this, they are working on ways to ensure a sufficient, consistent, clear and public system of legislation in customs area in line with international commitments. To better align with business modernization, the Vietnamese Customs Agency plans to automate, increase regional discretion, and integrate a more sophisticated computer system into their network. To date, there has been no evidentiary support to substantiate these goals and plans (Van, 2009).
As a member of WTO, Vietnam has an outstanding commitment to not dump goods into international markets. This is supported by its 2004 adoption of the "Ordinance on Anti-Dumping of Imports into Vietnam (Van, 2009)." In recent years, there has been evidence to suggest that Vietnamese exports are consistently facing aggressive international competition, which has limited their expansion efforts in the international atmosphere. In just the past two years, there have been more than 30 anti-dumping cases filed against Vietnam (Van, 2009). The majority of these cases were filed by key partners, such as the US, EU, and Canada. Products involved catfish, shrimp, and footwear. Because Vietnam is so ill-experienced with protocols of dumping procedure, offenders often face maximum fines and highest duties (Export Markets, 2010).
Import tariff on pharmaceuticals varies from 5-15%, 25-40% for medical supplements, and 0-5% for medical devices (Export Markets, 2010).Vietnamese officials routinely demand bribes which contributes to the instability of access to medicines. "Tariffs create opportunity for public officials to extract bribes" (Bate, 2006).
Vietnam's regulatory system (documents required, costs, and title frames) is easier to get through than those of Japan and China, especially for the medical device field where there is a high market growth rate. Foreign companies must make deals with hospitals via a local partner and the Ministry of Health requires imported pharmaceuticals to be registered before they're put on the domestic market where it takes on average 8 months to register a pharmaceutical/ supplement. Import licenses for medical devices takes 6 months or longer to register because of the required documentation: original catalogue, instructional manual/technical guide (originals & Vietnamese translations), manufacturer's quality certificate, free sales certificate and quality declaration letter. Packaging of imports is monitored by the Drug Administration Department of Vietnam (DAV). DAV also responsible for granting and removing licenses for all medicines in the country, but the Ministry of Finance is responsible for price control of medical products (Export Markets, 2010).
The ASEAN committee responsible for medical device regulatory harmonization is the Medical
Device Product Working Group (MDPWG). The MDPWG is one of 12 working groups of the ASEAN Consultative Committee on Standards and Quality (ACCSQ). The MDPWG's has a unified set of rules for medical devices. The overall regulation the MDPWG is crafting to harmonize medical device regulations is called the ASEAN Medical Device Directive (AMDD). The AMDD is not a law in ASEAN countries, but all member countries will be required to pass laws with the same provisions. The AMDD lays out basic requirements for medical device safety and performance (Minot & Gross, 2009).
Since Vietnam's admission to the World Trade Organization (WTO) in January 2007, the
country has seen numerous and diverse changes that have propelled it into double-digit growth.
In 2010, the Vietnamese market for medical equipment and supplies is estimated at $515 million
USD, or $6 US per capita. Furthermore, the Medical devices market is expected to expand at nearly 15.3% per year. If this number is accurate, it would take the Vietnamese market to over $1 billion USD in 2015, although the per capita rate will remain low. (British Library Cataloguing in Publication Data, July 30, 2010)
Local production is limited to basic items such as syringes and hospital beds, so Vietnam relies
heavily on imports. In fact, 86.5% of the medical device market is supplied by imports. In 2008, Singapore, China and Japan made up 43.1% of these imports. Vietnam also receives a large amount of international aid comes to Vietnam in the form donated medical equipment, much of which is funded by the World Bank and the EU (Minot & Gross, 2009).
The U.S. is the largest consumer of medical devices and leads the world in the production
of medical devices. The U.S. has a medical device market valued at more than $100 billion in2008, roughly 42 percent of the world's total. (Source: 2008 data compiled from tariff and trade data from the U.S. Department of Commerce and the U.S. International Trade Commission. ) Since the U.S. – Vietnam Bilateral Trade Agreement (BTA) was implemented in 2001, economic links between the two countries have strengthened, producing a rise in U.S. imports of medical devices and equipment. The best sales prospects for U.S. manufacturers of medical equipment are imaging diagnostic equipment (i.e., X-ray, CT Scanner, Color Ultrasound, M.R.I.), laboratory equipment, operating theaters and sterilizing equipment, patient monitoring equipment and emergency equipment (Vietnam Minister of Health, 2005).
In April 2008, Vietnam's Ministry of Health announced long-awaited plans to revamp the
country's healthcare infrastructure. This plan outlined a budget of nearly $1.1 billion USD to build and upgrade hospitals across the nation, equipping 1,200 hospitals and medical institutes with a total of 190,000 beds by the end of 2010, to be followed by a further 250,000 beds in that will immediately benefit from the five-year socioeconomic development plan (2006–2010) that aims to upgrade and develop medical centers and hospitals. The proposed investment in healthcare facilities opens up a myriad of opportunities across the entire healthcare ecosystem, particularly for suppliers of medical devices and equipment. Companies who wish to expand sales internationally will clearly benefit from this expansion (Solidiance, 2009).
Another reason for the market expansion is lifestyle changes that have caused poorer health and
led to changes in the market. According to research from the Center for Disease Control and Prevention (CDC), around 31% of non-accidental deaths are cardiovascular-related, while an estimated 16.3% of the northern population has been diagnosed with heart disease. CDC
continues to quote that Ho Chi Minh City alone has 9,000 patients waiting for heart operations. This clearly creates another need for more medical devises (Solidiance, 2009).
Vaccines
Vietnam has struggled to gain a stronghold or any type of regulation on the pricing of vaccines.
In 2003, the Ministry of Health attempted to implement a regulation that required all medicinal
products to have labels indicating their retail price. These stickers were to be visible on both the
boxes and the containers before they drug would be legal to sell. Due to the dissent expressed by
powerful doctors, the rule was not implemented (Lam, 2003).
The health ministry classifies drugs into three categories (Lam, 2003):
1. Drugs for national programs2. Essential medicines3. Popular drugs
There are more than 12,000 popular drugs sold in Vietnam and only about 4,500 of them are produced domestically in Vietnam. This represents only 38% of the popular drugs circulating in the country. Startlingly, 96% of the raw materials used to create the domestic drugs are imported (Lam, 2003).
Placing a pricing fix on drugs sold in Vietnam under the circumstances of such a large amount of importing would place drug retailers in a predicament. If foreign pharmaceutical companies or material providers increase their price while Vietnam is locked in to a certain retail price, margins are going to rapidly diminish. Le Van Truyen, the Chairman of the Association of the Production and Trading in Pharmaceutical Products (in 2003), noted that, "If they [retailers] sell at higher prices, they will break the law; and if they sell at the price printed on the package, they will lose money. Retailers can do nothing but break the law (under these circumstances)" (Lam, 2003).
In 2005, basic price control regulations were put into place with the 2005 Pharmaceutical Law of Vietnam. The regulations required all drug importers and distributors to have the Drug Administration of Vietnam (DAV) approve their price lists (Tamayo, 2008). In late 2007, the pricing regulations were supplemented with guidelines that granted about 200 drugs a slight price increase, while 600 others were denied. Within that year alone, the average price of vaccines rose 8.93% (Tamayo, 2008).
The same problem of drug price fluctuations continued to plague the pharmaceutical industry in Ho Chi Minh City through 2008 and 2009. Many retailers, hospitals and health clinics seemed to just ignore this regulation when, in 2009, the flu season came around, and customers had no choice but to pay the asking price to get the medication needed to treat sickness symptoms (Thai News Service, 2009).
A health officer at Ho Chi Minh city's Center for Hygiene and Epidemiology claimed that drug suppliers had "raised vaccine prices, forcing them to do the same" (Thai News Service, 2009). Despite the claim, they did not seek approval from the DAV, making every institution guilty of price hikes subject to potential punishment. To weasel out of sanctions, the health officer went on to say, "Due to the multiple levels of responsibility, the situation cannot be blamed entirely on our management," since the Ministry of Finance, the Ministry of Trade and the DAV all coordinate together to set price levels for vaccines.
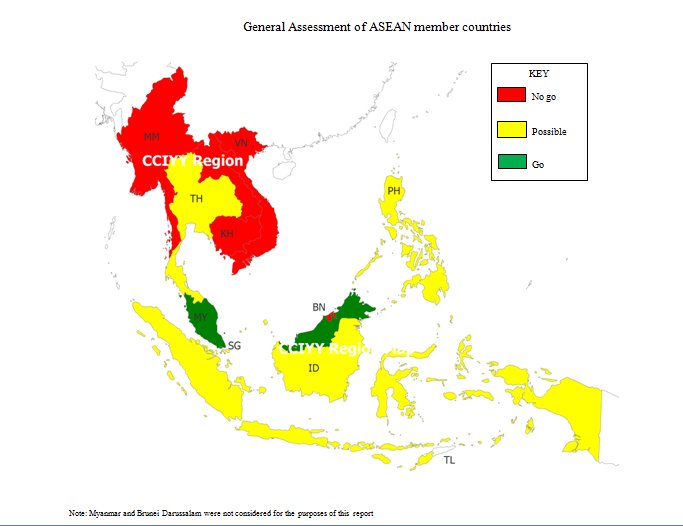
2. Sanofi-Aventis
4. GlaxoSmithKline
7. Johnson & Johnson
9. Bristol-Myers Squibb
10. Abbott Laboratories
DRUG MARKET PRICING
CAGR USD$
No regulations. Among highest in world, only 14%
of health care spending
Swissmedic regulates pharmaceutical pricing. The price structure for pharmaceuticals not covered
by reimbursement is determined by the Sanphar pricing code that governs manufacturer sel ing
prices, wholesale prices and retail prices.
The PBS al ows for a subsidised reduction of an individual's medicine costs and the subsidy is
calculated based on the total cost of products that the person entitled to benefits buys in one
year, commencing on the date of the first
Wholesaler and pharmacy margins are added to
the manufacturer's price to give a uniform retail price, with the system linking pharmacy profit to
product price levels. Retail double
manufacturer's cost, no reduced tax rate, 19% VAT applied
Legal y set own prices, reality the Comite Economique du Medicament negotiates prices
Controlled by Department of Health. Benchmark.
Food and Drug Administration (FDA)
Strong, patent 20 years or additional protection
Switzerland EU Haromonisation and
A Swiss patent has a lifespan of 20 years, but
owing to the time from the research and
development (R&D) stage to authorisation, the effective duration is 10-12 years
EU Harmonisation and Medical
EU and International Standards of 20 years,
Products Agency (MPA)
extend by 5 years up to 15 years
EU Directives and Institute for Drugs Since signing the European patent agreement, a
and Medical Devices (BfArM), and
manufacturer can now apply for both a national
the Paul Ehrlich Institute (PEI)
patent and, within a year, a European patent
EU Directives and Ministry of Health, EU and International Standards of 20 years,
extend by 5 years up to 15 years
EU and Medicines and Healthcare
EU and International Standards of 20 years,
Products Regulatory Agency (MHRA)
extend by 5 years up to 15 years
? Any person who deliberately and without right conduct shall be sentenced to
imprisonment of at least 1 month and/or a fine of at least Pr. 1,000,000 (one million rupiahs) or imprisonment of at most 7 years and/or a fine of at most Rp. 5,000,000,000 (five billion rupiahs)
? Any person who deliberately broadcasts, exhibits, distributes, or sells to the public
a work or goods resulting from an infringement of copyright or related rights shall be sentenced to imprisonment of at most 5 years and/or a fine of at most Rp. 500,000,000
? Any person who deliberately and without rights uses the Trade Secret of another
party, or conducts shall be sentenced to imprisonment of at most 2 year and or a fine of at most Rp. 300,000,000
? The criminal action as referred to in paragraph above shall constitute offence that
warrants complain
? Any person who deliberately and without right uses a Mark which is similar in its
entirety to a registered Mark of another party for the same kind so goods and/or services produced and/or traded shall be sentenced to imprisonment for a maximum period of 5 years and/or a fine of a maximum amount of Rp. 1,000,000,000
? Any person who deliberately and without right uses a Mark which is similar in its
essential part to a registered Mark of another party for the same kind of goods and/or services produced and/or traded shall be sentenced to imprisonment for a maximum period of 4 years and/or a fine of a maximum amount of Rp.800,000,000
Source: Indonesia: Licensing and Intellectual Property, EIU ViewsWire, 2010.
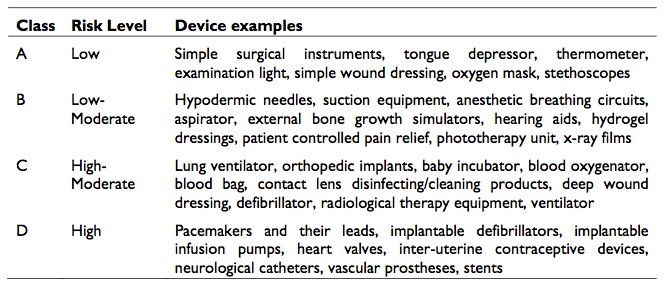
Medical Device Products in Malaysia
Catheters, rubber gloves (Specialized gloves
Electromedical equipment, cardiovascular
include low protein, powder free medical
products, orthopedic products, in-vitro
gloves, safety gloves and clean room gloves),
diagnostic devices, wound care products,
syringes, surgical equipment, blood transfusion ophthalmic products, home care product.
sets, blood pressure transducers, dialysis solutions, medical gases, hypodermic /spinal/
AV fistula needles, medical tubes & bags, diagnostic radiographic equipment, orthopedic products and procedural kitsData from http://www.mida.gov.my/
Figures provided by publication "Medical Devices Regulatory Framework in Malaysia"
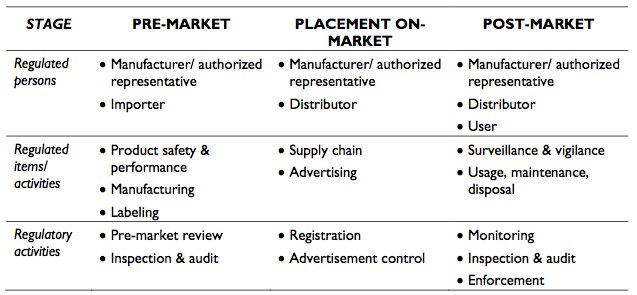
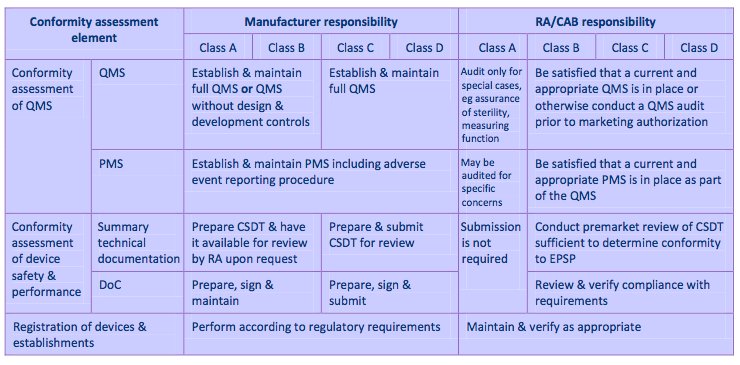
Figures provided by publication "Medical Devices Regulatory Framework in Malaysia"
Figure provided by "Placement of Medical Devices on the Malaysian Market"
Based on level of value-added, technology used, industrial linkages of "promoted
activities" or "promoted products"
Medical, surgical, dental or veterinary devices/equipment;
Activities and
Gauges or measuring apparatus; Surveying, hydrographic,
navigational, meteorological, hydrological or geophysical instruments; Testing equipment; Stainless steel cannulae or tubesfor needles; Research and development (R&D); Design and
prototyping; Technical or vocational training; Integrated logistic services; Integrated market support services; Integrated centralized utility facilities; Total chemical management system; Pharmaceutical goods; Clinical diagnostic reagents; Gelatine or gelatine products; Intravenous, dialysis or irrigating
solutions; Vaccines; Medicaments
activities-and-products: List of Promoted Activities & Products)
Pioneer Status
Investment Tax Allowance
Overview: Couples a partial exemption from Overview: Allows companies to offset 60%
payment of income tax with carry
of qualifying capital expenditure
forward treatment of unabsorbed
(factory, plant, machinery,
capital allowances as well as
approved equipment) against 70%
accumulated losses
of statutory income
5-year period to pay 30% of
Alternative to Pioneer; Unutilized
statutory income, investment in
qualifying expenditures may be
promoted areas (States of Sabah,
carried forward, companies in
Sarawak, Perlis, and "Eastern
promoted areas receive 100%
Corridor" receive 100%
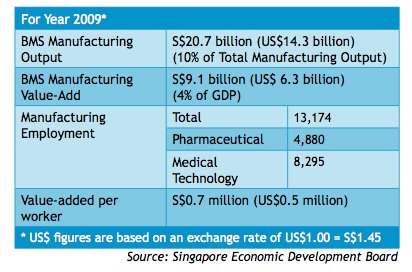
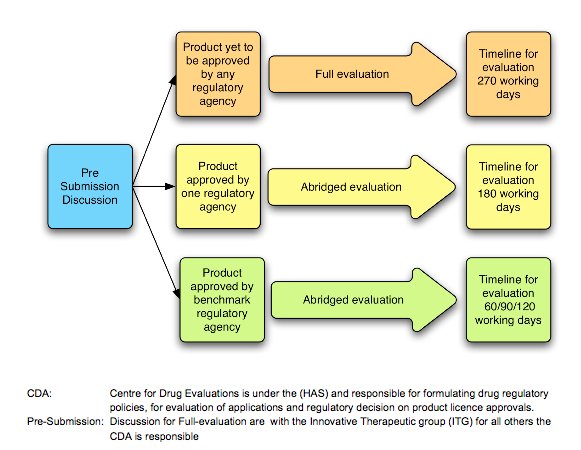
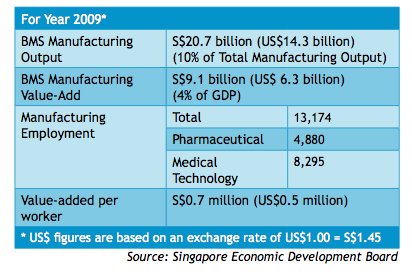
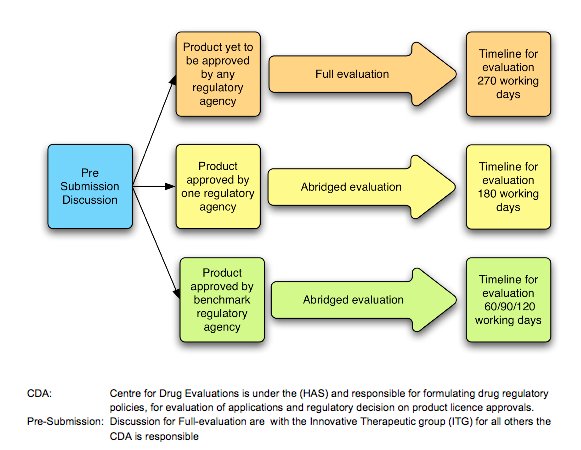
Source: Centre for Drug Administration, Singapore
Source: Centre for Drug Ad
Source: Health Sciences Authority, Singapore
Source: Health Sciences Authori
GOOD MANUFACTURING PRACTICE FOR MEDICIN AL PRODUCTS (GMP)
1.3. Good Manufacturing Practice is that part of Quality Assurance which ensures that products are consistently
produced and controlled to the quality standards appropriate to their intended use and as required by the
marketing authorisation or product specification.
Good Manufacturing Practice is concerned with both production and quality control. The basic requirements of
GMP are that: i. all manufacturing processes are clearly defined, systematically reviewed in the light of experience and
shown to be capable of consistently manufacturing medicinal products of the required quality and complying with their specifications:
ii. critical steps of manufacturing processes and significant changes to the process are validated; ii . al necessary facilities for GMP are provided including:
a. appropriately qualified and trained personnel;
b. adequate premises and space;
c. suitable equipment and services;
d. correct materials, containers and labels;
e. approved procedures and instructions;
f. suitable storage and transport;
iv. instructions and procedures are written in an instructional form in clear and unambiguous language,
specifically applicable to the facilities provided;
v. operators are trained to carry out procedures correctly;
vi. records are made, manual y an/or by recording instruments, during manufacture which demonstrate that
all the steps required by the defined procedures and instructions were in fact taken and that the
quantity and quality of the product was as expected. Any significant deviations are fully recorded and investigated;
vii. records of manufacture including distribution which enable the complete history of a batch to be traced,
are retained in a comprehensible and accessible form;
vii . the distribution (wholesaling) of the products minimises any risk to their quality; ix. a system is available to recall any batch of product, from sale or supply;
x. complaints about marketed products are examined, the causes of quality defects investigated and
appropriate measures taken in respect of the defective products and to prevent re-occurrence.
QUALITY CONTROL
1.4. Quality Control is that part of Good Manufacturing Practice which is concerned with sampling, specifications
and testing, and with the organisation, documentation and release procedures which ensure that the
necessary and relevant tests are actually carried out and that materials are not released for use, nor products released for sale or supply, until their quality has been judged to be satisfactory.
The basic requirements of Quality Control are that:
i. adequate facilities, trained personnel and approved procedures are available for sampling, inspecting and
testing starting materials, packaging materials, intermediate, bulk, and finished products, and where appropriate for monitoring environmental conditions for GMP purposes;
ii. samples of starting materials, packaging materials, intermediate products, bulk products and finished
products are taken by personnel and by methods approved by Quality Control;
ii . test methods are validated; iv. records are made, manually and/or by recording instruments which demonstrate that al the required
sampling, inspecting and testing procedures were actually carried out. Any deviations are fully recorded and investigated;
1 August 2006 Page 5 of 145 PE 009-5Chapter 1 Quality Management
v. the finished products contain active ingredients complying with the qualitative and quantitative
composition of the marketing authorisation, are of the purity required, and are enclosed within
their proper container and correctly labelled;
vi. records are made of the results of inspection and that testing of materials, intermediate, bulk, and
finished products is formal y assessed against specification. Product assessment includes a review
Source: T. Lithgow, "Six Steps to Pharmaceutical Packaging Sustainability," 2002.
Source: L. Hui-Keng, ASEAN Harmonisation: An Agency Perspective,
Source: L. Hui-Keng, ASEAN Harmonisation: An Agency Perspective,
Source: Yuppadee Javroongrit, P. D.
Source: Yuppadee Javroong
ASEAN Harmonization, 2006.
Source: Yuppadee Javroongrit, P. D.
Source: Yuppadee Javroong
ASEAN Harmonization, 2006.
Drugs that are to be used only in hospitals include:
1. Anticancer Drugs2. HIV-treated Drugs
3. Anti-Acne of Retinoid Group4. Cisapride5. Misoprostol
6. Ninoprostone7. Sulprostone
8. Comination of L-Tryptophan for Medicated Supplement9. Cloramphenicol for Human Use
Drugs to be used in clinics in hospitals only include:1. New Drug Approvals with Conditions2. Sildenafil
3. Caverject4. Muse
Source: ASEAN Labeling Requirements: Issues on Country Specific Requirements.
Intended to bring Thailand into conformity with international standards under TRIPS and
the Berne Convention. Effective in March 1995
Thai government is in the process of amending the copyright law in order to bring it in
line with the WIPO Copyright Treaty and the WIPO Performances and Phonograms Treaty (WIPO= World Intellectual Property Org)
Optical Disk Manufacturing Control bill in 2005 designed to enhance the authority and
capabilities of the Thai government to act against operators of illicit optical disk factories and to control production materials and machines of legal producers.
Book publishers concern that the existing copyright law is allowing extensive book
piracy, especially in the form of illegal photocopying.
Amended trademark law in 1992, increasing penalties for infringement and extending
protection to service, certification, and collective marks.
Additional amendments designed to bring Thailand's trademark law into compliance with
the TRIPS Agreement enacted in June 2000.
Created viable legal framework/improvements in enforcement for clothing, accessories,
and push toys, but infringement still remains a serious problem.
Penalties for proven trademark violations are insufficiently high to have a deterrent effect Geographic Indications Act passed by Thai Parliament in September 2003, into effect in
Trade Secrets Act 2002 allows government agencies under certain circumstances to
disclose trade secrets to protect any "public interest" not having commercial objectives
However, implementing regulations have yet to be approved
Thailand's patent office lacks resources to keep up with the volume of applications, and
patent examinations can take more than five years.
Parts of patent search for novelty and preparation of application has been outsourced to
academic institutions to speed up the registration process
2005 Thailand began preparations to accede to the Paris Convention and the Patent
Cooperation Treaty
Inconsistent enforcement efforts due to corruption and cultural climate of leniency Concerns of frequent raids compromised by leaks from police sources Establishment of the Department of Special Investigations in 2003 to focus on major
infringing production, warehousing and trafficking operations.
Establishment of a specialized intellectual property court in 1997, which improved
judicial procedures and imposed tougher penalties
Anti-Money Laundering Act Dec 2003: Allow police and other law enforcement officials
to seize and investigate funds and suspect bank accounts
Source: IPR Toolkit for the Kingdom of Thailand
(1978). Medicines Act (Chapter 176, Section 18 & 74) Medicines (Clinical Trials) Regulations.
A. G. M. f. E. Office. Singapore, Government of Singapore.
(1987). Medicines Act (Chapter 176, Section 44) Medicines (Labelling) Regulations. A. G. M. f.
E. Office. Singapore, Government of Singapore.
(2003), Thai Drug Control Division, Procedure of generic drugs registration Bangkok:
(2003). Thailand Food and Drug Administration. Pre-marketing control: licensing Bangkok:
(2004) Indonesia: Supply and Demand Survey on Pharmaceuticals and Natural Products.
International Trade Centre – South-South Trade Promotion Programme.
<http://www.intracen.org/TDC/SSTP/SUPPLYDEMANDSURVEYS/32145.pdf>
(2008, April 4). Thailand pharmaceuticals & healthcare report q1 2008 forecasts modest growth
over the period researched, with the value reaching us$2.62bn by 2012. Red Orbit: <www.redorbit.com/news/health/1326104/the_report_thailand_pharmaceuticals healthcare_report_q1_2008_forecasts/>
(2008). Thailand Food and Drug Administration. Guideline on procedural aspects regarding ma
(2009) Indonesia: Regulating registration and imports of pharmaceutical products. Global Trade
(2010). Indonesia: licensing and intellectual property, EIU ViewsWire:
(2010) Indonesia Pharmaceuticals & Healthcare Report Q4 2010. Business Monitor International
– Industry Report & Forecast Series. Business Monitor International (BMI) Ltd, <http://www.businessmonitorinternational.com>.
(2010). Medical device control in Thailand: <http://www.fda.moph.go.th/fda-
(2010) Thailand Pharmaceuticals and Healthcare Report Q4 2010. Business Monitor
International - Country Risk, Industry, Company Intelligence. Business Monitor International Ltd, n.d.: <http://www.businessmonitor.com>
Araneta, S. (2010). Nbi seizes p25-m counterfeit drugs . The Philippine Star, Retrieved from
ASEAN (August 1, 2006). ASEAN Sectoral Mutual Recognition Arrangement for Good
Manufacturing Practice (GMP) Inspection of Manufacturers of Medicinal Products. H. S. Authority. Singapore, ASEAN Secretariat.
ASEAN Background. ASEAN. N.p., <http://www.aseansec.org/about_ASEAN.html>
ASEAN Labeling Requirements: Issues on Country Specific Requirements. ASEAN. N.p.,
Barriers to trade. China.org.cn - China news, weather, business, travel & language courses.
Bate, R. & Boateng, K. Medicinal Malpractice; Improving Drug Access and Reducing
Corruption. AEI Outlook Series (Dec 2006) http://www.aei.org/outlook/25276
British Library Cataloguing in Publication Data. (July 30, 2010). Vietnam Medical Device Market Intelligence Report. UK: Espicom Business Intelligence.
Building GPP Pharmacies - Obstacles Still Exist. (2010, August 14). Retrieved from
Business Monitor International. (2009) Industry Forecast-Export and Import.BMI Supply Chain
Malaysia Pharmaceuticals and Healthcare Report. 1
Business Monitor International. (2010) Industry Analysis- Drugmakers Reminded of Production
Rules. BMI Malaysia Regulatory Pharmaceuticals and Healthcare. Asia Pacific Pharma and Healthcare Insights. 1-2
Business Monitor International. (2010) Industry Forecast- Generics. BMI Malaysia Regulatory
Pharmaceuticals and Healthcare. Malaysia Pharmaceuticals and Healthcare Report. 1-2
Business Monitor International (2010). Malaysia Pharmaceuticals and Healthcare Report Q4
Business Monitor International (2010). Singapore - Pharmaceuticals & Healthcare. BMI Industry
View - Singapore -Q4 2010, Business Monitor International.
Business Monitor International (2010). Vietnam Pharmaceuticals and Healthcare Report Q4
2010 (ISN 1748-2305). London, UK: Business Monitor International.
Central Intelligence Agency, (2010). The CIA world factbook Cambodia. Langley, VA:
Chase, M. (2004, August 12). Key AIDS study in Cambodia now in jeopardy. Wall Street
Clinical Trials in Vietnam Required for First-Time Device Import. (2009, April 01). Retrieved
Counterfeit Drugs Challenge Pharma Industry. (2010, October 20). Retrieved from
Curbing Corruption in Public Procurement in Asia and the Pacific. Asian Development Bank. N.p., <www.adb.org/Documents/Books/Public-Procurement-Asia-Pacific/tha.pdf>.
Deardorff, A. V. & Stern, R. M. (2005). A centennial of anti-dumping legislation and
implementation: introduction and overview. World Economy, 28, 633-640.
DGIPR (Indonesia), (n.d.). Enforcement of intellectual property in Indonesia: <http://www.ecap-
Ellison,S. F. & Snyder, C. M. (2010). Countervailing power in wholesale pharmaceuticals. The
Journal of Industrial Economics, 58, 32-53.
Endeshaw, A. (1998, September). Harmonization of intellectual property laws in ASEAN: issues and prospects. Retrieved from
Export Markets; Health and medical to Vietnam. Austrade, Australian Government, (last updated
Food and Drug administration - Thailand. (n.d.). Retrieved from
GHTF: overview and mission. (2007, November 20). Retrieved from
Gross, A. (2009, January 1). Asean regulatory medical device integration. Retrieved from
Gross, Ames, and Rachel Weintraub. Thailand Pharmaceutical and Medical Device Markets.
Pacific Bridge Medical: Asia Medical Consulting, Regulatory Affairs, Business Development. Pacific Bridge Medical.
Haq, A. S. M. ASEAN Pharmaceutical Harmonization Updates. ASEAN Pharmaceutical
Harmonization Updates, Ministry of Health, Malaysia.
Ho, Prudence (2006, 12 July). Kingmaker eyes Cambodia to sidestep duties. The Standard.
Factiva.com. Web. 15 October 2010.
Hollis, L. (2010, October 13). Top 10 Medical Devices: 2010. Retrieved from
HSA (2008). Health Product Regulation: Medical Advertisements & Sales Promotion. H. S.
Authority. Singapore, HSA.
HSA (2009). A Guide on Advertisements & Sales Promotion of Medicinal Products. H. S.
HSA (2010). Health Product Regulations: Clinical Trial Statistics. H. S. Authority. Singapore.
HSA (2010). Guideline on Application for Clinical Trial (CTC). H. S. Authority. Singapore,
HSA (2010). Import License (For Authorized Agent) and Wholesale Dealer's Licence for
Medicinal Products. H. S. Authority. Singapore.
Hui-Keng, L. (2009). ASEAN Harmonisation: An Agency Perspective. ASEAN Harmonization,
Singapore, Health Sciences Authority.
IDA. (2009). "Intellectual Property Rights." Retrieved November 6, 2010, from
Industries in Malaysia: medical devices industry. (2010, April 2). Retrieved from
Invest in Malaysia: incentives for investment: incentives for the manufacturing sector. (2010,
April 5). Retrieved from http://www.mida.gov.my/en_v2/index.php?page=manufacturing-sector-2
Invest in Malaysia: intellectual property protection. (2010, August 18). Retrieved from
IPR Toolkit for the Kingdom of Thailand."Retrieved 10 15, 2010, from US Commercial Service-
United States of America Department of Commerce: <http://www.buyusa.gov/thailand/en/ipr.html>
Ismail, Zakiah. (2009) Guidelines for Application if Clinical Trial Import License and Clinical
Trial Exemption in Malaysia. National Pharmaceuticals Control Bureau. http://portal.bpfk.gov.my/index.cfm?menuid=71&parentid=69
Kongkamee, B. (2005, March 14). Medical device regulations - Thailand. Retrieved from
development. Factica.com. Web. 21 November 2010.
Laetzel, R. (2007). Development of the ASEAN Pharmaceutical Harmonization Scheme: An
Example of Regional Integration.
Lätzel, R., (2007). Development of the asean pharmaceutical harmonisation scheme: an example
of regional integration. Unpublished manuscript, Drug Regulator Affairs, University of Bonn, Bonn, Germany: <http://www.dgra.de/studiengang/pdf/master_laetzel_r.pdf>
Lam, T.D.T. (20 October 2003). Health-Vietnam: Cure for rising drug prices hard to find. Inter
Press Service. Factiva doi: IPRS000020031020dzak0000a
Lithgow, T. (2002, March 12, 2002). "Six Steps to Pharmaceutical Packaging Sustainability."
Retrieved October 12, 2010, from http://www.pharma-iq.com/article.cfm?externalid=2062.
Lon, C. T., Tsuyuoka, S.et al.(2006). Counterfeit and substandard antimalarial drugs in
Malaysian Industrial Development Authority. (2008) Invest in Malaysia Taxation. 1-2.
Maskus, K. E. (2001). Parallel Imports in Pharmaceuticals: Implications for Competition and
Prices in Developing Countries. Final Report to World Intellectual Property Organization. Colorado, University of Colorado at Boulder.
Medical device bureau: ministry of health Malaysia - background. (2008, June 12). Retrieved
Medical device bureau: ministry of health Malaysia. (2010, November 10). Retrieved from
Minh, Nguyen, & Kien, Trinh. (2004). Medical, pharmaceutical, and cosmetic product
distribution in vietnam (Written for Tilleke & Gibbins Consultants Ltd.), Retrieved from http://www.tginfo.com/publications/VN_articles/pharma_dental_VN.pdf
Ministry of Health Malaysia, Medical Device Bureau. (n.d.). Placement of medical devices on
the Malaysian market Malaysia: Retrieved from http://www.mdb.gov.my/mdb/documents/placement.pdf
Ministry of Information and Communication. (2008).Vietnam customs. Retrieved from
Minot, J., & Gross, A. (2009, January/February). ASEAN Medical Device Regulatory
Integration. Retrieved November 15, 2010, from Medical Product Outsourcing: http://www.mpo-mag.com/articles/2009/01/asean-medical-device-regulatory-integration
Mokkhavesa, W. (2010, November 5). Bangkok post. Retrieved from
Muller-Langer, F. (July 2007). A Game Theoretic Analysis of Parallel Trade and the Pricing of
Pharmaceutical Products. German Working Papers in Law and Economics.
National List of Essential Medicines. (2008) Ministry of Health of the Republic of Indonesia.
National Pharmaceutical Controls Bureau. (2010) Drug Registration Guidance Document
Malaysia. Ministry of Health Malaysia. 40-44
National Pharmaceuticals Control Bureau. (2010) Analytical Validation Documents of Injections
Submission Guidelines. Ministry of Health. 1
New Drug Approval Process FDA. (2010, November 15). Retrieved from
Oktaviani, R., Rifin, A., & Reinhart, H. (2007). A review of regional tariffs and trade in the
ASEAN priority goods sector. Brick by Brick: The Building of an ASEAN Economic Community, 59-85.
Overview (n.d.). Retrieved 11 15, 2010, from Association of Southeast Asian Nations:
Park, P. (2010, July 27). Lethal counterfeits [Web log message]. Retrieved from
Partners, T. L. C. (August 28, 2001). "Copyright Law and Parallel Imports: Australia, the United
Philippines Pharmaceuticals & Healthcare Report Q1 2011. (2010, October). Retrieved October
19, 2010, from the Business Monitor International database.
Philippines Pharmaceuticals & Healthcare Report Q4 2010. (2010, August). Retrieved October
19, 2010, from the Business Monitor International database.
Pre-marketing control. Food and Drug Administration. Food and Drug Administration Thailand,
Quang, M. (2010, September 17). Counterfeit enforcement ineffectual, report says. Thanh Nien
Ratanawijitrasin, S. (2005). Drug Regulation and Incentives for Innovation: The Case of ASEAN. World health organization: <http://www.who.int/intellectualproperty/studies/Drugregulationincentives.pdf>
Research and Markets. (2010, July). Cambodia pharmaceutical and healthcare report q3 2010.
Roth, G. (2010, September 01). 2010 top 20 Pharmaceutical Companies. Retrieved from
Sanofi P., (2009). Asean: <www.adediem.com/docs/Adediem%2011fev%20-%20ASEAN%20-
Sauwakon Ratanawijitrasin, P. D. (2005). Drug Regulation and Incentives for Innovation: The Case of ASEAN.
SECRETARIAT, A. (2010). "AFTA & FTAs: TRADE." Retrieved October 8, 2010, from
SEDB (2010). Singapore - The Biopolis of Asia. S. E. D. Board. Singapore.
Solidiance. (2009, June). Vietnam: Medical Device industry. Retrieved Novemeber 15, 2009,
Stenson, B, Syhakhang, L, Eriksson, B, & Tomson, G. (2001). Real world pharmacy: assessing
the quality of private pharmacy practice in the Lao People's Democratic Republic. Soc Sci Med, 52(3), Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/11330774
Tamayo, M. (10 July 2008). Vietnam's Ministry of Health begins crackdown on illegal drug
price increases. Global Insight Daily Analysis. Factiva doi: WDAN000020080710e47a0004m
Taylor, Phil. Pharmelp counterfeit drug analysis project gains pace. Phnom Penh Post. 24
Thai News Service. (31 March 2009). Vietnam: Improper rises in vaccine prices may be
punished. Factiva doi: THAINS0020090330e53v0001m
Thailand Legal Basics. Tilleke & Gibbons. Tilleke & Gibbons International Ltd.,
Thailand. The Official Website of the Association of Southeast Asian Nations. ASEAN,
The medical device market: Malaysia - opportunities and challenges. (2010). Espicom, Retrieved
The Telegraph (2008, 17 November). Fake drugs worth more than £4m seized by Interpol.
The World Bank, (2008). Project appraisal on a proposed grant in the amount of us$12.35
Transparency International. (2010, October). Corruption perceptions index 2010 results.
U.S. Food and Administration, Centers and Offices. (2010, November 15). Retrieved from
USP revising medicare model guidelines. (2006). USP Press, 3(3), Retrieved from
Van, Le, & Tong, Sarah. (2009). Vietnam and anti-dumping: regulations, applications, and
responses. EAI, Retrieved from http://www.eai.nus.edu.sg/EWP146.pdf
Vietnam Black Market Value $ 0.6748 billion ($674.8 million). (2010, November 01). Retrieved
Vietnam Minister of Health. (2005). Vietnam Medical Device Market, Commercial Service
Report. Hanoi, Vietnam: Vietnam Ministry of Health.
Widespread Antibiotic Abuse in Vietnam Said Increasing Drug-Resistance. (2010, August 24).
Saigon Giai Phong, translated to English by BBC Monitoring Asia Pacific.
Wondemagegnehu, E. World Health Organization, (1991). Counterfeit and Standard Drugs in
Myanmar and Vietnam (WHO/EDM/QSM/99.3). Geneva, Switzerland: World Health Organization. Retrieved from http://apps.who.int/medicinedocs/pdf/s2276e/s2276e.pdf
WHO launches campaign against counterfeit medicines. Bulletin of the World Health
Organization 2003; 81 (12): pp. 921-22
Why Malaysia: supportive government policies. (2010, April 2). Retrieved from
Yang, Q. & Cheng, L. (2008). Import tariff, intellectual property right protection and foreign
merger. Economic Modelling, 25, 1225-1231.
Yeo, J. P. "A Brief Overview of the Pharmaceutical Industry in Singapore." The Journal of
European Medical Writers Association: 35-36.
Yuppadee Javroongrit, P. D. (2006). ASEAN Harmonization. The 2006 Symposium of APEC
Network on Pharmaceutical Regulatory Sciences, Tokyo, Japan.
Zaman, S. (2010). Interview with Senior Regulatory Specialist, HSA. E. Lee. Singapore.
Source: http://prema.or.th/uploads/premanews_detail/75_asean_harmonizationstandardizationbymasonschool.pdf
Vacumn Pumps : Bathmate Hydromax Pump - x30 Xtreme and kit Bathmate Hydromax Pump - x30 Xtreme and kit Bathmate Hydromax Pump - Xtreme X30 and Accesories Kit Hydromax Xtreme X30 and accessories kit has improved function and ease of use. Use after prostatectomy for improved penis health, size andfunction. For advanced pump users. *** SALE NOW ON ***
Heft 48 Juni 2014 Die digitale Zeitung für Lebens- und Sozialberater/innen Herausgeber: ÖGL Österreichische Gesellschaft für Lebensberatung in Kooperation mit BG LSB Wirtschaftskammer OÖ LIEBE KOLLEGINNEN!LIEBE KOLLEGEN!Nach einigen Unruhen im letzten Jahr über un- sere Ausübungsrechte, ist es nun Dank des








