Levitra enthält Vardenafil, das eine kürzere Wirkdauer als Tadalafil hat, dafür aber schnell einsetzt. Männer, die diskret bestellen möchten, suchen häufig nach levitra kaufen ohne rezept. Dabei spielt die rechtliche Lage in der Schweiz eine wichtige Rolle.
Regbio.yeditepe.edu.tr
HEMATOPOIESIS AND STEM CELLS
Meis1 regulates the metabolic phenotype and oxidant defense of hematopoieticstem cells
*Fatih Kocabas,1 *Junke Zheng,2,3 Suwannee Thet,1 Neal G. Copeland,4 Nancy A. Jenkins,4 Ralph J. DeBerardinis,5Chengcheng Zhang,2 and Hesham A. Sadek1
1Department of Internal Medicine, Division of Cardiology, and 2Departments of Physiology and Developmental Biology, UT Southwestern Medical Center, Dallas,
TX; 3Key Laboratory of Cell Differentiation and Apoptosis of Chinese Ministry of Education, Shanghai Jiao-Tong University School of Medicine, Shanghai, China;
4The Methodist Hospital Research Institute, Houston, TX; and 5Departments of Pediatrics and Genetics, UT Southwestern Medical Center, Dallas, TX
The role of Meis1 in leukemia is well
amined the effect of loss of Meis1 on HSC
duction, and apoptosis of HSCs. Finally,
established, but its role in hematopoietic
function and metabolism. Inducible Meis1
we demonstrate that the effect of Meis1
stem cells (HSCs) remains poorly under-
deletion in adult mouse HSCs resulted in
knockout on HSCs is entirely mediated
stood. Previously, we showed that HSCs
loss of HSC quiescence, and failure of
through reactive oxygen species where
use glycolytic metabolism to meet their
bone marrow repopulation after transplan-
treatment of the Meis1 knockout mice
energy demands. However, the mecha-
tation. While we previously showed that
with the scavenger N-acetylcystein re-
nism of regulation of HSC metabolism,
Meis1 regulates Hif-1␣
transcription in
stored HSC quiescence and rescued
and the importance of maintaining this
vitro, we demonstrate here that loss of
HSC function. These results uncover an
distinct metabolic phenotype on HSC
Meis1 results in down-regulation of both
important transcriptional network that
function has not been determined. More
Hif-1␣
and Hif-2␣
in HSCs. This resulted
regulates metabolism, oxidant defense,
importantly, the primary function of Meis1
in a shift to mitochondrial metabolism,
and maintenance of HSCs. (Blood. 2012;
in HSCs remains unknown. Here, we ex-
increased reactive oxygen species pro-
Hematopoietic stem cells (HSCs) are defined by their abilities to
ish NAD⫹. Anaerobic glycolysis produces 18 times less ATP than
self-renew and to differentiate into all blood cell types.1,2 Much of
mitochondrial oxidative phosphorylation,12 which may be well
the advancement in HSC therapy is credited to decades of
suited for quiescent cells, but certainly cannot sustain cells with
pioneering work that led to the development of HSC enrichment
techniques based on staining of cell-surface antigens or vital dyes
The energy advantage of mitochondrial oxidative phosphoryla-
followed by fluorescence-activated cell sorting (FACS).3-5 How-
tion over glycolysis is, unfortunately, not without deleterious
ever, little is known about metabolic characteristics of HSCs, its
consequences, as the mitochondrion is considered a major source
regulation, or how the metabolic phenotype may influence HSC
of reactive oxygen species (ROS) production.13,14 ROS are believed
to be important mediators of aging, and of numerous degenerative
In 1978, the concept of the special microenvironment, or niche,
diseases, including HSC dysfunction and senescence.15 In fact,
of HSCs was introduced.6 Since then, it has become clear that the
within the HSC compartment, the repopulation capacity is local-
niche plays a crucial role in self-renewal and differentiation of
ized to only those HSCs with low levels of free radicals.16
HSCs.7,8 One of the hallmarks of the HSC niche is its low oxygen
Therefore, the glycolytic metabolic phenotype of HSCs may not
tension, hence the term "hypoxic niche."9 Numerous studies
only protect them against hypoxic insults, but may also serve to
indicate that this low oxygen environment is not only tolerated by
minimize oxidant damage that result from mitochondrial oxidative
HSCs, but is also essential for their function.10 We recently
demonstrated that HSCs rely on glycolysis and have lower rates of
Hypoxia-inducible factor-1␣ (Hif-1␣) is a major transcriptional
oxygen consumption,11 which may be crucial for survival of HSCs
regulator of hypoxic response. Hif-1␣ mediates the metabolic
within hypoxic bone marrow niches.
switch from aerobic mitochondrial metabolism, to anaerobic cytoplas-
In the mitochondria, oxygen is used as the terminal electron
mic glycolysis17-19 by increasing both the expression,20 and kinetic
acceptor for the respiratory chain, and in the absence of oxygen the
rate21 of key glycolysis enzymes. Moreover, Hif-1␣ inhibits the use
proton gradient generated by the respiratory chain collapses and
of pyruvate by the mitochondria,22,23 and inhibits mitochondrial
mitochondrial ATP production declines. Under these hypoxic or
biogenesis.24 Takubo and colleagues recently demonstrated that
anoxic conditions, energy production is derived from cytoplasmic
Hif-1␣ is enriched in HSCs, and that loss of
Hif-1␣ results in HSC
glycolysis through the fermentation of glucose, and in the final step
dysfunction,25 while our group recently showed that Meis1 is required
of anaerobic glycolysis, pyruvate is converted to lactate to replen-
for optimum transcriptional activation of Hif-1␣ in HSCs ex vivo.11
Submitted May 30, 2012; accepted September 11, 2012. Prepublished online
The publication costs of this article were defrayed in part by page charge
as
Blood First Edition paper, September 20, 2012; DOI 10.1182/blood-2012-
payment. Therefore, and solely to indicate this fact, this article is hereby
marked ‘‘advertisement'' in accordance with 18 USC section 1734.
*F.K. and J.Z. contributed equally to this project.
The online version of this article contains a data supplement.
2012 by The American Society of Hematology
BLOOD, 13 DECEMBER 2012
䡠 VOLUME 120, NUMBER 25
BLOOD, 13 DECEMBER 2012 䡠 VOLUME 120, NUMBER 25
Meis1, which is a 3-amino-acid loop extension homeodomain
lineages was also performed to confirm multilineage reconstitution as
protein, plays an important role in leukemogenesis as well as
previously described.11
normal hematopoiesis. Meis1 was first identified as a common viral
For analyzing LT-HSCs in the peripheral blood by flow cytometry,
integration site in myeloid leukemic cells of BXH-2 mice,26 and it
peripheral blood of Meis1⫺/⫺ or Meis1⫹/⫹ mice was collected by retro-orbital bleeding and stained with LT-HSCs markers as described in the
is also frequently up-regulated in human primary acute myeloid
previous paragraph.
leukemia (AML) and acute lymphoblastic leukemia (ALL)
The cell-cycle analysis with Hoechst 33342 and pyronin Y staining was
samples.27 Moreover, overexpression of Meis1 accelerates the
performed as we described.36 Samples were immediately analyzed by flow
initiation of AML in murine models.28,29 In normal hematopoiesis,
cytometry (FACSAria; BD Biosciences). To examine the apoptosis,
Meis1 is expressed in the most primitive hematopoietic populations
Lin⫺Kit⫹Sca-1⫹ cells were stained with PE-conjugated anti–annexin V and
and is down-regulated on differentiation.30-32 Targeted
Meis1
7AAD according to the manufacturer's manual (BD Pharmingen). To study
knockout causes lethality by embryonic day 14.5 with multiple
the apoptosis in LT-HSCs, bone marrow cells were stained for HSC markers
hematopoietic and vascular defects.33,34 Moreover, Pbx-1, a cofac-
Sca-1-PE/Cy5.5, C-Kit-APC, CD34-PE, and Flk2-PE after lineage deplet-
tor of Meis1, has been shown to regulate self-renewal of HSCs by
ing and stained with FITC-conjugated anti–annexin V according to the
maintaining their quiescence.35 However, the role of Meis1 regulat-
manufacturer's manual (eBioscience).
ing the function and metabolism if HSCs remain poorly understood.
In the current report, we show that Meis1 regulates both HSC
Competitive reconstitution analysis
metabolism and oxidant stress response, through transcriptional
The indicated numbers of CD45.2 donor cells from Meis1⫹/⫹ or Meis1⫺/⫺
regulation of
Hif-1␣ and
Hif-2␣, respectively.
mice were mixed with 1 ⫻ 105 freshly isolated CD45.1 competitor BMcells and the mixture was injected intravenously via the retro-orbital routeinto each of a group of 6- to 8-week-old CD45.1 mice previously irradiatedwith a total dose of 10 Gy. To measure reconstitution of transplanted mice,
peripheral blood was collected at the indicated time points posttransplanta-tion, and the presence of CD45.1⫹ and CD45.2⫹ cells in lymphoid and
Mouse breeding and genotyping
myeloid compartments was measured as described.37
Meis1 knockout (KO) mice were genotyped with Meis1 For1: 5⬘-CCAAAGTAGCCACCAATATCATGA-3⬘ and Meis1 Rev: 5⬘-AGCGT-
CACTTGGAAAAGCAATGAT-3⬘ primers. Wild-type (WT) allele is de-termined by a 332-bp-long PCR product and mutant allele determined by
BM Lin⫺ cells were labeled with 5- (and -6) carboxyfluorescein succinimi-
a 440-bp long PCR product on 1.2% agarose gel. HSC-specific deletion of
dyl ester (CFSE), and 3 ⫻ 106 cells were transplanted into indicated strains
Meis1 was achieved by following crosses of Meis1f/f with Scl-Cre-ERT
of lethally irradiated mice. After 16 hours, the total number of CFSE⫹ cells
mice.4 Scl-Cre mice were genotyped using Scl-Cre-ER primer 1: 5⬘-
in the BM, spleen, or liver was determined by flow cytometry. When
GAACCTGAAGATGTTCGCGAT-3 and Scl-Cre-ER primer 2: 5⬘-
CFSE⫹ LSK (CFSE⫹Lin⫺Sca1⫹Kit⫹) cells (HSCs) were analyzed, the BM
ACCGTCAGTACGTGAGATATC-3. To generate Meis1⫺/⫺ mice, Meis1f/
cells were stained with a biotinylated lineage cocktail followed by
f;Scl-Cre-ERT⫹ mice were injected intraperitoneally with tamoxifen
streptavidin-PE/Cy5.5, anti–Sca-1–PE, and anti-Kit–APC before analysis,
(40 mg/kg, T5648-1G; Sigma-Aldrich) daily for 14 days. We used age-
matched tamoxifen-injected Meis1⫹/⫹;Scl-Cre-ERT(⫹) or Meis1f/f;Scl-Cre-ERT(⫺) mice as controls (Meis1⫹/⫹ mice). Genotyping of Cre-deleted
Meis1 locus (Meis1 exon8 deleted) was performed using Meis1 For2:5⬘-CATTGACTTAGGTGTATGGGTGTC-3⬘ and Meis1 Rev: 5⬘-
Normal BM cells were diluted to the indicated concentration in IMDM with
AGCGTCACTTGGAAAAGCAATGAT-3⬘ primers. Cre-deleted Meis1
2% FBS, and were then seeded into methylcellulose medium M3434
locus gives rise to a 261-bp product while wt and mutant (nondeleted)
(StemCell Technologies), for CFU-GM and BFU-E colony formation
shows no amplicon.
according to the manufacturer's instructions.
Dr Joseph A. Garcia (UT Southwestern) kindly provided the Hif-1␣
floxed mice. HSC-specific deletion of Hif-1␣ was achieved by following
crosses of Hif-1␣f/f with Scl-Cre-ERT mice and tamoxifen injections(40 mg/kg for 14 days). We used age-matched tamoxifen-injected Hif-1␣⫹/⫹;
Total RNA was isolated from Meis1⫺/⫺ and Meis1⫹/⫹ HSCs using the
Scl-Cre-ERT(⫹) or Hif-1␣f/f;Scl-Cre-ERT(⫺) mice as controls (Hif-1␣⫹/⫹ mice).
RNeasy Mini Kit (QIAGEN) according to the manufacturer's instructions.
cDNA was synthesized using SuperScript II RT (Invitrogen). Predesignedprimers (Table 1) from the National Institutes of Health (NIH) mouse
primer depot (http://mouseprimerdepot.nci.nih.gov/) were ordered from
Donor bone marrow (BM) cells were isolated from 8- to 12-week-old
Integrated DNA Technologies. Real-time PCR was performed with Syber-
Meis1⫹/⫹ or Meis1⫺/⫺ mice (or Hif-1␣⫹/⫹ and Hif-1␣⫺/⫺ mice).
Green (Applied Biosystems) on an ABI Prism 7700 Sequence Detector
Lin⫺Sca1⫹Kit⫹Flk2⫺CD34⫺ cells (long-term HSCs [LT-HSCs]) were
(Applied Biosystems). -actin was used as control to normalize results.
isolated by staining with a biotinylated lineage cocktail (anti-CD3, anti-CD5, anti-B220, anti–Mac-1, anti-Gr-1, anti-Ter119; StemCell Technolo-
PCR array
gies) followed by streptavidin-PE/Cy5.5, anti–Sca-1–FITC, anti–Kit-allophycocyanin (APC), anti–Flk-2–PE, and anti-CD34–PE. For analyzing
The Mouse Hypoxia Signaling Pathway RT2 Profiler PCR Array was
repopulation of mouse HSCs, peripheral blood cells of recipient CD45.1
performed as described previously.11 RNA was extracted from Meis1⫺/⫺
mice were collected by retro-orbital bleeding, followed by lysis of red blood
and Meis1⫹/⫹ LT-HSCs (Lin⫺Sca1⫹Kit⫹Flk2⫺CD34⫺cells) using the
cells and staining with anti-CD45.2–FITC, anti-CD45.1–PE, anti-
RNeasy Mini Kit (QIAGEN) according to manufacturer's instructions.
Thy1.2–PE (for T-lymphoid lineage), anti-B220–PE (for B-lymphoid
cDNA was retrotranscribed by using SuperScript II RT (Invitrogen). Mouse
lineage), anti–Mac-1–PE, or anti–Gr-1–PE (cells costaining with anti-
Real-Time Syber Green PCR Master Mix (SuperArray) and Cell Cycle
Mac–1 and anti–Gr-1 were deemed to be of the myeloid lineage)
primer sets (SABiosciences) used for real-time PCR on an ABI Prism
monoclonal antibodies (BD Pharmingen). The "percent repopulation"
7700 Sequence Detector (Applied Biosystems). The data were analyzed
shown in all figures was based on the staining results of anti-CD45.2–FITC
using the ⌬⌬Ct method. Fold change was calculated as difference in gene
and anti-CD45.1–PE. In all cases, FACS analysis of the above-listed
expression between Meis1⫺/⫺ and Meis1⫹/⫹ LT-HSC samples.
BLOOD, 13 DECEMBER 2012
Meis1 REGULATES Hif-1
䡠 VOLUME 120, NUMBER 25
␣ AND Hif-2␣ IN HSCs
Table 1. List of primers used for real-time PCR
Generation of luciferase reporter vectors
Measurement of ROS
Conserved Meis1 motifs in Hif-2␣ gene were determined using a genome
Bone marrow cells from Meis1⫹/⫹ and Meis1⫺/⫺ mice were isolated as
browser (http://genome.ucsc.edu/). A 779-bp-long DNA fragment con-
described in "Flow cytometry." After lineage depletion (lineage depletion
taining conserved Meis1 sites (located next to start codon sequence, human
kit; BD Biosciences), cells were incubated with 1M 5-(and-6)-carboxy-
chr2:46 525 052-46 525 064) from the Hif-2␣ promoter was amplified by
2⬘,7⬘-dichlorofluorescein diacetate (carboxy-DCFDA; Invitrogen) for
PCR from mouse genomic DNA with the following primers: conserved
30 minutes in a 37°C water bath in the dark. Then, cells were stained for
Meis1 site: pHif-2␣-F, 5⬘-GGGCTAAACGGAACTCCAGG-3⬘ and pHif-
HSC markers Sca-1-PE/Cy5.5, C-Kit-APC, CD34-PE, and Flk2-PE and
2␣-R, 5⬘-CATAGGAACGCTCTCGGAAAGAC-3⬘. PCR fragments were
assayed with a flow cytometer.
subcloned into the pCR2.1-TOPO vector (Invitrogen) according to themanufacturer's instructions. pHif2a-TOPO and E1b-pGL2 vectors were
NAC administration in vivo
digested with
XhoI and
KpnI. Then, the PCR fragments containing con-served Meis1 sites were cloned into E1b-pGL2 to generate the pHif2a-
Six-week-old Meis1f/f;Scl-Cre-ERT⫹ mice were injected intraperitoneally
pGL2 luciferase reporter vector. To test Meis1 site specificity, Meis1-
with tamoxifen (40 mg/kg) daily for 14 days. After tamoxifen injections,
binding sites (TGAC) at the
TGACAGC
TGACAA (Meis1 binding site is
animals were treated daily for 2 weeks with N-acetyl-L-cysteine (NAC;
indicated in bold) sequence were mutated to
TGGCGGC
CGCCAA (
NotI
100 mg/kg; Sigma-Aldrich) by subcutaneous administration or provided in
site insertion) using iProof High-Fidelity DNA Polymerase (Bio-Rad) from
drinking water (500 mg ⫻ kg⫺1 ⫻ d⫺1 in drinking water as described
the Hif-2␣-pGL2 vector with the following primers: Mut-Hif2a-F, 5⬘-
previously39,40) and were subsequently analyzed by flow cytometry. We
AAgcggccgcCAAGGAGAAAAAAAGGTAAGCGGG-3⬘ and Mut-
used age-matched tamoxifen-injected Meis1⫹/⫹;Scl-Cre-ERT(⫹) or
Hif2a-R, 5⬘-AAgcggccgcCATTGTCGCCGTGGCCCTC-3⬘. The Hif-2␣-
Meis1f/f;Scl-Cre-ERT(⫺) mice as controls (Meis1⫹/⫹ mice).
Mut-pGL2 reporter was generated after
NotI digestion and ligation of thePCR product. Transcriptional activation of Hif-2␣ by Meis1 was evaluated
Chromatin immunoprecipitation assay
using a luciferase reporter system (Promega) as described previously.11
Chromatin immunoprecipitation assay (ChIP) assays were performed to evaluate
the in vivo binding of Meis1 to its consensus sequence in the
Hif-2␣ gene. Theassays were done in Kasumi-1 cells, a hematopoietic progenitor cell line, using
Metabolic assays are carried out as described previously11 with some
the ChIP kit (Upstate) as described previously.11 Meis1 antibody (Santa Cruz
Biotechnology) and normal goat IgG (Santa Cruz Biotechnology) were used.
The DNA isolated from input chromatin fragments and from the precipitated
Oxygen consumption assays
chromatin fragments by anti-Meis1 antibody or control IgG was subjected toreal-time PCR using primers flanking the consensus Meis1-binding sites on
Meis1⫹/⫹ and Meis1⫺/⫺ HSCs were separated flowcytometrically as
described in "Flow cytometry." Equal numbers of cells (5-10 ⫻ 104 cells/
well) were incubated for 6 hours in the provided 384-well plate (BDOxygen Biosensor System) and sealed to prevent air exchange beforemeasurement. Culture media lacking cells was used as a negative control
Measurement of blood cell counts (CBC/differential)
and sodium sulfite (100mM) was used as a positive control. Oxygen
Approximately 50 L of peripheral blood collected by retro-orbital bleed-
consumption is presented as relative units.
ing from Meis1⫹/⫹ and Meis1⫺/⫺ mice post 1 month and 3 months oftamoxifen injections in K3-EDTA tubes. Samples are submitted to Univer-
ATP assays
sity of Texas Southwestern Medical Center Animal Resources Center
Meis1⫹/⫹ and Meis1⫺/⫺ HSCs were sorted as described and centrifuged at
(UTSW ARC) Diagnostic Laboratory and analyzed with the HemaVet
1200
g for 10 minutes. At least 50 000 cells were used for each single ATP
950FS analyzer. The following parameters are reported for the sample
measurement. Fifty microliters of ATP standards (10⫺6-10⫺12M) and 50 L
submitted: white blood count (WBC), neutrophil count, lymphocyte count,
of cell lysates were quantified using the ATP Bioluminescence Assay Kit
monocyte count, eosinophil count, basophil count, red blood cell count,
CLS II (Roche) using Fluostar Optima plate reader (BMG Labtech).
hemoglobin, HCT, MCV, MCH, MCHC, platelet count, and MPV.
Finally, data were normalized to cell count and protein content.
Generation of the Meisf/f;Scl-Creⴙ
; rtTA; TRE-Hif-1␣
mice and
Glycolytic flux assay
rescue analysis by viral Hif-1␣
and Hif-2␣
overexpression
13C-lactate production, end product of glycolysis, was measured as
To determine the role of Hif-1␣ in Meis1 KO HSCs, inducible HSC-
described previously11 using glycolytic flux medium supplemented with
specific, Hif-1␣ transgenic mice were generated. The transgenic construct
10mM D-[1-6-13C]-glucose (Cambridge Isotope Labs) to allow up to all of
was made by cloning
Hif-1␣ cDNA (Open Biosystems) into the pTRE-
the glucose-derived lactate pool to be labeled on C-3. A minimum of
Tight vector (Clontech), which makes it responsive to rtTA regulatory
50 000 cells were cultured in 40 L of flux medium overnight. Then, the
proteins in the Tet-On system. We crossed TRE-Hif-1␣ mice with rtTA mice
cells were pelleted and supernatant collected and prepared for gas
which has a stop-codon flanked by loxP sequence. Cre-mediated removal of
chromatography–mass spectrometry. Lactate abundance was determined
the stop region by deletion of the loxP flanked sequence allows expression
by monitoring m/z at 117 (unenriched), 118 (lactate containing 13C from
of rtTA as described.41 Therefore, sequential administration of tamoxifen
glucose), and 119 (internal standard) as described previously.11
will result in
Meis1 deletion, and concomitant deletion of the stop-codon,
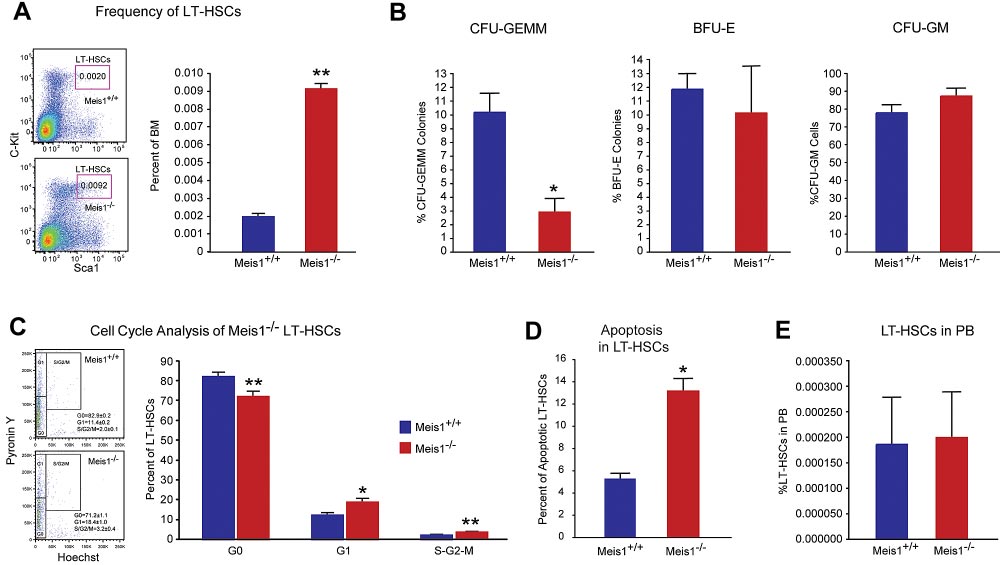
BLOOD, 13 DECEMBER 2012 䡠 VOLUME 120, NUMBER 25
Figure 1. Meis1 Deletion in LT-HSCs results in apoptosis and loss of quiescence. (A) Left panel: Representative flow cytometry profile of LT-HSCs (Lin⫺Sca-
1⫹Kit⫹Flk2⫺CD34⫺) of bone marrow (BM) cells are shown for control Meis1⫹/⫹ and mutant Meis1⫺/⫺ mice. Numbers in the FACS plots indicate percentages among total
BM cells. Right panel: Quantification of LT-HSCs demonstrates significantly higher number of HSCs in Meis1⫺/⫺ BM (n ⫽ 6). (B) The in vitro methylcellulose colony formation
assay was performed at the time of sacrifice after tamoxifen injections with BM cells of control and Meis1⫺/⫺ mice. CFU-GEMM colonies representing most undifferentiated
progenitors type of colonies derived from Meis1⫹/⫹ and Meis1⫺/⫺ BM cells demonstrates decreased percentage of CFU-GEMM. Quantification of BFU-E and CFU-GM colonies
derived from Meis1⫺/⫺ cells shows no differences (n ⫽ 3). (C) Left panel: Representative FACS analysis of Pyronin Y/Hoecst staining on LT-HSCs (Lin⫺Sca-
1⫹Kit⫹CD150⫹CD48⫺) of Meis1⫹/⫹ and Mesi1⫺/⫺ mice. Numbers in the FACS plots indicate percentages among LT-HSCs. Right panel, The quantification of G0, G1, or S/G2/M
phase in Meis1⫹/⫹ and Meis1⫺/⫺ LT-HSCs (n ⫽ 6). (D) Quantification of apoptosis in Meis1⫹/⫹ and Meis1⫺/⫺ LT-HSCs (n ⫽ 3). (E) Quantification of LT-HSCs in peripheral blood
(PB) of Meis1⫹/⫹ and Meis1⫺/⫺ mice (n ⫽ 6); *P ⬍ .05, **P ⬍ .01.
which allows for Hif-1␣ overexpression on administration of doxycycline.
We generated Meisf/f;Scl-Cre⫹; rtTA; TRE-Hif-1␣ mice by a series of
crosses to overexpress in Hif-1␣ in HSC-specific manner after 14 days oftamoxifen (1 mg/d/mice) and providing doxycycline in drinking water
Deletion of Meis1 in LT-HSCs
(500 mg/L). HSCs from these mice were either used for in vivo repopula-tion studies, or for in vitro colony forming assay.
Because the global loss of Meis1 is embryonic lethal,33,34 we sought
We also used a viral strategy to overexpress Hif-1␣ or Hif-2␣ in HSCs
to pursue an inducible deletion of Meis1 to study the role of Meis1
(generated by cloning into XZ201 vector) and lentivirus-expressing Hif-2␣
in adult HSCs. Meis1fl/fl mice with loxp flanking exon 8 were
(kindly provided by Dr Joseph Garcia, UT Southwestern). Fetal liver cells
crossed with transgenic mice expressing the tamoxifen-inducible
were harvested from Meis1f/f;Scl-Cre⫹ pups at P1 and infected with eitherlentivirus-expressing Hif-2␣ or retrovirus-expressing Hif-1␣ with IRES-
Cre recombinase under the control of stem cell leukemia (Scl) HSC
GFP. Then, infected cells were transplanted into CD45.1⫹ recipient host
enhancer, which drives deletion in HSCs.4 Upon tamoxifen treat-
mice. The repopulation was measured 2 months posttransplantation before
ment, exon 8 is deleted which results in the loss of Meis1
induction of deletion of Meis1 with tamoxifen injections. The repopulation
expression in HSCs (supplemental Figure 1A, available on the
analysis performed at indicated time points post-tamoxifen treatments
Blood Web site; see the Supplemental Materials link at the top of
either measuring GFP⫹ cells or CD45.2⫹ donor cells (n ⫽ 3-5).
the online article). To verify deletion of Meis1, we performed
Finally, in a separate set of experiments, we used cobalt chloride (CoCl2
100M), which is a known stabilizer of both Hif-1␣ and Hif-2␣, in
genotyping analysis and quantitative RT-PCR in peripheral blood
methocult culture after deletion of Meis1 to examine the effects of
cells and phenotypic LT-HSCs (Lin⫺Sca1⫹Kit⫹Flk2⫺CD34⫺) in
stabilization of Hif-1␣ and Hif-2␣ on colony formation assay after Meis1
the bone marrow after tamoxifen treatment. Meis1 in peripheral
deletion. After 14 days of tamoxifen injection into aged matched Meis1⫹/⫹;
blood cells was deleted 14 days after tamoxifen treatment, and
Scl-Cre⫹ (Control) and Meis1f/f;Scl-Cre⫹ (Meis1 KO) mice, we performed
Meis1 mRNA level in LT-HSCs was markedly decreased to
colony-forming assays using Methocult medium supplemented with 100M
approximately 10% of control values (supplemental Figure 1B).
CoCl2. Same number of bone marrow cells (20 ⫻ 103 cells/plate) wasplated as recommended by manufacturer and colonies were quantified after
Meis1 is required for the maintenance of LT-HSCs
12 days of culture.
To explore the role of Meis1 in LT-HSCs, we first examined the
frequencies of phenotypic LT-HSCs in control (Meis1⫹/⫹) and
Results are expressed as mean ⫾ SEM, and a 2-tailed Student t test was
Meis1 conditional KO (Meis1⫺/⫺) mice. As shown in Figure 1A,
used to determine the level of significance. P ⬍ .05 was considered
7 days after tamoxifen treatment, there was a 4.5-fold increase of
HSC frequency in Meis1⫺/⫺ mice compared with Meis1⫹/⫹ controls
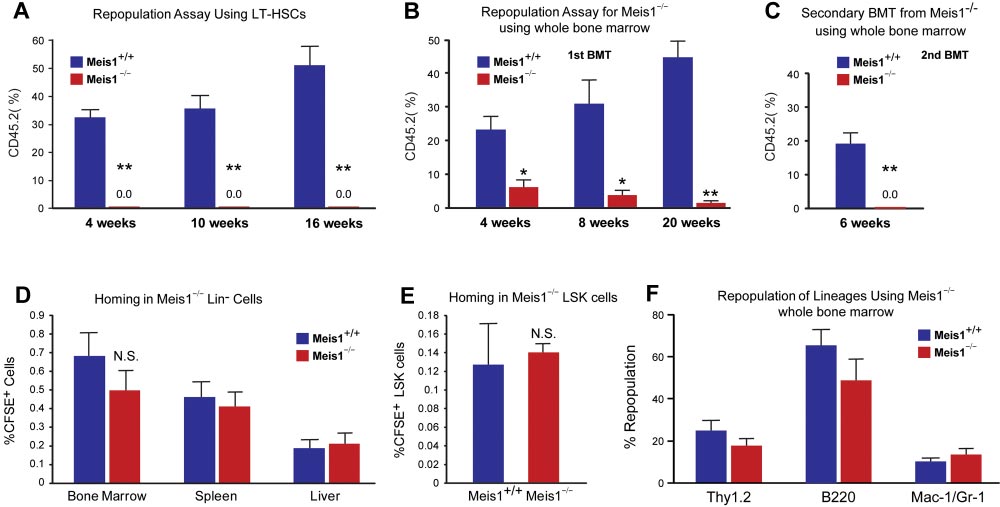
BLOOD, 13 DECEMBER 2012
Meis1 REGULATES Hif-1
䡠 VOLUME 120, NUMBER 25
␣ AND Hif-2␣ IN HSCs
Figure 2. Impaired repopulation in Meis1ⴚ/ⴚ LT-HSCs. Repopulation assays: (A) LT-HSCs (Lin⫺Sca1⫹Kit⫹Flk2⫺CD34⫺; 150 cells) from either control (Meis1⫹/⫹) or mutant
Meis1⫺/⫺ CD45.2 mice were transplanted into irradiated CD45.1 hosts in competition with BM from CD45.1 mice (1 ⫻ 105 cells). Quantification of flow cytometry profile of
peripheral blood of bone marrow recipient mice up to 16 weeks for percentage of CD45.2⫹ cells demonstrates total loss of bone marrow reconstitution of Meis1⫺/⫺ LT-HSCs
(n ⫽ 5). (B) Repopulation assay with whole bone marrow from either control Meis1⫹/⫹ or mutant Meis1⫺/⫺ CD45.2 mice were transplanted into irradiated CD45.1 mice
demonstrates significantly impaired repopulation in mice transplanted with Meis1⫺/⫺ cells (n ⫽ 5). (C) Analysis of repopulation after second bone marrow transplantation (BMT)
from first BMT mice demonstrates complete loss of repopulation (n ⫽ 5). Homing assays: (D) BM Lin⫺ cells (3 ⫻ 106 cells) were transplanted into irradiated mice and quantified
for CFSE⫹ cells in different tissues. Quantification of percentage of CFSE⫹ cells in BM, spleen, and liver show no difference between Meis1⫹/⫹ and Meis1⫺/⫺ mice (n ⫽ 5).
(E) Quantification of percentage of CFSE⫹ LSK cells in BM of Meis1⫹/⫹ and Meis1⫺/⫺ mice (n ⫽ 5). (F) Quantification of repopulation of lineages for Meis1⫺/⫺ using whole bone
marrow from first BMT mice demonstrates no defects in lineage repopulation (n ⫽ 5); *P ⬍ .05, **P ⬍ .01.
(0.0092% vs 0.002%). FACS analysis revealed robust reduction of
To further evaluate the function of Meis1 in HSCs in vivo, we
granulocyte-monocyte progenitors (GMPs) and common myeloid
performed competitive bone marrow transplantation with Meis1⫹/⫹
progenitors (CMPs) and mild reduction in the total number of
and Meis1⫺/⫺ HSCs. Two weeks after tamoxifen treatment,
megakaryocyte/erythroid progenitors (MEPs; supplemental Figure
150 Lin⫺Sca1⫹Kit⫹Flk2⫺CD34⫺ Meis1⫹/⫹ or Meis1⫺/⫺ CD45.2
1C). Meanwhile, by using a colony-forming assay, we demon-
HSCs, together with 1 ⫻ 105 CD45.1 competitors, were adminis-
strated that Meis1⫺/⫺ mice had much lower primitive myeloid
tered into CD45.1 recipients through retro-orbital injection. Repopu-
progenitor cells (CFU-GEMM), but no change in percentage of
lation was examined at 4, 10, 16 weeks after transplantation.
differentiated myeloid progenitor cells (CFU-GM), or erythroid
Strikingly, as shown in Figure 2A, we could not detect any
progenitor cells (BFU-E; Figure 1B, supplemental Figure 1D). No
engraftment with Meis1⫺/⫺ HSC donors, indicating a severe
difference was detected in the distribution of T, B, myeloid, and
impairment of HSC repopulation ability after loss of Meis1. A
erythroid lineages either in BM or peripheral blood of Meis1⫺/⫺
repeat transplantation experiment with total BM cells showed
(supplemental Figure 1E-F).
similar results (Figure 2B). The impaired repopulation of Meis1-
The increase of HSC frequency may result from cell-
deficient LT-HSCs (Figure 2A) and progressive decline in repopu-
autonomously accelerated proliferation or the compensatory effect
lation of Meis1-deficient cells in primary transplant recipients
of increased apoptosis or mobilization in Meis1⫺/⫺ HSCs. To
(Figure 2B) suggested a defect in self-renewal. Therefore, we
address this concern, we first examined the cell cycle of HSCs by
performed secondary transplantation experiments to confirm the
using Hoechest 33342 and pyronin Y staining, and we found that
reduction of function Meis1-deficient LT-HSCs in the bone marrow
only approximately 71% of Meis1⫺/⫺ LT-HSCs were in the G0
of primary transplant recipients. Eight weeks after primary trans-
compartment, which is much lower than that of Meis1⫹/⫹LT-HSCs
plantation, total bone marrow from primary recipients from whole
(⬃ 83%; Figure 1C). This indicated that Meis1⫺/⫺ HSCs were
transplanted mice were harvested and transplanted into secondary
much less quiescent and prone to proliferation. Next, we examined
recipients. Meis1-deficient cells from primary recipients (0.5-
apoptosis status in LT-HSCs (Lin⫺Sca1⫹Kit⫹Flk2⫺CD34⫺) by
1 ⫻ 106 cells) were unable to repopulate secondary recipients
using annexin V. We detected more apoptotic cells in Meis1⫺/⫺
(Figure 2C). The decreased repopulation may result from defects in
LT-HSCs compared with Meis1⫹/⫹ counterpart (Figure 1D, supple-
HSC maintenance, or homing, or increased apoptosis. To exclude
mental Figure 1G for LSK cells). Finally, to examine HSC
the possibility that the decreased engraftment is caused by defect of
mobilization, we performed LT-HSC staining in peripheral blood in
homing in Meis1⫺/⫺ HSCs, we labeled Meis1⫹/⫹ or Meis1⫺/⫺ Lin⫺
Meis1⫹/⫹ and Meis1⫺/⫺ mice, which did not show any difference in
cells with CFSE and injected into lethally irradiated recipients.
HSC frequency (Figure 1E). These data suggest that the increased
Sixteen hours later, we examined CFSE⫹ or LSK CFSE⫹ cells in
apoptosis in Meis1⫺/⫺ LT-HSCs may result in increase of HSC
spleen, liver, and BM. No significant difference in homed total
frequency and decrease of quiescence in BM.
CFSE⫹ or LSK CFSE⫹ cells was detected between Meis1⫹/⫹and
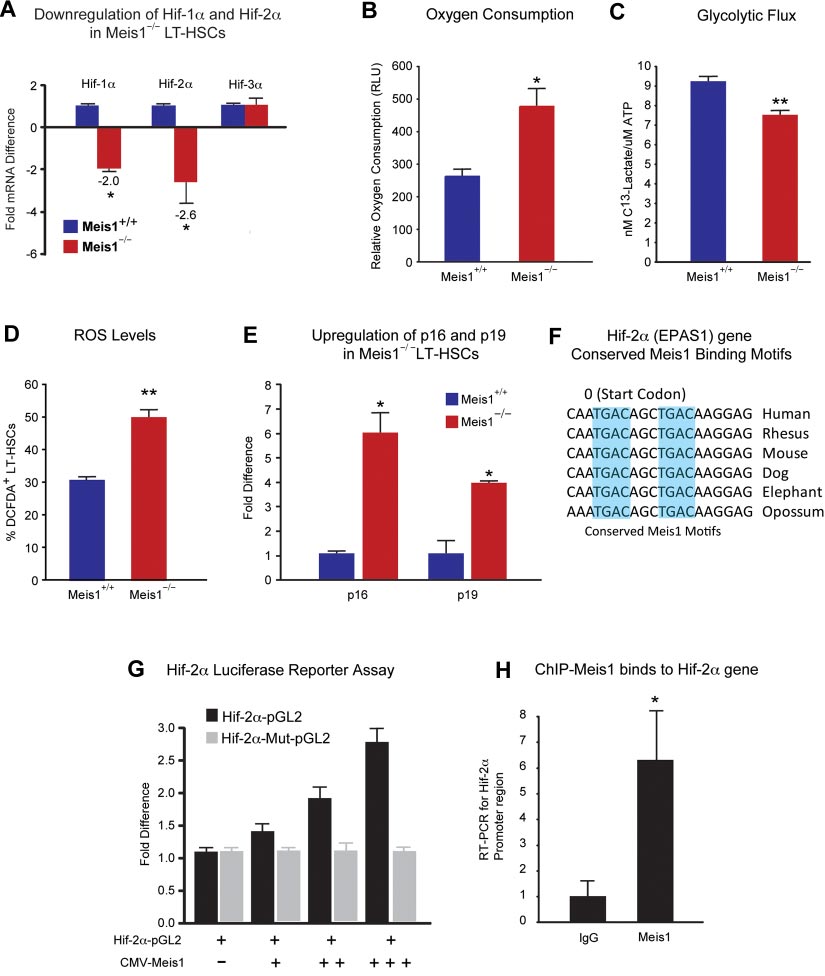
BLOOD, 13 DECEMBER 2012 䡠 VOLUME 120, NUMBER 25
Figure 3. Metabolic regulation of LT-HSCs by Meis1. (A) RT-PCR histogram demonstrates the significant down-regulation of Hif-1␣ and Hif-2␣ (EPAS1), but not Hif-3␣ after
Meis1 deletion in LT-HSCs (n ⫽ 3). (B) Measurement of oxygen consumption rate for 6 hours demonstrates significantly higher aerobic phosphorylation in Meis1⫺/⫺ LT-HSCs
compared with Meis1⫹/⫹ LT-HSCs (n ⫽ 3). (C) Quantification of labeled lactated in glycolytic flux assay demonstrates that Meis1⫺/⫺ LT-HSCs are less glycolytic (n ⫽ 3).
(D) Measurement of reactive oxygen species (ROS) in LT-HSCs as determined by quantification of the percentage of DCFDA⫹ LT-HSCs in Meis1⫹/⫹ and Meis1⫺/⫺ mice
(n ⫽ 3). (E) RT-PCR of p16 and p19 demonstrates association of higher level of ROS with up-regulation of p16 and p19 in Meis1⫺/⫺ LT-HSCs (n ⫽ 3). (F) Figure shows
conserved consensus Meis1 motifs found on Hif-2␣ (EPAS1) gene. Note the duplex Meis1-binding motifs found next to each other and conserved till Opossum. (G) Luciferase
reporter assays demonstrate dose-dependent transcriptional activation of Hif-2␣ by Meis1 (n ⫽ 3). (H) Real-time PCR with primers flanking the consensus Meis1-binding
sequence after ChIP assay demonstrating in vivo binding of Meis1 to Hif-2␣ promoter (n ⫽ 3); *P ⬍ .05, **P ⬍ .01.
Meis1⫺/⫺ donors (Figure 2D-E). In addition, quantification of
hypoxia-inducible factors (Hif-1␣, Hif-2␣, and Hif-3␣) are regu-
lineage repopulation of Meis1⫹/⫹ and Meis1⫺/⫺ donors demon-
lated by Meis1 in LT-HSCs. Quantitative real-time PCR analysis
strates no lineage-specific defects (Figure 2F). Taken together,
demonstrated that mRNA levels of both Hif-1␣ and Hif-2␣ were
these data provide strong evidence that Meis1 plays a crucial role in
down-regulated but no change in Hif-3␣ levels was observed in
the maintenance of HSCs and prevention of apoptosis in HSCs.
Meis1⫺/⫺ LT-HSCs (Figure 3A). Moreover, we found that severaldownstream targets of Hif-1␣ and/or Hif-2␣ are down-regulated in
Role of Meis1 in regulating Hif-1␣ for glycolytic metabolism of
Meis1⫺/⫺ LT-HSCs as determined by quantitative PCR (qPCR)
array (supplemental Figure 2). To explore the role of Hif-1␣ in the
We have shown previously that the Meis1 gene acts upstream of
regulation of metabolism of LT-HSCs, we used the same inducible
Hif-1␣ by regulating its transcriptional activity by an enhancer
Cre system to delete Hif-1␣ specifically in HSCs (supplemental
located in the first intron of Hif-1␣.11 We further examined whether
Figure 3A). Hif-1␣fl/fl mice with loxp flanking exon 2 were crossed
BLOOD, 13 DECEMBER 2012
Meis1 REGULATES Hif-1
䡠 VOLUME 120, NUMBER 25
␣ AND Hif-2␣ IN HSCs
with transgenic mice expressing the tamoxifen-inducible Cre
activation of Hif-2␣ in LT-HSCs. We also confirmed the in vivo
recombinase under the control of stem cell leukemia (Scl) HSC
binding of Meis1 to its conserved sequences in the Hif-2␣ gene by
enhancer. Upon tamoxifen treatment, exon 2 is deleted resulting in
ChIP assays in Kasumi1 cells as determined by real-time PCR after
the loss of Hif-1␣ expression in HSCs (supplemental Figure 3A).
immunoprecipitation with Meis1 antibody (Figure 3H).
To verify deletion of Hif-1␣, we performed quantitative RT-PCR inLT-HSCs (Lin⫺Sca1⫹Kit⫹ Flk2⫺CD34⫺) in bone marrow after
Effect of ROS scavenging on the Meis1ⴚ/ⴚ phenotype
tamoxifen treatment. Hif-1␣ mRNA level in LT-HSCs was dramati-
To study effects of ROS observed in Meis1⫺/⫺ mice, we used NAC,
cally decreased, approximately 400-fold lower than that of control
an antioxidant, in an attempt to rescue the Meis1 phenotype by
(Hif-1⫹/⫹) after 14 days of tamoxifen treatment (supplemental
scavenging of ROS in Meis1 HSCs. After 14 days of tamoxifen
Figure 3B). We showed previously that LT-HSCs primarily rely on
injection, we administered NAC intraperitoneally for 12 days
cytoplasmic glycolysis rather than mitochondrial oxidative phos-
(Figure 4A). Mice where then harvested and HSCs were isolated
phorylation.11 However, the role of Hif-1␣ in HSC metabolism was
for analysis of HSC frequency, HSC cell-cycle status, apoptosis
not examined before. Here, we demonstrate that HSC-specific
rate, ROS levels, and expression of redox-sensitive cell-cycle
deletion of Hif-1␣ results in increased mitochondrial respiration as
regulators p16Ink4a and p19Arf. Here, we show that NAC administra-
shown by increased oxygen consumption (supplemental Figure
tion rescues the Meis1⫺/⫺ phenotype. We found that frequency of
3C), and decreased glycolytic flux measured by the rate of
HSCs in Meis1⫺/⫺ mice become similar to Meis1⫹/⫹ mice (Figure
glucose-derived 13C lactate production (supplemental Figure 3D).
4B). Flow cytometric analysis of cell cycle of Meis1⫹/⫹ and
To further examine the role of Hif-1␣ in HSCs, we examined
Mesi1⫺/⫺ LT-HSCs, which were both injected with NAC, shows
expression of Hif-2␣ and Hif-3␣ in Hif-1␣⫺/⫺ LT-HSCs. We found
similar numbers of G
that deletion of Hif-1␣ in LT-HSCs results in a profound increase in
0 cells in Meis1⫺/⫺ LT-HSCs (Figure 4C).
Quantification of apoptosis in Meis1⫹/⫹ and Meis1⫺/⫺ mice shows
Hif-2␣ mRNA levels (⬎ 120-fold), with no significant change in
an increased apoptosis in Meis1⫺/⫺ mice; however, this increase in
the levels of Hif-3␣ mRNA (supplemental Figure 3E). This marked
the number of apoptotic cells was not statistically significant
compensatory up-regulation of Hif-2␣ in Hif-1␣⫺/⫺ LT-HSCs is in
(P ⫽ .051; Figure 4D). In addition, quantification of ROS after
stark contrast to the down-regulation of Hif-2␣ in the Meis1⫺/⫺
NAC treatments restored ROS to Meis1⫹/⫹ control levels in
Meis1⫺/⫺ LT-HSCs (Figure 4E). Finally, scavenging ROS restored
HSC-specific deletion of Hif-1␣ also demonstrated hematopoi-
the transcript levels of p16Ink4a and p19Arf in HSC to Meis1⫹/⫹
etic defects similar (supplemental Figure 4) to global deletion
control levels (Figure 4F). While these effects are most likely
(Mx-1-Cre) in the bone marrow shown by Takubo and colleagues,25
attributable to the antioxidant effect of NAC, other unknown
including the increased frequency of HSCs (supplemental Figure
mechanisms of NAC may also contribute to this rescue.
4A), decreased myeloid progenitors (supplemental Figure 4B), loss
To further evaluate the effect of ROS in engraftment defect
of quiescence (supplemental Figure 4C), and similar multilineage
observed after Meis1 deletion, we performed another bone marrow
defects (supplemental Figure 4F-G).
transplantation from Meis1⫹/⫹ and Meis1⫺/⫺ mice treated with and
Meis1 is a transcriptional activator of Hif-2␣
without NAC. Two weeks after tamoxifen treatment and NACadministration, 1 ⫻ 105 BM cells from Meis1⫹/⫹ or Meis1⫺/⫺
Down-regulation of Hif-1␣ and Hif-2␣ in Meis1⫺/⫺ LT-HSCs
CD45.2, together with 1 ⫻ 105 CD45.1 competitors, were injected
(Figure 3A) led us to examine their metabolic profile and ROS
into CD45.1 recipients through the retro-orbital complex (Figure
levels. We found that Meis1⫺/⫺ LT-HSCs have increased rates of
4G). After bone marrow transplantation, NAC is provided in
oxygen consumption (Figure 3B) and decreased glycolytic flux
drinking water for 2 weeks and injected daily for 2 more weeks.
(Figure 3C). In addition, Meis1⫺/⫺ LT-HSCs showed significantly
Repopulation was examined at 4 weeks posttransplantation. As
higher ROS levels compared with control Meis1⫹/⫹ LT-HSCs
shown in Figure 4H, scavenging of ROS by NAC administration
(Figure 3D). This increased ROS was associated with increased
restored engraftment defects in Meis1⫺/⫺ donors. In conclusion,
expression of p16Ink4a and p19Arf in Meis1⫺/⫺ LT-HSCs, which are
scavenging ROS, through administration of NAC, rescues the
known to induce HSC senescence and apoptosis, respectively
Meis1 phenotype in HSC by decreasing levels of ROS, thereby
(Figure 3E). Therefore, the increased ROS production in Meis1⫺/⫺
normalizing p16Ink4a and p19Arf expression and restoring HSC
LT-HSCs is compounded by the down-regulation of the master
quiescence and repopulation defects.
antioxidant gene Hif-2␣. Moreover, measurement of peripheralblood counts of Meis1⫺/⫺ mice post 1 month and 3 months of
Effect of HIFs on stem cell function in Meis1ⴚ/ⴚ phenotype
tamoxifen treatments demonstrates decreased red blood cells,white blood cells, as well as platelets, similar to the phenotype
Meis1 KO BM cells treated with 100M CoCl2 resulted in
observed in Hif-2␣⫺/⫺ mice42 (supplemental Figure 5). To deter-
restoration of total number of colonies to the WT levels (supplemen-
mine the mechanism of down-regulation of Hif-2␣ in Meis1⫺/⫺
tal Figure 6A). Moreover, we found that Hif-1␣ overexpression
LT-HSCs, we identified 2 Meis1 consensus-binding motifs in the
using the Meis1f/f;Scl-Cre⫹; rtTA; TRE-Hif-1␣ mice strategy,
promoter of the Hif-2␣ gene (Figure 3F). Using a Hif-2␣–pGL2
which resulted in marked up-regulation of Hif-1␣ in HSCs,
reporter which includes the conserved Meis1-binding sites, we
resulted in restoration of the BFU-E and total number of colonies to
demonstrate a dose-dependent activation of Hif-2␣ by Meis1
the WT levels, as well as partial restoration of number of mixed
expression vector (CMV-Meis1; Figure 3G). In addition, this
colonies (CFU-GEMM) which is a measure of most undifferenti-
activation demonstrates dependence on binding of Meis1 to its
ated hematopoietic progenitors (supplemental Figure 6C). In
consensus-binding sequences in the Hif-2␣ promoter because
addition, we attempted to rescue repopulation defect in Meis1⫺/⫺
mutation of the seed sequences (Hif-2␣–Mut-pGL2) completely
HSCs using viruses that express Hif-1␣ and Hif-2␣ (supplemental
abolished the activation of Hif-2␣ by Meis1. Given down-
Figure 6D) or using Meisf/f;Scl-Cre⫹; rtTA; TRE-Hif-1␣ (supple-
regulation of Hif-2␣ in Meis1⫺/⫺ LT-HSCs (Figure 3A), these
mental Figure 6E); however, these strategies failed to rescue the in
results indicate that Meis1 is required for optimal transcriptional
vivo reconstitution capacity up to 4 months after Meis1 deletion. In
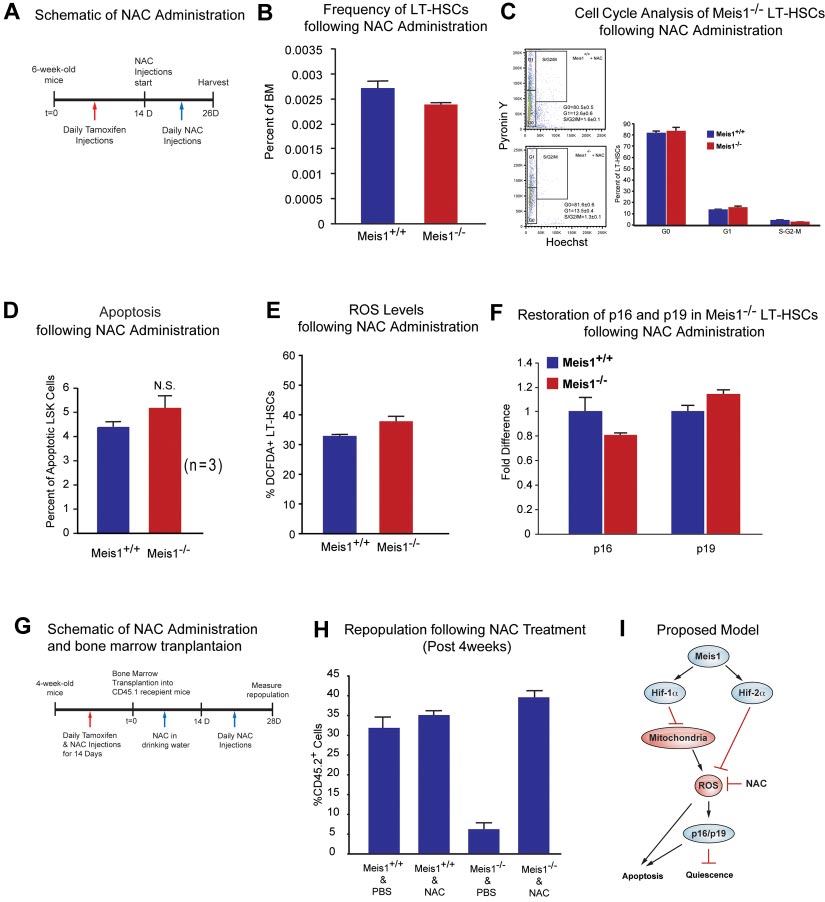
BLOOD, 13 DECEMBER 2012 䡠 VOLUME 120, NUMBER 25
Figure 4. Effect of ROS scavenging on the Meis1ⴚ/ⴚ phenotype. (A) Schematic of NAC administration. We performed daily IP injections for tamoxifen for 14 days followed
by daily NAC injections up to 12 days. (B) Flow cytometry profile of LT-HSCs (Lin⫺Sca-1⫹Kit⫹Flk2⫺CD34⫺) of Meis1⫹/⫹ and Meis1⫺/⫺ mice after 12 days NAC administration.
Note the number of HSCs in Meis1⫺/⫺ mice is now similar to Meis1⫹/⫹ values (n ⫽ 3). (C) Left panel, FACS plot of Pyronin Y/Hoechst staining of LT-HSCs. Right panel:
Quantification of flow cytometric analysis of cell cycle of Meis1⫹/⫹ and Mesi1⫺/⫺ LT-HSCs demonstrates restored numbers of G0 cells in Meis1⫺/⫺ cells which indicates restored
quiescence of LT-HSCs (n ⫽ 3). (D) Quantification of apoptosis in Meis1⫹/⫹ and Meis1⫺/⫺ LSK cells (Lin⫺Sca-1⫹Kit⫹) showing persistent trend toward an increase in the
number of apoptotic cells, which was not statistically significant (P ⫽ .059; n ⫽ 3). (E) Quantification of ROS in Meis1⫹/⫹ and Meis1⫺/⫺ LT-HSCs (Lin⫺Sca-1⫹Kit⫹Flk2⫺CD34⫺)
after NAC treatment showing only a modest increase in ROS in Meis1⫺/⫺ HSCs (n ⫽ 3). (F) Real-time PCR of HSCs isolated from Meis1⫹/⫹ and Meis1⫺/⫺ HSCs after NAC
treatment demonstrating no change in p16 and p19 transcripts (n ⫽ 3). (G) Schematic of NAC administration and bone marrow transplantations. We performed daily IP
injections for tamoxifen and NAC for 14 days followed by bone marrow transplantation. Then, NAC is provided in drinking water for 2 weeks and administrated another
2 weeks. Repopulation was examined at 4 weeks after transplantation. (H) Analysis of repopulation after NAC treatments of BMTs from Meis1⫹/⫹ and Meis1⫺/⫺ mice
demonstrates restoration of repopulation defect after Meis1 deletion (n ⫽ 5). (I) Schematic of proposed model is demonstrating how Meis1 regulates metabolism and
maintenance of HSCs through its role on Hif-1␣ and Hif-2␣.
retrospect, these results are not entirely surprising in light of recent
Hif-2␣, and as a result Meis1 deletion results in HSC dysfunction
results demonstrating a narrow beneficial dose range of Hif-1␣ on
and apoptosis.
HSC function, where overstabilization of Hif-1␣ results in worsen-ing HSC function43 which might explain worsening of the HSCrepopulation defect in Meis1 KO HSCs in vivo (supplemental
Figure 6D-E).
In summary, we demonstrate that Meis1 deletion results in a
In the current report, we demonstrate that Meis1 functions up-
shift in HSC metabolism toward oxygen consumption, with the
stream of a transcriptional network that regulates HSC metabolism
resultant increase in ROS production. This phenotype is com-
and oxidant defense. The Meis1 deletion-induced metabolic shift
pounded by down-regulation of the oxidant stress response gene
and oxidant injury is compounded by the down-regulation of
BLOOD, 13 DECEMBER 2012
Meis1 REGULATES Hif-1
䡠 VOLUME 120, NUMBER 25
␣ AND Hif-2␣ IN HSCs
Hif-2␣, which is in stark contrast to the marked up-regulation of
phenotype after Meis1 deletion, there certainly may be additional
Hif-2␣ in HSC after Hif-1␣ deletion. This phenotype is associated
ROS modulators that are targeted by Meis1.
with up-regulation of p16Ink4a and p19Arf, loss of quiescence,
In the current report, we highlight the role of Meis1 in a
increased apoptosis, and marked HSC dysfunction.
transcriptional network that regulates HSC metabolism and antioxi-
Endothelial PAS domain protein 1 (EPAS1), also known as
dant defense. These results implicate Meis1 as an important
Hif-2␣,44 is closely related to Hif-1␣ in structure and is likewise
regulator of the redox state of HSCs, which may be echoed by its
activated during hypoxia.20 While Hif-1␣ is a master regulator of
role in leukemogenesis. Therefore, it would be important for future
metabolism, Hif-2␣ is a master regulator of oxidant stress re-
studies to determine the role of Meis1 in regulation of leukemia
sponse45 and is induced by ROS.46 It is involved in regulation of
stem cell metabolism, survival, and self-renewal. Our findings
numerous antioxidant genes that minimize the oxidant damage that
suggest that HSCs are endowed with redundant mechanisms for
results from mitochondrial respiration.45 Hif-2␣⫺/⫺ mice are pancy-
regulation of their metabolic phenotype, rather than being solely
topenic,42,47 and have high levels of oxidative stress,45 which
dependent on environmental signals, such as the hypoxic microen-vironment. Deciphering the role of these transcriptional networks
suggests that Hif-2␣ is required for normal hematopoiesis. Our
in regulating HSC fate and function may provide valuable clues for
results indicate that Hif-2␣ is markedly up-regulated after Hif-1␣
understanding HSC disease and malignancies.
deletion, which is an indication of a robust antioxidant response toHif-1␣ deletion, likely secondary to a shift toward oxidativemetabolism, with the subsequent increase in ROS.46 In stark
contrast, we show that Hif-2␣ is down-regulated after Meis1 KO inHSCs, which may partially explain the severity of the Meis1⫺/⫺
The authors thank Dr Keith Humphries, Michelle Miller, and Patty
phenotype compared with the Hif-1␣⫺/⫺ phenotype.
Rosen for sharing some initial data on characterization of the KO
Delicate control of ROS levels in HSCs is crucial for HSC
line genotyping, Dr Joachim R. Goethert at Universitaetsklinikum
maintenance, where elevated ROS levels in HSCs is associated
Essen for providing the Scl-Cre-ERT mice, and Dr Joseph A. Garcia
with defects in HSC self-renewal and increased apoptosis. Regula-
for Hif-1␣ floxed mice.
tion of ROS in HSCs is a highly complex process that involves
This work is supported by grants from the American Heart
regulation of the metabolic phenotype of HSCs, as well as
Association (AHA; GIA12060240 [H.A.S.]), the Gilead Research
regulation of antioxidant defense mechanisms. The contribution of
Scholars Program in Cardiovascular Disease (H.A.S.), National
oxidative metabolism to ROS production has been extensively
Institutes of Health (NIH) grant R01HL115275 (H.A.S.), NIH
studied.48 It is estimated that 2% of all electrons flowing through
grant K01 CA 120099 (C.Z.), and Cancer Prevention and Research
the mitochondrial respiratory chain result in the formation of
Institute of Texas (CPRIT) RP100402 (C.Z.).
oxygen free radicals. Electrons leaking from the respiratory chaininteract with oxygen, partially reducing it to superoxide anion
(O2⫺䡠).48 Even though O2⫺䡠 itself is not a strong oxidant, it is theprecursor of most other ROS.14 ROS overwhelm the natural
Contribution: F.K. designed and performed the research, analyzed
antioxidant defense mechanisms over time, and result in wide-
data, and wrote the manuscript; J.Z., S.T., N.G.C., N.A.J., and
spread cellular damage.48 ROS can also induce the cell-cycle
R.J.D. performed research and analyzed data; and C.Z. and H.A.S.
regulators p16Ink4a and p19Arf, which cause loss of quiescence and
designed and supervised research and wrote the manuscript.
apoptosis of HSCs.49 Our results indicate that Meis1 regulates ROS
Conflict-of-interest disclosure: The authors declare no compet-
production in HSC through regulation of both the metabolic
ing financial interests.
phenotype (through Hif-1␣) and oxidant defense mechanisms
The current affiliation for F.K. is Texas Institute of Biotechnol-
(through Hif-2␣). Finally, although our ROS scavenging studies
ogy, Edu&Res, North American College, Houston, TX.
indicate that the effect of loss of Meis1 on HSC cell cycle and
Correspondence: Hesham A. Sadek, MD, PhD, Department of
engraftment defect are entirely mediated through an increase in
Internal Medicine, Division of Cardiology, UT Southwestern
ROS production, rescue studies using Hif-1␣ and/or Hif-2␣
Medical Center, NB10.222, 6000 Harry Hines Blvd, Dallas, TX
overexpression or stabilization failed to fully rescue the phenotype.
75390; e-mail: [email protected]; or Chengcheng
These results support previous reports which indicate that the
Zhang, PhD, Departments of Physiology and Developmental Biology,
precise dose of Hif-1␣ is necessary for optimal HSC function.25
UT Southwestern Medical Center, NB10.222, 6000 Harry Hines Blvd,
Although our results implicate Hif-1␣ and Hif-2␣ in the HSC
Dallas, TX 75390; e-mail: [email protected].
1. Abramson S, Miller RG, Phillips RA. The identifi-
contribute to adult hematopoiesis. Blood. 2005;
the neighbors: stem cells and their niche. Cell.
cation in adult bone marrow of pluripotent and
restricted stem cells of the myeloid and lymphoid
5. Kiel MJ, Yilmaz OH, Iwashita T, Yilmaz OH,
9. Eliasson P, Jonsson JI. The hematopoietic stem
systems. J Exp Med. 1997;145(6):1567-1579.
Terhorst C, Morrison SJ. SLAM family receptors
cell niche: low in oxygen but a nice place to be.
2. Jordan CT, McKearn JP, Lemischka IR. Cellular
distinguish hematopoietic stem and progenitor
J Cell Physiol. 2010;222(1):17-22.
and developmental properties of fetal hematopoi-
cells and reveal endothelial niches for stem cells.
10. Cipolleschi MG, Dello Sbarba P, Olivotto M. The
etic stem cells. Cell. 1990;61(6):953-963.
role of hypoxia in the maintenance of hematopoi-
3. Osawa M, Hanada K, Hamada H, Nakauchi H.
6. Schofield R. The relationship between the spleen
etic stem cells. Blood. 1993;82(7):2031-2037.
Long-term lymphohematopoietic reconstitution by
colony-forming cell and the haemopoietic stem
11. Simsek T, et al. The distinct metabolic profile of
a single CD34-low/negative hematopoietic stem
cell. Blood Cells. 1978;4(1-2):7-25.
hematopoietic stem cells reflects their location in
7. Spradling A, Drummond-Barbosa D, Kai T. Stem
a hypoxic niche. Cell Stem Cell. 2010;7(3):380-
4. Go¨thert JR, Gustin SE, Hall MA, et al. In vivo fate-
cells find their niche. Nature. 2001;414(6859):98-
tracing studies using the Scl stem cell enhancer:
12. Semenza GL. Life with oxygen. Science. 2007;
embryonic hematopoietic stem cells significantly
8. Fuchs E, Tumbar T, Guasch G. Socializing with
BLOOD, 13 DECEMBER 2012 䡠 VOLUME 120, NUMBER 25
13. Miquel J, et al. Mitochondrial role in cell aging.
level is essential for hematopoietic stem cells.
neic transplantation. Cell Stem Cell. 2011;9(2):
Exp Gerontol. 1980;15(6):575-591.
Cell Stem Cell. 2010;7(3):391-402.
14. Turrens JF. Mitochondrial formation of reactive
26. Moskow JJ, et al. Meis1, a PBX1-related homeo-
38. Xu Y, et al. SUMO-specific protease 1 regulates
oxygen species. J Physiol. 2003;552(Pt 2):335-
box gene involved in myeloid leukemia in BXH-2
the in vitro and in vivo growth of colon cancer
mice. Mol Cell Biol. 1995;15(10):5434-5443.
cells with the upregulated expression of CDK in-
15. Ergen AV, Goodell MA. Mechanisms of hemato-
hibitors. Cancer Lett. 2011;309(1):78-84.
27. Rozovskaia T, et al. Upregulation of Meis1 and
poietic stem cell aging. Exp Gerontol. 2010;45(4):
HoxA9 in acute lymphocytic leukemias with the
39. Barresi MJ, et al. The development of the Can-
t(4:11) abnormality. Oncogene. 2001;20(7):874-
berra symptom scorecard: a tool to monitor thephysical symptoms of patients with advanced tu-
16. Jang YY, Sharkis SJ. A low level of reactive oxy-
mours. BMC Cancer. 2003;3:32.
gen species selects for primitive hematopoietic
28. Pineault N, et al. Differential and common leuke-
stem cells that may reside in the low-oxygenic
40. Li HY, et al. Reprogramming induced pluripotent
mogenic potentials of multiple NUP98-Hox fusion
niche. Blood. 2007;110(8):3056-3063.
stem cells in the absence of c-Myc for differentia-
proteins alone or with Meis1. Mol Cell Biol. 2004;
tion into hepatocyte-like cells. Biomaterials. 2011;
17. Elvidge GP, et al. Concordant regulation of gene
expression by hypoxia and 2-oxoglutarate-
29. Fischbach NA, et al. HOXB6 overexpression in
dependent dioxygenase inhibition: the role of
41. Belteki G, et al. Conditional and inducible trans-
murine bone marrow immortalizes a myelomono-
HIF-1alpha, HIF-2alpha, and other pathways.
gene expression in mice through the combinato-
cytic precursor in vitro and causes hematopoietic
J Biol Chem. 2006;281(22):15215-15226.
rial use of Cre-mediated recombination and tetra-
stem cell expansion and acute myeloid leukemia
cycline induction. Nucleic Acids Res. 2005;33(5):
18. Hagg M, Wennstrom S. Activation of hypoxia-
in vivo. Blood. 2005;105(4):1456-1466.
induced transcription in normoxia. Exp Cell Res.
30. Argiropoulos B, Yung E, Humphries RK. Unravel-
42. Scortegagna M, et al. The HIF family member
ing the crucial roles of Meis1 in leukemogenesis
EPAS1/HIF-2alpha is required for normal hema-
19. Maxwell PJ, et al. HIF-1 and NF-kappaB-mediated
and normal hematopoiesis. Genes Dev. 2007;
topoiesis in mice. Blood. 2003;102(5):1634-1640.
upregulation of CXCR1 and CXCR2 expression pro-
43. Takubo K, et al. Regulation of the HIF-1alpha
motes cell survival in hypoxic prostate cancer cells.
31. Argiropoulos B, Humphries RK. Hox genes in he-
level is essential for hematopoietic stem cells.
matopoiesis and leukemogenesis. Oncogene.
Cell Stem Cell. 2010;7(3):391-402.
20. Wang GL, et al. Hypoxia-inducible factor 1 is a
44. Tian H, McKnight SL, Russell DW. Endothelial
basic-helix-loop-helix-PAS heterodimer regulated
32. Imamura T, et al. Frequent co-expression of
PAS domain protein 1 (EPAS1), a transcription
by cellular O2 tension. Proc Natl Acad Sci U S A.
HoxA9 and Meis1 genes in infant acute lympho-
factor selectively expressed in endothelial cells.
blastic leukaemia with MLL rearrangement. Br J
Genes Dev. 1997;11(1):72-82.
21. Marin-Hernandez, Gallardo-Pe´rez JC, Ralph SJ,
45. Scortegagna M, et al. Multiple organ pathology,
Rodríguez-Enríquez S, Moreno-Sa´nchez R. HIF-
metabolic abnormalities and impaired homeosta-
1alpha modulates energy metabolism in cancer
33. Azcoitia V, et al. The homeodomain protein Meis1
sis of reactive oxygen species in Epas1⫺/⫺
cells by inducing over-expression of specific gly-
is essential for definitive hematopoiesis and vas-
mice. Nat Genet. 2003;35(4):331-340.
colytic isoforms. Mini Rev Med Chem. 2009;9(9):
cular patterning in the mouse embryo. Dev Biol.
46. Wiesener MS, et al. Induction of endothelial PAS
domain protein-1 by hypoxia: characterization
22. Kim JW, et al. HIF-1-mediated expression of py-
34. Hisa T, et al. Hematopoietic, angiogenic and eye
and comparison with hypoxia-inducible factor-
ruvate dehydrogenase kinase: a metabolic switch
defects in Meis1 mutant animals. EMBO J. 2004;
1alpha. Blood. 1998;92(7):2260-2268.
required for cellular adaptation to hypoxia. Cell
47. Scortegagna M, et al. HIF-2alpha regulates mu-
35. Ficara F, et al. Pbx1 regulates self-renewal of
rine hematopoietic development in an
23. Papandreou I, et al. HIF-1 mediates adaptation to
long-term hematopoietic stem cells by maintain-
erythropoietin-dependent manner. Blood. 2005;
hypoxia by actively downregulating mitochondrial
ing their quiescence. Cell Stem Cell. 2008;2(5):
oxygen consumption. Cell Metab. 2006;3(3):187-
48. Orrenius S, Gogvadze V, Zhivotovsky B. Mito-
36. Zheng J, et al. Angiopoietin-like protein 3 sup-
chondrial oxidative stress: implications for cell
24. Zhang H, et al. HIF-1 inhibits mitochondrial bio-
ports the activity of hematopoietic stem cells in
death. Ann Rev Pharmacol Toxicol. 2007;47:143-
genesis and cellular respiration in VHL-deficient
the bone marrow niche. Blood. 2011;117(2):470-
renal cell carcinoma by repression of C-MYC ac-
49. Ito K, et al. Reactive oxygen species act through
tivity. Cancer Cell. 2007;11(5):407-420.
37. Zheng J, et al. Ex vivo expanded hematopoietic
p38 MAPK to limit the lifespan of hematopoietic
25. Takubo K, et al. Regulation of the HIF-1alpha
stem cells overcome the MHC barrier in alloge-
stem cells. Nat Med. 2006;12(4):446-451.
2012 120: 4963-4972
originally published
doi:10.1182/blood-2012-05-432260online September 20, 2012
Meis1 regulates the metabolic phenotype and oxidant defense of
hematopoietic stem cells
Fatih Kocabas, Junke Zheng, Suwannee Thet, Neal G. Copeland, Nancy A. Jenkins, Ralph J.
DeBerardinis, Chengcheng Zhang and Hesham A. Sadek
Updated information and services can be found at:
Articles on similar topics can be found in the following Blood collections
Information about reproducing this article in parts or in its entirety may be found online at:
Information about ordering reprints may be found online at:
Information about subscriptions and ASH membership may be found online at:
Blood (print ISSN 0006-4971, online ISSN 1528-0020), is published weekly by the American Societyof Hematology, 2021 L St, NW, Suite 900, Washington DC 20036.
Source: http://regbio.yeditepe.edu.tr/uploads/6/5/1/4/6514592/blood.pdf
Manual de Ceremonial y Protocolo UNIVERSIDAD TECNICA FEDERICO SANTA MARIA Los actos y ceremonias que se realizan en la USM, requieren estar dotados de uni- formidad y revestidos de la solemnidad correspondiente a su prestigio e imagen institucional. Por ello, la Dirección General de Comunicaciones (DGC) presenta el Manual de Ceremonial y Protocolo, con el objetivo de ordenar y orientar su elabora-
Dopage au quotidien Un document de base d'Addiction Suisse 1. Introduction A en croire les médias, on assiste à une augmentation de la tendance à recourir aux artifices les plus divers pour augmenter sa performance au travail et dans la vie privée. Même des personnes en parfaite santé n'hésitent pas à intervenir dans le fonctionnement de leur corps. Une pratique qui n'a rien de nouveau. Dans le domaine du physique ce souci d'optimisation, nourri par l'obsession contemporaine de la beauté et de la performance, se traduit déjà, du moins dans certains milieux, par la chirurgie esthétique, la médecine sportive et les médicaments « lifestyle » (par exemple les produits anti-âge). En matière de sport, le dopage a déjà provoqué un vaste débat éthique et philosophique, non seulement en raison des risques qu'il comporte pour la santé, mais surtout du manque de fair-play dont les sportifs de compétition font preuve, de l'avis général, en se procurant ainsi des avantages injustifiés sur leurs adversaires. Cette controverse a débouché sur l'établissement de règles concernant l'utilisation des produits dopants. En revanche, la discussion sur l'emploi de ces derniers dans le cadre de la vie privée et professionnel e ne fait que commencer.




