Levitra enthält Vardenafil, das eine kürzere Wirkdauer als Tadalafil hat, dafür aber schnell einsetzt. Männer, die diskret bestellen möchten, suchen häufig nach levitra kaufen ohne rezept. Dabei spielt die rechtliche Lage in der Schweiz eine wichtige Rolle.
Model of pathogenesis of psoriasis. part 1. systemic psoriatic process.


Part 1. Systemic psoriatic process
Edition e4.0
Mikhail Peslyak
Moscow, 2012
UDC 616.5:616-092
Mikhail Yuryevich Peslyak
Model of pathogenesis of psoriasis. Part 1. Systemic psoriatic process.
Edition e4.0 (revised and updated), Russia, Moscow, MYPE, 2012.– 84 p.
ISBN 978-5-905504-02-0
Copyright 2009-2012, Mikhail Peslyak
Electronic Publication Dates
in Eco-trends (ISBN 978-5-88405-101-0):e2.0: 2009,Dec 30; e3.1: 2010,Dec 21;
in MYPE e3.2: 2011, Jul 12; r4.0: 2012, Apr 14;
Part 1 (edition e4.0) and Part 2 (edition e1.3) are in agreement.
Web-mail:
It is allowed to use of unchanged materials of the e-book for non-commercial objectives,
specifying authorship, name of the book, edition number, ISBN and Web. This e-book is
distributed free of charge.
Creative Commons Lic
The given book is the authorized translation of the book, published in Russian.
Translation has been carried out with active support of .
The author thanks Vladimir Turbin for the help with the proofreading.
Periodic reprinting at occurrence of new materials or detection of serious errors
is occured. Further, updated texts (Russian and English) of the monograph will be
regularly placed in the Internet.
Details and links on
2009-2012, Peslyak MY, Model of pathogenesis of psoriasis. Part 1. Systemic psoriatic process. e4.0a. 2
Abstract
Review and analytical study of results of experimental and theoretical works on etiology and
pathogenesis of psoriatic disease was conducted. Psoriasis is dermal implication of systemic
psoriatic process (SPP). New model of pathogenesis (hereinafter Y-model) explaining results
of clinical and laboratory experiments was formulated. According to Y-model there are two
main factors: hyperpermeability of small intestine for bacterial products and colonization of its
walls by Gram+ bacteria (incl. psoriagenic bacteria PsB) and Gram(-) TLR4-active bacteria.
Inside SPP there is a vicious cycle which is supported by disturbance of production and-or
circulation of bile acids.
SPP central subprocess is PAMP-nemia, namely chronic kPAMP-load on blood phagocytes
(neutrophiles, monocytes and dendritic cells). The load results in increase of blood kPAMP
level. The major key PAMP (kPAMP) are LPS and PG (incl. PG-Y – peptidoglycan of psoriagenic
bacteria). Chronically increased kPAMP-load possibly provides tolerization of some
neutrophils Neu, monocytes Mo and dendritic cells DC in blood flow.
Chemostatus of tolerized blood Neu-T in process of their aging changes similarly to
chemostatus nonactivated Neu and, hence, they carry endocytosed content from blood flow
into bone marrow. Chemostatuses of tolerized Mo-T and DC-T are similar to nonactivated ones.
So they don't bring endocytosed content to lymph nodes or spleen and remain in blood.
Tolerized phagocytes degrade endocytosed fragments of bacterial products containing
kPAMP slowly and incompletely. Tolerized phagocytes appeared to be (PG-Y)-carriers are
named by R-phagocytes and are designated as Neu-R, Mo-R and DC-R.
SPP severity predetermines possibility of psoriasis initialization and maintenance because
Mo-R and DC-R along with normal Mo and DC participate in homeostatic and inflammatory
renewal of pool of dermal macrophages and DC of non-resident origin. Mo-R and DC-R enter
derma and they can transform to mature maDC-Y (in particular – to TipDC), presenting
Y-antigen to specific TL-Y. Local processes in derma and epidermis will be described in details
in the second part of the monograph.
The given book is the authorized translation of the book, published in Russian
(ISBN 978-5-905504-01-3; edition r4.0).
Keywords
Systemic psoriatic process, psoriasis, psoriatic arthritis, intestinal permeability, intestine
microflora, bile acids, psoriagenic bacteria, bacterial products, peptidoglycan, muramyl
dipeptide, lipopolysaccharide, endotoxin, PAMP-nemia, neutrophils, monocytes, dendritic
cells, chemostatus, tolerization, tolerized phagocytes, R-phagocytes, kPAMP-carriage,
(PG-Y)-carriage.
2009-2012, Peslyak MY, Model of pathogenesis of psoriasis. Part 1. Systemic psoriatic process. e4.0a. 3
Content
2009-2012, Peslyak MY, Model of pathogenesis of psoriasis. Part 1. Systemic psoriatic process. e4.0a. 4
List of figures
2009-2012, Peslyak MY, Model of pathogenesis of psoriasis. Part 1. Systemic psoriatic process. e4.0a. 5
«Tank with kerosene was strapped to fireman's shoulders…»
Ray Bradbury, «Fahrenheit 451»
Introduction
Epidermis self-renewal is regular process. New cells are born in basal layer. They mature, vary,
migrate outside and form external horny layer. Then they die away and exfoliate. Standard duration of epidermis cell life (renewal period) for areas of skin with average thickness is 20-25 days. Psoriasis accelerates self-renewal. Cells live 4-10 days ). Cells migrating outside have no time to differentiate and they aren't quite functional. Psoriatic plaques have red shade. They are tender, they are covered by white flakes due to intensive lost of cells and they are much thicker.
Psoriasis isn't contagious. There are various types of psoriasis: vulgaris or plaque (L40.0),
flexural or inverse (L40.83-4), erythrodermic (L40.85), pustular (L40.1-3, L40.82), guttate (L40.4). Codes of diseases are given according to ICD-10. Chronic plaque psoriasis (CPs) is the most frequent type (more than 80% of total number of cases). Up to 15% of psoriatics also suffer from psoriatic arthritis (L40.5). Psoriasis strikes about 2-3% of population (120-180 million people). New diagnosis of psoriasis gets 4-6 million people every year. Disease appears after birth or in extreme old age. Psoriasis is a chronic disease so there are periods of aggravation and remission. Sometimes there is no cause for period change and sometimes aggravation can be decreased as a result of treatment. Serious psoriasis can result in disability. Psoriasis course is similar in men and women. Afro-Americans, Indians, Chineses and Japaneses suffer from psoriasis less frequently and Eskimos don't suffer from psoriasis at all .
Psoriasis is registered in "Online Mendelian Inheritance in Man" at number OMIM*177900.
Psoriasis is disease with hereditary predisposition: concordance of uniovular twins is 70%. If one parent suffers from psoriasis children are diagnosed the disease in 15-25% of cases; if both parents suffer from psoriasis children are diagnosed the disease in more than 40-60% of cases. The interrelation of allele HLA-Cw*0602 (chromosome 6p21) and psoriasis of the first type which is characterized by early beginning is proved . This allele is found in more than 60% of psoriatics (not more than 15% of healthy people). Locuses of other chromosomes have weaker interrelations ). Psoriasis can't begin only in presence of genetical deflections. External exposure is necessary for beginning and maintenance of psoriasis. Infections, skin traumas, stresses, reaction to medications, climatic changes and other causes can provoke onset of psoriasis or its aggravation
Accelerated proliferation of keratinocytes is likely to be caused by erroneous actions of
mechanisms of skin antibacterial protection. Influence of beta-hemolitic streptococci (first of all causing tonsillar infections) on initialization and aggravations of psoriasis is avowed , . There is no uniform point of view on etiology and pathogenesis of psoriasis. Researches offer various models , .
Note. contains the list of all essential changes and additions (in comparison with the pregoing edition) and the reference to particular places. In the text the significant new or revised fragments are marke d with a vertical line on the right. New works included into the bibliography are marked the same way.
2009-2012, Peslyak MY, Model of pathogenesis of psoriasis. Part 1. Systemic psoriatic process. e4.0a. 6
Objective
Present work is review and analytical study of interrelation of disfunction of small intestine,
hepatobiliary system, intestinal microflora and blood phagocytes (neutrophiles, monocytes and dendritic cells). The aim of the study was substantiation of new model of psoriasis pathogenesis (further Y-model). Model of pathogenesis of psoriasis was formulated in previous work . It was based on colonization of intestine by beta-hemolitic streptococci (BS) associated with hyperpermeability of intestinal walls for BS-proteins. Appreciable part of new results confirms this model. However results of new studies of psoriasis , , , and mechanisms of cellular immune protection , demanded specification, development and more detailed formulation of model.
Publications with subject of examination of GIT and hepatobiliary system, investigation of
intestine and biliary microflora or antiendotoxic and antistreptococcal immunity in psoriatics were searched and analyzed. Attention was also paid to publications with subject of prepsoriatic skin investigation and evaluation of events initiating the beginning of psoriatic plaque. Publications were searched in and Russian publications were searched in Bibliography includes about 25% from total number of analyzed publications. Review and/or analysis study results are given in description of subprocesses. Real or prospective mutual influence of subprocesses is given at the end of description of dependent subprocess.
Abbreviations and terms
There are two tables in appendices: "Abbreviations" and "New terms"
There are links or references on works where these terms are well described in the tables.
Novelty and hypotheses
There are references where the subprocess is proved and/or described in details or hypothesis
Hypothesises about fractionation of blood phagocytes under chronic PAMP-load
and about chemostatus of tolerized phagocytes are essentially new. Suggested systemic psoriatic process SPP is essentially new also.
2009-2012, Peslyak MY, Model of pathogenesis of psoriasis. Part 1. Systemic psoriatic process. e4.0a. 7
Model of pathogenesis. Systemic process.
Review of models of pathogenesis
The majority of studies on psoriasis state that principal cause of the disease is in skin itself.
There is far less number of authors, who don't restrict themselves with investigation of only local processes, but conduct search and find of evidences, proving that psoriasis is local sign of systemic psoriatic process SPP , , . In work suggested largely simplified model of traffic of activated T-lymphocytes moving from gut-associated lymphoid tissue to skin and/or joints. It results in initialization and maintenance of psoriasis and/or psoriatic arthritis (it was suggested in 1999 for the first time). There were no assumptions on the mechanism of process in publications of the authors. On the contrary the authors of work thought that systemic process is caused only by disorder of production and circulation of bile acids . The authors are undoubtedly right, but it isn't the only one and main disorder which causes systemic process. It is discussed in details considering new model.
In the work a complex model of pathogenesis of the chronic dermatoses based
on trigger role of blastocystosis (BLC) was offered. The blastocystosis can entail serious dysbiotic deviations in microflora of intestine and disturbance of its barrier function which occurs because of both blastocystosis and disbacteriosis of parietal microflora ). As a result too many toxic products of vital activity of blastocystes and microflora (it is not specified, which exactly) get to blood flow and support chronic endointoxication. The chronic endointoxication in its turn breaks the work of immune system and the balance of the oxidizers-antioxidants system. There is hyperactivation of processes of free-radical oxidation and decrease of antioxidatic activity. The author assumes that disturbance of work of these systems appears to be sufficient for the initialization and support of chronic dermatoses, including psoriasis.
Professor Korotkii NG and I suggested model of SPP pathogenesis in 2005 for the first time
model supposed that products of beta-streptococci moved to skin directly from blood. The products move to blood from intestine with hyperpermeability .
However B.Baker et al. proved presence of BSPG (beta-streptococcal peptidoglycan) in psoriatic
plaques only inside dermal and epidermal monocytes Mo and possibly in dendritic cells . On the other hand, other scientists proved that attraction of monocytes, their activation and
secretion of TNF-alpha was defining link of escalation of psoriatic plaques.
In his book-atlas Lionel Fry surveyed the role of BS in the initialization and support of guttate and
chronic psoriasis and analysed the ways of BSPG getting to skin from the streptococci localized in tonsils (Fry 2005). A year later Barbara Baker, Anne Powles and Lionel Fry offered the model of pathogenesis (further BF-model) based on the new role of peptidoglycan (further PG) Process of initialization of temporary guttate psoriasis is simulated on right part of figure. Streptococci temporary situated in tonsils produce toxins-superantigens. They activate TL of tonsils or skin lymph nodes. PG-specific TL are selected due to contacts with PG+Mo (transformed to PG+MoDC). Other TL become anergy or apoptotic.
Similar sequence of events can be observed in chronic psoriasis (left part of figure) if
streptococci and/or streptococcal antigens stay in tonsils and/or intestine for a long time. Plaques appear after PG+Mo and PG-specific TL enter in derma. Autoantigen (e.g. keratin) has aggravating effect. BF-model doesn't give answers to the next two questions:
1. Why do PG+Mo appear in skin though PG was endocytosed by Mo in other place of
organism?
2. Why do PG+Mo become PG+MoDC and present PG?
The first question is connected to disorder of traffic of immune cells. Let's answer this question.
The answer to the second question will be given while discussing local processes .
2009-2012, Peslyak MY, Model of pathogenesis of psoriasis. Part 1. Systemic psoriatic process. e4.0a. 8
In the works proved that dermal TipDC are main factors of psoriasis
maintenance. TipDC are mature dendritic cells maDC which actively secrete iNOS and TNF-alpha and present unknown antigen Y.
Baker proposes that Y are parts of BSPG interpeptide bridge The authors of work showed that 90% of TipDC are CD11c+BDCA-1(-)DC of non-
resident origin while 10% of TipDC are CD11c+BDCA-1+DC of resident origin.
Blood monocytes Mo and/or dendritic cells DC were proved to be precursors of larger part
BDCA-1(-)TipDC. However two questions remain; which Mo and-or DC fraction is it; and why does this fraction act in such a way in case of psoriasis? TipDC is present in visible healthy prepsoriatic skin and in healthy people skin, but its quantity is rather less than in psoriatic derma. It means that TipDC-precursors moving from blood and their transformation to TipDC take place in homeostasis also.
Part PG+ dermal cells expresses CD68 However increased expression CD68 is
available at dermal inflammatory BDCA-1(-)DC in comparison with BDCA-1(+)DC I.e. some from CD68+PG+ cells can be BDCA-1(-)DC.
Results received by Baker and Zaba correlate with each other. It is possible to assume that
TipDC-precursors are PG+Mo.
BF-model has been developed recently in work Authors recognize role
BSPG, but as well as earlier assume that, the basic antigens are parts of beta-streptococcal M-protein (BSMP). As the area of chronic localization of BS only tonsils are admitted. The essential role in their model is played by cross-reactivity between BSMP and autoantigens - peptides of epidermal keratins K14, K16 and K17.
In 2005-7 works of Garaeva ZS et al. were published. The authors showed that majority of
psoriatics had high blood LPS level and also increased LPS-load on blood Mo and DC , ).
TLR2 and TLR4 are membranous PAMP ligands, while NOD1 and NOD2 are intracellular PAMP
ligands . As fragments of peptidoglycan PG (BLP (bacterial lipoprotein), LTA (lipoteichoic acids), MDP (muramyl dipeptide), DAP (diaminopimelic acid)) are also active PAMP so blood Mo and DC in SPP have combined (LPS and PG) PAMP-load giving synergic effect , . Term "load" hereafter means linkage, endocytosis and/or contact with PAMP. Abovementioned facts and recently published studies on action and transformation of blood Mo and DC ) forced to reconsider model 2005 and to assume that unknown antigen move to skin mainly inside involved Mo and/or DC ).
Hyperpermeability of small intestine for bacterial products (subprocess SP1) and growth of
bacterial populations (including beta-streptococci) on its walls (subprocess SP2) are main factors (as before) ). Let's describe systemic psoriatic process SPP.
2009-2012, Peslyak MY, Model of pathogenesis of psoriasis. Part 1. Systemic psoriatic process. e4.0a. 9
Systemic psoriatic process SPP.
Increased kPAMP-carriage of tolerized phagocytes.
Increased (PG-Y)-carriage of R-phagocytes.
The process was partly studied earlier. However its name and influence on psoriasis
development are suggested for the first time. Systemic psoriatic process SPP was partly (SP1, SP2) suggested in . The process involves GIT, hepatobiliary, vascular and immune systems, elimination organs; F - fragments of bacterial products containing PAMP (including kPAMP) chronically enter the blood as part of this process. Therefore PAMP-load (contact, linkage, endocytosis) on blood phagocytes (including Mo and DC) becomes constant, so tolerized phagocytes appear.
Term "phagocytes" hereinafter is used not only for neutrophils and monocytes (macrophages), but also for
dendritic cells. Immature dendritic cells are also professional phagocytes .
Phagocytes (neutrophils Neu, monocytes Mo, dendritic cells DC) exposed to chronic PAMP-load (contact,
linkage, endocytosis) can be tolerized .
For SPP defining role play two properties of tolerized phagocytes (Neu-T, Mo-T, DC-T):
Property 1. Despite contact, linkage with PAMP and endocytosis of PAMP (as a part of endocytosed F-content) their chemostatuses are similar to nonactivated; Property 2. As tolerization occurs under chronic kPAMP-load then there is kPAMP in endocytosed F. Its degradation occurs slowly and not completely, i.e. kPAMP-carriage takes place;.
Tolerized phagocytes appeared to be (PG-Y)-carriers are named by R-phagocytes and are designated as
Neu-R, Mo-R and DC-R. All R-phagocytes possess properties 1, 2 and 3.
Property 3. In endocytosed F-content is PG-Y. Its degradation occurs slowly and not completely, i.e. takes place (PG-Y)-carriage.
Property 1 - and properties 2 and 3 - Mo-T and DC-T (incl. Mo-R and DC-R) migrate under the influence of chemokines as nonactivated Mo and DC
(including during renewal of pool of tissue Mo and DC) because their chemostatuses are similar to nonactivated ones. Mo-T and DC-T (incl. Mo-R and DC-R) preserve earlier endocytosed F-content some time after entering the tissue and participate in one of two scripts, in particular, depending on level of cytokines -deprogrammers :
1. Low level of cytokines-deprogrammers: Mo-T and DC-T (incl. Mo-R and DC-R) mainly preserve tolerance to
F-content, gradually degrading it.
2. Increased level of cytokines-deprogrammers: Mo-R and DC-R quickly lose tolerance to F-content. They are
activated, they mature and they are transformed: Mo-T in MF-T or MoDC-T (incl. Mo-R in MF-R or MoDC-R). Thus only DC-R and MoDC-R can mature and be transformed into maDC-Y
Until full degradation of PG-Y (inside of Mo-R and DC-R) can enter the second script during the first one
(including maDC-Y formation).
Features of certain tolerized phagocyte:
depth of tolerization which is defined by previous and present PAMP-load;
volume and assortment of kPAMP (within F), containing in it;
Features of certain R-phagocyte:
depth of tolerization which is defined by previous and present PAMP-load;
volume and assortment of kPAMP (within F), containing in it;
volume of PG-Y (within F), containing in it;
Volume of total (PG-Y)-carriage of all blood R-phagocytes is the important factor for SPP. This index depends
on part of R-phagocytes among total number of blood phagocytes and from volumes of PG-Y containing in single R-phagocytes. Low part of R-phagocytes can be combined with high volumes of PG-Y containing in single R-phagocytes and, on the contrary, reprogrammed state of all blood phagocytes can be combined with small volumes of PG-Y containing in single R-phagocytes (SP7).
2009-2012, Peslyak MY, Model of pathogenesis of psoriasis. Part 1. Systemic psoriatic process. e4.0a. 10
SPP severity is proportional to total (PG-Y)-carriage of Mo-R and DC-R in blood flow. SPP severity also depends on total kPAMP-carriage of tolerized phagocytes in blood flow.
Chronically increased blood level LPS is called endotoxemia and chronically increased LPS-load
on phagocytes is the main sign of endointoxication. Let's name set of these deflections (in case of influence of several PAMP) "PAMP-nemia" on the analogy of term "endotoxinemia" , and set of PAMP causing these deflections - key PAMP, i.e. kPAMP. Major kPAMP in psoriasis are PG (including PG-Y) and LPS (see SP4).
Significant percent of tolerized Mo-T and DC-T appear among blood Mo and DC due to chronic
kPAMP-load (SP8). Mo-T and DC-T can respond to homeostatic and proinflammatory chemokines and participate in homeostatic and intensive inflammatory renewal of pool of tissue MF and DC due to chemostatuses similar to nonactivated ones.
Initialization and maintenance of psoriasis with R-phagocyte provide carriage of PG-Y –
peptidoglycan from psoriagenic bacteria. Normal skin becomes prepsoriatic skin due to Mo-R and DC-R participation in homeostatic (inflammatory) renewal of pool of dermal MF and DC. It is the main cause of initialization and maintenance of psoriatic plaque in local inflammation.
Source of PG-Y entering the blood can be not only GIT microflora, but also temporary local
infections, for example tonsillar infection (SP6). Genetical deflection can be the cause of absence of respond to Mo and DC on PG-content in rare cases. So episodic and low level of (PG-Y)-load can be enough for initialization and maintenance of psoriasis (SP7).
All subprocesses forming process SPP are on scheme Subprocesses SP2, SP4 and SP8 make SPP-basis. Internal dependencies of SPP-basis and
separation on two components are on scheme
2009-2012, Peslyak MY, Model of pathogenesis of psoriasis. Part 1. Systemic psoriatic process. e4.0a. 11
Subprocess SP1. Hyperpermeability of intestinal walls for F-content
Subprocess is known; its influence on psoriasis was investigated. F-content constantly moves to blood through intestinal wall. It is proved by blood tests of healthy
people . F-content consists of macromolecules so small volumes move through inter-enterocytes contacts under control of barrier function. As a result of genetical predisposition or gastroenterologic diseases barrier function of inter-enterocytes transmission is interrupted and volume of F-content increases . Long-term therapy of glucocorticoids (hydrocortizone, betamethasone, etc.) or cytostatics (methotrexate, cyclophosphamide, etc.) also can promote the process. Additionally permeability for F-content can increase as a result of disturbed mechanism of transcytosis around Peyer's patches and lymphoid follicles through associated epithelium ).
Ovalbumin (OVA) test was used to evaluate the level of intestinal permeability in children with psoriasis
. Standard blood OVA-level before OVA-load (chicken egg proteins) is close to zero and it shouldn't exceed 1 ng/ml after 3 hours of OVA-load. Initial average OVA-level of 30 children was 1,13 ng/ml and average OVA-level after OVA-load was 15,5 ng/ml (maximum - 104 ng/ml). Average OVA-level in children with advanced psoriasis was 35,4 ng/ml, in children with stable psoriasis - 5,1 ng/ml.
OVA-permeability depends on disease duration in children with advanced psoriasis. It sharply increases for
first four months and then doesn't essentially vary. OVA-permeability decreased from 43,2 ng/ml to 23,1 ng/ml during treatment in patients with subacute psoriasis. There was no obvious correlation between OVA-permeability and psoriasis severity (index PASI).
In work in vitro the accelerated proliferation of enteric enterocytes has been shown. In research
there were 5 psoriatics (index of proliferation LI=20,26,29,36) and 5 patients from the control (LI = 13,17,23,26) at incubation time (0,5; 1; 2; 3 hours). That is probably caused by removing LPS to intestine from blood ( Such acceleration defines incomplete differentiation of enterocytes at renewal of epithelium and, as consequence, disturbance of trans-enterocyte permeability.
100 patients with only psoriasis were examined. Decrease of acid production in stomach in stimulated phase
was observed in 63% of patients . The more psoriasis duration the more was deterioration of D-xylose and fats adsorption in small intestine. Decrease of trans-enterocyte adsorption of monosaccharides (D-xylose, Mannitolum), as a rule, correlates with increased inter-enterocyte permeability for macromolecules, including F-content. 60% (33/55) of psoriatics and only 3% (2/65) of control group patients had low D-xylose absorption in other study . 2 patients with low D-xylose absorption suffered from celiac disease and 7 patients suffered from SIBO.
The subject of works was also relations between malabsorption syndrome (SM) and psoriasis
. SM grade can be measured in grams of D-xylose excreted with urine within 5 hours after oral taking. SM was diagnosed in 83 psoriatics and 20 persons of control group. It was rather lower in psoriatics (average value SM=1,0) in comparison with standard (average value SM=1,8). They found inverse relationship between SM and severity (PASI) and type of psoriasis: vulgar (SM=1,2, PASI=14), exudative or arthropat hic (SM=1,0; PASI=18), erythrodermic (SM=0,8; PASI=39). Also they found that the lower SM, the longer is disease duration. These results are represented in more details and with a larger group of psoriatics (103 patients).
Phytalbumin gluten is part of many graminoids. If the patient has predisposition (16% psoriatics) gluten has an
adverse effect on enterocytes. Villuses atrophy and permeability of intestinal walls increases . In average, level of IgA to tissue transglutaminase and gliadine in case of psoriasis is increased. Level of antibodies to gliadine was 14,8 (67 psoriatics) vs. 5,7 (85 – control group). This is a sign of latent celiac disease . It is likely to be the result of beneficial influence of gluten-free diet on psoriasis course in patients involved by this sign .
SP1 depends on SP2. Some intestine bacteria are capable to influence function of inter-
enterocyte transmission and transmission through LPS-influence and excrete toxins , . So increased inter-enterocyte permeability can be a direct result of composition of enteric microflora, especially in SIBO , including Gram(-)TLR4-active microflora.
SP1 depends on SP3. Inter-enterocyte transmission depends on quantitative and qualitative
composition of bile entering the intestine ). Chronic insufficiency of its entering interrupts barrier function of inter-enterocyte contacts and increases inter-enterocyte permeability, including for F-conten
SP1 depends on SP4. Significant rising of blood LPS level interrupts intestine barrier function
SP1 depends on SP5.1
2009-2012, Peslyak MY, Model of pathogenesis of psoriasis. Part 1. Systemic psoriatic process. e4.0a. 12
Subprocess SP2. Growth of populations of Gram(-) TLR4-active and
Gram+ bacteria on small intestine mucosa.
Subprocess is known; its influence on psoriasis was partly investigated. Subprocess, as a rule, takes place within the limits of SIBO (small intestine bacterial overgrowth)
). However, subprocess
SP2 may also not be accompanied with SIBO. Probably, the growth of parietal microflora frames conditions for SIBO.
Detailed researches of transient enteric microflora at psoriatics have been made for the first time
by authors of the following works
Their results have confirmed
the presence of serious dysbiotic deviations at BLC+ psoriatics (with blastocystosis) and at BLC(-) psoriatics (without blastocystosis). These results are given in detail in App. 6.
The information about blastocystosis and its role in the change of colic microflora can be found
in the following work
Within the limits of subprocess SP2 special subprocess SP2.1 takes place
SP2.1. Growth of populations of psoriagenic PsB
The results of work forced to reconsider the exclusiveness of BS
(beta-streptococci) offered in previous editions of this book, and to determine a more exact term, namely PsB - psoriagenic bacteria. For the definition of this term we will enter the following symbols:
IB-Y - interpeptide bridges of peptidoglycan Str.pyogenes: L-Ala(2-3) or L-Ala-L-Ser
p. 107-9). Bacteria Str.pyogenes are skin pathogens causing streptoderma, cellulitis, erypsipelas etc. (more than 100 million cases of skin forms of diseases annually) IB-Y - is marked in red
Y-antigen - part(s) of interpeptide bridge IB-Y.
PG-Y - peptidoglycan of type A3alpha , p. 396) containing interpeptide bridges of
type IB-Y (L-Ala (2-3) and-or L-Ala-L-Ser), but probably some others, too. The muropeptides formed at PG-Y degradation, contain entirely and/or fragmentary interpeptide bridges IB-Y. A good analysis of the set of such muropeptides is given in work
PsB - psoriagenic bacteria - Gram+ bacteria with peptidoglycan of type PG-Y are marked
in red color). All BS - the beta-streptococci having peptidoglycan PG-Y, also are PsB. The more share of interpeptide bridges of type IB-Y is in PG-Y, the stronger psoriagenity of PsB is. Only such PsB can show their psoriagenity, which are able to form stable colonies in such place of human body from which products of bacterial disintegration constantly get to blood flow. Tonsils and mucous small intestines are unfortunately the most suitable to this place.
nPsB – non-psoriagenic bacteria = Gram+ bacteria, or having PG type distinct from A3alpha, or
having PG type A3alpha, but without interpeptide bridges of type IB-Y.
The left part ofcontains the grouped information on Gram+ microflora from,
added with statistics on carriage of particular bacterial species from other works. The right part of this table contains the general statistics Gram+ SSTI (skin and soft tissue infections) among all hospitalized patients from the USA, France, Germany, Italy and Spain for 2001 . It is possible to assume that the role of particular bacterial species at SSTI among the population as a whole is similar.
2009-2012, Peslyak MY, Model of pathogenesis of psoriasis. Part 1. Systemic psoriatic process. e4.0a. 13
Tabl.1. Psoriagenic bacteria - priming and enteric carriage
Enteric carriage
SSTI shares = % of priming among
types of interpeptide
B.animalis subgr.
distinct from IB-Y
Enterococcus sp.
distinct from IB-Y
Gram+ bacteria with IB distinct from IB-Y
2,54 D-Asp (basically)
Gly(5-6); L-Ala -
2,70 Gly(4-5); Gly(2-3) -
Clostridium spp.
2009-2012, Peslyak MY, Model of pathogenesis of psoriasis. Part 1. Systemic psoriatic process. e4.0a. 14
Notes to Tab. 1.
CFU – colony-forming unit; nd - there is no data;
The information on interpeptide bridges bases mostly on but also was specified and completed from later works.
VGS = Viridans group streptococci, found both at SSTI and in intestine, are basically Str.mutans, Str.salivarius,
Str.sanguinis, Str.mitis, Str.anginosus , p. 299; p. 24). They have the peptidoglycans containing IB of type L-Ala(1-3) (but also others) Tab. 20 and 22; ab. 1).
CoNS = Coagulase-negative staphylococcus, found at SSTI, are basically S.epidermidis, S.lugdunensis,
S.haemolyticus, S.saprophyticus, S.p. 385).
# - the initialization and support of psoriasis is possible.
In the absence of the data in work on the percentage of carriage of bacterial species in small intestines, the data on carriage in excrements of healthy adults is presented:
[1] - to this specie belong 23% of the strains which have been found in the samples taken from 30 healthy adults
[2] - it is found in 44 of 48 (92%) samples at ; [2, 3] - p. 327); [4] - to this specie belong 4% of the strains which have been found on mucous large intestine at 30 healthy adults
[5] - Enterococcus spp. in samples of adults (29 persons) from . The quantity of E.faecalis as a rule
exceeded 10-100 times the quantity E.faecium.
[6] - Estimated calculation was performed. Enterococcus spp. are found at 65% of psoriatics
and E.faecium - at 25% of psoriatics but the exact data on E.faecalis is absent. As as a rule there is simultaneous carriage of both E.faecalis and E.faecium, while E.faecalis itself is apparently found at more than 40% of psoriatics (65-25=40).
If in the term prior to the occurrence of psoriasis:
firstly, there occurs Gram+ priming of dermal immune system by one of species of
psoriagenic bacteria (during SSTI, Tab.1 columns) and, as consequence, the pool of mTL-Y is
formed in priming places and also in derma and epidermis, and
secondly, on the mucous of small intestines one or several species of psoriagenic bacteria
form significant colonies (lines of Tab. 1),
there appears a possibility of initialization and support of psoriasis (corresponding cells of
Tab. 1 are noted # ).
Whether this possibility becomes reality or not - depends on the intensity of the SPP
psoriatic process as a whole and on the genetic predisposition. Particular places of
plaques are defined by local processes. How exactly? Assumptions will be made in .
Other species of streptococci also have peptidoglycan PG-Y: Str.pneumonia, Str.thermophilis,
Str.equi, Str.dysgalactiae, Str.equisimilis, Str.zooepidemicus Tab. 20 and 22), however they are practically not found in GIT.
There is a probability to find in GIT the representatives of order Lactobacillales (Weissella spp.,
Leuconostoc spp.), part of which contain peptidoglycan PG-Y p. 274-5) or representatives of family Micrococcaceae (Micrococcus spp., Arthrobacter spp., Kocuria spp., Rothia spp., Stomatococcus spp.), part of which are also containing peptidoglycan PG-Y p. 947, 965, 979).
contains the list of cores PsB, but in each specific case of psoriasis it is necessary to define
not only E.faecalis, VGS and Str.pyogenes and the listed species Bifidobacterium spp., but also to carry out search of other significant colonies of PsB in small intestines.
It is necessary to notice that in work transient microflora was investigated,
therefore it is only possible to make an indirect conclusion on the condition of parietal microflora. I.e. the absence of significant colonies of particular species in transient microflora most likely means the absence of its significant colonies also in parietal one and, on the contrary, detection of significant colonies of particular species in transient microflora means the probability of presence of its significant colonies also as a part of the parietal. But the absolute value (CFU/ml) may differ essentially.
On the other hand there are indirect and/or direct evidences of PsBP presence in skin and/or bloods
in the absence of local PsB-infections in psoriasis and psoriatic arthritis . However the authors didn't think that intestine
2009-2012, Peslyak MY, Model of pathogenesis of psoriasis. Part 1. Systemic psoriatic process. e4.0a. 15
microflora is potential source of PsBP, so they didn't investigate it. Long-term PsB-infection with subsequent long persistence and/or deposition PsBP for example, in tonsillar tissue or directly in skin, was usually supposed. The authors of work were the first, who supposed that psoriagenic BS can be a part of intestine microbiocenosis.
In addition about SP2.
Enteric Gram(-) bacteria are E.coli, Bacteroides spp., Proteus spp., Acinetobacter spp.,
Klebsiella spp., Moraxella spp.
and also Pseudomonada aeruginosa, Enterobacter spp., etc.
The role of particular Gram(-) bacteria in creation of chronic LPS-load (SP4) defines their activity in relation to human phagocytic receptor TLR4. TLR4-activity, in particular, is defined by structure of lipid A (fragment LPS), and namely by phosphatic groups, length and number of acylic chains. So, for example, LPS of E.coli (six acylic chains in lipid) have the greatest TLR4-activity, and LPS of Bacteroides spp. and Pseudomonada aeruginosa (five acylic chains in lipid) activates TLR4 substantially less. Also the interaction of particular LPS with LBP, CD14 (membranous and soluble) and with coreceptor MD2, necessary for effective TLR4-activation , The growth of colonies of Gram(-) TLR4-active bacteria on small intestine mucous defines income growth of F-content with TLR4-active LPS in blood flow.
Some influence of Gram(-) on psoriasis was proved in works ,
Apart from bacteria entering small intestine together with nutrition (more often transit), there are
two more constant sources of enteric populations: microflora of upper respiratory tracts moving in the case of interruption of stomach-acid barrier and microflora from distal parts moving in the case of interrupted peristalsis . Growth of enteric populations confines by: antibacterial action of bile acids (SP3); acidity of gastric juice ; peristalsis providing fast moving of microorganisms to distal parts ; release of immunoglobulins; enzymatic activity; state of intestine epithelium and slime excreted by goblet cells (it contains inhibitors of microorganism growth).
Indirect evidence of PsB presence in upper parts of small intestine is their pathogenic presence
in gallbladder microflora at its various diseases. In unsterile bile Streptococcus spp. are present in 15% of cases, E.coli – in 20%, Enterococcus spp. – in 18% , .
Interesting results were received in study . The authors supposed that chronic subclinical
streptococcal infection (obscure localization) is responsible for chronic psoriasis. 30 patients with moderate-to-serious psoriasis were examined. The majority of patients have been suffering from psoriasis for more than 5 years (21/30). They were treated with various methods without essential recover and they had frequent recur rences. Initial ASLO titer more than 200 IU/ml was found in 15 patients; positive reaction to C-reactive protein in culture of smear from fauces: (2 - GAS; 6 - Str. viridians) was found in 7 patients. Studies lasted two years. The patients were administered benzathine penicillin (1,2 mln U) i/m every fortnight for the first 24 weeks. During 25-48 weeks they were administered benzathine penicillin (1,2 mln U) every month. Marked recover was observed after 12 weeks (average initial PASI – 32,7; after 12 weeks – 19,1; after 24 weeks – 8,7; after 36 weeks – 3,5; after 48 weeks – 1,5). Patients were followed up for 2 years and all of them had remission.
The same group of researchers studied the effect of long oral reception of azithromycin. 30
of 50 patients (with psoriasis severity from moderated to serious) received azithromycin during 24 courses (two weeks each course). For 4 days - oral dose of 500 mg unitary, then 10 days break, i.e. totally 48 weeks. The other 20 patients received tablet of vitamin C. Appreciable recovery by PASI index has been detected from the 12th week at the majority of the patients receiving azithromycin. In the end of the 48th week, 18 patients (60%) had excellent recovery, 6 patients (20%) – good, and 4 patients – moderate. PASI 75 (decrease of not less than 75% from the initial level) was 80% (i.e. it was observed at 24 patients). No essential changes have been found in the control group.
2009-2012, Peslyak MY, Model of pathogenesis of psoriasis. Part 1. Systemic psoriatic process. e4.0a. 16
SP1 and SP2 should be considered together because their combination does influence SP4. In
particular, SP4 can be observed at significant SP1 level and insignificant SP2 level and inversely.
Possible influence of changed intestine microflora on immune system at various diseases
(microflora hypothesis) is analyzed in works Subprocess SP2 in SPP plays a main role, that corresponds to the given hypothesis.
SP2 depends on SP3. Bile has bactericidal properties to many non-commensal enteric bacteria
and ability to inactivate F-content or to degrade it to non-toxic fragments thereby reducing level of entering the blood . Decrease of production or quality of bile and/or irregularity of its secretion result in decrease of bile bactericidal properties. It promotes bacterial growth in small intestine
SP2 depends on SP5.1. SP2 depends on SP6. PsB (streptococci, enterococci and bifidobacteria) are facultative
nonpathogenic inhabitants of small intestine mucosa , . Some of them move from oral cavity and fauces mucosa (where they are commensals) to upper parts of small intestine. They can also move in case of gingivitis ) or tonsillar infections
Subprocess SP3. Disturbance of production and/or circulation of bile acids (BA).
Subprocess is well-known. It was investigated in patients with psoriasis ,
. Subprocess SP3 is essential part of vicious cycle (letter A) because it directly influences SP1 and SP2.
Disturbance of enterohepatic circulation can be a result of weakening of hepatic function of
extraction and conjugation of BA from portal blood. So some part of BA constantly enters peripheral blood. BA pool is reduced, if liver possibilities on BA generation are limited. So the level of BA in bile is low. High blood BA level can be toxic for tissues and cause rising of permeability of membranes and local inflammation. Cholanic acid derivatives can interrupt integrity of blood vessel walls, increase their permeability and dilate lumens of vessels of derma papillary layer (i.e. to influence rate of entering tissues by phagocytes).
SP3 depends on SP5.2. Chronic diseases or congenital defects of hepatobiliary organs result in
depression of BA production. Obstructive jaundice or gallbladder excision completely stops their income to intestine. Chronic overload of liver by F-content recycling also reduces BA production.
2009-2012, Peslyak MY, Model of pathogenesis of psoriasis. Part 1. Systemic psoriatic process. e4.0a. 17
Subprocess SP4. PAMP-nemia. Increased kPAMP-load on blood phagocytes.
Increased kPAMP level in blood.
The major kPAMP are PG and LPS.
Subprocess is well-known for LPS (including in psoriasis). But there were only few studies on
PG and none on psoriasis . Note that in work ) the aim of current investigations was announced: to define both: PG-level and frequency of PG+Mo in the blood of psoriatics.
Previously endotoxins were considered as any products of bacterial degradation (as against
exotoxins - toxic secreted products of bacterium vital activity). But now term "endotoxin" means LPS, term "endotoxemia" means increased blood LPS level and term "endotoxicosis" means increased LPS-load on phagocytes . LAL-test is used for measuring blood free LPS level. Standard values are in range from 0 to 1 Eu/ml (0,1 Eu/ml or 10 pg/ml on average). It depends on rate of LPS entry from intestine into portal blood and quality of LPS-elimination made by hepatobiliary system (up to 95% of LPS are destroyed by system of hepatic macrophages before they enter systemic blood flow and excreted with bile). Additionally it depends on porto-caval shunts (portal blood entry in systemic blood flow, i.e. not through liver) and activity of antiendotoxic immunity .
There are data which allow supposing that obligate kPAMP in psoriasis is PG (including PG-Y)
PAMP-nemia and endotoxinemia have the same cause. The cause is combined action of SP1 and SP2. Besides, overload and/or disturbance of detoxication systems (SP5) influence its level. Thus, detoxication systems do not have enough time to eliminate excess volume of F-content entering the blood, or they are weakened because of diseases and they don't stand usual load. It is important that kPAMP-load increases to such extent, that it results in reprogramming of significant percent of blood phagocytes. Chronically increase results in their tolerance to kPAMP-load . Actually PAMP-receptors (TLR4 in LPS-load, TLR2 and NOD2 in PG-load) of tolerized phagocytes are temporarily blocked .
PAMP-load on blood phagocytes increases while blood PAMP-level raises slowly during initial
stage of PAMP-nemia. PAMP-load can be critical for psoriasis possibility at this stage. PAMP-consumption (phagocyte-dependent and phagocyte-independent) can't stand PAMP-income at the second stage. Blood PAMP-level becomes even higher and PAMP-level can be critical for psoriatic arthritis possibility.
Endotoxinemia can accompany any Gram(-) infections and become the cause of many systemic
diseases , but not psoriasis. LPS-load on phagocytes influences volume and F-content composition (SP8) and, as a result, influences the volume and types of cytokines secreted after possible loss of tolerance (deprogramming) from R-phagocytes in tissues, including because of synergy between LPS and PG . Although TLR4 is membranous receptor, but linkage with LPS and subsequent endocytosis make complex TLR4-LPS able to enter endosomes and cell ). However LPS-load itself can't provide local psoriatic process, so LPS has aggravating effect in Y-model of pathogenesis.
Severity of PAMP-nemia also can be estimated on shares of fractions of tolerized Mo-T and
DC-T. Than more these shares are - the more severity of PAMP-).
Within the limits of subprocess SP4 special subprocess SP4.1 takes place
2009-2012, Peslyak MY, Model of pathogenesis of psoriasis. Part 1. Systemic psoriatic process. e4.0a. 18
SP4.1. (PG-Y)-nemia.
(PG-Y)-nemia is called raised (PG-Y)-load on blood phagocytes in combination to increased level
PG-Y in blood. All populations of Gram(+) and Gram(-) bacteria (not only PsB) of zones of hyperpermeability of intestine mucosa, define total PG-income into blood and total PG-load on phagocytes. But only growth of PsB populations (SP2.1) is at the bottom of (PG-Y)-nemia.
Existence of fractions of tolerized Mo-T and DC-T is necessary, but not sufficient condition of
occurrence of subfractions Mo-R and DC-R (SP8.1). Only essential level of chronic (PG-Y)-load can entail the occurrence of such subfractions, and also provide necessary level of total (PG-Y)-carriage of Mo-R and DC-R for possible initialization and support of psoriasis. SP4 depends on SP1 and SP2. If there are no local and/or systemic bacterial infections the main
source of F-content entering the blood is the microflora of small intestine mucosa.
SP4 depends on SP5. Normal state of detoxication systems inhibits PAMP-nemia increase and,
on the contrary, their diseases and/or overload promote its increase. Complex therapy of psoriasis always includes investigation of hepatobiliary organs, kidneys and urinary tract and their treatment if it is required.
SP4.1 depends on SP2.1. (PG-Y)-nemia severity is proportional to growth of PsB-populations on
small intestine mucous.
SP4.1 depends on SP6. Tonsillar PsB-infection (as well as any other local PsB-infection) results
in temporary (PG-Y)-nemia: temporary increase of (PG-Y)-level and (PG-Y)-load.
2009-2012, Peslyak MY, Model of pathogenesis of psoriasis. Part 1. Systemic psoriatic process. e4.0a. 19
Subprocess SP5. Overload and/or disorders of detoxication systems.
Subprocess is well-known . Detoxication systems role in
psoriasis was described earlier ; also Taking into account various influence of the components of the given subprocess on other subprocesses, we will allocate disorders in intestine (SP5.1) and in hepatobiliary system (SP5.2):
SP5.1. Intestine
Researches show that functional and structural disorders in intestine aggravate psoriasis, and
the combined treatment aimed at normalization of its functioning lead to good and long-term results
helminthiasis aggravates the current of psoriasis, in particular, opisthorchiasis
Successful treatment of helminthiases and/or parasitic
diseases and correction of dysbiotic deviations lead to much more successful and stable results in psoriasis treatment.
SP5.2. Hepatobiliary system
Chronic endotoxinemia in psoriasis
can result in dysfunction of liver.
Dysfunction severity depends on its level, duration and concomitant diseases
organic pathology of biliary tract and/or its functional disorders aggravate psoriasis, and cholestasia degree correlates w
It is well known that liver diseases aggravate psoriasis and make its treatment more
complicated. For example, symptoms of non-alcoholic fatty liver disease (NAFLD) have been found at 47% of psoriatics (of 130), while in the control group of healthy - only at 28% (of 260). Psoriatics with NALFD symptoms have higher PASI (14,2 against 9,6), than psoriatics without NALFD symptoms
). The review of works with similar results has been published recently
should be mentioned that implication of NALFD can correlate with the disturbance of circulation and transportation of bile aci
In addition about detoxicating
Percent of LPS linkage in liver decreases from 90-95% (in healthy patients) to 24% in patients with obstructive
jaundice. Experimental study on influence of endotoxinemia on liver and kidneys of experimental rats is described in monograph . Increscent irreversible changes in structure and function of these organs caused by increase of endotoxin injection dose (LPS E. coli) were studied.
The authors of work showed that enterocytes and colonocytes are important factors of LPS
excretion from organism. The process was simulated and studied in details on experimental rats. Rats were intravenously injected high dose of LPS. Previously excretion of LPS absorbed by Kupffer cells of liver along with bile to intestine was studied. Authors of the same work also studied the mechanism of LPS excretion (free or absorbed by macrophages) to intestine by translocation through own plate of mucosa (lamina propria) and basolateral basis directly to enterocytes (colonocytes). Enterocytes (colonocytes) moved from crypts to the top of villus after 4-5 days. They are exfoliated, taking collected LPS away to intestine lumen. Translocation of LPS injected in intestine to the blood was investigated. The results showed that translocation isn't made through enterocytes or inter -enterocyte space.
SP5 depends on SP4. PAMP-nemia provides constant load on all detoxication systems.
2009-2012, Peslyak MY, Model of pathogenesis of psoriasis. Part 1. Systemic psoriatic process. e4.0a. 20
Subprocess SP6. Tonsillar PsB-infection
Subprocess is well-known; it was repeatedly investigated for BS in psoriasis. Work
represents the modern detailed review of the facts and assumptions of the influence of streptococcal infection on psoriasis.
Tonsillar PsB-infection (as well as other local PsB-infection) provides temporary, but significant
PsBP (including PG-Y) entry in blood. The detailed analysis of these events for BS-infection may be found at )
208 patients with plaque psoriasis were observed for a year. Researches took swabs from fauces in the
beginning of follow-up and during each aggravation of psoriasis or throat inflammation (61 cases). 20,7% of patients (percent corresponds to average value) were asymptomatic carriers of BS with M-protein of A, C and G groups. BS provoked throat inflammation with subsequent aggravation of psoriasis in 9% of cases if inflammation lasted not less than 4 days. BS didn't cause throat inflammation and didn't provoke psoriasis aggravation in 20% of cases
Questionnaire of 74 psoriatics who underwent tonsillectomy has shown that within 4.5 years after this
operation, one third of psoriatics had full purification of skin, and another third of psoriatics had significant remission Three patients recovered from psoriasis after tonsillectomy. Identical TL-receptors of TL in tonsils and psoriatic plaques was previously shown . The authors of review work carried out analysis of influence of antibiotics and tonsillectomy on psoriasis course. They concluded that despite variety of positive results additional control studies are needed.
Interesting results have been received in work . It has been proved that L-forms of bacteria (i.e.
bacteria at which there cellular wall is partly or completely absent) are defined in smear from fauces at 74% (92 of 124) psoriatics (65 - with guttate, 59 - with chronic), only at 24% (19 of 81) patients only with adenoid disease and at 6% (5 of 79) healthy of control group. L-forms Gram+ and Gram(-) bacteria have been found, at 79% (73 of 92) psoriatics dominant species were Gram(-) bacilli (Chryseomonas luteola, Burkholderia cepacia, Enterobacter cloacae etc.), and at 21% (19 of 92) psoriatics Gram+ coccuses, mainly Str.pyogenes, were dominant species, After treatment by antibiotics only 9% of psoriatics still had carriage of L-forms of bacteria. These authors have not published the data about the correlation of the condition of psoriasis with carriage of L-forms of bacteria yet, neither about condition of patients before and after treatment .
About 30% of patients with primary guttate psoriasis recover spontaneously. However it
regenerates to chronic plaque psoriasis at once or after remission in 70% of cases Probably tonsillar PsB-infection causing primary guttate psoriasis, also becomes a source of stable PsB-populations in upper parts of small intestine (SP2). It can result in development of chronic psoriasis. Chronic psoriasis aggravation during tonsillar PsB-infections is caused by because significant additional PsBP (including PG-Y) entry in blood.
The authors of work discussed preventive streptococcal vaccination for risk groups
(genetic or family signs) if CPs hasn't yet begun.
2009-2012, Peslyak MY, Model of pathogenesis of psoriasis. Part 1. Systemic psoriatic process. e4.0a. 21
Subprocess SP7. Deviation in intracellular signal path "from MDP recognition
through NOD2-ligand to chemostatus change" (<1%).
MDP is PG fragment formed after its intracellular degradation. MDP is the main ligand of
intracellular receptor NOD2 ). "Wrong" blood phagocytes appear when intracellular signal path of bone marrow stem MoDP (and blood Mo and DC) is weakened or interrupted: from contact between MDP and NOD2 to cell chemostatus change . DNA changes cause congenital or acquired deviation (as a result of mutagenesis).
"Wrong" phagocyte without chronically increased PG-load acts as tolerized phagocyte because
endocytosis small volume of PG doesn't change its chemostatus. If MoDP has such deviation psoriasis can develop at low (PG-Y)-load as formed in blood flow PG-Y(+)Mo and PG-Y(+)DC can keep nonactivated chemostatuses and participate in subprocess LP1.1.
Deviation disappears after allogenic transplantation of bone marrow when MoDP (stem
precursors DC and Mo) are replaced. But transplantation is made for more serious indications than psoriasis. Blood level of "wrong" phagocytes of recipient decreases and blood level of normal phagocytes of donor increases.
Course of serious disease which was the cause of transplantation is relieved and psoriasis
subsides forever or until the moment when kPAMP-load (SP4) and/or (PG-Y)-load (SP4.1) will increase and SP8 will begin.
The authors of work reported 8 patients with long and full remission of psoriasis after
allogenic bone marrow transplantation. They also reported that psoriasis or psoriatic arthritis developed in recipients after similar transplantation from donors with these diseases. The authors of work described 3 similar patients with serious psoriasis and leukemia. The authors consider that remission of these diseases was due to elimination of genetic deviations, including chromosomal instability and defect of DNA reparation of hemopoietic cells.
50000 bone marrow transplantations are made in the world every year; about 30% of them are allogenic (data
of 2002). Prevalence of psoriasis among recipients is 2-3% (not low than in population). If each allogenic bone marrow transplantation result in remission of psoriasis the number of such cases would be not less than 300-450 in a year. It wouldn't remain unnoticed, but such cases are single (less than 1%). Therefore genetic deviations of cells of bone marrow origin aren't the cause of psoriasis in overwhelming number of cases.
NOD2-mutations provoke susceptibility to Crohn's disease . NOD2-mutations can be the cause
of increased risk (more than 10%) of psoriasis development in patients with Crohn's disease .
In work statistically significant differences (P=0,003) for intracellular protein IRAK-1 in
rs3027898 polymorphism between patients with psoriatic arthritis (29 people) and the control group of healthy (66 people) have been found for the first time.
In the following works are described recently found some significant differences
for protein A20 (=TNFAIP3) and binding it protein TNIP1 in polymorphisms rs610604 (OR=1,19, p = 9*10−12) and rs17728338 (OR = 1.59, p =1*10−20) between psoriatics (more than 5000) and control group of healthy people (more than 5000). Normally, interaction of these proteins blocks signal paths TRAF6, RIP1 and RIP2 for prevention of the superfluous inflammatory response The found out differences may promote increase in share of blood Mo-R and DC-R at the same level of PAMP-load that raises probability of occurrence and support of psoriasis.
2009-2012, Peslyak MY, Model of pathogenesis of psoriasis. Part 1. Systemic psoriatic process. e4.0a. 22
Subprocess SP8. Growth of tolerized fractions Mo-T and DC-T.
Increased kPAMP-carriage.
This subprocess (incl. SP8.1) is the final stage of systemic process SPP and it is formulated
here for the first time. Chronically increased kPAMP-load provides tolerization (reprogramming) of some Mo and DC. They stop to recognize F-content (fragments of bacterial products containing PAMP, including kPAMP) as pathogenic material and become F-carriers.
Mo-T and DC-T are abbreviations of tolerized Mo and DC.
Assumption 1: Tolerized Mo-T and DC-T continue to participate in renewal of pool of tissue
MF and DC (LP1.1), degrading F-content slowly and incompletely.
Why can it happen? Nonactivated Mo and DC under temporary kPAMP-load activate, secrete
more TNF-alpha and their chemostatuses temporarily undergoes significant changes . However activated Mo and DC tolerize and almost stop secreting of TNF-alpha under chronically increased PAMP-load. So, may be chemostatuses of tolerized Mo and DC became similar to nonactivated?
Assumption 2: Chemostatuses of Mo-T and DC-T are similar to chemostatuses of
nonactivated Mo and DC.
Possible intracellular signal paths from PAMP-receptors to TNF-alpha secretion and
CCR7-expression are given in Appendix:
Transformation of certain nonactivated Mo in tolerized Mo-T (and, in particular, in Mo-R)
depends on time of stay in systemic blood flow under chronic PAMP-load. At first the small number of occurrings with F-content converts nonactivated Mo into activated, and then the subsequent occurrings convert activated Mo into tolerized Mo-T
The more certain Mo (or DC) meets F-content, the more of formed intracellular protein IRAK-M
occures. IRAK-M temporarily blocks intracellular signal paths going from concrete PAMP-receptors (TLR4 at LPS-load, TLR2, NOD1 and NOD2 at PG-load) .
The share of fractions tolerized Mo-T and DC-T in blood flow is proportional to total kPAMP-load
on Mo and DC. This kPAMP-load takes place in blood flow (SP4) and, possibly, in bone marrow (SP10).
Within the limits of subprocess SP8 special subprocess SP8.1 takes place
SP8.1. Growth of subfractions Mo-R and DC-R. Increased (PG-Y)-carriage.
Subprocess SP8.1 occurs only if SP4.1 operates, i.e. when as a part of kPAMP-load on blood
phagocytes (SP4) is (PG-Y)-load.
Mo-R is PG-Y(+)Mo-T. Similarly DC-R is PG-Y(+)DC-T. Thereby Mo-R and DC-R are subfractions
of fractions of tolerized Mo-T and DC-T. Mo-R and DC-R can be also carriers of others PAMP (besides PG-Y). Total (PG-Y)-cariage of Mo-R and DC-R in blood flow is proportional to:
(a) shares of fractions tolerized Mo-T and DC-T and (b) total (PG-Y)-load on Mo and DC in blood flow (SP4.1) and, possibly, in bone marrow (SP10). SPP severity is proportional to total (PG-Y)-carriage of Mo-R and DC-R in blood flow. Total (PG-Y)-carriage should be normed on total amount of blood of the patient. Such norming will
allow to compare SPP severity at psoriatics of various weight. I.e. SPP severity is proportional to total (PG-Y)-carriage of Mo-R and DC-R in one ml of blood. Y-antigen is part of the interpeptide bridges IB-Y necessarily containing in PG-Y.
Therefore SPP severity also is proportional to total Y-carriage of Mo-R and DC-R in one ml of blood.
2009-2012, Peslyak MY, Model of pathogenesis of psoriasis. Part 1. Systemic psoriatic process. e4.0a. 23
In addition about SP8.
According to part of PG+Mo among total quantity not more than 5-10% of blood
monocytes are Mo-R in psoriasis. Blood CD14+CD16+Mo are more likely to be reprogrammed. Possible sequence of events leading to tolerization (reprogramming) and formation of CD14+CD16+Mo-R is given at scheme.
It is supposed that as a part of kPAMP-load is (PG-Y)- Let S2 = {CCR1, CCR2, CCR5, CXCR4} - assortment of the chemokine receptors
CD14+CD16+Mo which is responsible for attraction in tissue.
Then S2+ = {CCR1+, CCR2+, CCR5+, CXCR4+} - at inactive or at tolerized chemostatus, and
S2(-) = {CCR1(-), CCR2(-), CCR5(-), CXCR4(-)} - at active chemostatus.
Normal CCR7(low)S2+Mo has chances to accept kPAMP-load many times (1) because low
CCR7 expression ambiguously influences its action. It can bring F-content to lymph node (2) or it can degrade F-content, remaining in blood and return to initial chemostatus (3). However the level of intracellular blocking protein IRAK-M will slightly increase.
If subsequent kPAMP-load takes place in the near future each cycle of transformations (1,3) will
increase IRAK-M level. (IRAK-M level gradually decreases in the absence of kPAMP-load). As soon as IRAK-M level becomes blocking, transformation (4) to F+CCR7(-)S2+Mo-R will take place. Such Mo-R is mainly attracted to non-lymphatic tissues (5).
Monocytes CD14+CD16+Mo has lower CCR7 expression than CD14++Mo does, but high
potential of phagocytosis remains . They well express CCR2, CCR5, CXCR4 and CX3CR1 , most actively secretes TNF-alpha and iNOS and also is more capable (in comparison with CD14++Mo and CD14(-)CD16+Mo) to be quickly transformed (without division) to MoDC . CD14+CD16+Mo-R seem to be precursors of BDCA-1(-)TipDC (90% of total number of TipDC )).
This assumption correlates with data that many inflammatory BDCA-1(-)DC in psoriatic derma
coexpress monocytic markers CD14 and CD16. These markers can remain during fast transformation CD14+CD16+Mo into BDCA-1(-)DC after their attraction into derma from blood flow ).
The results of transcriptome of skin BDCA-1(-) DC research have shown the increased level of
CCR2, CD14, CD64 that quite corresponds to such assumption The essential expression of CD14 available at BDCA-1(-)DC, makes it improbable that they originate from blood DC which practically do not express this receptor On the other hand assumption of their origination from CD14++Mo, which practically lose FPRL1 receptor at the transformation in MoDC, is
Blood CCR2+Mo are precursors of TipDC during intracellular Gram+L.monocytogenes-infection
. Mo (and their derivatives MoDC) are activated only through intracellular receptor NOD2. Similar activation is likely to arise during transformation PG+Mo-R in MF-R and in MoDC-R.
Transformation (activation or even tolerization) of Mo and DC can begin during their
hemopoiesis in bone marrow (SP10). Senescent neutrophils Neu-R coming back to bone marrow to perish (SP9) Neu-R can bring in bone marrow F-content endocytosed in blood. F-content is situated in extracellular space after their apoptosis Reprogramming of bone marrow phagocytes finishes in 1-3 hours after LPS-load Details are given in Appendix.
If SP7 operates, all Mo and DC can be considered reprogrammed. Endocytosis PG-Y (within F-
content) R-phagocytes increase (PG-Y)-carriage level. (PG-Y)-load should be increased in case of SP4 and can be episodical in case of SP7 to make total (PG-Y)-carriage significant. Sufficient rate of (PG-Y)-entering inside Mo-R and DC-R into derma will be supported for initialization and maintenance of psoriasis only under these conditions.
2009-2012, Peslyak MY, Model of pathogenesis of psoriasis. Part 1. Systemic psoriatic process. e4.0a. 24
When Mo-R and DC-R enter tissues their action also essentially differs from action of normal Mo
and DC. They are «delayed-action mines» because they contain non-degraded PG-Y
Tolerance of R-phagocytes to the major kPAMP (e.g. LPS) can cause tolerance to minor kPAMP
(e.g. flagellin) if intracellular signal path of minor kPAMP completely coincides with one of such ways of the major kPAMP . I.e. endocytosed material can contain not only major kPAMP, but also minor kPAMP and chemostatuses of tolerized phagocytes became similar to nonactivated also
Blood tolerized Mo-T and DC-T chronically contact and endocytose LPS (free or bound). Their
chemostatuses became similar to nonactivated and they don't bring endocytosed content to lymph nodes and/or spleen. Thereof level of T-independent humoral immune response to LPS decreases
Lowered expression HLA-DR at blood monocytes is a sign of immune supression. It happens because of their
reprogramming, for example during CARS chapter 15). Expression HLA-DR also is lowered and at psoriatic Mo (it is essential at CD14++ Mo and slightly less at CD16+Mo) . The expression is restored by an incubation of monocytes with IFN-gamma.
And as IFN-gamma is cytokine-deprogrammer these results confirm reprogramming of psoriatic Mo.
Hypersensitivity of peripheral blood Mo to low LPS concentration results in intensive secretion of TNF-alpha, IL-1beta and IL-6 in psoriatics (in comparison with healthy people) .
Blood psoriatic Mo spontaneously secreted 2-4-fold levels of IL-1alpha, IL-1beta, IL-6 and IL-8
. They reached maximum after 48 hours . Blood psoriatic Mo spontaneously secreted the following quantities of cytokines (after 20 hours in supernatant): in patients with serious psoriasis - TNF-alpha (150 pg/ml) and IL-6 (184 pg/ml), in patients with moderated psoriasis - TNF-alpha (63 pg/ml) and IL-6 (30 pg/ml) against in healthy people - TNF-alpha (37 pg/ml) and IL-6 (24 pg/ml)
Blood Mo have similar status in atopic dermatitis when 2-10-fold levels of IL-6, IL-10, TNF-alpha are
spontaneously secreted . PAMP-load (especially under PG-load) always results in increase of part of blood CD14+ Mo (in psoriatics - 90-99% against in healthy people - 85%) .
The facts listed above mean that blood Mo at psoriasis basically are activated. However it does
not exclude existence of other fractions of monocytes, as, in particular tolerized fraction (including subfraction Mo-R).
Treatment for psoriasis with MDP-immunomodulators - licopid and GMDP -
gives positive results. They activate monocytes and neutrophils, increase their englobing and destroying properties, microbicidal function . Since MDP is PG-fragment kPAMP-load on blood Mo and DC significantly increases after MDP-immunomodulator taking (half-life - 4,3 hours) in comparison with previous chronic PG-load. It activates not only usual Mo and DC, but part of already tolerized Mo-T and/or Mo being tolerized . Reprogramming stops for a while and/or completely and all Mo and DC degrade F-content faster. Increase of blood level of cytokines-deprogrammers IFN-gamma, IL-12, GM-CSF, also caused by MDP, prevents tolerization As a result part of reprogrammed Mo-R and DC-R and F-content volume decreases. According Y-model this process causes psoriasis remission.
HIV (human immunodeficiency virus) is known to increase risk of development of serious and extended
psoriasis . TLR2-receptor activation increases CCR5-expression in HIV+Mo more, than in normal Mo . Taking into account increased CCL5 level in psoriatic skin in comparison with prepsoriatic (where it is also increased) it is possible to assume that increased PG-load on HIV+Mo results in more active attraction of HIV+Mo to prepsoriatic and/or psoriatic skin (including Mo-R). On the other hand HIV-infection significantly changes proportions of monocyte fractions, in particular 40% of monocyte pool is CD14(low)CD16(high) fraction (while in healthy people it doesn't exceed 15 -20%) . Abovementioned assumption that CD14+CD16+Mo are the most probable precursors of Mo-R makes clear increased risk of psoriasis initialization and severity in HIV+ patients. Increase of fraction of potential precursors CD14+CD16+Mo raises fraction CD14+CD16+Mo-R .
In overwhelming majority of cases subprocess SP7 is not present. For total (PG-Y)-carriage
becoming significant, (PG-Y)-load should be chronically increased (share of Mo-T and DC-T is insignificant). However, if SP7 operates (and shares of Mo-T and DC-T are conditionally possible to assume as equal to 100%), it is weak or incidental (PG-Y)-load enough for support of SP8.1.
Rate of (PG-Y)-entry (inside Mo-R and DC-R) into derma is the main factor of psoriatic plaque
2009-2012, Peslyak MY, Model of pathogenesis of psoriasis. Part 1. Systemic psoriatic process. e4.0a. 25
SP8 depends on SP4. The share of fractions Mo-T and DC-T depends on kPAMP-load level in
SP8.1 depends from SP4. The share of subfractions Mo-R and DC-R depends on kPAMP-load
level in blood flow.
SP8.1 depends from SP4.1. The share of subfractions Mo-R and DC-R and their level
(PG-Y)-carriage depend on level of (PG-Y)-load in blood flow. Really share of fractions PG-Y(+)Mo and PG-Y(+)DC and their level of (PG-Y)-carriage obviously depend of SP4.1 And as subfractions Mo-R and DC-R are crossing of tolerized fractions and fractions PG-Y(+)Mo and PG-Y(+)DC, so their share depends on the size of each of crossed fractions.
SP8 depends on SP7 (instead of dependence on SP4). Deviation grade in functioning of
intracellular signal path influences level of «tolerization» of blood Mo and DC.
SP8 depends from SP10. Truly, if there exists relay vf fractions Mo-T
and DC-T depends on level of bone marrow kPAMP-load. Total (PG-Y)-carriage of Mo-R and DC-R depends on level of bone marrow (PG-Y)-load.
2009-2012, Peslyak MY, Model of pathogenesis of psoriasis. Part 1. Systemic psoriatic process. e4.0a. 26
Local subprocess LP1.1. Attraction of Mo and DC,
Mo-T and DC-T (incl. Mo-R and DC-R) from blood.
Homeostatic or inflammatory renewal of pool of dermal macrophages MF and dendritic cells of
non-resident origin.
Moving to derma, only Mo-R and DC-R can transform to mature maDC-Y, presenting unknown
Y-antigen to specific TL-Y derma). According this antigen is a part of interpeptide bridge (IB) of BSPG. According this antigen is a part of BSMP.
Here it is supposed that Y is part of interpeptide bridge IB-Y of psoriagenic bacteria PsB (in
particular some of BS). These transformations depend on type of derma: psoriatic or prepsoriatic ).
In renewal of pool of dermal macrophages MF and dendritic cells DC of non-resident origin are
participating all Mo-T and DC-T. Having arrived in psoriatic derma Mo-T and DC-T appear under the influence of cytokines-deprogrammers, so they lose tolerance and secrete proinflammatory cytokines, chemokines and AMP and, thereby, promote aggravation of psoriatic inflammation. Thus Mo-T will be transformed in MoDC-T or in MF-rma).
Activity of such secretion is defined by quantity and assortment of kPAMP (except PG-Y) which
carriers they (and cells derived from them) are.
At the description of subprocess SP8.1 it is offered to estimate SPP severity through total
(PG-Y)-carriage of Mo-R and DC-R in one ml of blood. To consider influence of secretion of Mo-T and DC-T, caused by others kPAMP (except PG-Y), it is necessary to apply raising factor. This factor will be proportional to total kPAMP-carriage (except PG-Y) of Mo-T and DC-T in one ml of blood. And summation should be lead with correction multipliers for each of kPAMP, depending on their activity.
Such correction of SPP severity considers all kPAMP (and not just PG-Y) that corresponds to
direct correlation (R=0,46) between degree of expression SIBO and PASI To pick up correction multipliers it will be possible only after carrying out of researches of fractions of tolerized Mo-T and DC-T at psoriatics.
Subprocess LP1.1 is a component of complex of the local processes which are taking place in
prepsoriatic and in psoriatic skin and is in detail stated
Systemic psoriatic process SPP defined by set of interconnected subprocesses
Hyperpermeability of intestinal walls for F-content (SP1)
Specific small intestine disbacteriosis (SP2)
Disorder of production and/or circulation of bile acids (BA) (SP3).
Overload and/or disorder of detoxication systems (SP5)
Chronic PAMP-nemia (SP4)
SPP severity is proportional to total (PG-Y)-carriage of Mo-R and DC-R in blood
flow (SP8.1).
Each of these subprocesses can be caused or strengthened by genetic
) and/or functional deviations.
2009-2012, Peslyak MY, Model of pathogenesis of psoriasis. Part 1. Systemic psoriatic process. e4.0a. 27
Discussion
Systemic psoriatic process SPP is a dynamic interaction of all listed subprocesses. The chronic
increased PAMP-load (SP4) lays constant impact on blood immune system and includes chronic proinflammatory and antiinflammatory (in particular SP8) mechanisms. The result of it is the dynamic equilibrium condition of blood immune system. It is possible to draw an analogy with the interaction between SIRS (systemic inflammatory response syndrome) and CARS (compensatory anti-inflammatory response syndrome) which take place for more serious reasons than SP4. Tolerization (reprogramming) monocytes and dendritic cells (SP8) is limited by the compartment of systemic blood flow (and probably bone marrow) and corresponds to the definition of CARS , ).
SPP affects all organs because tolerized phagocytes participate in homeostatic and/or
inflammatory renewal of pool of any tissue phagocytes. However problems can begin if F-content brought by R-phagocytes contains enough antigenic material wrongly accepted by local immune system as proof of presence of pathogenic bacteria. Antigenic material in psoriasis is PG-Y (in F-content) accepted by local immune system as proof of S.pyogenes presence in skin.
The SPP-basis is made by three obligatory subprocesses SP2, SP4 and SP8. Without any of
them SPP will be incomplete. The SPP-basis can be parted on two components: tolerization of phagocytes and (PG-Y)-carriage of phagocy
Tolerization of phagocytes assumes growth in small intestine of bacterial populations without
PsB (SP2 without SP2.1). As a result PAMP-nemia without participation of PG-Y (SP4 without SP4.1) takes place. Growth of fractions Mo-T and DC-T in blood flow will be a consequence of joint LPS-load and PG-load on blood phagocytes (SP8 without SP8.1). And the main contribution is done, as a rule, by LPS-load.
(PG-Y)-carriage of phagocytes assumes growth on small intestine mucous of PsB populations
only (SP2.1). Thus arises (PG-Y)-nemia (SP4.1) and in blood flow there are fractions PG-Y(+)Mo and PG-Y(+)DC. But (PG-Y)-load is not enough for occurrence of fractions Mo-T and DC-T as activating (tolerizing) abilities of PG are essentially lower, than such LPS abilities. Occurrence of tolerized fractions only because of (PG-Y)-load is theoretically possible at essential growth of PsB-populations only (in the absence of any others). In Fig. 8 this variant is represented as questionable.
Each of these two components alone, is not enough for occurrence of subfractions Mo-R and
DC-R in blood flow (SP8.1). Each of components separately is incomplete SPP-basis (pre-SPP) and can precede development SPP in the concrete patient.
Only their joint action (tolerization and (PG-Y)-carriage of phagocytes) provides growth of
subfractions Mo-R and DC-R (SP8.1) – the final subprocess of SPP. Without subprocess SP8.1 systemic psoriatic process SPP is incomplete and cannot entail initialization and support of psoriasis.
If SPP completely operates, local conditions are necessary for occurrence and support of any
psoriatic plaque. In particular for giving Y-antigen (in PG-Y) time to be presented before its complete degradation. So Mo-R should be transformed to MoDC-R and DC-R, MoDC-R should be transformed to maDC-Y before full degradation of PG-Y. For detail information see (Part 2).
Basic hypotheses of this work):
Systemic psoriatic process SPP as the main factor of psoriasis initialization
and maintenance
Psoriagenic bacteria PsB as a key factor (SP2.1)
(PG-Y)-nemia as key factor (SP4.1)
Growth of tolerized fractions Mo-T and DC-T under chronic PAMP-load
(
Increased (PG-Y)-carriage of blood Mo-R and DC-R (SP8.1)
2009-2012, Peslyak MY, Model of pathogenesis of psoriasis. Part 1. Systemic psoriatic process. e4.0a. 28
10-15% of patients with psoriasis have psoriatic arthritis ,
). There is no doubt now that LPS and blood MF under PG-load ) have aggravating effect on atherosclerosis pathogenesis. Probably new effect of tolerized phagocytes (in particular R-phagocytes) on pathogenesis of other diseases will explain why patients with psoriasis are at high risk of their development.
The results of statistical study conducted in 2008 are given at The study included data
of 16851 psoriatics and 48681 control group patients without psoriasis . 13% of psoriatics suffered from diabetes, against 7,3% in control group. 27,5% of psoriatics suffered from arterial hypertension, against 14,4% of control group. 8,4% of psoriatics suffered from obesity, against 3,6% of control group. 14,2% of psoriatics suffered from coronary heart disease, against 7,1% of control group. Similar study (46095 psoriatics) also showed increased comorbidity of diabetes (OR=1,27; 95%CI 1,1-1,48) and atherosclerosis (OR=1,28; 95%CI 1,04-1,59) . The results are of high statistical reliability p <0.001 because of large scale sample group.
The detailed analysis of conditions of comorbidity for cases of obesity and for cardiovascular
diseases is executed
Further tests for psoriatics and control group patients will check abovementioned SPP model. Apart from well -
known LAL-test, allowing to determine endotoxinemia level and its modification realized in it is necessary to use SLP (silkworm larvae plasma) - test for determination of blood peptidoglycan level
Phagocyte-dependent PAMP-load can be evaluated by blood neutrophils condition because they take up
essential part of this PAMP-load. Intensive LPS entry to blood is known to increase LPS+Neu count up to 100% (standard count doesn't exceed 10%) . Not only percent of LPS+Neu should be determined, but also volume of LPS they brought.
Test for determination of level of intracellular protein IRAK-M in psoriatic blood
Mo, DC and Neu and control healthy group of patients would determine share of Mo-R, DC-R and Neu-R fractions among normal Mo, DC and Neu. According to SPP model IRAK-M level in Mo-R, DC-R and Neu-R should be increased. Recently increased level IRAK-M has been found out in Neu during a .
Only comparative qualitative and quantitative monitoring of intestine parietal microflora can prove SIBO effect
and to determine specific types of psoriagenic and arthritogenic bacteria (SP2). Respiratory tests (hydrogen with glucose; hydrogen with lactulose; 14S-D-xylose or 13C-D-xylose) give possibility for indirect evaluation. Bacteriological analysis of aspirate and scrape of small intestine is direct method . Introduction of perspective techniques can provide effective monitoring and, as consequence, it will promote direct correction of intestine parietal microflora.
I hope that the publication will stimulate new cooperative studies of dermatologists,
rheumatologists, gastroenterologists and microbiologists and will give possibility to solve a puzzle of psoriatic disease. I believe that future treatment for psoriatic disease will not be aimed at cosmetic and/or anti-inflammatory correction of local symptoms only, but at elimination and/or alleviation of underlying causes, i.e. at treatment for intestine dysfunctions in most cases (SP1, SP2). Efficiency of such treatment will depend mainly on patient's wish and possibility of lifestyle and diet control. So remission will be long-term (or life-long!). It can be supported by regular or periodic drug administration (bacteriophages, pre - and probiotics).
The proposed systemic process SPP is a component of Y-model of pathogenesis of psoriasis.
Description of Y-model for prepsoriatic and psoriatic skin will be the content of the subsequent
The author declares no conflict of interest.
2009-2012, Peslyak MY, Model of pathogenesis of psoriasis. Part 1. Systemic psoriatic process. e4.0a. 29
Appendices
Appendix 1. Abbreviations
Term and comments
M-protein of beta-hemolitic streptococci
f beta-hemolitic streptococci
- component of Gram(-) PG, ligand
Reprogrammed (tolerized) and repleted by PG-Y
dendritic cells are subfraction of tolerized fraction DC-T.
Fragments of bacterial products with PAMP (including
Interpeptide bridges bind stem peptides attached to
glycan chains of peptidoglycan
Interpeptide bridges from peptidoglycan of Str.pyogenes:
L-Ala(2-3) or L-Ala-L-Ser
Mature DC derived from DC-R or from MoDC-R, which
present Y-antigen
- component of Gram+ and Gram(-)
2009-2012, Peslyak MY, Model of pathogenesis of psoriasis. Part 1. Systemic psoriatic process. e4.0a. 30
Abbreviations (continuation)
Term and comments
Reprogrammed (tolerized) and repleted by PG-Y
monocytes are subfraction of tolerized fraction Mo-T.
DC, derived from Mo
DC-T, derived from Mo-T
DC-R, derived from Mo-R
CD34+ cells-precursors of monocytes and immature
dendritic cells of bone marrow
Macrophages, derived from Mo-T
Macrophages, derived from Mo-R
Reprogrammed (tolerized) and repleted by PG-Y
neutrophiles are subfraction of tolerized fraction Neu-T.
Psoriagenic bacteria = Gram+ bacteria with
peptidoglycan PG-Y.
Products of vital activity and-or disintegration of
psoriagenic bacteria
in particular PG-Y)
Peptidoglycan A3alpha with interpeptide bridges IB-Y
(but can contain and others also)
Systemic psoriatic process
Mature dendritic cells actively secreting TNF-alpha and
iNOS (in particular maDC-Y)
2009-2012, Peslyak MY, Model of pathogenesis of psoriasis. Part 1. Systemic psoriatic process. e4.0a. 31
Abbreviations (continuation)
Term and comments
Model of pathogenesis of psoriasis proposed
in this monograph
Y-antigen = part(s) of interpeptide bridge IB-Y
YM-antigen = part(s) of BSMP
* - new abbreviation; images: #1 -, #2 - Links are given fo
2009-2012, Peslyak MY, Model of pathogenesis of psoriasis. Part 1. Systemic psoriatic process. e4.0a. 32
Appendix 2. New and specified terms
Description
Chemostatus Range of chemokine receptors expressed by cell (CCR1, CCR2,
CCR3 etc.) including the amount of each. Chemostatus of cell
defines its behavior in reply to homeostatic and/or inflammatory
Phagocyte-dependent consumption (linkage, endocytosis) of
PAMP and contact with PAMP.
Phagocytes under weak PAMP-load are not activated. Their
chemostatuses remain nonactivated.
Nonactivated phagocytes under temporarily increased
PAMP-load are activated. Particularly, the secretion of TNF-alpha
raises and their chemostatuses change significantly.
The activated phagocytes under chronically increased
PAMP-load are tolerized (reprogrammed).
Tolerized phagocytes are designated as Neu-T, Mo-T and DC-T.
It is supposed that tolerized phagocytes (except known
properties) have:
Property 1. Despite contact, linkage with PAMP and
endocytosis of PAMP (as a part of endocytosed F-content)
their chemostatuses are similar to nonactivated;
Property 2. As tolerization occurs under chronic kPAMP-load
then there is kPAMP in endocytosed F-content. Its
degradation occurs slowly and not completely, i.e.
kPAMP-carriage takes place;
Properties 1 and 2 seem to depend on the blocking intracellular
protein IRAK-M. Level of this protein in tolerized phagocytes
becomes more than critical as a result of chronically increased
PAMP-load.
Property 2 depends on parity between rate of endocytosis F and
intracellular proteolytic activity.
In process of aging chemostatus of tolerized Neu changes
similarly to chemostatus of nonactivated Neu.
Those and others start to express actively CXCR4 that provides
their homing into bone marrow (SP9).
R-phagocytes are tolerized phagocytes which also are
(PG-Y)-carriers. They are designated as Neu-R, Mo-R and DC-R
and have additional:
Property 3. There is PG-Y in endocytosed F-content.
Its degradation occurs slowly and not completely, i.e. takes
place (PG-Y)-carriage.
Property 3 (as well as Property 2) depends on parity between rate of endocytosis F and intracellular proteolytic activity. As endocytosis and conservation of PG-Y (as a part of F-content) by phagocytes occurs in a random way, also subfraction R-phagocytes in tolerized fraction is formed in a random way.
2009-2012, Peslyak MY, Model of pathogenesis of psoriasis. Part 1. Systemic psoriatic process. e4.0a. 33
New and specified terms (continuation)
Description
Not all PAMP but only key PAMP (kPAMP) provide tolerization of
phagocytes. Tolerized phagocytes were likely to contact with kPAMP than with other PAMP and/or endocytosed F-content contained more kPAMP than other PAMP.
All kPAMP can be conditionally divided to major and minor
PAMP. Only major kPAMP-load can help to achieve critical level of IRAK-M in tolerized phagocytes because major kPAMP form the greater part of chronic load. The major kPAMP for systemic psoriatic process are LPS (TLR4-ligand) and PG (PG-fragments BLP, LTA - TLR2-ligands, MDP - NOD2-ligand and DAP - NOD1-ligand).
Minor kPAMP also positively influence IRAK-M level in
phagocytes. However they bring essentially lesser contribution, their load can be neither chronic nor increased. Besides minor kPAMP-load is not necessary for tolerized phagocyte formation though the load enlarges depth of their reprogramming. Minor kPAMP bring contribution because their intracellular signal paths completely coincide with paths used by major kPAMP. Minor kPAMP for systemic psoriatic process seem to be flagellin (TLR5-ligand), CpG DNA (TLR9-ligand) and others.
Phagocyte-dependent consumption (linkage, endocytosis) of
kPAMP and contact with kPAM
PAMP-nemia Chronic increasing of kPAMP-load on blood phagocytes resulting
in a) formation of essential share of tolerized phagocytes; b) increased kPAMP-carriage of tolerized phagocytes; c) kPAMP-level increasing in blood;
Normal phagocytes aren't tolerized (reprogrammed) and tolerized
phagocytes (incl. R-phagocytes) quickly lose tolerance
(deprogrammed) in the presence of such cytokines.
IFN-gamma, GM-CSF and IL-12 are known. IL-12 stimulates non-
monocytic cells to produce IFN-gamma.
Appendix 2a. Properties of tolerized phagocytes and R-phagocytes
Tolerized phagocytes
R-phagocytes (subfractions of
tolerized fractions)
1. Chemostatuses are similar to
nonactivated 2. kPAMP-carriage
3. (PG-Y)-carriage
2009-2012, Peslyak MY, Model of pathogenesis of psoriasis. Part 1. Systemic psoriatic process. e4.0a. 34
Appendix 3. (Sub)processes, references and hypotheses
References with facts
Process or
With psoriasis
Hypotheses and
subprocess
psoriasis
comments
SPP Systemic psoriatic
SPP-H1. Fractions of
SPP-H2:, tolerized phagocytes
exist at psoriasis;
kPAMP-carriage of
tolerized phagocytes.
(PG-Y)-carriage of
(PG-Y)-carriage of
, R-phagocytes is
necessary for psoriasis
, initialization and
Hyperpermeability of ,
Permeability for
intestinal walls for
D-xylose, lactulose,
mannitolum etc. were
investigated by many
scientists. SP1-H1. These data correlate with F-content permeability.
populations of Gram+ ,
PsB-populations define
, psoriasis possibility.
Gram(-)TLR4-active ,
bacteria on small
SP2-H2. Y-antigen
intestine mucosa.
define psoriagenity of
SP2.1 Growth of
production and/or
subprocess worsens
circulation of bile
SP4-H1. (PG-Y)-nemia
is present and it plays
kPAMP-load on blood
essential role in
level in blood. The
major kPAMP are PG
SP4.1 (PG-Y)-nemia
2009-2012, Peslyak MY, Model of pathogenesis of psoriasis. Part 1. Systemic psoriatic process. e4.0a. 35
(Sub)processes, references and hypotheses (continuation)
References with facts
Process or
With psoriasis
Hypotheses and
subprocess
psoriasis
comments
detoxication systems
SP5.1 Intestine
SP5.2 Hepatobiliary system ,
SP7-H1. Possibility of
intracellular signal
path "from MDP
recognition through
NOD2-ligand to chemostatus change" (<1%).
Growth of tolerized
SP8-H1. Defining role
fractions Mo-T and
of the subprocess in
psoriasis maintenance.
SP8-H2. SPP severity
SP8.1 Growth of fractions
is proportional to total
(PG-Y)-carriage of
Mo-R and DC-R in
(PG-Y)-carriage.
2009-2012, Peslyak MY, Model of pathogenesis of psoriasis. Part 1. Systemic psoriatic process. e4.0a. 36
(Sub)processes, references and hypotheses (continuation)
References with facts
Additional subpro
SP9-H1. Neu transform
carriage of Neu-T.
Increased (PG-Y)-
carriage of Neu-R.
SP9-H2. Senescent
Return of senescent
Neu from blood to
senescent Neu – they
go to die in bone
their apoptosis.
SP9-H3. Role of the
subprocess in psoriasis maintenance.
SP10 Transformation of Mo
activated under relay
hemopoiesis in bone
kPAMP-load, that
promotes their tolerization after exit into blood flow; SP10-H2. Role of this subprocess in psoriasis maintenance.
*- references are available; SPn-Hm = hypothesis Hm, proposed in subprocess SPn.
2009-2012, Peslyak MY, Model of pathogenesis of psoriasis. Part 1. Systemic psoriatic process. e4.0a. 37
Appendix 4. Substantiation of hypothesis about chemostatus of tolerized phagocytes.
Functional F-carriage of blood Mo and DC is standard. It arises occasionally at low changeable
PAMP-load due to single endocytosis of F-content. Episodic PAMP-load activate blood Mo (DC) and changes their chemostatuses. Expression of proinflammatory and homeostatic chemokine receptors (CCR1, CCR2, CCR5, CXCR4) is reduced to prevent endocytosed F-content (probably pathogenic) translocation made by phagocytes from blood to tissues.
DC is transformed to licensed (semimature) liDC which move to spleen and/or lymph nodes
under the influence of chemokines CCL19 and CCL21 (CCR7 - ligands), preserving endocytosed F-content. Then they finally mature, simultaneously degrading and presenting F-content brought from blood. So they activate and support humoral immune response , . Blood CD16+ Mo seem to behave similarly, supporting the T-independent humoral response . However blood CD14++Mo reduce expression of all chemokine receptors (getting reduced chemostatus) for time necessary for degradation of endocytosed content under the influence of episodic PAMP-load. Then they restore expression of chemokine receptors (coming back to normal chemostatus), remaining in blood all the time: CCR1, CCR2, CCR5 CXCR4 and CCR5 ; CCR2 ); CCR1 and CCR2 .
Work is the state-of-the-art review of the publications devoted to condition of ET -
endotoxic tolerance. Intracellular signal paths are in details illustrated, the short review of influence of ET on expression of chemokine receptors at monocytes/macrophages is made, and series of important open questions is formulated. Assuming the existence of R-phagocytes (as a part of tolerized fraction) we can foresee answers (certainly incomplete) to some of them:
It would be worth investigating whether
Yes it does, to be exact, the level of expression
downregulation of other monocyte/macrophage
CCR7 raises insufficiently (though it should rise
chemokine receptors (except those mentioned
essentially). It occurs during the chronically
in this work) occurs during ET and thereby it
increased PAMP-load (in particular at ET).
has a role in mediating immunosuppression?
Traffic F+DC and F+CD16+Mo into spleen
and/or into lymph nodes is thus reduced and, as consequence, the level of the humoral answer decreases. That is a characteristic sign of immunosuppression. It is confirmed by recent researche
Besides tuning-down of inflammatory responses Yes, the chronically increased PAMP-load (as and modulating phagocytosis, does ET have a
well as at ET) modulates chemotactic
regulatory role over other aspects of
characteristics of blood
monocyte/macrophage functions?
monocytes/macrophages. It makes their
chemostatus almost unchangeable.
What similar phenotype have endotoxin-
For example the same as at R-monocytes (see
tolerized monocytes/macrophages at different
What other immune cells and non-immune cells
During the chronically increased PAMP-load (as
may also be affected by ET?
well as at ET) the condition of other blood
phagocytes also changes. In this monograph the author analyses its influence on neutrophils and dendritic cells.
Mo and DC are tolerized under the chronic kPAMP-load to subsequent influence of kPAMP
. In particular they stopping TNF-alpha secretion.
Changes of chemostatuses of PAMP-tolerized Mo and DC were not investigated until recently.
In work the chemotactic behavior of mouse bone marrow DC after LPS-load of various duration was studied. Expression CCR7 (and other chemokine receptors) was studied, and also the
2009-2012, Peslyak MY, Model of pathogenesis of psoriasis. Part 1. Systemic psoriatic process. e4.0a. 38
ability of DC to migrate in lymph nodes. It is shown that the longer LPS-load lasts, in lesser degree DC expresses CCR7, and the worse their migration goes. The opposite interrelation between the level of intracellular blocking protein IRAK-M and the level of expression CCR7 is proved. Therefore it is possible to assume that signal paths providing PAMP-load influence on TNF-alpha secretion partially coincide with signal paths providing influence on expression of CCR7 in DC
One of the mechanisms of tolerization is increasing of level of blocking intracellular proteins
IRAK-M, Tollip, SOCS-1. Chronic LPS-load (TLR4-ligand) or PG-load (in the form of fragments BLP and/or LTA) on phagocytes leads to increase of IRAK-M. IRAK-M can block link IRAK-1, IRAK-4 . In particular low LPS-load only raises IRAK-M level while higher LPS-load also reduces IRAK-1 level . IRAK-M production is similarly increased due to chronic load of PG-fragments MDP or DAP (NOD2 and NOD1 ligands respectively) on phagocytes. However the exact mechanism of blocking of RIP2 link (and/or subsequent links) is unknown ). It is known that at chronic MDP-load tolerization occurs because of level decrease of phosphorylated TAK1, but how it is reached, is not known ). The fragment of intracellular signal paths of monocyte from kPAMP-load to CCR7 and CD163 expression and TNF-alpha secretion is schematically presented).
The activated MF and DC located in psoriatic derma have increased expression of CD163
receptor which appeared to be characteristic for the definition of these cells among the others ). It is possible to assume that such increased expression occurs for the same reason, as the active secretion of TNF-alpha. I.e. due to the action of cytokines-deprogrammers on dermal MF-T, MoDC-T and DC-T (incl. MF-R, MoDC-R and DC-R) and the subsequent loss of their tolerance to F-content derma). Receptor CD163 belongs to the family of scavengers and promotes binding and endocytosis of bacteria. Blood monocytes express it in reply to the single PAMP-load, thus its essential part is disconnected from cellular membrane (shed by cell) to pass into the soluble form sCD163. After a while after shedding, its expression is restored in much larger volume than it was before the PAMP-load Intracellular signal paths defining the expression of CD163 after the PAMP-load partly coincide with the similar ways defining secretion of TNF-alpha It is possible to assume that blood Mo-R and DC-R being reprogrammed keep the expression of CD163 at the usual level though they have F-content. But if they appear in the environment of cytokines - deprogrammers (i.e. in psoriatic derma), they begin the active expression of this receptor.
Chronic kPAMP-load tolerizes Mo and DC and leads to decrease of secretion of proinflammatory
cytokines (first of all TNF-alpha), simultaneously reduces changes of chemokine receptors which can be observed at single PAMP-load. Under chronic kPAMP-load chemostatuses of Mo and DC became similar to nonactivated.
Transformation of certain nonactivated phagocyte into tolerized one (and, in particular, into
R-phagocyte) depends on time of stay in blood flow under the chronic PAMP-load. The small number of occurrings with F-content converts nonactivated phagocyte into activated, and large number - converts the activated phagocyte into tolerized one (for monocytes - see
R-phagocytes remain those if predominant volume of PAMP-load on them is kPAMP-load
(including obligatory (PG-Y)-load). Proportion of blood tolerized phagocytes among all phagocytes depends on level of chronic kPAMP-load. And share of Mo-R and DC-R among Mo and DC in blood flow also depends on level of chronic (PG-Y)-load.
Mo-T and DC-T along with nonactivated DC and Mo participate in homeostatic and/or
inflammatory traffic due to their chemostatuses similar to nonactivated. As a result Mo-T and DC-T (incl. Mo-R and DC-R) move to tissues. It is very dangerous if there is local inflammation. Mo-T (Mo-R) can transform into MF-T (MF-R) and aggravate any inflammation. Being (PG-Y)-carriers Mo-R and DC-R can transform into maDC-Y providing complete maintenance of psoriatic inflammation.
Phagocytes containing F-content can be found in tissues and/or blood of patients with different
diseases: rheumatoid and psoriatic arthritis, atherosclerosis and CNS diseases , including in psoriatics .
2009-2012, Peslyak MY, Model of pathogenesis of psoriasis. Part 1. Systemic psoriatic process. e4.0a. 39
Appendix 5. Relay variant. Transformation of Mo and DC by means of apoptotic Neu in bone
marrow.
The subject of the appendix is the possible role of neutrophils in Mo and DC activation and
reprogramming (full or partial) in hemopoiesis. Subprocesses SP9 and SP10 as an addition to subprocess SP8, especially in DC reprogramming are offered.
Chronic kPAMP-load in blood is enough for tolerized Neu-T and Mo-T formation because, as a
rule, endocytosis of F-content doesn't promote these phagocytes to leave blood. However blood DC change of chemostatus at once and express CCR7 under impact of kPAMP after linkage and endocytosis of F (including kPAMP) . Then F+DC respond to chemokines CCL19 and CCL21 and move to the nearest lymph node or spleen to provide the humoral immune response. I.e. the certain DC may have no time to undergo chronic kPAMP impact in blood, which is necessary for its tolerization because, as a rule, the DC leaves blood after the first kPAMP impact.
Subprocess SP9. Increased kPAMP-carriage of Neu-T. Increased (PG-Y)-carriage
of Neu-R. Return of senescent Neu from blood to bone marrow and their apoptosis.
Subprocess components are partly known, but they haven't been investigated in psoriatics. LPS+Neu percent can be increased up to 100% during endotoxinemia (in norm no more than
10%) ), depending on LPS-load. On blood Neu lays the largest part of work on PG utilization and degradation, and for that purpose they actively produce lysozyme and PRGP2 . Neu status in PG-nemia hasn't been investigated, but it is possible (similarly to LPS) that PG+Neu percent also significantly increases.
In work human neutrophils were investigated ex vivo. It is found out that NOD1 is
not practically expressed at Neu, and, as consequence, they are not activated after the influence of DAP. At the same time NOD2 is well expressed at Neu, and consequently Neu are activated after the influence of MDP.
Recently in experiments on mice it has been proved that fragments of PG get from intestine
bacteria (containing labeled DAP) to systemic blood flow. Then Neu endocytose these fragments, and subsequently senescent Neu bring them into bone marrow
Chronic LPS-load provides Neu tolerization (reprogramming) , in particular it
decreases their response to LPS. It is possible that chronic kPAMP-load (LPS- or PG-load) also results in Neu tolerization (reprogramming), in particular it decreases level of degradation endocytosed PG.
Significant percent of tolerized (reprogrammed) neutrophils Neu-T containing F-content
(including kPAMP) occur among blood Neu due to increased kPAMP-load. Neu are formed from cells-precursors in bone marrow and then move to blood to perform "fast reaction" function both in blood and in tissues where they migrate in the case of inflammation ). Migrated Neu are lost in tissues. Remained Neu senesce in blood, express CXCR4 and above all they come back to die in bone marrow . Senescent Neu can bring material, endocytosed in blood, to bone marrow. This material appears in extracellular space after Neu apoptosis
It is supposed that in process of aging chemostatus of tolerized Neu-T changes like chemostatus
of nonactivated Neu. Both of them start to express actively CXCR4 that provides their homing into bone marrow. Whether CXCR4 express activated kPAMP+Neu in process of aging it is not known. Tolerized Neu-T appeared (PG-Y)-carriers are designated Neu-R. Neu-R make subfraction of fraction tolerized Neu-T.
Neu role in psoriasis is generalized in reviews . Experimental decrease
of Neu level in psoriatic skin isn't beneficial for psoriasis course . So skin Neu aren't necessary for support of local psoriatic plaque.
On the other hand, it is known a special case of neutropenia (caused by medications), leading
to full disappearance of psoriasis, which plaques started to reappear again after the renewal of the Neu level in blood ).
2009-2012, Peslyak MY, Model of pathogenesis of psoriasis. Part 1. Systemic psoriatic process. e4.0a. 40
In work tic activity of Neu at psoriatics (183 people, PASI >= 18) and in
control group of healthy (52) people has been defined. Results: phagocytic indicator (42,2% against 56,7%), phagocytic number (6,8 against 5,9) and indicator of completeness of phagocytosis (38,6% against 42,6%). Function of killing at psoriatic Neu also has appeared weakened. It correlates with changes of phagocytic activity of Neu under LPS influence).
SP9 depends on SP4. Level of chronic kPAMP-load defines share of fraction of tolerized Neu-T
in blood flow and level of their kPAMP-carriage. Level of chronic (PG-Y)-load defines share of subfraction Neu-R in blood flow and level of their (PG-Y)-carriage.
Subprocess SP10. Mo and DC transformation during hemopoiesis in bone marrow.
Part of DC and Mo can already leave bone marrow being already activated or tolerized (and, in
particular, reprogrammed DC-R and Mo-R). These transformations can occur during their development in bone marrow when DC and Mo are trained, endocytosing apoptotic bodies of various cells and including dying Neu-R Activation of DC and Mo occurs, if the relay PAMP-load (transmitted through apoptotic bodies Neu-R) in the period before the exit of Mo and DC from bone marrow, takes place incidentally. Transformation of activated DC in DC-R, and activated Mo into Mo-R, can occur, if the relay PAMP-load in the same period appears constant and essential
Mo and DC are formed from MoDP - mutual CD34+ cells-precursors in bone marrow
. DC are formed longer than Mo. Blood Mo can turn to MoDC. For example, it takes place in renewal of pool of epidermal dendritic cells LC
Reprogramming of bone marrow phagocytes takes place in 1-3 hours after essential LPS-load
) which simultaneously increases formed Mo number due to reduction of formed DC number .
Activated or tolerized Mo and DC leave bone marrow containing some non-degraded F-content
(endocytosed together with apoptotic bodies Neu-R), including kPAMP. After entering the blood they are again under kPAMP-load. They continue contact, linkage and endocytosis of F-content and support and/or strengthen their own activated or tolerized status.
It is known that in bone marrow of psoriatics the activity of monocytopoiesis is raised and the
phagocytic hyperplasia takes place At psoriasis peripheric blood and bone marrow stromal cells secrete cytokines abnormally and express receptors , ). It breaks the normal functioning of bone marrow hemopoietic microenvironment. It shall be mentioned that the chronic LPS-load can be one of the reasons of bone marrow hyperplasia ). It is possible to assume that the given subprocess is component of such disturbances.
SP10 depends on SP9. Without formation of Neu-T, their aging and apoptosis in bone marrow it
is impossible relay kPAMP-load on Mo and DC during hemopoiesis. This load activates Mo and DC already in bone marrow that accelerates their activation and subsequent tolerization after exit into blood flow.
Without formation of Neu-R, their aging and apoptosis in bone marrow is impossible relay
(PG-Y)-load on Mo and DC during hemopoiesis. This load provides occurrence of PG-Y(+)Mo and PG-Y(+)DC already in bone marrow that promotes increasing level of their (PG-Y)-carriage after exit into blood flow
2009-2012, Peslyak MY, Model of pathogenesis of psoriasis. Part 1. Systemic psoriatic process. e4.0a. 41
Appendix 6. Small intestine microflora in SIBO (without psoriasis) and in psoriasis.
In norm total bacterial count of duodenum and upper parts of small intestine does not exceed
104-105 CFU/ml. There are lactobacillus, bifidobacteria, bacteroides, enterococci, streptococci,
yeast-like fungi, etc. Their count in ileum is 108 CFU/ml of chymus. About 10% of obligate microflora is
Gram(-) microflora .
The research of small intestines microflora is a difficult procedure. Materials for such research
are received by capture of aspirate during intestinoscopy. Usually this procedure is prescribed at GIT diseases for the purpose of diagnosis specification
). It is seldom carried out in the
absence of diseases, or at the diseases which etiology traditionally isn't bound with GIT diseases.
The report of the results of the researches of transient microflora of the small intestines
described in two works is shown in
In work ) the proximal department of
jejunum at 63 patients having various diseases of GIT was surveyed. At 55 from them SIBO was
found (total bacterial count more than 105 CFU/ml). The microflora of various departments of small
intestines at the group of healthy volunteers was studied in work The data
presented in Tab. 2 allows to make a comparison with the results received recently
(* - in the first column) and healthy v
Density of parietal microflora of small intestine (especially in distal part) is comparable with
density of parietal microflora of large intestine - 1011 CFU/ml F - fragments of
bacterial products of parietal microflora first of all penetrate through intestinal walls and enter the
blood. Therefore PAMP blood level and kPAMP-load on phagocytes depend on count and types of
parietal bacteria.
The author of dissertation uses original method of bacteria count evaluation in
small intestine mucosa by means of proteolytic activity of coprofiltrates. Lymphoid system of small intestine mucosa (Peyer's patches) responds to bacterial antigens of intestinal microbiocenosis. Antibodies (sIgA, IgG) are transferred to intestine through mucosa epithelium by means of endocytosis ). Bacteria actively produce proteinases, which are capable to break antibodies . The quantity of bacterial proteinases in excrements corresponds to volume of bacterial colonies of intestine mucosa. Psoriatics had 4–5 times higher than normal level of thiolic proteinases before treatment. 3–4 times higher than normal level of thiolic proteinases was found after standard method of psoriasis treatment and less than 1,5 times higher than normal level was found after complex treatment. It was the feature of complex method efficiency. Therefore the method provided reduction of volume of bacterial colonization of small intestine and, as a result, longer remission of psoriasis. Thereby they proved influence of volume of bacterial colonization of small intestine mucosa on psoriasis.
2009-2012, Peslyak MY, Model of pathogenesis of psoriasis. Part 1. Systemic psoriatic process. e4.0a. 42
Tabl.2. Transient microflora of small intestine at patients with SIBO (without psoriasis)
and at healthy person
SIBO - 55 pers.
Microflora
proximal +
proximal
proximal
JIT diseases
(22 pers.)
(22 pers.)
(20 pers.)
Acinetobacter spp
Bacteroides spp (pig)
Bacteroides spp (npg)
Bifidobacterium spp
Clostridium ramosum
Clostridium spp (gel-)
Clostridium spp (gel+)
Corynebacterium spp
Enterobacter spp
Enterococcus spp
Enterococcus faecalis
Fusobacterium spp
Lactobacillus spp
Leptotrichia spp
Peptostreptococcus spp
Propionibacterium spp
Staphylococcus spp
Staphylococcus spp (coag+)
Staphylococcus sp (coag-)
= CNS Streptococcus spp
Str.viridans group = SVG
Total bacterial count (TBC)
Notes to Table 2: nd - no data; pig = pigmented; npg = nonpigmented; gel+ = gelatinase positive; gel- = gelatinase negative; coag- = coagulase negative; coag+ - coagulase positive;
2009-2012, Peslyak MY, Model of pathogenesis of psoriasis. Part 1. Systemic psoriatic process. e4.0a. 43
Positive dynamics of psoriasis in children as a result of treatment with method of interval
normobaric hypoxia proved that psoriasis associated with aerobic microflora (streptococci, enterobacteria)
Researches of microflora of large intestine is carried out by research of microflora of faeces - so
the transient microflora is defined, or by research of the materials taken with mucous at colonoscopy - so the parietal microflora is defined. Such researches allow to define dysbiotic deviations in large intestine, but cannot reflect the condition of microflora of small intestines qualitatively or quantitatively. It is proved by the special comparative researches of the enteric and colic microflora which review of those is presented in Researches show that dysbiotic deviations in large intestine as a rule precede and may be one of principal causes of dysbiotic deviations in small intestines.
In several dissertations the comparative analysis of fecal microflora in particular has been
carried out. In work ) 152 psoriatics and 80 healthy people were surveyed. There have been established dysbiotic changes of microflora of the large intestine mostly expressed at exacerbation of widespread psoriasis and psoriasis followed with arthropathy. These changes were aggravated with the increase in duration of disease. In work 140 psoriatics and 30 healthy people were surveyed. It has been established that the maximum disturbances of colic microflora are observed at 75% of psoriatics with moderate and at 97% of psoriatics with serious psoriasis. Less appreciable dysbiotic shifts are found at 47% of psoriatics with the limited psoriasis. Severity of disturbances of microflora correlates with increase in index PASI (to 26 and more), duration of disease over 5 years and presence of psoriatic arthropathy.
In work ) the intestine microflora at patients with various dermatoses (psoriasis,
eczema, atopic dermatitis and planus) and their BLC-carrier states was investigated. In particular 193 psoriatics have been surveyed (PASI>18, average PASI = 37,9), among them has appeared 146 (79,8%) BLC+ psoriatics. The expressed dysbiotic changes in large intestine have been found out in majority BLC(-) and BLC+ patients in comparison with healthy people. These deviations appeared to be most essential at BLC+ patients. Investigation of enteric microflora at patients with chronic dermatoses has been carried, in 69,6% BLC+ patients is found out SIBO (the specific data on psoriatics not present).
Than in the work was represent the first investigation of transient
microflora of small intestine. They examined 80 psoriatics and 20 healthy persons of control group.
70% of psoriatics were diagnosed SIBO (more than 105 CFU/ml). 21% of psoriatics had SIBO severity
level III and 35% of psoriatics had SIBO severity level II. All patients with SIBO had Gram(-) E.coli,
Bacteroides and Gram+ Clostridium. So colon microflora supposed to be migrating to small intestine
and cause increasing of LPS and PG level entering the blood (SP4). 25% of psoriatics had
Enterococcus faecium, 10% psoriatics had Klebsiella pneumonia and 5% psoriatics had Proteus
vulgaris. There were no SIBO and pathogenic flora in control group. Evaluation of biopsy materials
from distal duodenum of all psoriatics with SIBO showed morphological signs of chronic inflammation.
Correlation analysis shown direct relationship between SIBO grade and PASI value (correlation
coefficient R=0,46) and between SIBO grade and disease duration (R=0,43). Similar results are
presentedhere the quantity of the surveyed psoriatics is 100 persons.
In the dissertation the results of previous researches are generalized 121
psoriatics were surveyed: 52 people with moderate psoriasis (PASI in range 20-30) and 69 people with severe psoriasis (PASI more than 30). At all patients the psoriasis was in progressing stage. 43 healthy persons have been included into the control group.
Let's notice that psoriatics with PASI >= 20 make approximately 12% of the whole contingent of
psoriatics. This estimation can be received from the statistical data presented for 2260 psoriatics ). I.e. the results received in works characterize BLC-carriage and condition of intestine microflora concerning a small subgroup (about 12%) of the whole contingent of psoriatics. However it is these patients who have psoriasis in the most severe form (moderate and serious degree), and many of them also have psoriatic arthritis.
2009-2012, Peslyak MY, Model of pathogenesis of psoriasis. Part 1. Systemic psoriatic process. e4.0a. 44
At treatment of BLC+ patients with psoriatic arthritis under the combined scheme which (in
addition to the standard scheme) included Tinidazolum, Intestibacteriophage, Enterosgel, Linex and Hilak-forte, appreciable improvement has come at 46,8% of patients. At treatment under the standard scheme - only at 36
Summary results of researches of transient microflora of proximal department of small intestines
) are presen. Level SIBO more than 105 CFU/ml (TBC> 5) was found
at 95 (78,5%) psoriatics. TBC (total bacterial count) for psoriatics has made average 3x106 CFU/ml
that is much more than in the control group - 1,1x103 CFU/ml. The correlation between SIBO level and
type, severity and duration of psoriasis disease has been found.
At 93% of psoriatics Bifidobacterium spp. was found - on average 2x105 CFU/ml (in the control
group at 40%, on average 250 CFU/ml). At 84% of psoriatics Lactobacillus spp. was found, on
average 4,6x104 CFU/ml; (in the control group at 19%, on average 350 CFU/ml). At 65% of psoriatics
Enterococcus spp. was found - on average 2x105 CFU/ml (in the control group not found at all). At part
of psoriatics Str.pyogenes (9%) and Str.viridans (30%) were found (in the control group not found).
Maximum (100 to 10000 times) excess took place at BLC+ psoriatics, at BLC(-) psoriatics the
excess over the control group was also essential (10 to 100 times).
Various researchers tried to estimate influence of Helicobacter pylori (HP) on the condition of
psoriasis as well as on the permeability of intestine. The authors of work conducted comparative study of two groups of psoriatics (38 and 12 patients) who had or had no Helicobacter pylori (HP). 55% of HP+ psoriatics suffered from intensive itch, while the HP(-) psoriatics didn't. Psoriatic lesions of nail plates were observed in 47% of HP+ psoriatics against 17% of HP(-) psoriatics. 21% of HP+ psoriatics complained of periodic mild pains in joints. Average PASI value for HP+ psoriatics was 20% more. In work 50 psoriatics and 50 healthy people were surveyed. Among the psoriatics there were 40% HP+, while among the healthy people 5% only. In work 33 patients were surveyed: 15 HP+ and 18 HP(-). It was shown that the average permeability of intestine defined by sucrose test at HP+ patients is 345 mg/g against 59 mg/g at HP(-), i.e. is increased more than 5 times. Others refer to this work trying to explain the remission of psoriasis at HP+ patient who received complex treatment, including anti-Helicobacter therapy
2009-2012, Peslyak MY, Model of pathogenesis of psoriasis. Part 1. Systemic psoriatic process. e4.0a. 45
Tabl.3. Transient microflora of proximal small intestine at psoriatics and at control
healthy
Psoriatics (121 pers.)
Control healthy (43 pers.)
Microflora
Bifidobacterium spp.
Lactobacillus spp.
Bacteroides spp.
lactose-negative E.coli hemolytic
Enterococcus spp.
Acinetobacter spp.
Clostridium spp.
Total bacterial count
The note: The analysis and comparison of the data resulted in various tables ( allows to
assume that the average level of bacterial carriage (lg CFU/ml) was defined by calculation of the arithmetic average of logarithmic value of carriers only, and to define the average TBC – the arithmetic average of logarithmic value of all patients was calculated. A similar way of averaging is also used at This way of averaging of the absolute value (CFU/ml) means the calculation of their geometrical average instead of their arithmetic average. The average value received in such way is underestimated, and the error (arithmetic average minus geometrical average) is proportional to the disorder of averaged absolute value.
2009-2012, Peslyak MY, Model of pathogenesis of psoriasis. Part 1. Systemic psoriatic process. e4.0a. 46
Appendix 7. BA in psoriasis. Study results.
The authors of monograph chapter 3) summarized results of evaluation of
functional condition of hepatobiliary system in psoriatics. 213 psoriatics were examined. Diseases of GIT, liver and bile ducts were diagnosed in 88 patients (58 patients suffered from chronic cholecystitis-cholangitis). Bile of 67 psoriatics was received with duodenal intubation and analyzed for composition. 1st group (28 persons) included psoriatics with hepatobiliary system disorder; 2nd group (39 persons) included psoriatics without such disorder. Both groups (in comparison with control group of 15 persons) had significant reduction of bile acid level in bile. Results of 2nd group are most interesting: portion B: 11,9 against 27,4 g/l, portion C: 3,8 against 6,0 g/l. The level of 3-OH- and 2-OH-acids and bilirubin was reduced similarly. Cholatocholesterol coefficient also was decreased for portion B: 3,14 against 7,47.
Total blood level of BA and their fractions was determined with thin-layer chromatography
The level was increased 50 times higher than normal and more. There was a correlation between reduction of bile BA level and increasing of BA blood level. The results show disturbance of enterohepatic circulation of BA, cholestasis and diffuse damage of liver parenchyma in psoriatics. Hepatobiliary system disorders haven't been diagnosed in these psoriatics before now.
Similar results are summarized in work . 50 psoriatics were examined. Blood
BA-fractions – deoxycholic acid (DC), cholic acid (C), glycochenodeoxycholic acid along with glycodeoxycholic acid (GCDC + GDC) and glycocholic acid (GC) - were determined before and after hepatotropic therapy. High BA blood level (10-20 times higher than standard) correlated with disease severity and stability Free secondary DC-BA in blood is a sign of disorder of its enterohepatic circulation because the main site of its formation under the influence of microflora is small intestine. Increase of primary C-BA concentration, synthesized by hepatic cells is a sign of conjugation disorder and/or disorder of patency of bile ducts of the functional or organic genesis promoting cholestasis.
There was apparently no GC-BA in blood of patients with complications (erythrosis, arthropathy)
before and after treatment. It is likely to characterize more serious disorders of BA pool formation, than those found in patients with chronic psoriasis only. Decrease of blood fractions of free BA, psoriasis remission, itch alleviation - were found after complex hepatotropic therapy.
Similar results were received for studies of bile lithogenicity . Sample of
average values for control group (15 persons) and psoriatics (45 patients) is given Levels of bile acids and phospholipids in bile were significantly decreased and lithogenic indexes were 2-4 times increased in psoriatics.
Authors of work examined 30 children with psoriasis. Dyskinesia of bile
ducts was the most frequent concomitant disease of GIT (72%).
Authors of work supposed that deficiency of bile acids (BA) raised LPS
volume moving from intestine to blood and aggravated psoriasis. Test group included 551 psoriatics (average PASI = 19,1). All patients were administered oral dehydrocholic acid. 434 psoriatics (78,8%) were asymptomatic and 117 psoriatics had significant improvement (average PASI = 2,7) after treatment. Control group included 249 psoriatics. They were administered standard therapy. Only 62 psoriatics (24,9%) were asymptomatic after the therapy. 319 of 551 (57,9%) test group psoriatics against 15 of 249 (6%) control group psoriatics were asymptomatic two years later. Dehydrocholic acid (DA) temporary increases BA (including DA) volume. So volume LPS entering blood through intestinal walls decreases. Authors of work explain that the results are associated with BA destructive ability in relation to LPS and don't take into account BA bactericidal action.
Similar results were obtained in psoriatics who took ursodeoxycholic acid every day for 5-12
months Three patients were administered the drug to treat liver disease and the drug improved psoriasis. However the authors don't think that this BA can influence intestine microflora.
2009-2012, Peslyak MY, Model of pathogenesis of psoriasis. Part 1. Systemic psoriatic process. e4.0a. 47
Tab. 4. Average blood BA level (mkg/ml) in psoriatics (in aggravation phase)
Pathology of Quan- Gene-
(TC - taurocholic, TCDC - taurochenodeoxycholic, TDC - taurodeoxycholic, TLC - taurolithocholic)
Tab. 5. Average blood BA level (mkg/ml) in healthy patients, patients with chronic
hepatitis and psoriatics before and after hepatotropic therapy
(healthy) Chronic
hepatitis Psoriasis
stage stationary
stage complicated
(DC – deoxycholic, C – cholic, GCDC – glycochenodeoxycholic, GDC – glycodeoxycholic, GC – glycocholic)
Tab. 6. Biochemical structure and indicators of lithogenicity gallbladder (B) and
hepatic
Psoriatics (n=45)
Bile acids (mmol/l)
Cholesterol (mmol/l)
Bilirubin (mmol/l)
Total lipids(g/l)
Phospholipids (mmol/l)
Cholatocholesterol coefficient
Lithogenic index (Rubens)
Lithogenic index (Thomas-Hofmann)
2009-2012, Peslyak MY, Model of pathogenesis of psoriasis. Part 1. Systemic psoriatic process. e4.0a. 48
Appendix 8. PAMP-nemia in psoriasis. Study results.
The author of dissertation showed that total blood LPS-level (free and bound
LPS) correlated with psoriasis severity. Plasma samples were dissolved in purified water in ratio of 1:10 and incubated in water bath 30 minutes to release endotoxin from complexes with LPS-binding proteins. Such modification of LAL-test allows estimating LPS-load on phagocytes better. LPS-load is realized also in the form of complexes with LPS-binding proteins LBP and sCD14
Total LPS-level for 16 patients with moderate psoriasis (average PASI = 16,5) was 7,2 Eu/ml.
Total LPS-level for 30 patients with serious psoriasis (average PASI = 24) was 35,8 Eu/ml. Total LPS-level for patients with erythrodermic and exudative psoriasis was from 1000 to 2800 Eu/ml. Total LPS-level in control group (112 healthy persons) was about 0,1 Eu/ml. The authors of this work also investigated antiendotoxic immunity. They determined the level of antibodies to glycolipid (structural part of LPS consisting of lipid A and R-core). Humoral antiendotoxic immunity was decreased from 2 to 15 times and, as a rule, it had inverse relationship with blood LPS-level.
Authors of work ) offered complex method of treatment including not only
standard, but specific therapy according to condition of antiendotoxic immunity and intestinal microflora. The authors received good results: average PASI in patients with serious psoriasis decreased from 24,5 to 1, with exudative psoriasis - from 26 to 3,2 and with stationary psoriasis - from 16,4 to 0. Significant decrease of blood LPS-level was observed before beneficial changes. Average duration of remission after complex method (in comparison with standard method) increased from 6 months to 1,5 years.
However there was no direct dependence between total LPS-level and psoriasis severity in
some cases. So the patients with localized form of psoriasis (palmar, plantar or psoriasis on hairy part of head) had low PASI level (average 1,9) and higher LPS-level (average 39 Eu/ml), than patients with stationary psoriasis (7,2 Eu/ml) with much higher PASI (average 16,4). It means that not only total LPS-level influences psoriasis severity. These results are summarized in the work .
Pagano regimen is sometimes associated with temporary aggravation before remission,
so-called Herxheimer reaction . Psoriasis aggravation is due to mass destruction among populations of psoriagenic bacteria in intestine. Thus we can observe high, but temporary growth of (PG-Y)-load on phagocytes. The condition is transient and, as a rule, there is no need to treat it.
Subprocesses SP4 and SP5 have dynamic interaction. Normalization of antiendotoxic immunity (in particular level of humoral immune response for
LPS) became possible after blood LPS-level was normalized. The process was observed in 38 psoriatics after complex treatment . LPS-level decreased simultaneously with decrease of LPS-load on phagocytes. It resulted in decrease of tolerized phagocytes (incl. R-phagocytes) part and provided long remission.
2009-2012, Peslyak MY, Model of pathogenesis of psoriasis. Part 1. Systemic psoriatic process. e4.0a. 49
Appendix 9. Definition of concept of PAMP-load on phagocytes
Let's use functions PI(t) - PAMP-income and PC(t) - PAMP-consumption per unit of time to
understand correlation between blood PAMP-level and PAMP-load on phagocytes (neutrophils, monocytes, dendritic cells). Blood PAMP-level in specific moment T is defined as
PL(T) = ∫ (PI(t) - PC(t))dt + PL(T0),
where integral is taken on interval from T0 to T, and PL (T0) - PAMP-level at initial moment T0.
PAMP-consumption PC(t) depends on PI(t) and PL(t). The more income and level, the more is consumption. Degradation, linkage, endocytosis and elimination of PAMP at moderate rate of PAMP-income seem to be carried out so that PL(t) doesn't exceed certain admissible level.
PAMP-consumption can be presented as
PC(t) = PCi(t) +PCd(t),
where PCi (t) - phagocyte-independent PAMP-consumption, and PCd (t) - phagocyte-dependent
PAMP-consumption (contact and linkage, endocytosis). Phagocyte-independent PAMP-consumption provide degrading enzymes (for example, lysozyme and PGPR2 for PG), proteins and antibodies binding PAMP in complexes, elimination organs etc. PAMP-consumption is completely phagocyte-independent if its PRR-ligandic properties are fully lost without participation of phagocytes.
PAMP-consumption is phagocyte-dependent, if PAMP (as a part of F-content, complexes or
fragments) is endocytosed (is bound) by blood phagocytes and PAMP still has PRR-ligandic properties to the moment of endocytosis (binding).
Binding of free PAMP into complexes and/or its degradation by enzymes at conservation of any
PRR-ligandic properties can result in phagocyte-dependent consumption.
PAMP-load on phagocytes depends on phagocyte-dependent PAMP-consumption. Part of
tolerized phagocytes (of total number of phagocytes), depth of their reprogramming and kPAMP-carriage level depends on duration and intensity of PAMP-load.
PAMP-income and PUMP-consumption can be presented as container with untight
(non-hermetical) walls (systemic circulation). Water enters container through a pipe (PI) and it is filtered outside through walls - consumed (PCi, PCd)
Rates of PAMP-income and consumption to container correspond to length and number of white
arrows. They are 2 times larger on scheme B than on scheme A, however PAMP-level raised less than in 2 times (calculation by formula for such container). The functional interrelation of PAMP-level and PAMP-load in blood seems to be similar (graph C).
The consumption depends on water level in container PC(T)=FUN(PL(T)). This dependence is
calculated by formula (3).
PC(T) = A*PL(T)**(3/2),
For example, if rate of income is constant PC (T) = PICO, then equation (1) will be transformed
in (4), and then in (5) after differentiation.
PL(T) = ∫ (PICO – FUN(PL(T))dt + PL(T0),
PL´(T) = PICO – FUN(PL(T))
If equation (5) at T -> infinity has solution PLB, it can be found from equation (6) as PL´(T) aims
to 0 at stabilization PL(T).
PICO = FUN(PLB), i.e.
PLB = FIN(PICO), where FIN – inverse function for FUN.
For example, if FUN is dependence of type (3), than PLB is calculated by formula (8). In
particular it means that if PICO increases in 2 times PLB increases only in 2**(2/3) =1,6 times.
PLB = (PICO/A)**(2/3).
Thus water level fluctuates near PLB.
2009-2012, Peslyak MY, Model of pathogenesis of psoriasis. Part 1. Systemic psoriatic process. e4.0a. 50
Appendix 10. Fractionation of blood monocytes under chronic PAMP-load
After the exit from bone marrow monocytes stay in blood flow before they will be involved into
tissues or lymph nodes. Leaving from blood flow of certain monocyte occurs in a random way. kes place for time of stay of monocytes in blood flow (9)
F(T) = LAM*EXP(-LAM*T), where LAM = ln(2)/Thalf
Calculations and estimations of different authors for Thalf - time of half-life of monocytes in
human blood flow are re
Tab. 7. Time of half-life of monocytes in blood flow
In ) the quantity of monocytes, in systemic blood
table and text on
flow for 8 people surveyed (middle age 57 years) on the average
p. 254 (with ref.
is estimated as 8,0*E+7/kg, the same value is specified in
Now it is well-known that averagely it is nearby
2,8*E+7/kg. Marks of own monocytes were carried out by
Tmid=Thalf/ln(2)
3H-DPF in vitro, and then their injection was carried out
(autohemotrasfusion). Authors note the loss of up to 40% of
marked monocytes within the first 1-2 hours after injection,
assuming their apoptosis owing to damages at reception, marks
in vitro and injections. Authors consider that main reason of
abbreviation of number of marked monocytes in blood flow in the
next hours, is their attraction in tissues or lymph nodes. They completely neglect possible essential apoptotic loss of marked monocytes in blood flow in the next hours. Also have received as underestimated Thalf, as much essential this loss was.
Average time of stay of monocytes in blood flow Tmid =12-48
hours is resulted.
Thalf=Tmid*ln(2)=0,5*(12+48)*ln(2)=17,3 hours
executed here. References are given on ) and on (Javorkovsky LI, 1987).
p.7; In these works average time of stay of monocytes in blood flow
Tmid = 20-40 hours is resulted.
Thalf=Tmid*ln(2)=0,5*(20+40)*ln(2)=21 hour
executed here. Comments and references are not present.
7 patients of hospital (middle age of 61 years)
without hematological problems are surveyed. Marks of blood
p. 255 (both with
monocytes in vivo occurred during their bone marrow division
owing to injection of tritiated thymidine. Marked monocytes have
appeared in blood flow every other day, their quantity has
reached a maximum in 3 days, and then began to decrease.
Owing to injection neutrophils were marked also. As the full cycle
p. of their bone marrow development reaches 4-6 days, so marked 31.
neutrophils have appeared in blood flow only on 5th day, and
their quantity has reached maximum on 8th day. Authors certainly could not consider recently opened effect of return of senescent neutrophils back into the bone marrow with their subsequent apoptosis ). This effect (since 5 days) could affect the additional relay marks of bone marrow monocytes and by that on essential increase of Thalf.
It is easy to notice, what even with modern researchers estimations of Thalf strongly differ. In
work two extreme value, based on results ) and ) are
2009-2012, Peslyak MY, Model of pathogenesis of psoriasis. Part 1. Systemic psoriatic process. e4.0a. 51
resulted. Surprisingly, but from 1972-3 any group of researchers did not execute similar experiments on humans. And, hence, untill now there are no proofs present, which of these values (8,4 hours or 71 hour) are closer to reality.
In later years experiments by definition of Thalf were executed only for the experimental animal
(mice, rats, rabbits or monkeys). The comparative review of techniques and results contains in ). In the same work the stay of monocytes in blood flow for rabbits (marks by BrdU of donor monocytes in vivo and their subsequent injection to recipients) is investigated and are received Thalf(rabbits)=12,7 hours. According to authors their result correlates with received earlier by other researchers: Thalf(mice)=17,4 hours, Thalf(rats) =42 hours and Thalf(human) =71 hour.
On the basis of results it is possible to make calculation and to receive Thalf
(macaques-rhesus)=63 hours.
Which Thalf is closer to reality does not influence a sense of the assumptions stated
further about the fractionation of blood monocytes under chronic PAMP-load. But to accurately formulate these assumptions, it is necessary to take concrete value, for example, average between the extremes.
(10) Thalf = (8,4+71)/2 = 39,7 hours = 1,65 days
The graph of exponential distribution is executed at Thalf, defined under the formula
Rate of production of monocytes at serious psoriasis is above the norm in Ka=1,6, and their
quantity in blood flow is above the norm in Kb=1,9 Proceeding from it at serious psoriasis
(11) GAM1 = GAM*Ka/Kb, where GAM = 1 - EXP(-LAM) and GAM1 = 1 - EXP(-LAM1)
(12) Thalf1 = ln(2)/LAM1 = 2,04 days
Graphs of exand executed at Thalf1, defined under the
The total PAMP-load on certain monocyte is directly proportional to time of its stay in blood flow.
Staying in blood flow under chronic PAMP-load the certain monocyte at first is activated, and then if yet has not left blood flow, is tolerized.
It is possible to assume that at chronic PAMP-load (a) in blood flow three fractions of monocytes simultaneously coexist: nonactivated, activated
(b) at SPP activated fraction is the biggest. Assumptions (a) and (b) allow to compound the facts about activated blood Mo at psoriasis
and hypothesis about existence of tolerized Mo-T (SP8).
At SPP nonactivated fraction basically consists of monocytes which have recently left bone
marrow. These are monocytes of short-term stayAellow zone).
At SPP activated fraction consists of monocytes of intermediate-term stay and constantly
replenishes with monocytes from nonactivated fraction (it is possible and from bone marrow - Nonactivated monocytes during the first events of essential PAMP-load (contacts, linkage, endocytosis) become activ-k zone).
Activated monocytes secrete TNF-alpha more strongly, than nonactivated (as it is spontaneous
and in reply to the subsequent PAMP-load). Activated monocytes change chemostatus, they reduce the expression of the basic chemokine receptors S which are responsible for their attraction in tissue. S is (CCR1, CCR2, CCR5, CXCR4) for CD14+CD16+Mo ) and (CCR1, CCR2, CXCR3, CXCR4) for CD14++Mo.
probable fractionation of blood CD14+CD16+Mo under chronic
PAMP-load is represented. Distribution and fractionation of all blood monocytes under chronic PAMP-load is supposed to be similar.
Monocytes with increased expression of CCR7 carry endocytosed F-content into lymph nodes,
while in blood flow mainly remain monocytes with lowered expression of CCR7.
2009-2012, Peslyak MY, Model of pathogenesis of psoriasis. Part 1. Systemic psoriatic process. e4.0a. 52
In activated fraction there are monocytes F(+)TNF-alpha(+)S(-)Mo which not completely
degraded F-content. But is also TNF-alpha(+)S(-)Mo with the lowered F-content which were activated mainly due to contacts and linkage with PAMP and-or already thoroughly degraded the endocytosed earlier F-content.
At SPP tolerized fraction consists of monocytes of long-term stay and constantly replenishes
with monocytes from activated fraction. Activated monocytes after the long essential PAMP-load become tolerized. It occurs at achievement IRAK-M of blocking level Tolerized monocytes secrete TNF-alpha more weakly, than nonactivated (as it is spontaneous and in reply to the subsequent PAMP-load). Tolerized monocytes change chemostatus, restoring expression of the basic chemokine receptors S which is responsible for attraction into the tissues. Tolerized monocytes essentially reduce expression CCR7. As a result chemostatus of tolerized monocytes becomes similar to chemostatus of nonactivated monocytes (Property 1). For this reason, under the influence of chemokines they migrate similarly to nonactivated monocytes, including at renewal of pool of tissue monocytes.
In tolerized fraction there are monocytes PG-Y(+)F(+)Mo-R which are keeping essential part of
PG-Y. But as well there are also PG-Y(-)F(+)Mo-T which were tolerized mainly due to contacts and linkage with PAMP different from PG-Y and-or already degraded endocytosed earlier PG-Y.
All tolerized fraction of monocytes has Properties 1 and 2, then they are CCR7(-)S(+). But only
the part of tolerized fraction has simultaneously Properties 1, 2 and 3 and there are R-monocytes PG-Y(+)F(+)Mo-R.
Under the influence of chemokines the part of monocytes constantly leaves all three fractions,
going into tissues or lymph nodes.
On A) distribution on fractions of blood monocytes is represented. Here it is supposed
that bone marrow activation is absent, i.e. all monocytes leave bone marrow while being nonactivated. Conditionally it is supposed that level of chronic PAMP-load is such, that monocytes which have stayed in blood flow more than 2,5 days, become activated. Transition from nonactivated condition (yellow zone) to activated (pink zone) occurs gradually. Also conditionally it is supposed that for all monocytes the period of stay in blood flow from 5 till 8 days is transitive from activated condition (pink zone) to tolerized (green zone).
The areas of each of three zones correspond to share of each fraction: nonactivated ( 39%),
activated ( 51%) and tolerized ( 10%).
In Fig. 15-B possible graphs of five characteristics (F, IRAK-M, CCR7, S, TNF-alpha) which
change in process of stay of monocytes in blood flow under chronic PAMP-load are represented.
is represented the distribution by fractions of blood monocytes in the assumption that
bone marrow activation takes place ). In this case the part of monocytes leaves bone marrow already being activated (conditionally 50%). It is supposed that events occur in advance of 1,5 days before (in comparison w-A).
Monocytes which have stayed in blood flow more, than 1 day, become activated. Transition from
nonactivated condition (yellow zone) to activated (pink zone) occurs gradually. It is supposed that for all monocytes the period of stay in blood flow from 3,5 till 6,5 days is transitive from activated condition (pink zone) to tolerized (green zone). Areas of each of three zones correspond to share of each fraction: nonactivated ( 8%), activated ( 75%) and tolerized ( 17%).
I.e., at bone marrow activation the essential increase in shares of activated and tolerized
fractions takes place.
How to check up the reality of assumptions of fractionation of monocytes at SPP? For
representative group of psoriatics to execute the flowing cytometry of blood monocytes on several of characteristics set forth above (Fig. 15-B). As at SPP the main kPAMP are LPS and PG (incl. PG-Y) and it is necessary to take them as representatives of F-content.
For pair of PG-Y and IRAK-M expected distribution is represented. It is necessary to assume that at SPP similar fractionation and distribution
takes place also for the blood dendritic cells.
2009-2012, Peslyak MY, Model of pathogenesis of psoriasis. Part 1. Systemic psoriatic process. e4.0a. 53
Appendix 11. List of essential changes and additions
This appendix is intended for readers who are familiar with the previous edition of this book
(e3.2) and allows to get briefly acquainted only with essential changes and additions ). In the text the significant new or revised fragments are marked with a vertical line on the right. The new works included into the bibliography are also marked the same way.
Tab. 8. Changes and additions
There, where is represented materials concerning phagocytes of
various types (for example about Mo and DC) discrepancy is corrected: the word "chemostatus" is used in plural. All figures are moved to "Figures" section.
have exchanged in places. re corrected and added so that to become identical about fig. 2-1 and fig. 2-2 of Part 2. added. re changed. Tolerized phagocytes are designated Neu-T, Mo-T and DC-T.
Also derived from Mo-T macrophages and dendritic cells are designated MF-T and MoDC-T. For all tolerized phagocytes special images are offered) . In figures these images as a rule are used for PG-Y(-) tolerized phagocytes. Concerning chemostatus of senescent Neu-T specification is made. Definition of tolerized phagocytes is specified:
kPAMP-carriage is recognized by obligatory (Property 2).
Definition of R-phagocytes is specified: (PG-Y)-carriage is recognized by obligatory (Property 3). As a result there was actually renaming entities: Before: a) tolerized b) R-phagocytes and c) (PG-Y)+ R-phagocytes; Now: a) and b) tolerized and c) R-phagocytes. It has entailed the most part from changes listed further. In all fragments where earlier there were materials about
R-phagocytes (Mo-R and DC-R) corrections were made: Before: "R-phagocytes", Now: «Tolerized phagocytes (incl. R-phagocytes)». Before: «Mo-R and DC-R», now: «Mo-T and DC-T (incl. Mo-R and DC-R)», etc.
Before: «….essential share of R-phagocytes …»;
Now: «… essential share of tolerized phagocytes …» Similarly, in some other places the term "R-phagocyte" is replaced with «tolerized phagocyte».
Increased kPAMP-carriage of R-phagocytes.
Increased kPAMP-carriage of tolerized phagocytes.
Increased (PG-Y)-carriage of R-phagocytes .
2009-2012, Peslyak MY, Model of pathogenesis of psoriasis. Part 1. Systemic psoriatic process. e4.0a. 54
Changes and additions (continuation)
Subprocess SP4. The name is corrected.:
«PAMP-nemia. Increased kPAMP-load on blood phagocytes (Mo
and DC). Increased kPAMP level in blood. The major kPAMP are
PG (including PG-Y) and LPS.» Now: «PAMP-nemia. Increased kPAMP-load on blood phagocytes. Increased kPAMP level in blood. The major kPAMP are PG and LPS.» In subprocess SP4 is allocated: «SP4.1. (PG-Y)-nemia». It is made for more accurate formulation of dependences and in connection with change of formulation SP8.
«Reprogramming blood Mo-R and DC-R and their increased kPAMP-carriage (certainly with PG-Y).» Now: «Growth of tolerized fractions Mo-T and DC-T. Increased kPAMP-carriage.» In subprocess SP8 is allocated: «SP8.1. Growth of subfractions Mo-R and DC-R. Increased (PG-Y)-carriage.»
«Attraction of Mo, Mo-R, DC, DC-R from blood.» Now: «Attraction of Mo and DC, Mo-T and DC-T (incl. Mo-R and DC-R) from blood.»
2009-2012, Peslyak MY, Model of pathogenesis of psoriasis. Part 1. Systemic psoriatic process. e4.0a. 55
Changes and additions (continuation)
«Increased kPAMP-carriage of blood Neu-R. Return of senescent Neu-R from blood to bone marrow and their apoptosis.» Now: «Increased kPAMP-carriage of Neu-T. Increased (PG-Y)-carriage of Neu-R. Return of senescent Neu from blood to bone marrow and their apoptosis.»
c_Titul c_Imprint c_List_of_figures c_Abstract c_Keywords c_Bibliography c_Content c_Figures c_Discussion
LP1 c_Model_of_pathogenesis
PR1 PR2 PR3 PR4 PR5 PR6 PR7 PR8 PR9 PR10 PR11
c_Review_of_models
SP1 SP10 SP2 SP2_1SP3 SP4 SP4_1 SP5 SP6 SP7 SP8 SP8_1 SP9
2009-2012, Peslyak MY, Model of pathogenesis of psoriasis. Part 1. Systemic psoriatic process. e4.0a. 56
Fig. 1. Model of pathogenesis of psoria
2009-2012, Peslyak MY, Model of pathogenesis of psoriasis. Part 1. Systemic psoriatic process. e4.0a. 57
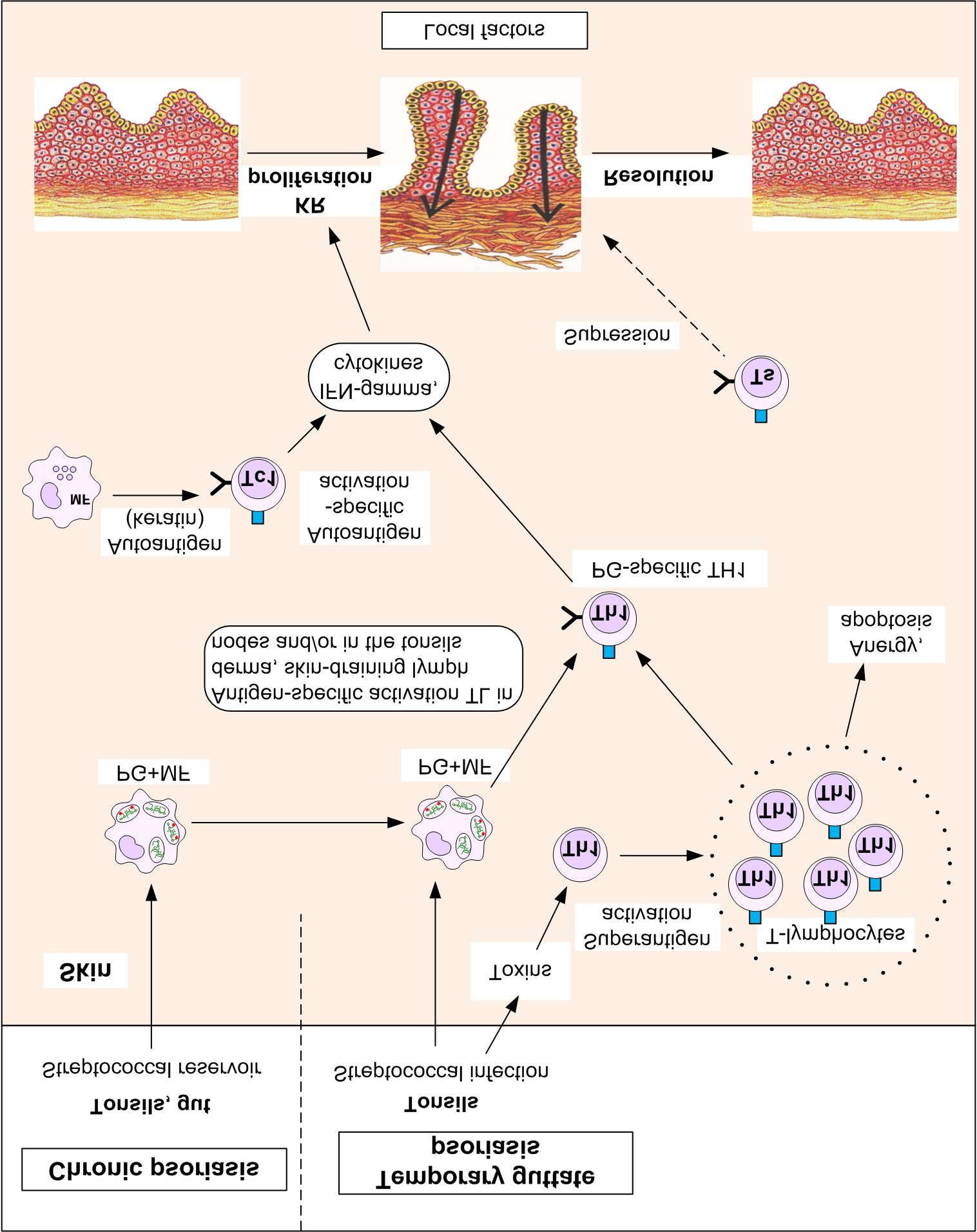
Fig. 2. PG-induced TL (T-lymphocytes) activation in temporary guttate psoriasis (GP) and chronic
psoriasis (CPs).
In GP, streptococci in the tonsils produce toxins (e.g. SPE-C) that function as superantigens and activate
TL(Th1). Superantigens also induce TL expression of the skin-homing receptor, CLA (blue). Most of these
activated TL become anergic to further activation, or die (by apoptosis), whereas a subset PG-specific TL
are rescued by stimulation by PG+MF (PG-carrying macrophages) have migrated from the tonsils.
Cytokines, including IFN-gamma, produced by these PG-specific TL induce keratinocyte (KR) proliferation
and skin lesion development. The resolution of skin lesions is mediated by T-suppressor cells (Ts) and
local factors by unknown mechanisms. In CPs, streptococci and/or streptococcal antigens persist in the
tonsils and/or gut. PG+MF migrate to the skin to activate pathogenic PG-specific Th1. The disease
process is maintained by cytokines produced by Tc1 cells (CD8+TL that produce IFN-gamma but not
IL-4), activated locally by skin-derived antigen (e.g. keratin), preventing resolution. Mainly based on Fig.2
from
2009-2012, Peslyak MY, Model of pathogenesis of psoriasis. Part 1. Systemic psoriatic process. e4.0a. 58
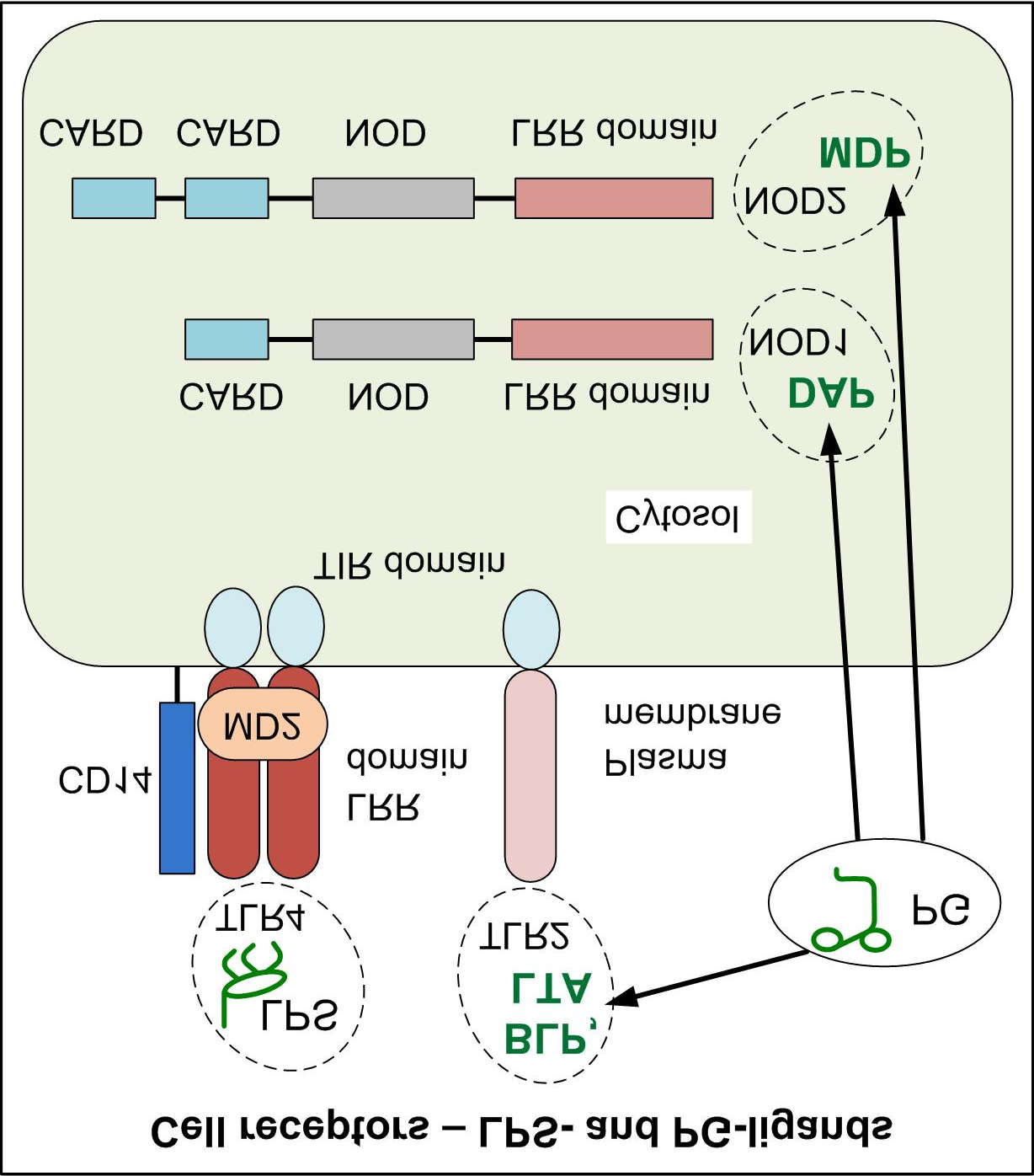
Fig. 3. Structure and cell's localization of TLR2, TLR4, NOD1 and NOD2.
TLR4 – LPS-ligand; Receptors for PG(peptidoglycan)-fragments: TLR2 – ligand of BLP and LTA; NOD1 – ligand of DAP; NOD2 – ligand of MDP. Based
2009-2012, Peslyak MY, Model of pathogenesis of psoriasis. Part 1. Systemic psoriatic process. e4.0a. 59
Fig. 4. Bacteria, bacterial products, viruses and tissue cells (symbols).
2009-2012, Peslyak MY, Model of pathogenesis of psoriasis. Part 1. Systemic psoriatic process. e4.0a. 60
Fig. 5. Immune cells (symbols).
2009-2012, Peslyak MY, Model of pathogenesis of psoriasis. Part 1. Systemic psoriatic process. e4.0a. 61
Fig. 6. SPP (main subprocesses) and some local processes. Illustration.
2009-2012, Peslyak MY, Model of pathogenesis of psoriasis. Part 1. Systemic psoriatic process. e4.0a. 62
Fig. 7. SPP and local subprocess LP1.1. The scheme of dependencies.
Letters A - vicious cycle. Subprocesses SP2, SP4 and SP8 (green contour) make SPP-basis . Relay variant (SP9, SP10) -
2009-2012, Peslyak MY, Model of pathogenesis of psoriasis. Part 1. Systemic psoriatic process. e4.0a. 63
Fig. 8. SPP-basis. Obligatory subprocesses SP2, SP4 and SP8.
Two components of SPP-basis: tolerization of phagocytes and (PG-Y)-carriage of phagocytes.
2009-2012, Peslyak MY, Model of pathogenesis of psoriasis. Part 1. Systemic psoriatic process. e4.0a. 64
Fig. 9. CD14+CD16+Mo-R (= F+CCR7(-)S2+Mo-R) formation.
S2+ - nonactive (or tolerized) chemostatus, S2(-) – active chemostatus of CD14+CD16+Mo. There are main chemokine receptors. Black arrows - transformation, other - traffic. At LP1.1 non-lymphatic tissues are derma. Increasing of chronic kPAMP-load and (PG-Y)-load levels is likely to enlarge part of CD14+CD16+Mo-R up to 5-10% among all CD14+CD16+Mo. It is supposed that in F-content contains PG-Y. In the absence of PG-Y it is possible to replace designation Mo-R on Mo-T.
2009-2012, Peslyak MY, Model of pathogenesis of psoriasis. Part 1. Systemic psoriatic process. e4.0a. 65
Fig. 10. Results of two large-scale statistical studies:
- more than 48000 psoriat- more than 16000 psoriatics.
2009-2012, Peslyak MY, Model of pathogenesis of psoriasis. Part 1. Systemic psoriatic process. e4.0a. 66
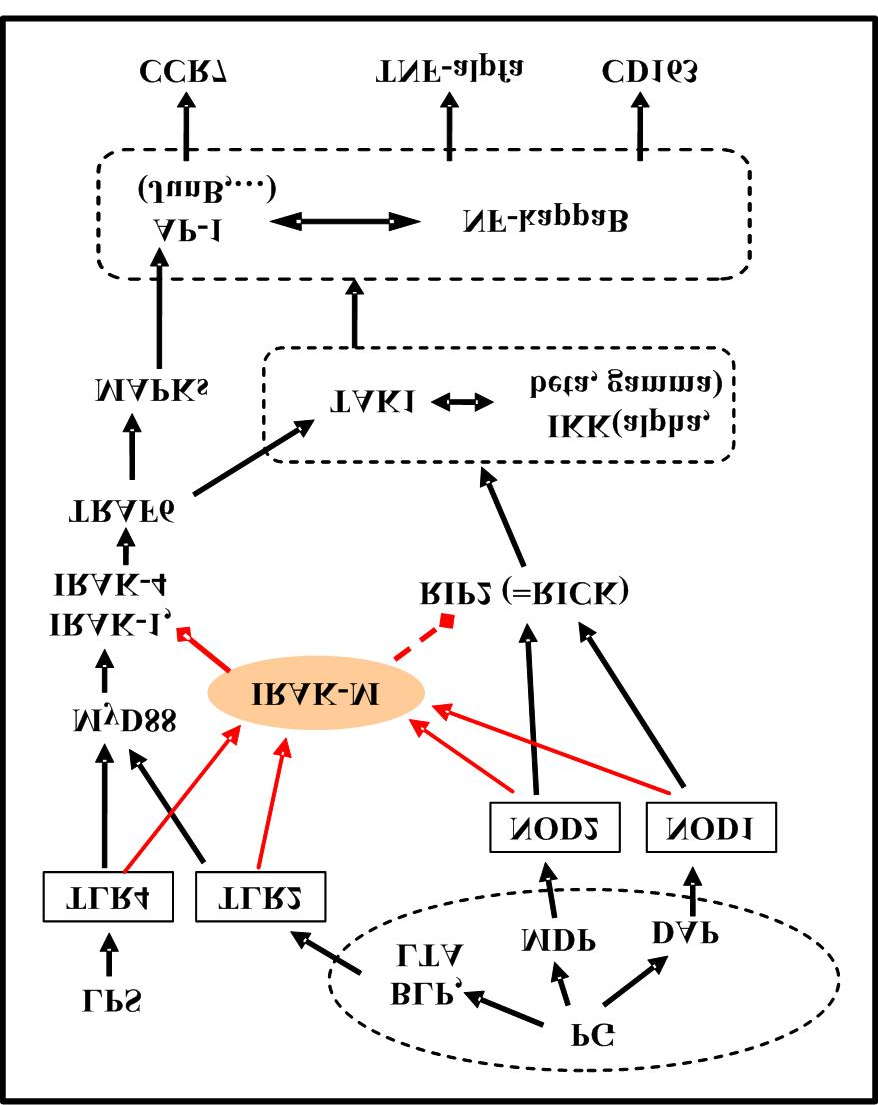
Fig. 11. Fragment of signal paths from kPAMP-load to CCR7 and CD163 expression and TNF-alpha
secretion.
Red arrows - production increasing, red rhombuses – blocking, red dot-dash - presumable influence.
2009-2012, Peslyak MY, Model of pathogenesis of psoriasis. Part 1. Systemic psoriatic process. e4.0a. 67
Fig. 12. Bone marrow transformation Mo and DC with of Neu participation.
2009-2012, Peslyak MY, Model of pathogenesis of psoriasis. Part 1. Systemic psoriatic process. e4.0a. 68
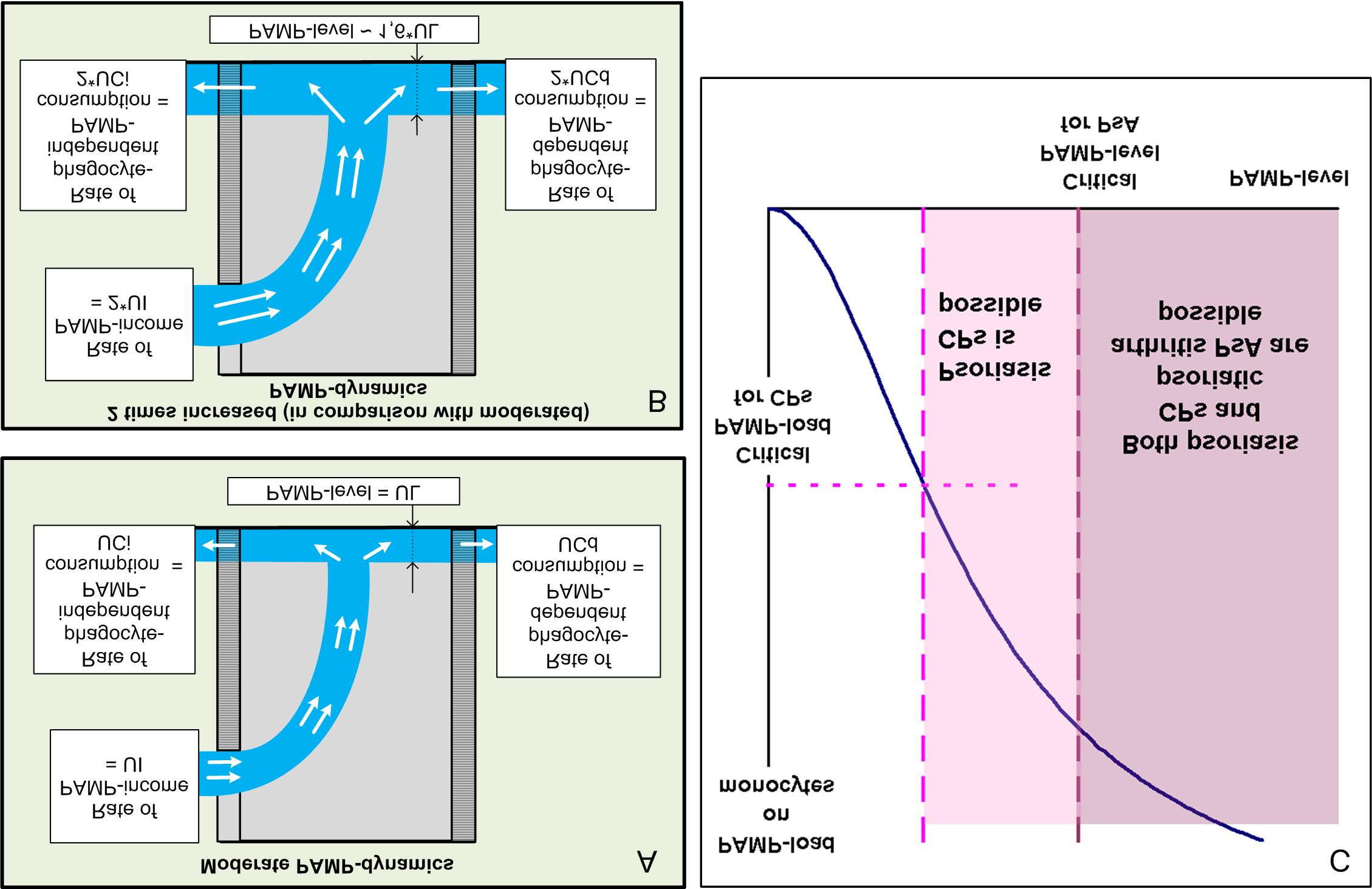
Fig. 13. PAMP-income and PAMP-consumption.
Blood flow = container with non-hermetic walls (phagocyte-independent PAMP-consumption is made through left wall, phagocyte-dependent PAMP-consumption is made through right wall). Rates of PAMP-income and RAMP-consumption correspond to length and number of white arrows. They are 2 times larger on scheme B than on scheme A, however PAMP-level raised less than in 2 times (calculation by formula for such container). (C) Critical PAMP-load for CPs possibility is reached earlier than critical PAMP-level for PsA possibility.
2009-2012, Peslyak MY, Model of pathogenesis of psoriasis. Part 1. Systemic psoriatic process. e4.0a. 69
Fig. 14. Exponential distribution of time of stay of monocytes in blood flow in norm.
The chronic PAMP-load is absent..
2009-2012, Peslyak MY, Model of pathogenesis of psoriasis. Part 1. Systemic psoriatic process. e4.0a. 70
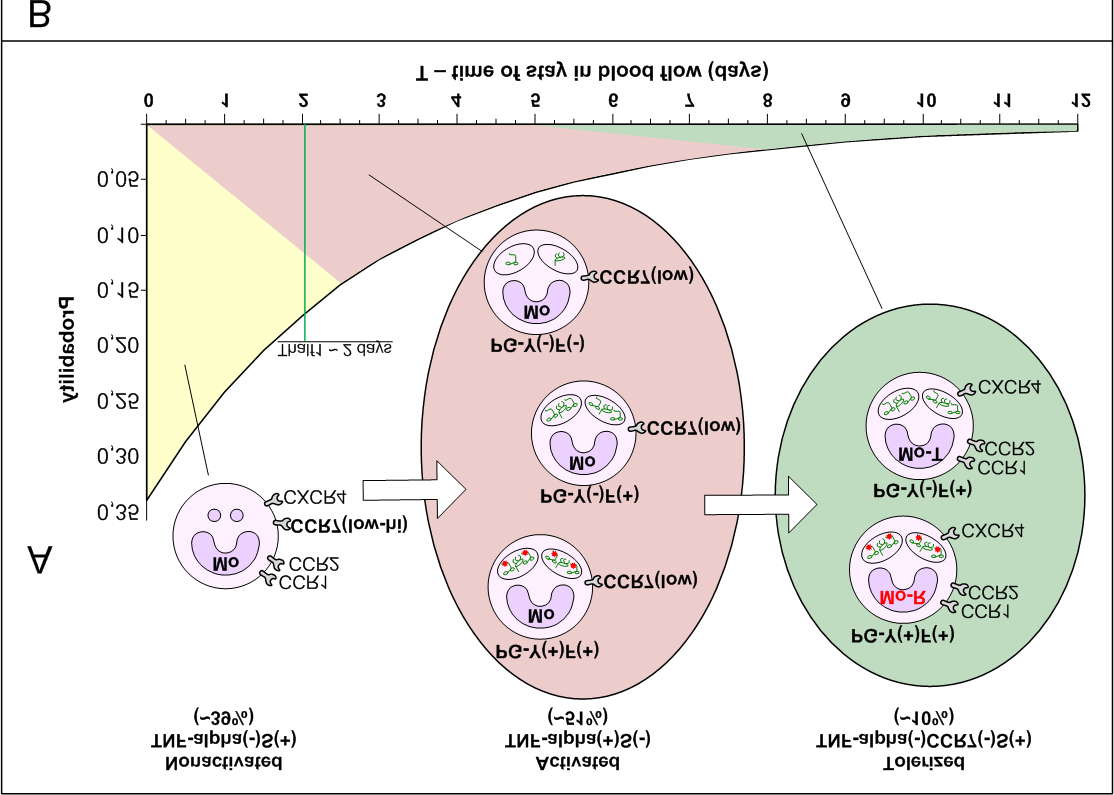
Fig. 15. Fractionation of blood monocytes without bone marrow activation and possible graphs at SPP.
Fractionation at SPP (A). Possible graphs of expression and secretion of proteins, chemokine receptors and cytokines at blood monocytes depending on time of stay in blood flow (B).
2009-2012, Peslyak MY, Model of pathogenesis of psoriasis. Part 1. Systemic psoriatic process. e4.0a. 71
Fig. 16. Fractionation of blood monocytes at SPP in case of bone marrow activation.
Thalf1=2,04 days.
2009-2012, Peslyak MY, Model of pathogenesis of psoriasis. Part 1. Systemic psoriatic process. e4.0a. 72
Fig. 17. Expected (PG-Y, IRAK-M)-distribution of blood monocytes at SPP.
2009-2012, Peslyak MY, Model of pathogenesis of psoriasis. Part 1. Systemic psoriatic process. e4.0a. 73
Bibliography
Abdulkadyrov KM. Hematology. Newest directory. Eksmo, 2004, 928 p. (Rus). ISBN 5699050744.
Abenavoli L, Leggio L, Gasbarrini G, Addolorato G. Celiac disease and skin: Psoriasis association. World J
Gastroenterol. 2007 Apr 14;13(14):2138-
Adcock IM, Ito K, Barnes PJ. Glucocorticoids Effects on Gene Transcription. Proc Am Thorac Soc.
Adib-Conquy M, Cavaillon JM. Gamma interferon and granulocyte/monocyte colony-stimulating factor prevent
endotoxin tolerance in human monocytes by promoting interleukin-1 receptor-associated kinase expression and its association to MyD88 and not by modulating TLR4 expression. J Biol Chem . 2002 Aug 2;277(31):27927-34.
Adib-Conquy M, Cavaillon JM. Compensatory anti-inflammatory response syndrome. Thromb Haemost. 2009
Altmeyer P, Munz DL, Chilf G. et al. Morphological and functional findings of fixed phagocytes in psoriatics.
Arch Dermatol Res. 1983;275(2):95-
Ancuta P, Rao R, Moses A. et al. Fractalkine preferentially mediates arrest and migration of CD16+
monocytes. J Exp Med. 2003 Jun 16;197(12):1701-
Assimakopoulos SF, Scopa CD, Vagianos CE. Pathophysiology of increased intestinal permeability in
obstructive jaundice. World J Gastroenterol. 2007 Dec 28;13(48):6458-
Auffray C, Sieweke MH, Geissmann F. Blood monocytes: development, heterogeneity, and relationship with
Bachmann MF, Kopf M, Marsland BJ. Chemokines: more than just road signs. Nat Rev Immunol. 2006
a. Baker BS, Laman JD, Powles AV. et al, Peptidoglycan and peptidoglycan -specific Th1 cells in psoriatic skin
lesions, J Pathol 2006 Jun;209(2):174-
Baker BS, Ovigne JM, Fischetti VA, Powles A, Fry L. Selective Response of Dermal Th-1 Cells to 20-50 kDa
Streptococcal Cell-Wall Proteins in Chronic Plaque Psoriasis, Scand J Immunol. 2003 Sep; 58(3): 335 -.
b. Baker BS, Powles A, Fry L. Peptidoglycan: a major aetiological factor for psoriasis? Trends Immunol. 2006
Baker BS. Recent Advances in PSORIASIS: The Role of the Immune System. ICP Imperial College Press,
2000, 180 p. ISBN 1860941206.
Baltabaev MK, Khamidov SA, Valikhanov UA, Khamidov FS. Psoriasis and bile acids metabolism, Vestn
Dermatol Venerol 2005;(4):25-28. (Rus), ISSN 0042-460
Bartz H, Avalos NM, Baetz A, Heeg K, Dalpke AH. Involvement of suppressors of cytokine signaling in toll-like
receptor-mediated block of dendritic cell differentiation. Blood. 2006 Dec 15;108(13):4102-
Begley M, Gahan CG, Hill C. The interaction between bacteria and bile. FEMS Microbiol Rev. 2005
Berger DP, Engelhardt M, Henb H. Mertelsmann R. Concise Manual of Hematology and Oncology, Springer,
Berthelot JM. Psoriatic arthritis as a mountain, Reumatismo, 2003; 55(1):6-
Bevelacqua V, Libra M, Mazzarino MC et al. Long pentraxin 3: a marker of inflammation in untreated psoriatic
patients. Int J Mol Med. 2006 Sep;18(3):415-23.
Billot-Klein D, Gutmann L, Bryant D. et al. Peptidoglycan synthesis and structure in Staphylococcus
haemolyticus expressing increasing levels of resistance to glycopeptide antibiotics. J Bacteriol. 1996 Aug;178(15):4696-
Biswas SK, Lopez-Collazo E. Endotoxin tolerance: new mechanisms, molecules and clinical significance.
Trends Immunol. 2009 Oct;30(10):475-
Blander JM, Medzhitov R. Toll-dependent selection of microbial antigens for presentation by dendritic cells.
Nature. 2006 Apr 6;440(7085):808-
Blander JM. Signalling and phagocytosis in the orchestration of host defence. Cell Microbiol. 2007
Bondarenko VM, Lykova EA, Matsulevich TV. Microecological aspects of small intestinal bacterial overgrowth
syndrome. Zh Mikrobiol Epidemiol Immunobiol. 2006 Sep-Oct;(6):57-63.
2009-2012, Peslyak MY, Model of pathogenesis of psoriasis. Part 1. Systemic psoriatic process. e4.0a. 74
Bos JD, de Rie MA, Teunissen MB, Piskin G. Psoriasis: dysregulation of innate immunity, Br J Dermatol. 2005
Jun;152(6):1098-
Bouhnik Y, Alain S, Attar A. et al. Bacterial populations contaminating the upper gut in patients with small
intestinal bacterial overgrowth syndrome. Am J Gastroenterol. 1999 May;94(5):1327-3
Boyman O, Conrad C, Tonel G, Gilliet M, Nestle FO. The pathogenic role of tissue-resident immune cells in
psoriasis. Trends Immunol. 2007 Feb;28(2):51-
Boyman O, Hefti HP, Conrad C, Nickoloff BJ, Suter M, Nestle FO. Spontaneous development of psoriasis in a
new animal model shows an essential role for resident T cells and tumor necrosis factor--alpha., J Exp Med. 2004 Mar 1;199(5):731-
Buckley JM, Wang JH, Redmond HP. Cellular reprogramming by gram-positive bacterial components: a
review. J Leukoc Biol. 2006 Oct;80(4):731-4
Bures J, Cyrany J, Kohoutova D et al. Small intestinal bacterial overgrowth syndrome. World J Gastroenterol.
2010 Jun 28;16(24):2978-90.
Cai YH, Lu ZY, Shi RF et al. Enhanced Proliferation and Activation of Peripheral Blood Mononuclear Cells in
Patients with Psoriasis Vulgaris Mediated by Streptococcal Antigen with Bacterial DNA. J Invest Dermatol. 2009 Nov;129(11):2653-
Canto E, Moga E, Ricart E. et al. MDP-Induced selective tolerance to TLR4 ligands: impairment in NOD2
mutant Crohn's disease patients. Inflamm Bowel Dis. 2009 Nov;15(11):1686-.
Carapetis JR, Steer AC, Mulholland EK, Weber M. The global burden of group A streptococcal diseases.
Lancet Infect Dis. 2005 Nov;5(11):685-
Cavaillon JM, Adib-Conquy M. Bench-to-bedside review: endotoxin tolerance as a model of leukocyte
reprogramming in sepsis. Crit Care. 2006;10(5
Cavaillon JM, Adrie C. Sepsis and Non-infectious Systemic Inflammation: From Biology to Critical Care,
Wiley-VCH, 2008, 446 p. ISBN 9783527319350.
Chatzikyriakidou A, Voulgari PV, Georgiou I, Drosos AA. The role of microRNA-146a (miR-146a) and its
target IL-1R-associated kinase (IRAK1) in psoriatic arthritis susceptibility. Scand J Immunol. 2010 May;71(5):382-
Chin AC, Flynn AN, Fedwick JP, Buret AG. The role of caspase-3 in lipopolysaccharide-mediated disruption
of intestinal epithelial tight junctions. Can J Physiol Pharmacol. 2006 Oct;84(10):1043-
Christophers E. Comorbidities in psoriasis. JEADV 2006, 20 (Suppl. 2), 52 –
Ciampolini M, Bini S, Orsi A. Microflora persistence on duodenum-jejunal flat or normal mucosa in time after a
meal in children. Physiol Behav. 1996 Dec;60(6):1551-
Clark RA, Kupper TS. Misbehaving macrophages in the pathogenesis of psoriasis., J Clin Invest. 2006,
Aug;116(8):2084-
Clarke TB, Davis KM, Lysenko ES et al. Recognition of peptidoglycan from the microbiota by Nod1 enhances
systemic innate immunity. Nat Med. 2010 Feb;16(2):228-
Cohen AD, Sherf M, Vidavsky L. et al. Association between psoriasis and the metabolic syndrome. A cross-
sectional study. Dermatology. 2008;216(2):152-
Coll RC, O'Neill LA. New insights into the regulation of signalling by toll -like receptors and nod-like receptors.
J Innate Immun. 2010;2(5):406-
Conte MP, Schippa S, Zamboni I. et al. Gut-associated bacterial microbiota in paediatric patients with
inflammatory bowel disease. Gut. 2006 Dec;55(12):1760-
Chesnokova NP, Mihajlov AV, Ponukalina EV, et al. Infectious process. "Natural sciences academy", 2006,
Chapter 1.3, (Rus), ISBN 5986540190.
Crossley KB, Archer G, Jefferson K, Fowler V. Staphylococci in Human Disease. John Wiley and Sons, 2009,
640 p. ISBN 9781405163323.
Damasiewicz-Bodzek A, Wielkoszyński T. Serologic markers of celiac disease in psoriatic patients. J Eur
Acad Dermatol Venereol. 2008 Sep;22(9):1055-
Danilenko DM. Review paper: preclinical models of psoriasis. Vet Pathol. 2008 Jul;45(4):563-
Davidovici BB, Sattar N, Prinz JC et al. Psoriasis and Systemic Inflammatory Diseases: Potential Mechanistic
Links between Skin Disease and Co-Morbid Conditions. J Invest Dermatol. 2010 Jul;130(7):1785-96.
Degos L, Linch D, Lowenberg B. Textbook of malignant haematology, Taylor & Francis, 1999, 944
2009-2012, Peslyak MY, Model of pathogenesis of psoriasis. Part 1. Systemic psoriatic process. e4.0a. 75
De Groot M, Teunissen MB, Ortonne JP. et al. Expression of the chemokine receptor CCR5 in psoriasis and
results of a randomized placebo controlled trial with a CCR5 inhibitor. Arch Dermatol Res. 2007 Sep;299(7):305-
De Jongh GJ, Zeeuwen PL, Kucharekova M. et al. High expression levels of keratinocyte antimicrobial
proteins in psoriasis compared with atopic dermatitis J Invest Dermatol. 2005 Dec;125(6):1163-73.
De Vos AF, Pater JM, van den Pangaart PS et al. In vivo lipopolysaccharide exposure of human blood
leukocytes induces cross-tolerance to multiple TLR ligands. J Immunol. 2009 Jul 1;183(1):533-42.
De Waele B, Van Nieuwenhove Y, Lauwers S, Delvaux G. Biliary tract infection in patients with acute biliary
pancreatitis, Surg Infect (Larchmt). 2003 Fall;4(3):241-
Diluvio L, Vollmer S, Besgen P, Ellwart JW, Chimenti S, Prinz JC. Identical TCR beta-chain rearrangements in
streptococcal angina and skin lesions of patients with psoriasis vulgaris. J Immunol. 2006 Jun 1;176(11):7104-
a. Dworkin M, Falkow S. The prokaryotes: a handbook on the biology of bacteria, Volume 3, Science, 2006,
1143 p. ISBN 9780387254937.
b. Dworkin M, Falkow S. The prokaryotes: a handbook on the biology of bacteria, Volume 4, Science, 2006,
1140 p. ISBN 9780387254944.
Eckburg PB, Bik EM, Bernstein CN et al. Diversity of the human intestinal microbial flora. Science. 2005 Jun
10; 308(5728): 1635-
Ekman AK, Cardell LO. The expression and function of Nod-like receptors in neutrophils. Immunology. 2010
El-Rachkidy RG, Hales JM, Freestone PP. et al. Increased Blood Levels of IgG Reactive with Secreted
Streptococcus pyogenes Proteins in Chronic Plaque Psoriasis. J Invest Dermatol. 2007 Jun;127(6):1337-.
Erridge C. The roles of pathogen-associated molecular patterns in atherosclerosis. Trends Cardiovasc Med.
2008 Feb;18(2):52-.
. Falova OE. Features of microflora of intestine at psoriasis against Blastocystis hominis invasion, Dissertation.
Ulyanovsk, 2004, 180 p. (Rus)
Fabriek BO, van Bruggen R, Deng DM et al. The macrophage scavenger receptor CD163 functions as an
innate immune sensor for bacteria. Blood. 2009 Jan 22;113(4):887-
Fasano A, Nataro JP. Intestinal epithelial tight junctions as targets for enteric bacteria-derived toxins. Adv
Drug Deliv Rev. 2004 Apr 19;56(6):795-
Fasano A, Shea-Donohue T. Mechanisms of disease: the role of intestinal barrier function in the pathogenesis
of gastrointestinal autoimmune diseases. Nat Clin Pract Gastroenterol Hepatol. 2005 Sep;2(9):416-22.
Fitting C, Dhawan S, Cavaillon JM. Compartmentalization of tolerance to endotoxin. J Infect Dis. 2004 Apr
Fry L. An Atlas of Psoriasis, Second Edition (Encyclopedia of Visual Medicine Series), Taylor & Francis, 2005,
108 p. ISBN 1842142372.
a. Fry L, Baker BS, Powles AV. Psoriasis - A possible candidate for vaccination. Autoimmun Rev. 2007
b. Fry L, Baker BS. Triggering psoriasis: the role of infections and medications. Clin Dermatol. 2007 Nov-
Fuentes-Duculan J, Suárez-Fariñas M, Zaba LC et al. A Subpopulation of CD163-Positive Macrophages Is
Classically Activated in Psoriasis. Journal of Investigative Dermatology 2010 Oct; 130:2412-2422.
Fukuda Y, Bamba H, Okui M. et al. Helicobacter pylori infection increases mucosal permeability of the
stomach and intestine. Digestion. 2001;63 Suppl 1:93-
Garaeva ZS. Clinical significance of levels of antiendotoxic and antibacterial immunity in psoriatics.
Dissertation, Kazan, 2005, 142 p. (Rus).
Garaeva ZS, Safina NA, Kuklin VT, Bilduk EV, Zinkevich OD. Particular qualities of humoral antibacterial
immunity in psoriatics, Kazan Medical Journal, 2001;(5):359-61. (Rus), ISSN 0368-4
Geisel J, Kahl F, Müller M et al. IL-6 and maturation govern TLR2 and TLR4 induced TLR agonist tolerance
and cross-tolerance in dendritic cells. J Immunol. 2007 Nov 1;179(9):5811-
2009-2012, Peslyak MY, Model of pathogenesis of psoriasis. Part 1. Systemic psoriatic process. e4.0a. 76
Giardina E, Sinibaldi C, Novelli G. The Psoriasis Genetics as a Model of Complex Disease, Current Drug
Targets - Inflammation & Allergy, 2004, 3, 129-
Ginhoux F, Tacke F, Angeli V. et al. Langerhans cells arise from monocytes in vivo. Nat Immunol. 2006
Girardin SE, Boneca IG, Viala J. et al. Nod2 is a general sensor of peptidoglycan through muramyl dipeptide
(MDP) detection. J Biol Chem. 2003 Mar 14;278(11):8869-.
Gisondi P, Targher G, Zoppini G, Girolomoni G. Non-alcoholic fatty liver disease in patients with chronic
plaque psoriasis. J Hepatol. 2009 Oct;51(4):758-
Gladman DD. Clinical, radiological, and functional assessment in psoriatic arthritis: is it different from other
inflammatory joint diseases? Ann Rheum Dis. 2006 Nov;65 Suppl 3:iii22-
Glebova NS. Changes of intestine microbiocenosis under the influence of destabilizing action of blastocystic
invasion. Bulletin OGU, 2007 (5):155-160. (Rus).
Grashin RA. Correction of proliferation of keratinocytes with the use of liposomes and its clinical and
laboratory estimation as a direction in the external therapy of psoriasis. Dissertation. St.-Petersburg, 2009, 231 p. (Rus).
Gubina LK, Kuralesina VP, Udenkova SN, Mikhailova ES, Rusanova TA. Influence of oral cavity professional
hygiene on quantity of beta-hemolitic streptococci of oral liquid and tooth-gingival sulcus, Voronezh State Medical Academy, Applied informative aspects of medicine, 2004; 7(2). (R
Goldman E. Green L. Practical handbook of microbiology. CRC Press, 2008, 874 p. ISBN 9780849393655.
Goto Y, Hogg JC, Suwa T. et al. A novel method to quantify the turnover and release of monocytes from the
bone marrow using the thymidine analog 5'-bromo-2'-deoxyuridine. Am J Physiol Cell Physiol. 2003 Aug;285(2):C253-
Grebowska A, Moran AP, Matusiak A. et al. Anti-phagocytic activity of Helicobacter pylori lipopolysaccharide
(LPS)--possible modulation of the innate immune response to these bacteria. Pol J Microbiol. 2008;57(3):185-
Gudjonsson JE, Johnston A. Current understanding of the genetic basis of psoriasis. Expert Rev Clin
Immunol. 2009 Jul;5(4):433-
Gudjonsson JE, Johnston A, Sigmundsdottir H, Valdimarsson H. Immunopathogenic mechanisms in
psoriasis, Clin Exp Immunol., 2004 Jan;135(1):1-
Gudjonsson JE, Thorarinsson AM, Sigurgeirsson B, Kristinsson KG, Valdimarsson H. Streptococcal throat
infections and exacerbation of chronic plaque psoriasis: a prospective study., Br J Dermatol. 2003 Sep;149(3):530-4.
a. Gumayunova NG. Syndrome of small intestine bacterial overgrowth at psoriatic disease against blastocystic
invasion. Dissertation, Chelyabinsk, 2009, 169 p, (Rus),
b. Gumayunova NG, Potaturkina-Nesterova NI, Nesterov AS, Magomedov MA. New approaches to diagnosis
intestinal dysbiosis of patients who have psoriatic disease. Bulletin of Russian Peoples Friendship University. Medicine. 2009;(2):93-
c. Gumayunova NG. Revealing of small intestine bacterial overgrowth at psoriatic disease. The postgraduate
bulletin of the Volga region, 2009 (3-4):162-4. (Rus).
Gunn JS. Mechanisms of bacterial resistance and response to bile. Microbes Infect. 2000 Jul;2(8):907-13.
Gyurcsovics K., Bertok L. Pathophysiology of psoriasis: coping endotoxins with bile acid therapy,
Pathophysiology. 2003 Dec;10(1):57-
Hadley JS, Wang JE, Foster SJ, Thiemermann C, Hinds CJ. Peptidoglycan of Staphylococcus aureus
upregulates monocyte expression of CD14, Toll-like receptor 2 (TLR2), and TLR4 in human blood: possible implications for priming of lipopolysaccharide signaling. Infect Immun. 2005 Nov;73(11):7613-9.
Havkin AI. Role of microflora in development of immune system of gastrointestinal tract. Consilium Medicum,
Pediatrics, 2008;(3). (Rus), ISSN 0031-
Harkov EI, Prohorenkov VI, Shiryaeva YuA. Indices of functional activity of small intestine in patients with
psoriasis. Siberian Medical Review, 2008;(6):55-
Harkov EI, Shiryaeva YuA, Teryoshina DS. Malabsoption syndrome and psoriasis: the method of correction.
Siberian Medical Journal (Irkutsk), 2006;(7):61-63. (R
Harkov EI, Shiryaeva YuA. Malabsorption syndrome in psoriasis: clinical-laboratory parallels. Siberian Medical
Review, 2005;(2-3):62-64. (R
2009-2012, Peslyak MY, Model of pathogenesis of psoriasis. Part 1. Systemic psoriatic process. e4.0a. 77
Hasegawa A, Liu H, Ling B. et al. The level of monocyte turnover predicts disease progression in the
macaque model of AIDS. Blood. 2009 Oct 1;114(14):2917-
Hayashi H, Takahashi R, Nishi T, Sakamoto M, Benno Y. Molecular analysis of jejunal, ileal, caecal and recto -
sigmoidal human colonic microbiota using 16S rRNA gene libraries and terminal restriction fragment length polymorphism. J Med Microbiol. 2005 Nov;54(Pt 11):1093-
He YL, Lu XJ, Qiu JY, Zhu TJ. Severe vulgaris psoriatic patients with acute myelogenous leukaemia and
resolution after allogeneic bone marrow transplantation/peripheral blood stem cell transplantation. Chin Med J (Engl). 2005 May 20;118(10):861-
Hedl M, Li J, Cho JH, Abraham C. Chronic stimulation of Nod2 mediates tolerance to bacterial products. Proc
Natl Acad Sci U S A. 2007 Dec 4;104(49):19440-
Heggelund L, Damås JK, Yndestad A et al. Stimulation of toll-like receptor 2 in mononuclear cells from HIV-
infected patients induces chemokine responses: possible pathogenic consequences. Clin Exp Immunol. 2004 Oct;138(1):116-
Hendel L, Larsen JK, Ammitzbøll T, Asboe-Hansen G. A study of cell proliferation kinetics in the small
intestinal epithelium of psoriasis patients. Clin Exp Dermatol. 1984 Jul;9(4):329 -
Hirahata T, Bjorkman D, Chamberlain JK. Endotoxin: a twofold effect on bone marrow ultrastructure.
Scanning Microsc. 1987 Sep;1(3):1349-.
Hoffbrand V, Moss P, Pettit J. Essential Haematology, 5 edition, Wiley-Blackwell, 2005, 380 p.
Hofmann AF, Eckmann L. How bile acids confer gut mucosal protection against bacteria. Proc Natl Acad Sci
U S A. 2006 Mar 21;103(12):4333-
Hoijer MA, Melief MJ, Calafat J et al. Expression and intracellular localization of the human N -acetylmuramyl-
L-alanine amidase, a bacterial cell wall-degrading enzyme. Blood. 1997 Aug 1;90(3):1246-
Huffnagle G, Noverr M. GI Microbiota and Regulation of the Immune System. Landes Bioscience and
Springer Science+Business Media, LLC, 2008, 165 p. ISBN 9780387095493.
Hughes-Jones NC, Wickramasinghe SN, Hatton C. Lecture notes: Haematology. Edition 8,
Wiley-Blackwell, 2008, 216 p.
Husebye E. The pathogenesis of gastrointestinal bacterial overgrowth. Chemotherapy. 2005;51 Suppl 1:1-22.
Husebye H, Halaas O, Stenmark H. et al. Endocytic pathways regulate Toll-like receptor 4 signaling and link
innate and adaptive immunity. EMBO J. 2006 Feb 22;25(4):683-
Iakovlev MIu, Elements of endotoxin theory in human physiology and pathology, Fiziol Cheloveka.
2003;29(4):98-109. (Rus), 13
Ibliyaminova AA, Khismatullina ZR, Krukova AJ. Clinical and functional condition of psoriatics biliary tract.
Modern questions of dermatovenerology, immunology and medical cosmetology. 2009; 3:39-43. (Rus).
Ibrahim G, Waxman R, Helliwell PS. The prevalence of psoriatic arthritis in people with psoriasis. Arthritis
Rheum. 2009 Sep 29;61(10):1373-
Iizuka H, Takahashi H, Ishida-Yamamoto A. Psoriatic architecture constructed by epidermal remodeling.
Journal of Dermatological Science 2004; (35):93-
Itoh S, Kono M, Akimoto T. Psoriasis treated with ursodeoxycholic acid: three case reports. Clin Exp
Dermatol. 2007 Jul;32(4):398-
Jones ME, Karlowsky JA, Draghi DC et al. Epidemiology and antibiotic susceptibility of bacteria causing skin
and soft tissue infections in the USA and Europe: a guide to appropriate antimicrobial therapy. Int J Antimicrob Agents. 2003 Oct;22(4):406-
Johnson-Huang LM, Pensabene CA, Shah KR et al. Post-Therapeutic Relapse of Psoriasis after CD11a
Blockade Is Associated with T Cells and Inflammatory Myeloid DCs. PLoS One. 2012;7(2):e30308. Epub
Juffermans NP, Weijer S, Verbon A, Speelman P, van der Poll T. Expression of human immunodeficiency
virus coreceptors CXC chemokine receptor 4 and CC chemokine receptor 5 on monocytes is down-regulated during human endotoxemia. J Infect Dis. 2002 Apr 1;185(7):986-
Kanamori H, Tanaka M, Kawaguchi H. et al. Resolution of psoriasis following allogeneic bone marrow
transplantation for chronic myelogenous leukemia: case report and review of the literature.Am J Hematol. 2002 Sep;71(1):41-
2009-2012, Peslyak MY, Model of pathogenesis of psoriasis. Part 1. Systemic psoriatic process. e4.0a. 78
Kilpper-Balz R, Wenzig P, Schleifer KH. Molecular Relationships and Classification of Some Viridans
Streptococci as Streptococcus oralis and Emended Description Streptococcus ovalis. International Journal of Systematic Bacteriology, Oct. 1985, p. 482-488.
Karsonova MI, Il'inskaia AN, L'vov VL, Pinegin BV. Changes in functional activity of macrophages caused by
bacterial muramylpeptides. Zh Mikrobiol Epidemiol Immunobiol. 2007;(3):34-
Khairutdinov VR, Samtsov AV, Moshkalov AV, Imianitov EN. Current views of immune mechanisms
underlying the development of psoriasis. Vestn Dermatol Venerol, 2007;(1):3-7. (Rus), ISSN 0042-4609.
Khardikova SA, Beloborodova EI, Pesterev PN. Psoriasis, intestinal adsorption. Tomsk, NTL, 2000, 120 p.
(Rus), ISBN 589503084x.
Khardikova CA, Beloborodova EI, Pesterev PN, Nepomniashchikh GI. Morphofunctional changes in
gastrointestinal tract in patients with psoriasis accompanied by chronic opisthorchosis, Klin Med (Mosk), 2005;(1):43-46. (Rus), 15759490, ISSN 0023-
Kitchens RL, Thompson PA. Modulatory effects of sCD14 and LBP on LPS-host cell interactions. J Endotoxin
Res. 2005;11(4):225-
Kobayashi T, Tani T, Yokota T, Kodama M. Detection of peptidoglycan in human plasma using the silkworm
larvae plasma test. FEMS Immunol Med Microbiol. 2000 May;28(1):49-
Korotkii NG, Peslyak MY. Psoriasis as a consequence of incorporation of beta-streptococci into the
microbiocenosis of highly permeable intestines (a pathogenic conce pt), Vestn Dermatol Venerol, 2005;(1): 9-18. (Rus), ISSN 0042-
Korotkii NG, Udzhuhu VJ, Abdulaeva AA, Kubylinsky AA. Complex immunomodulating therapy for psoriatics,
Russian Journal of Skin and Sexually Transmitted Diseases, 2001;(1):14-6. (Rus), ISSN 1560-9588.
Krappmann D, Wegener E, Sunami Y et al. The IkappaB kinase complex and NF-kappaB act as master
regulators of lipopolysaccharide-induced gene expression and control subordinate activation of AP-1. Mol Cell Biol. 2004 Jul;24(14):6488-
Kullberg BJ, Ferwerda G, de Jong DJ et al. Crohn's disease patients homozygous for the 3020insC NOD2
mutation have a defective NOD2/TLR4 cross-tolerance to intestinal stimuli. Immunology. 2008 Apr;123(4):600-
Kuranova NYu, Khardikova SA, Beloborodova YeI, Kalyuzhina MI. Influence od dehelmithization on bile
lithogenity indices in patients having psoriasis combined with chronic opisthorchiasis. Siberian Medical Journal (Tomsk), 2009;(1):49-
Laman JD, Schoneveld AH, Moll FL, van Meurs M, Pasterkamp G. Significance of peptidoglycan, a
proinflammatory bacterial antigen in atherosclerotic arteries and its association with vulnerable plaques, Am J Cardiol. 2002 Jul 15;90(2):119-
Lande R, Gregorio J, Facchinetti V. et al. Plasmacytoid dendritic cells sense self -DNA coupled with
antimicrobial peptide. Nature. 2007 Oct 4;449(7162):564-
Leal L, Ribera M, Daudén E. Psoriasis and HIV Infection. Actas Dermosifiliogr. 2008 Dec;99(10):753-63.
Lowes MA, Bowcock AM, Krueger JG. Pathogenesis and therapy of psoriasis. Nature. 2007 Feb
22;445(7130):866-.
Maev IV, Samsonov AA. Therapeutic tactics in patients with SIBO. Consilium-Medicum 2007; 9(7):45-9.
Majewski S, Jablonska S. Possible involvement of epidermodysplasia verruciformis human papillomaviruses
in the immunopathogenesis of psoriasis: a proposed hypothesis. Exp Dermatol. 2003 Dec;12(6):721-8.
Male D, Brostoff J, Roth D, Roitt I. Immunology, 7 edition, Mosby, 2006, 544 p. ISBN 0323033997.
Mandron M, Ariès MF, Boralevi F. et al. Age-Related Differences in Sensitivity of Peripheral Blood Monocytes
to Lipopolysaccharide and Staphylococcus Aureus Toxin B in Atopic Dermatitis. J Invest Dermatol. 2008 Apr;128(4):882-
Martin C, Burdon PC, Bridger G, Gutierrez-Ramos JC, Williams TJ, Rankin SM. Chemokines acting via
CXCR2 and CXCR4 control the release of neutrophils from the bone marrow and their return following senescence. Immunity. 2003 Oct;19(4):583-9
Matin MA, Kunitomo K, Yada S, Miyoshi Y, Matsumura T, Komi N. Biliary stones and bacteriae in bile study in
211 consecutive cases.,Tokushima J Exp Med. 1989 Jun;36(1-2):11-
Matsuki T, Watanabe K, Tanaka R et al. Distribution of Bifidobacterial Species in Human Intestinal Microflora.
Appl Environ Microbiol. 1999 Oct;65(10):4506-
2009-2012, Peslyak MY, Model of pathogenesis of psoriasis. Part 1. Systemic psoriatic process. e4.0a. 79
Matusevich SL, Kungurov NV, Filimonkova NN, Gerasimova NM. Psoriasis and opisthorchiasis, Tyumen,
2000, 232 p. (Rus). ISBN 5881311337.
Matsulevich TV, Bondarenko VM. Intestine dysbacteriosis as a clinical and labo ratory syndrome. Current state
of the problem. GEOTAR-MEDIA, 2007, 304 p. ISBN: 9785970404300. (Rus)
McInturff JE, Modlin RL, Kim J. The role of toll-like receptors in the pathogenesis and treatment of
dermatological disease. J Invest Dermatol. 2005 Jul;125(1):1-
Medvedev AE, Sabroe I, Hasday JD, Vogel SN. Tolerance to microbial TLR ligands: molecular mechanisms
and relevance to disease. J Endotoxin Res. 2006;12(3):133-
Merad M, Ginhoux F. Dendritic cell genealogy: a new stem or just another branch? Nature Immunology 2007
Mishnyov OD, Shchegolev AI, Lysova NL, Tinkova IO. Liver and kidney at endotoxinemia, Moscow, 2003, 212
p. (Rus). ISBN 5859410573.
Meuret G, Bammert J, Hoffmann G. Kinetics of Human Monocytopoiesis. Blood. 1974 Dec;44(6):801-16.
Meuret G, Hoffmann G. Monocyte kinetic studies in normal and disease states. Br J Haematol. 1973
Meuret G, Schmitt E, Hagedorn M. Monocytopoiesis in chronic eczematous diseases, psoriasis vulgaris, and
mycosis fungoides.J Invest Dermatol. 1976 Jan;66(1):22-
Mizutani H, Ohmoto Y, Tanaka H, Shimizu M, Psoriatic monocytes respond sensitively to lipopolysaccharide
but with limited inflammatory cytokine production, Archives of Dermatological Research, 1997 Oct;289(11):657-
Møller AS, Øvstebø R, Westvik AB et al. Effects of bacterial cell wall components (PAMPs) on the expression
of monocyte chemoattractant protein-1 (MCP-1), macrophage inflammatory protein-1alpha (MIP-1alpha) and the chemokine receptor CCR2 by purified human blood monocytes. J Endotoxin Res. 2003;9(6):349-.
Molochkov VA, Badokin VV, Albanova VI, Volnuhin VA. Psoriasis and psoriatic arthtitis, Moscow 2007, 332 p.
(Rus), ISBN 9785873173921.
Munford RS. Sensing Gram-Negative Bacterial Lipopolysaccharides: a Human Disease Determinant? Infect
Immun. 2008 Feb;76(2):454-
Munz OH, Sela S, Baker BS et al. Evidence for the presence of bacteria in the blood of psoriasis patients.
Arch Dermatol Res. 2010 Sep;302(7):495-8.
Myhre AE, Aasen AO, Thiemermann C, Wang JE. Peptidoglycan--an endotoxin in its own right? Shock. 2006
Nagl M, Kacani L, Müllauer B. et al. Phagocytosis and Killing of Bacteria by Professional Phagocytes and
Dendritic Cells. Clin Diagn Lab Immunol. 2002, Nov;9(6):1165-
Nair RP, Ding J, Duffin KC. et al. Psoriasis Bench to Bedside – Genetics Meets Immunology. Arch Dermatol.
2009 Apr;145(4):462-4.
Nakatani T, Tsuchida K, Sugimura K, Yoshimura R, Takemoto Y. Response of peripheral blood mononuclear
cells in hemodialyzed patients against endotoxin and muramyldipeptide. Int J Mol Med. 2002 Oct;10(4):469-7
Nesterov AS. Features of pathogenesis and therapy of chronic dermatoses at blastocystic invasion.
Dissertation, St.-Petersburg, 2009, 298 p. (Rus),.
a. Nestle F, Conrad C, Tun-Kyi A. et al. Plasmacytoid predendritic cells initiate psoriasis through interferon --
alpha production. J Exp Med. 2005 Jul 4;202(1):135-
b. Nestle F, Gilliet M. Defining Upstream Elements of Psoriasis Pathogenesis: An Emerging Role For Interferon
alfa. J Invest Dermatol. 2005 Nov;125(5):xiv-.
Nickoloff BJ, von den Driesch P, Raychaudhuri SP. et al. Is psoriasis a T-cell disease?, Exp Dermatol 2000
Nijhuis MM. Peptidoglycan in atherosclerotic plaque formation and vulnerability. Dissertation. Utrecht
Noble CJ. Carriage of group D streptococci in the human bowel. J Clin Pathol. 1978 Dec;31(12):1182-6.
Noverr MC, Huffnagle GB. Does the microbiota regulate immune responses outside the gut? Trends
Microbiol. 2004 Dec;12(12):562-8.
Numerof RP, Asadullah K. Cytokine and anti-cytokine therapies for psoriasis and atopic dermatitis. BioDrugs.
2009-2012, Peslyak MY, Model of pathogenesis of psoriasis. Part 1. Systemic psoriatic process. e4.0a. 80
Nyfors A, Rasmussen PA, Lemholt K, Eriksen B. Improvement of refractory psoriasis vulgaris after
tonsillectomy. Dermatologica. 1975;151(4):216-2
O'Dwyer ST, Michie HR, Ziegler TR. et al. A single dose of endotoxin increases intestinal permeability in
healthy humans, Arch Surg. 1988 Dec;123(12): 1459-
O'Hara JR, Buret AG. Mechanisms of intestinal tight junctional disruption during infection. Front Biosci. 2008
Ojetti V, De Simone C, Aguilar Sanchez J. et al. Malabsorption in psoriatic patients: cause or consequence?
Scand J Gastroenterol. 2006 Nov;41(11):1267-
Okubo Y, Koga M. Peripheral blood monocytes in psoriatic patients overproduce cytokines. J Dermatol Sci.
1998, Jul;17(3):223-
Okubo Y, Oki N, Takeda H, Amaya M. et al. Increased microorganisms DNA levels in peripheral blood
monocytes from psoriatic patients using PCR with universal ribosomal RNA primers. J Dermatol. 2002 Sep;29(9):547-
Osipov GA, Parfenov AI, Bogomolov PO. Comparative study of chromatography-mass spectrography of
microorganism's chemical markers in blood and intestinal mucosa bioptats, Ross Gastroenterol Zh. 2001;(1):54-69. (Rus), 1560-
Ott S, Musfeldt M, Ullmann U, Hampe J, Schreiber S. Quantification of Intestinal Bacterial Populations by
Real-Time PCR with a Universal Primer Set and Minor Groove Binder Probes: a Global Approach to the Enteric Flora, Journal of clinical microbiology, 2004 Jun;42(6):2566-
Pagano J. Healing psoriasis: The natural alternative., 2008, 352 p, ISBN: 9780470267264
Parfenov AI, Ekisenina NI, Mazo VK, Gmoshinskii IB, Safonova SA. Barrier function of gastrointestinal tract,
Ter Arkh. 2000;72(2):64-6. (Rus), 10717933, ISSN 0040-
Parfenov AI, Mazo VK, Gmoshinskii IV, Safonova SA, Ekisenina NI. Clinical significance of evaluation of
ovalbumin blood level after oral load dose of chicken egg proteins, Russian Journal of Gastroenterology, 1999;(2). (Rus).
Parfenov AI, Osipov GA, Bogomolov PO. Intestinal disba
Parker LC, Whyte MK, Vogel SN, Dower SK, Sabroe I. Toll-like receptor (TLR)2 and TLR4 agonists regulate
CCR expression in human monocytic cells. J Immunol. 2004 Apr 15;172(8):4977-
Pavlenok NV, Mahnovets EN. Features of the clinical picture of psoriasis vulgaris in chronic H. pylori infection.
Bulletin of new medical technologies, 2007;(3):106-
Peslyak MY. Model of pathogenesis of psoriasis. Part 2. Local processes. Moscow, MYPE, 2012, 110 p.,
Piccioli D, Tavarini S, Borgogni E et al. Functional specialization of human circulating CD16 and CD1c
myeloid dendritic-cell subsets. Blood. 2007 Jun 15;109(12):5371-
Pietrzak A, Jastrzebska I, Chodorowska G. et al. Psoriasis vulgaris and digestive system disorders: Is there a
linkage? Folia Histochem Cytobiol. 2009 Jan 1;47(3):517-
Pinegin BV, Andronov TM. Some theoretical and practical problems of immunomodulator licopid application in
clinical practice. Immunology, 1998;(4):60-63. (Rus), ISSN 0206-
Piruzian AL, Abdeev RM. The molecular genetics of psoriasis. Vestn Ross Akad Med Nauk. 2006;(3):33-43.
Piruzian ES, Ishkin AA, Nikol'skaia TA, Abdeev RM, Bruskin SA. The comparative analysis of psoriasis and
Crohn disease molecular-genetical processes under pathological conditions. Mol Biol (Mosk). 2009;(1):175-9.
Pokrovskii VI, Briko NI, Ryapis LA. Streptococci and infectious diseases caused by streptococci, Geotar -
Media, 2006, 544 p. (Rus), ISBN 5970401838.
Prinz JC. The role of streptococci in psoriasis. Hautarzt. 2009 Feb;60(2):109-
Qayoom S, Ahmad QM. Psoriasis and Helicobacter pylori. Indian J Dermatol Venereol Leprol. 2003 Mar-
a. Randolph GJ, Jakubzick C, Qu C. Antigen presentation by monocytes and monocyte-derived cells. Curr Opin
Immunol. 2008 Feb;20(1):52-
b. Randolph GJ, Ochando J, Partida-Sanchez S. Migration of Dendritic Cell Subsets and their Precursors. Annu
Rev Immunol. 2008;26:293-3
Randow F, Döcke WD, Bundschuh DS. et al. In vitro prevention and reversal of lipopolysaccharide
desensitization by IFN-gamma, IL-12, and granulocyte-macrophage colony-stimulating factor. J Immunol. 1997 Mar 15;158(6):2911-
2009-2012, Peslyak MY, Model of pathogenesis of psoriasis. Part 1. Systemic psoriatic process. e4.0a. 81
Rankin SM. The bone marrow: a site of neutrophil clearance. J Leukoc Biol. 2010 Aug;88(2):241-51.
Rantakokko K, Rimpilainen M, Uksila J, Jansen C. et al., Antibodies to streptococcal cell wall in psoriatic
arthritis and cutaneous psoriasis, Clin. Exp. Rheumatol. 1997 Jul-Aug;15(4):399-
Raychaudhuri SP, Jiang WY, Farber EM, Schall TJ, Ruff MR, Pert CB. Upregulation of RANTES in psoriatic
keratinocytes: a possible pathogenic mechanism for psoriasis. Acta Derm Venereol. 1999 Jan;79(1):9 -.
Reddy RC, Standiford TJ. Effects of sepsis on neutrophil chemotaxis. Curr Opin Hematol. 2010 Jan;17(1):18-
Ritchlin C. Psoriatic disease--from skin to bone. Nat Clin Pract Rheumatol. 2007 Dec;3(12):698-706.
Roa I, Ibacache G, Carvallo J. et al. Microbiological study of gallbladder bile in a high risk zone for gallbladder
cancer, Rev Med Chil. 1999 Sep;127(9):1049-
Round JL, O'Connell RM, Mazmanian SK. Coordination of tolerogenic immune responses by the commensal
microbiota. J Autoimmun. 2010 May;34(3):J220-
Rudkovskaya ZV, Clinical and laboratory monitoring of efficiency of application of method of interval
normobaric hypoxia in complex treatment of psoriasis in children. Dissertation, Moscow, 2003, 137 p. (Rus).
Sabat R, Philipp S, Höflich C, Kreutzer S, Wallace E, Asadullah K, Volk HD, Sterry W, Wolk K.
Immunopathogenesis of psoriasis. Exp Dermatol. 2007 Oct;16(10):779-
Savina A, Amigorena S. Phagocytosis and antigen presentation in dendritic cells. Immunol Rev. 2007
Savitskaia KI, Mel'nikova EF, Vorob'ev AA, Zagal'skaia NV. Biliary microflora of patients with chronic
pancreatitis. Zh Mikrobiol Epidemiol Immunobiol. 2003;(1):14-
Saxena V, Dogra J. Long-term oral azithromycin in chronic plaque psoriasis: a controlled trial. Eur J Dermatol.
2010 May-Jun;20(3):329-
Saxena V, Dogra J. Long-term use of penicillin for the treatment of chronic plaque psoriasis. Eur J Dermatol.
2005 Sep-Oct;15(5):359-
Scarpa R, Ayala F, Caporaso N, Olivieri I. Psoriasis, Psoriatic Arthritis, or Psoriatic Disease? The Journal of
Rheumatology 2006 Feb;33(2):210-
Serbina NV, Jia T, Hohl TM, Pamer EG. Monocyte-mediated defense against microbial pathogens. Annu Rev
Immunol. 2008;26:421-
Serbina NV, Salazar-Mather TP, Biron CA et al. TNF/iNOS -producing dendritic cells mediate innate immune
defense against bacterial infection. Immunity. 2003 Jul;19(1):59-
Schleifer KH, Kandler O. Peptidoglycan types of bacterial cell walls and their taxonomic implications. Bacteriol
Rev. 1972 Dec;36(4):407-77.
Shagova YV. Clinical and prognostic value of functional and structural changes of intestine at psoriasis.
Dissertation, Saratov, 2009, 160 p. (Rus),
Shapiro J, Cohen AD, David M et al. The association between psoriasis, diabetes mel litus, and
atherosclerosis in Israel: a case-control study. J Am Acad Dermatol. 2007 Apr;56(4):629-
Sharapova GI, Korotkii NG, Molodenkov MN, Psoriasis. Moscow. Medicine, 1993, 223 p, (Rus).
Shenderov BA. Medical microbial ecology and functional nutrition. 3 vol, Moscow, Grant, 1998 -2001, 286 p;
412 p; 286 p, (Rus), ISBN 5891350025.
Shiryaeva YuA. Malabsorption syndrome at psoriatics. Dissertation, Krasnoyarsk, 2007, 150 p. (Rus),
Sica A, Saccani A, Borsatti A. et al. Bacterial lipopolysaccharide rapidly inhibits expression of C -C chemokine
receptors in human monocytes. J Exp Med. 1997 Mar 3;185(5):969-
Sozzani S. Dendritic cell trafficking: more than just chemokines. Cytokine Growth Factor Rev. 2005
Stenina MA, Kulagin VI, Rudkovskaya ZV et al, Role of disturbances of intestine barrier function in
pathogenesis of psoriasis in children, Russian Journal of Skin and Sexually Transmitted Diseases, 2003;(2):20-23. (Rus), ISSN 1560-9588.
Stoll LL, Denning GM, Weintraub NL. Potential role of endotoxin as a proinflammatory mediator of
atherosclerosis. Arterioscler Thromb Vasc Biol. 2004 Dec;24(12):2227-
Strober W, Murray PJ, Kitani A, Watanabe T. Signalling pathways and molecular interactions of NOD1 and
NOD2. Nat Rev Immunol. 2006 Jan;6(1):9-.
2009-2012, Peslyak MY, Model of pathogenesis of psoriasis. Part 1. Systemic psoriatic process. e4.0a. 82
Suau A., Bonnet R., Sutren M. et al. Direct Analysis of Genes Encoding 16S rRNA from Complex
Communities Reveals Many Novel Molecular Species within the Human Gut, Applied and Environmental Microbiology, 1999 Nov;65(11):4799-
Sullivan A, Törnblom H, Lindberg G. et al. The micro-flora of the small bowel in health and disease. Anaerobe.
2003 Feb;9(1):11-
Tabolin VA, Iakovlev MY, Ilina AJ, Lazareva SI, Likhoded VG,. Pathogenetic mechanisms and clinical aspects
of action of thermostable endotoxin of intestine microflora, RMJ, 2003; 11(3):126-8. (R
Tacke F, Ginhoux F, Jakubzick C, van Rooijen N, Merad M, Randolph GJ. Immature monocytes acquire
antigens from other cells in the bone marrow and present them to T cells after maturing in the periphery. J Exp Med. 2006 Mar 20;203(3):583-
Tan KS. New Insights on Classification, Identification, and Clinical Relevance of Blastocystis spp. Clin
Microbiol Rev. 2008 Oct;21(4):639-
Teranishi Y, Mizutani H, Murata M, Shimizu M, Matsushima K. Increased spontaneous production of IL-8 in
peripheral blood monocytes from the psoriatic patient: relation to focal infection and response to treatments. J Dermatol Sci. 1995 Jul;10(1):8-
Thieblemont N, Weiss L, Sadeghi HM et al. CD14lowCD16high: a cytokine-producing monocyte subset which
expands during human immunodeficiency virus infection. Eur J Immunol. 1995 Dec;25(12):3418-24.
Toichi E, Tachibana T, Furukawa F. Rapid improvement of psoriasis vulgaris during drug-induced
agranulocytosis. J Am Acad Dermatol. 2000 Aug;43(2 Pt 2):391-
Toivanen P. Normal intestinal microbiota in the aetiopathogenesis of rheumatoid arthritis. Ann Rheum Dis.
2003 Sep;62(9):807-
Traub S, von Aulock S, Hartung T, Hermann C. MDP and other muropeptides - direct and synergistic effects
on the immune system. J Endotoxin Res. 2006;12(2):69-
Trauner M, Claudel T, Fickert P et al. Bile acids as regulators of hepatic lipid and glucose metabolism. Dig
Dis. 2010;28(1):220-
Trent MS, Stead CM, Tran AX, Hankins JV. Diversity of endotoxin and its impact on pathogenesis. J
Endotoxin Res. 2006;12(4):205-
Turnis ME, Song XT, Bear A. et al. IRAK-M Removal Counteracts Dendritic Cell Vaccine Deficits in Migration
and Longevity. J I
Turroni F, Foroni E, Pizzetti P et al. Exploring the diversity of the bifidobacterial population in the human
intestinal tract. Appl Environ Microbiol. 2009 Mar;75(6):1534-
Valdimarsson H, Thorleifsdottir RH, Sigurdardottir SL, Gudjonsson JE, Johnston A. Psoriasis - as an
autoimmune disease caused by molecular mimicry. Trends Immunol. 2009 Oct;30(10):494-501.
van't Veer C, van den Pangaart PS, van Zoelen MA et al. Induction of IRAK-M is associated with
lipopolysaccharide tolerance in a human endotoxemia model. J Immunol. 2007 Nov 15;179(10):7110-20.
Vorob'ev AI. Manual to Hematology, vol.1, Moscow, Nyudiamed, 2002, 280 p. (Rus). ISBN 5881070380.
Vollmer W, Blanot D, de Pedro MA. Peptidoglycan structure and architecture. FEMS Microbiol Rev. 2008
Waksman G, Caparon M, Hultgren S. Structural Biology of Bacterial Pathogenesis, 2005, 273 p. ISBN
Wang GL, Li XY, Wang MY et al. Cell-wall-deficient bacteria: a major etiological factor for psoriasis. Chin Med
J (Engl). 2009 Dec;122(24):3011-
Wang M, Ahrné S, Jeppsson B, Molin G. Comparison of bacterial diversity along the human intestinal tract by
direct cloning and sequencing of 16S rRNA genes. FEMS Microbiol Ecol. 2005 Oct 1;54(2):219 -31.
Wang X, Quinn PJ. Lipopolysaccharide: Biosynthetic pathway and structure modification. Prog Lipid Res.
2010 Apr;49(2):97-
Weaver LK, Pioli PA, Wardwell K et al. Up-regulation of human monocyte CD163 upon activation of cell-
surface Toll-like receptors. J Leukoc Biol. 2007 Mar;81(3):663-
Weinstein GD, McCullough JL, Ross PA., Cell kinetic basis for pathophysiology of psoriasis., J Invest
Dermatol. 1985 Dec; 85(6): 579-
Weisenseel P, Laumbacher B, Besgen P. et al., Streptococcal infection distinguishes different types of
psoriasis, J. Med. Genet. 2002 Oct;39(10):767-
2009-2012, Peslyak MY, Model of pathogenesis of psoriasis. Part 1. Systemic psoriatic process. e4.0a. 83
Weisenseel P, Prinz JC. Incidental detection of S. pyogenes-DNA in psoriatic skin by PCR. Arch Dermatol
Res. 2005 Jun;296(12):573-
Wenk K, Arrington K, Ehrlich A. Psoriasis and non-alcoholic fatty liver disease. J Eur Acad Dermatol
Whitelaw DM. Observations on human monocyte kinetics after pulse labeling. Cell Tissue Kinet. 1972
Wiedermann CJ, Kiechl S, Dunzendorfer S. et al. Association of endotoxemia with carotid atherosclerosis and
cardiovascular disease: prospective results from the Bruneck Study. J Am Coll Cardiol. 1999 Dec;34(7):1975-
Williams RC, McKenzie AW, Roger JH, Joysey VC., HL-A antigens in patients with guttate psoriasis, Br J
Dermatol. 1976 Aug;95(2):163-
Williamson D, Chawla M, Marks R. GMDP for psoriasis. L
Wilson JK, Al-Suwaidan SN, Krowchuk D, Feldman SR. Treatment of psoriasis in children: is there a role for
antibiotic therapy and tonsillectomy? Pediatr Dermatol. 2003 Jan-Feb;20(1):11-
Windheim M, Lang C, Peggie M, Plater LA, Cohen P. Molecular mechanisms involved in the regulation of
cytokine production by muramyl dipeptide. Biochem J. 2007 Jun 1;404(2):179-
Wintrobe MM, Lee GR. Wintrobe's Clinical Hematology. Lippincott Williams & Wilkins; Twelfth Edition
Wolters M., Diet and psoriasis: experimental data and clinical evidence, British Journal of Dermatology 2005
Yang D, Chen Q, Le Y et al. Differential regulation of formyl peptide receptor-like 1 expression during the
differentiation of monocytes to dendritic cells and macrophages. J Immunol. 2001 Mar 15;166(6):4092-8.
Yimin Ge, Robert M. Ezzell, H. Shaw Warren, Localization of Endotoxin in the Rat Intestinal Epithelium, The
Journal of Infectious Diseases 2000 Sep;182(3):873-
a. Zaba LC, Fuentes-Duculan J, Eungdamrong NJ et al. Psoriasis Is Characterized by Accumulation of
Immunostimulatory and Th1/Th17 Cell-Polarizing Myeloid Dendritic Cells. J Invest Dermatol. 2009 Jan;129(1):79-
b. Zaba LC, Krueger JG, Lowes MA. Resident and "Inflammatory" Dendritic Cells in Human Skin. J Invest
Dermatol. 2009 Feb;129(2):302-
Zaba LC, Fuentes-Duculan J, Eungdamrong NJ et al. Identification of TNF-related apoptosis-inducing ligand
and other molecules that distinguish inflammatory from resident dendritic cells in patients with psoriasis. J Allergy Clin Immunol. 2010 Jun; 125(6):1261-
a. Zhang K, Hou R, Niu X et al. Decreased colony formation of HPP-CFC and CFU-GM and increased Hes-1
expression in bone marrow mononuclear cells from psoriatic patients. Br J Dermatol. 2010 July, 163(1): 93-
b. Zhang K, Liu R, Yin G et al. Differential cytokine secretion of cultured bone marrow stromal cells from patients
with psoriasis and healthy volunteers. Eur J Dermatol. 2010 Jan-Feb;20(1):49-
Zheng M, Mrowietz U. Phenotypic differences between human blood monocyte subpopulations in psoriasis
and atopic dermatitis. J Dermatol. 1997 Jun;24(6):370-8.
Ziegler-Heitbrock L. The CD14+ CD16+ blood monocytes: their role in infection and inflammation. J Leukoc
Biol. 2007 Mar;81(3):584-92.
Zilberstein B, Quintanilha AG, Santos MA et al. Digestive tract microbiota in healthy volunteers. Clinics (Sao
Paulo). 2007 Feb;62(1):47-5.
2009-2012, Peslyak MY, Model of pathogenesis of psoriasis. Part 1. Systemic psoriatic process. e4.0a. 84
Source: http://www.psorias.info/9785905504020_MPP_Part1_Systemic_psoriatic_process_eng.pdf
KEPPRA® (levetiracetam) Rx 250 mg, 500 mg, 750 mg, and 1000 mg tablets 100 mg/mL oral solution DESCRIPTION KEPPRA is an antiepileptic drug available as 250 mg (blue), 500 mg (yellow), 750 mg (orange), and 1000 mg (white) tablets and as a clear, colorless, grape-flavored liquid (100 mg/mL) for oral administration. The chemical name of levetiracetam, a single enantiomer, is (-)-(S)-α-ethyl-2-oxo-1-pyrrolidine acetamide, its molecular formula is C8H14N2O2 and its molecular weight is 170.21.
Carbamate and Pyrethroid Resistance in the Leafminer J. A. ROSENHEIM,' AND B. E. T ABASHNIK Department of Entomology, University of Hawaii at Manoa, Honolulu, Hawaii 96822 J. Econ.Entomol.83(6): 2153-2158 (1990) ABSTRACT Populations of D1glyphus begini (Ashmead), a parasitoid of Lirlomyza leafminers, showed resistance to oxamyl, methomyl, fenvalerate, and permethrin in labo-ratory bioassays. Relative to a susceptible strain from California, maximum resistance ratiosfor these pesticides were 20, 21, 17, and 13, respectively. Three populations that had beentreated frequently with insecticides were significantly more resistant to all four insecticidescompared with an untreated Hawaii population and a California population with an unknownspray history. Parasitoids from a heavily sprayed tomato greenhouse on the island of Hawaiihad LC",'s for permethrin and fenvalerate that were 10 and 29 times higher than the fieldrate, respectively. Populations resistant to oxamyl and methomyl had LC",'s two- and sixfoldbelow the field rate, respectively. D. begini is one of the few parasitoids resistant to pyre-throids, with LC",'s exceeding field application rates. Resistant D. begini may be useful forcontrolling leafminers in management programs that integrate biological and chemical con-trols.







