Levitra enthält Vardenafil, das eine kürzere Wirkdauer als Tadalafil hat, dafür aber schnell einsetzt. Männer, die diskret bestellen möchten, suchen häufig nach levitra kaufen ohne rezept. Dabei spielt die rechtliche Lage in der Schweiz eine wichtige Rolle.
Pt040000296p
0022-3565/00/2931-0296$03.00/0THE JOURNAL OF PHARMACOLOGY AND EXPERIMENTAL THERAPEUTICS
Copyright 2000 by The American Society for Pharmacology and Experimental Therapeutics
Printed in U.S.A.
JPET 293:296–303, 2000
Allopurinol Prevents Early Alcohol-Induced Liver Injury in Rats1
HIROSHI KONO, IVAN RUSYN, BLAIR U. BRADFORD, HENRY D. CONNOR, RONALD P. MASON, andRONALD G. THURMAN
Laboratory of Hepatobiology and Toxicology, Department of Pharmacology (H.K., I.R., B.U.B., H.D.C., R.G.T.), and Curriculum in Toxicology(I.R., R.P.M., R.G.T.), University of North Carolina at Chapel Hill, Chapel Hill, North Carolina; and Laboratory of Pharmacology and Chemistry,National Institute of Environmental Health Sciences, Research Triangle Park, North Carolina (H.D.C., R.P.M.)
Accepted for publication January 4, 2000
This paper is available online at http://www.jpet.org
ABSTRACT
Free radical formation caused by chronic ethanol administra-
the control group, serum aspartate aminotransferase and ala-
tion could activate transcription factors such as nuclear fac-
nine aminotransferase levels were ⬃40 I.U./l and 25 U/l, re-
tor-B (NF-B), which regulates production of inflammatory
spectively. Administration of enteral ethanol for 4 weeks in-
cytokines. Xanthine oxidase is one potential source of reactive
creased serum transaminases ⬃5-fold. Allopurinol blunted
oxygen species. Therefore, the purpose of this study is to
these increases significantly by ⬃50%. Ethanol treatment also
determine whether allopurinol, a xanthine oxidase inhibitor and
caused severe fatty infiltration, mild inflammation, and necrosis.
scavenger of free radicals, would affect free radical formation,
These pathological changes also were blunted significantly by
NF-B activation, and early alcohol-induced liver injury in rats.
allopurinol. Furthermore, enteral ethanol caused free radical
Male Wistar rats were fed a high-fat diet with or without ethanol
adduct formation, values that were reduced by ⬃40% by allo-
(10 –16 g/kg/day) continuously for up to 4 weeks with the
purinol. NF-B binding was minimal in the control group but
Tsukamoto-French enteral protocol. Either allopurinol or saline
was increased significantly nearly 2.5-fold by ethanol. This
vehicle was administered daily. Allopurinol had no effect on
increase was blunted to similar values as control by allopurinol.
body weight or the cyclic pattern of ethanol in urine. Mean urine
These results indicate that allopurinol prevents early alcohol-
ethanol concentrations were 271 ⫾ 38 and 252 ⫾ 33 mg/dl in
induced liver injury, most likely by preventing oxidant-depen-
ethanol- and ethanol ⫹ allopurinol-treated rats, respectively. In
dent activation of NF-B.
The establishment of a continuous intragastric in vivo
treatment prevents free radical formation and early alcohol-
enteral feeding protocol in the rat by Tsukamoto and French
induced liver injury in the enteral alcohol model (Adachi et
(Tsukamoto et al., 1984) represented a major development in
alcohol research (French et al., 1986; Tsukamoto et al., 1990).
Furthermore, inactivation of Kupffer cells with GdCl pre-
With this model, not only is steatosis observed, which is
vents the hypermetabolic state caused by acute ethanol
characteristic of several animal models, but also inflamma-
(swift increase in alcohol metabolism, SIAM) (Bradford et al.,
tion and necrosis occur in ⬃2 to 4 weeks and fibrosis begins
1993). Additionally, sterilization of the gut with antibiotics
to develop in 12 to 16 weeks.
blocks SIAM (Rivera et al., 1998) and it has recently been
Gram-negative bacterial species are a major source of en-
reported that the cyclooxygenase inhibitor indomethacin
dotoxin in the gut microflora (Bode et al., 1984) and blood
blocks SIAM, supporting the hypothesis that mediators of the
endotoxin levels increase with alcohol (Fukui et al., 1991).
Kupffer cell such as prostaglandin E (Bradford et al., 1999)
Endotoxin activates Kupffer cells that produce free radicals
are necessary for increasing the oxygen gradient in the liver
(e.g., superoxide and nitric oxide) (Decker et al., 1989), lead-
after alcohol. Collectively, these data are consistent with the
ing to liver injury (Knecht et al., 1995). Indeed, intestinal
hypothesis that oxidants from Kupffer cells activated by gut-
sterilization with antibiotics (Adachi et al., 1995) and sup-
derived endotoxin are involved in early alcohol-induced liver
pression of endotoxin production with lactobacillus feeding
injury (Thurman, 1998).
(Nanji et al., 1994) minimize alcohol-induced liver injury in
It is known that if Kupffer cells are destroyed with GdCl ,
the Tsukamoto-French enteral model. Moreover, GdCl
generation of ␣-hydroxyethyl radicals is blocked in the en-teral ethanol model in vivo (Knecht et al., 1995). Tumor
Received for publication August 26, 1999.
1
necrosis factor-␣ (TNF-␣) has been shown to cause alcohol-
This work was supported in part by grants from the National Institute of
Alcohol Abuse and Alcoholism.
induced liver injury based on studies with anti-TNF antibody
ABBREVIATIONS: SIAM, swift increase in alcohol metabolism; TNF-␣, tumor necrosis factor-␣; NF-B, nuclear factor-B; AST, aspartate
aminotransferase; ALT, alanine aminotransferase; ESR, electron spin resonance; POBN, ␣-(4-pyridyl-1-oxide)-
N-
t-butylnitrone; DPI, diphenyle-
neiodonium; ICAM-1, intercellular adhesion molecule.
Allopurinol Prevents Early Alcohol-Induced Liver Injury
(Iimuro et al., 1997a) and in TNF receptor-1 knockout mice
was analyzed by gas chromatography to verify that levels were not
(Yin et al., 1999). However, activation of nuclear factor-B
different between the groups when experiments were initiated
(NF-B), which regulates production of inflammatory cyto-
(Glassman et al., 1989). Rats were anesthetized with pentobarbital
kines (i.e., TNF-␣) (Thurman, 1998) has not been examined
(75 mg/kg) and the proximal bile duct was cannulated with polyehty-
in relation to the generation of free radicals. Because Kupffer
lene-10 tubing. After the spin trapping reagent ␣-(4-pyridyl-1-oxide)-
N-
t-butylnitrone (POBN, 1 g/kg b.wt.; Sigma Chemical Co.) was
cells contain xanthine oxidase, which is one potential source
injected slowly into the tail vain, bile samples were collected at
of free radicals, the purpose of this study was to determine
30-min intervals for 3 h into 35 l of 0.5 mM Desferal (deferoxamine
whether allopurinol, a compound shown to be both an inhib-
mesylate; Sigma Chemical Co.) to prevent ex vivo radical formation.
itor of xanthine oxidase and a free radical scavenger
Samples were stored at ⫺80°C until analysis of free radical adducts
(Wiezorek et al., 1994) would alter free radical production,
by ESR spectroscopy as described elsewhere (Knecht et al., 1995).
NF-B activity, and early alcohol-induced injury in rats. Al-
Specifically, samples were thawed, transferred in a quartz flat cell,
lopurinol is shown herein to have a protective effect. Prelim-
and ESR spectra were obtained with a Varian E-109 spectrometer
inary accounts of this work have appeared elsewhere (Kono
equipped with a TM110 cavity. Instrument conditions were as fol-
et al., 1998).
lows: 20-mW microwave power, 1.0-G modulation amplitude, 80-Gscan width, 16-min scan, and 1-s time constant. Spectral data werestored on an IBM-compatible computer and were analyzed for ESR
Materials and Methods
hyperfine coupling constants by computer simulation (Duling, 1994).
ESR signal intensity was determined from the amplitude of the high
Animals and Diets. Male Wistar rats were fed high-fat liquid
field member of the low field doublet (second line from the left) of the
diets with or without ethanol (10 –16 g/kg/day) continuously for up to
ESR spectra and expressed in arbitrary units (1 unit ⫽ 1 mm of chart
4 weeks with the intragastric enteral feeding protocol developed by
Tsukamoto and French (Tsukamoto et al., 1984; French et al., 1986).
Nuclear Protein Extraction and Gel Mobility Shift Assay.
Either allopurinol (100 mg/kg/day as a bolus and 100 mg/kg/day in
Binding conditions for NF-B were characterized and electrophoretic
diet; Sigma Chemical Co., St. Louis, MO) or vehicle (saline, 0.5
mobility shift assays were performed as described in detail elsewhere
ml/day) was administered daily based on protocols developed by
(Zabel et al., 1991). Briefly, nuclear extracts (40 g) from liver
Cohen (1992) and Karwinski et al. (1991). All animals received
tissues were preincubated for 10 min on ice with 1 g of poly(dI-dC)
humane care in compliance with institutional guidelines.
and 20 g of BSA (both from Pharmacia Biotech, Piscataway, NJ) in
A liquid diet described by Thompson and Reitz (1978) supple-
a buffer that contained 1 mM HEPES (pH 7.6), 40 mM MgCl , 0.1 M
mented with lipotropes as described by Morimoto et al. (1994) was
NaCl, 8% glycerol, 0.1 mM dithiothreitol, 0.05 mM EDTA, and 2 l of
used. It contained corn oil as a source of fat (37% of total calories),
a 32P-labeled DNA probe (10,000 cpm/l; Cerenkov) that contained
protein (23%), carbohydrate (5%), minerals, and vitamins, plus ei-
0.4 ng of double-stranded oligonucleotide was added and mixtures
ther ethanol (35– 40% of total calories) or isocaloric maltose-dextrin
were incubated for 20 min on ice and resolved on 5% polyacrylamide
(control diet) as described elsewhere (Tsukamoto et al., 1990).
(29:1 cross-linking) and 0.4⫻ Tris/boric acid/EDTA gel. After electro-
Urine Collection and Ethanol Assay. Ethanol concentration in
phoresis, gels were dried and exposed to Kodak film. Specificity of
urine, which is representative of blood alcohol levels (Badger et al.,
NF-B binding was verified by the competition assay and ability of
1993), was measured daily. Rats were housed in metabolic cages that
specific antibodies to supershift protein-DNA complexes. In the com-
separated urine from feces, and urine was collected over 24 h in
petition assay, a 200-fold excess of the unlabeled oligonucleotide was
bottles containing mineral oil to prevent evaporation. Each day at
added 10 min before addition of the labeled probe. In supershift
9:00 AM, urine collection bottles were changed and a 1-ml sample
experiments, 1 g of rabbit antisera against p50 or p65 protein (a
was stored at ⫺20°C for later analysis. Ethanol concentration was
kind gift of Dr. N. R. Rice, Advanced Bioscience Laboratories, Na-
determined by measuring absorbance at 366 nm, resulting from the
tional Cancer Institute) was added to the reaction mixture after
reduction of NAD⫹ to NADH by alcohol dehydrogenase as described
incubation with labeled probe, which was further incubated at room
elsewhere (Bergmeyer, 1988).
temperature for 30 min. Labeled and unlabeled oligonucleotides
Blood Collection and Transaminase Determinations. Blood
contained the consensus sequence for NF-B (top strand: 5⬘-GCA-
was collected via the tail vein once a week and centrifuged. Serum
GAGGGGACTTTCCGGA-3⬘; bottom strand: 5⬘-GTCTGCCAAAGTC-
was stored at ⫺20°C until assayed for aspartate aminotransferase
CCCTCTG-3⬘) (Baeuerle and Baltimore, 1989). Data were quanti-
(AST) and alanine aminotransferase (ALT) by standard enzymatic
tated by scanning autoradiograms with GelScan XL (Pharmacia
procedures (Bergmeyer, 1988).
LKB, Uppsala, Sweden).
Pathological Evaluation. After 4 weeks of ethanol treatment,
Statistics. ANOVA was used for the determination of statistical
animals were sacrificed; livers were removed, sectioned, and fixed in
significance as appropriate. For comparison of pathological scores,
formalin. Paraffin-embedded sections were stained with hematoxy-
the Mann-Whitney rank sum test was used. A
P value ⬍.05 was
lin-eosin for histological evaluation of steatosis, inflammation, and
selected before the study as the level of significance.
necrosis. Liver pathology was scored as described by Nanji et al.
(1989) as follows: steatosis (the percentage of liver cells containingfat): ⬍25% ⫽ 1⫹, ⬍50% ⫽ 2⫹, ⬍75% ⫽ 3⫹, 75%⬎ ⫽ 4⫹; inflamma-
tion and necrosis: 1 focus per low-power field ⫽ 1⫹; 2 or more foci ⫽2⫹. Pathology was scored in a blinded manner by one of the authors
Body Weight. To allow for full recovery from surgery,
and by an outside expert in rodent liver pathology.
liquid diets were initiated after 1 week. In spite of develop-
The number of neutrophils in liver sections was determined after
ment of greater hepatic injury in ethanol-treated groups, all
4 weeks by counting cells in three high-power fields (400⫻) per slide.
rats grew steadily, making nutritional complications an un-
Fat accumulation causes ballooning of hepatocytes and narrowing of
likely explanation for these results. Animals treated with
the sinusoidal space. This could affect the number of hepatocytes and
allopurinol had no complications during the experimental
sinusoidal space in each field; therefore, the number of hepatocytesalso was counted and the number of neutrophils was expressed per
period. The mean body weight gains were 3.9 ⫾ 0.4 g/day for
100 hepatocytes. The mean values were used for statistical analysis.
the ethanol group and 3.8 ⫾ 0.2 g/day for the ethanol ⫹
Collection of Bile and Detection of Free Radical by Elec-
allopurinol group (Fig. 1). There were no significant differ-
tron Spin Resonance (ESR). Ethanol concentration in the breath
ences in body-weight gains between the groups.
Kono et al.
Fig. 1. Effect of chronic enteral ethanol and allopurinol on body weight of
rats. Male Wistar rats (275–300 g) were used in this study. Body weight
was measured once a week. Data represent means ⫾ S.E. (
n ⫽ 6). E,
high-fat control diet; 䡺, high-fat control diet with allopurinol; F, high-fat
ethanol-containing diet; and f, high-fat ethanol-containing diet with
allopurinol.
Ethanol Concentrations in Urine. As it was reported
previously by several groups (Tsukamoto et al., 1985; Adachiet al., 1994; Nanji et al., 1997), alcohol levels fluctuate in acyclic pattern from zero to ⬎500 mg/dl for unknown reasons.
Allopurinol had no effect on this cyclic pattern of ethanol inurine. There were no significant differences in mean urinealcohol concentrations between rats given ethanol (Fig. 2,top; 271 ⫾ 38 mg/dl) and ethanol ⫹ allopurinol (bottom;252 ⫾ 33 mg/dl).
Serum Transaminase Levels. In control groups, serum
AST and ALT levels were ⬃60 and 25 U/l, respectively, after4 weeks of high-fat control diet (Fig. 3). Administration ofenteral ethanol for 4 weeks caused a 5-fold increase intransaminase levels. Allopurinol blunted this increase signif-icantly by ⬃50%.
Pathological Evaluation. In control groups, there were
no pathological changes in the liver after 4 weeks on a high-
Fig. 2. Representative plots of daily urine alcohol concentrations. Urine
fat diet (Fig. 4). As expected, severe fat accumulation, mild
alcohol concentrations were measured daily as described in
Materials and
inflammation, and necrosis were observed after 4 weeks of
Methods. Typical urine alcohol concentrations.
enteral ethanol feeding, resulting in a total pathology score of5.3 ⫾ 0.3 (Fig. 5). Increases in the pathology scores were
(data not shown). In contrast, treatment with enteral ethanol
blunted significantly by ⬃60% by allopurinol (total pathology
for 4 weeks caused significantly free radical formation (Fig.
score 2.5 ⫾ 0.4).
7). However, this increase was blunted significantly by allo-
The number of infiltrating neutrophils in the liver was
purinol. ESR hyperfine coupling constants were
aN ⫽ 15.70
minimal and not different between the groups in the absence
G, and
a H
⫽ 2.72 G, characteristic of the ␣-hydroxyethyl
of ethanol; however, enteral ethanol for 4 weeks increased
radical adduct (Knecht et al., 1995). ESR signal intensity was
this number ⬃3-fold over control values (Fig. 6). This in-
determined from the amplitude of the high field member of
crease was significantly blunted by ⬃50% by allopurinol.
the low field doublet (second line from the left) of the ESR
Effects of Chronic Ethanol and Allopurinol on Free
spectrum (Fig. 8). The intensity of these signals was in-
Radical Formation. POBN reacts with free radicals such as
creased significantly by enteral ethanol but was blunted by
␣-hydroxyethyl radical from ethanol to produce nitroxides
60% by allopurinol.
detectable by ESR. Radical adducts were barely detectable in
Effect of Chronic Ethanol and Allopurinol on He-
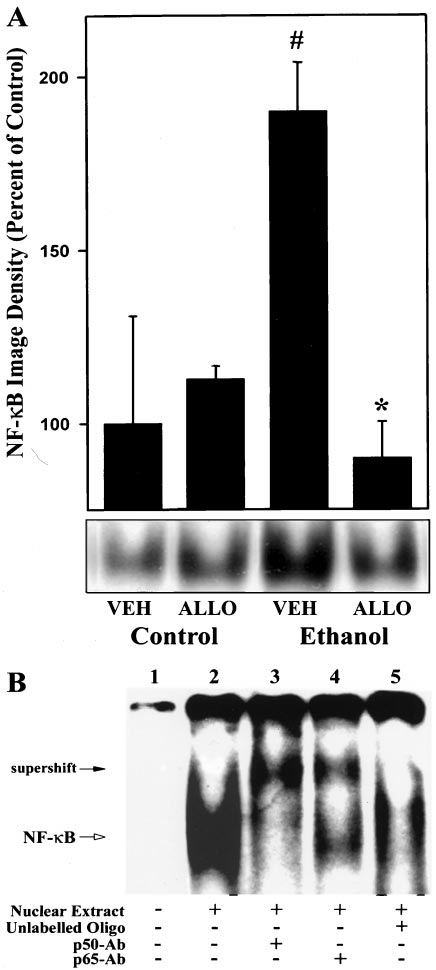
rats fed an ethanol-free, high-fat control diet in both groups
patic NF-
B Activity. NF-B activity was minimal after 4
Allopurinol Prevents Early Alcohol-Induced Liver Injury
quence for NF-B binding, or with antibodies specific for p50or p65 subunit (Fig. 9B). In the absence of nuclear proteins,no protein-DNA complex was detected (lane 1). NF-B DNAbinding activity was assessed from electrophoretic mobilityshift assays with nuclear extracts prepared from liver of a ratfed high-fat ethanol-containing diet (lane 2). Furthermore,addition of anti-p50 or -p65 antiserum reduced the intensityof the complex and produced supershifted complexes with ahigher molecular mass, respectively (lanes 3 and 4). More-over, unlabeled oligonucleotide that contained the NF-B-binding site could effectively compete for DNA binding with32P-labeled probe (lane 5).
Possible Mechanism of Effect of Allopurinol on Early
Alcohol-Induced Liver Injury. Xanthine oxidase gener-
ates reactive oxygen species such as superoxide anion and
hydrogen peroxide (Roy and McCord, 1983). In the liver,
Kupffer cells and endothelial cells, as well as hepatocytes,
contain xanthine dehydrogenase that is readily converted
into xanthine oxidase (Brass et al., 1991). Importantly, hyp-
oxia occurs in the liver during enteral ethanol feeding (Arteel
et al., 1997). Enteral ethanol has been shown to cause a
steepening of the oxygen gradient across the liver lobule and
to cause hypoxia in downstream pericentral regions (Arteel
et al., 1997). During hypoxia, xanthine dehydrogenase is
converted into xanthine oxidase significantly faster in
Kupffer cells than that in other cell types. Upon reoxygen-
ation, xanthine oxidase reacts with molecular oxygen to pro-
duce a burst of superoxide radicals that mediate subsequent
tissue injury (Zhong et al., 1989). Thus, xanthine oxidase in
Kupffer cells could be a potent source of oxidants in the
enteral alcohol model.
Allopurinol, a xanthine oxidase inhibitor and a free radical
scavenger (Wiezorek et al., 1994), prevents liver injury due toischemia-reperfusion by inhibition of free radical formation(Marotto et al., 1988). Moreover, it was reported recentlyfrom this laboratory that peroxisome proliferators (e.g., WY-14,643 and monoethylhexylphthalate) activate Kupffer cellsdirectly and that oxidants from Kupffer cells play a centralrole in NF-B activation and cell proliferation caused byperoxisome proliferators (Rose et al., 1999). Importantly, al-lopurinol blocks NF-B activation caused by WY-14 643 inthe liver (Rusyn et al., 1998). Thus, allopurinol preventsoxidant-dependent activation of NF-B. Oxidants producedduring enteral ethanol are most likely involved in the patho-
Fig. 3. Effect of chronic enteral ethanol and allopurinol on serum aspar-
genesis of early alcohol-induced liver injury (Thurman,
tate and aminotransferase levels. Blood samples were collected via thetail vein at 4 weeks and AST and ALT were measured as described in
1998). Indeed, free radical formation was increased in bile
Materials and Methods. Data represent means ⫾ S.E. (
n ⫽ 6). *
P ⬍ .05
from rats fed enteral ethanol (Knecht et al., 1995). Further-
compared with rats fed high-fat control diet; #
P ⬍ .05 compared with
more, the antioxidant diphenyleneiodonium (DPI) prevented
high-fat ethanol-containing diet by ANOVA and Bonferroni's post hoc
free radical formation and liver injury nearly completely in
the enteral alcohol model (Kono et al., 1999). DPI is an
weeks of high-fat control diet without ethanol (Fig. 9). After
inhibitor of NADPH oxidase that is present in high levels in
4 weeks of enteral ethanol, however, NF-B activity was
Kupffer cells and is a major source of superoxide in this cell
increased significantly nearly 2.5-fold over control values.
type. In this study, allopurinol also blunted free radical for-
This increase was blunted to similar values as control by
mation in the liver (Figs. 7 and 8). Importantly, the effect of
allopurinol on changes in pathology and free radical forma-
To confirm that protein binding to labeled oligonucleotide
tion showed a significant correlation (
r2 ⫽ 0.998). Thus, it is
probe was specific for the active form NF-B, gel shift assays
concluded that allopurinol prevents liver injury, most likely
were carried out either in the presence of an excess of unla-
by inhibition of xanthine oxidase and/or scavenging of free
beled double-stranded oligonucleotide with a consensus se-
radicals (Fig. 10).
Kono et al.
Fig. 4. Photomicrographs of livers after ethanol treatment. Livers from rats given high-fat control or high-fat ethanol-containing diets are shown.
Original magnification, 100⫻. Representative photomicrographs of high-fat control diet ⫹ vehicle (A), high-fat control diet ⫹ allopurinol (B), high-fat
ethanol-containing diet ⫹ vehicle (C), and high-fat ethanol-containing diet ⫹ allopurinol (D). With higher magnification (200⫻), E and F show
inflammation (open arrow) and necrosis (filled arrow) in rats fed high-fat ethanol-containing diet ⫹ vehicle, and G and H depict histology without
inflammation and necrosis in rats fed high-fat ethanol-containing diet ⫹ allopurinol.
Role of NF-
B in Early Alcohol-Induced Liver Injury.
fied within the regulatory elements of genes for several in-
NF-B is rapidly activated in response to immunological
flammatory cytokines such as TNF-␣. Thus, NF-B plays an
stimuli such as lipopolysaccharide, cytokines, and oxidants
important role in regulation of inflammatory responses. In-
(Baldwin, 1996). Binding sites for NF-B have been identi-
creases in production of inflammatory cytokines and adhe-
Allopurinol Prevents Early Alcohol-Induced Liver Injury
Fig. 5. Effect of chronic enteral ethanol and allopurinol on hepatic pa-
thology scores. Pathology was scored as described in
Materials and Meth-
ods. Steatosis, inflammation, and necrosis are shown individually. Data
represent means ⫾ S.E. (
n ⫽ 5). E, high-fat control diet; 䡺, high-fat
control diet with allopurinol; F, high-fat ethanol-containing diet; f, and
high-fat ethanol-containing diet with allopurinol. *
P ⬍ .05 compared with
rats fed high-fat control diet; #
P ⬍ .05 compared with rats fed high-fat
ethanol-containing diet by the Mann-Whitney rank sum test.
Fig. 7. Effect of chronic enteral ethanol and allopurinol on ESR spectra.
Rats were fed enteral liquid diets for 4 weeks intragastrically. After the
spin trap reagent (POBN injection, 1 g/kg i.v.), bile was collected into
Desferal (deferoxamine mesylate, 0.5mM) and analysis of ESR spectra
was performed as described in
Materials and Methods. Representative
ESR spectra.
Fig. 6. Effect of chronic enteral ethanol and allopurinol on the number of
neutrophils in the liver. The number of neutrophils observed in H&E
sections of liver in control and ethanol-fed rats are shown. Values were
determined by counting neutrophils in three high power fields (400⫻) per
slide. The number of hepatocytes also was counted in each field and the
number of cells was expressed per 100 hepatocytes. Data represent
means ⫾ S.E. (
n ⫽ 5). *
P ⬍ .05 compared with rats fed high-fat control
diet; #
P ⬍ .05 compared with rats fed high-fat ethanol-containing diet
with vehicle by ANOVA with Bonferroni's post hoc test.
Fig. 8. Effect of chronic enteral ethanol and allopurinol on average
radical adduct signal intensity. Conditions were the same as for Fig. 7.
sion molecules by NF-B activated by oxidants could be one
ESR signal intensity was determined from the amplitude of the high field
explanation for the pathogenesis of early alcohol-induced
member of the low field doublet (second line from the left) of the ESR
liver injury. Indeed, NF-B activation was increased signifi-
spectra and was averaged for rats treated as described in
Materials andMethods. Data represent means ⫾ S.E. (
n ⫽ 4). VEH, vehicle; ALLO,
cantly by enteral ethanol (Kono et al., 1999). Furthermore,
allopurinol. *
P ⬍ .05 compared with rats fed high-fat ethanol-containing
DPI prevented free radical formation, NF-B activation, and
diet with vehicle by ANOVA and Bonferroni's post hoc test.
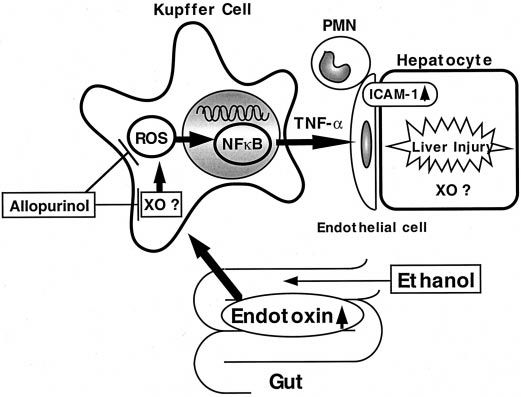
Kono et al.
Fig. 10. Working hypothesis. Chronic ethanol administration causes free
radical formation in the liver. These radicals could be involved in trig-
gering liver injury by increasing transcription factors such as NF-B,
which induce inflammatory cytokine productions. Kupffer cells play an
important role in this process because xanthine oxidase in Kupffer cells
responds to stress much faster than in hepatocytes. In this study, allo-
purinol, a xanthine oxidase inhibitor and a free radical scavenger, pre-
vented NF-B activity and early alcohol-induced liver injury, most likely
by inhibition of free radical formation or scavenging of radical species.
0.622). These results indicate that oxidant-dependent activa-tion of NF-B plays an important role in early alcohol-in-duced liver injury (Fig. 10).
NF-B could increase TNF-␣ production (Watanabe et al.,
1996), which plays an important role in the inflammatorycytokine cascade (Decker et al., 1989). Recent evidence hasaccumulated supporting the hypothesis that TNF-␣ plays anessential role in early alcohol-induced liver injury. Indeed,ethanol increases TNF-␣ mRNA expression in the liver in theTsukamoto-French model (Iimuro et al., 1997a,b). Further-more, anti-TNF-␣ antibody reduces inflammatory cell infil-tration and necrosis in the enteral alcohol model (Iimuro etal., 1997a). Moreover, alcohol-induced liver injury in wild-type mice fed ethanol is prevented in TNF receptor-1 knock-out mice (Yin et al., 1999). TNF-␣ stimulates endothelial cellsto synthesize adhesion molecules [e.g., intercellular adhesionmolecule (ICAM-1)], leading to liver injury (Bevilacqua et al.,
Fig. 9. Effect of chronic enteral ethanol and allopurinol on hepatic NF-B
1987; Dustin and Springer, 1988; Yu et al., 1995). Indeed,
activity. A, nuclear extracts (40 g of total protein in each line) were
expression of TNF-␣ mRNA and ICAM-1 as well as the num-
prepared from frozen livers and used for gel shift assays as described in
ber of neutrophils in the liver are increased in the Tsuka-
Materials and Methods. Data shown are results of densitometric analysisof the NF-B/DNA complex images. Density of the NF-B/DNA complex
moto-French enteral model (Fig. 6) (Iimuro et al., 1997a,b).
image in livers of male rats fed high-fat control diet ⫹ vehicle was set to
Furthermore, alcohol-induced liver injury in wild-type mice
100%. Data represent means ⫾ S.E. (n ⫽ 4). VEH, vehicle; ALLO, allo-
fed enteral ethanol was prevented in ICAM-1 knockout mice
purinol. *P ⬍ .05 compared with rats given high-fat control diet; #P ⬍ .05compared with male rats given ethanol-containing diet by ANOVA with
(data not shown). Taken together, TNF-␣ plays an essential
Bonferroni's post hoc test. B, lane 1 shows labeled probe with no nuclear
role in the mechanism of early alcohol-induced liver injury.
extract NF-B added. NF-B DNA-binding activity was assessed from
Allopurinol most likely inhibits TNF-␣ production by oxi-
electrophoretic mobility shift assays with nuclear extracts prepared fromlivers from of rats fed high-fat control diet (lane 2). Nuclear extracts from
dant-dependent NF-B activation in the liver (See Fig. 10).
rats fed high-fat ethanol-containing diet were used for supershift exper-iments with antibodies specific for p50 or p65 subunit as described inMaterials and Methods (lanes 3 and 4). In competition assays, 200-fold
excess of the unlabeled oligonucleotide was used (lane 5).
We propose that allopurinol prevents early alcohol-induced
liver injury in the enteral alcohol model. Moreover, allopuri-
liver injury by preventing NF-B activation, most likely by
nol blunted these increases significantly in this study (Figs.
inhibiting xanthine oxidase and/or scavenging oxidants.
5, 7, and 9). Furthermore, the effect of allopurinol on the
Kupffer cells most likely play a pivotal role in this process,
activation of NF-B by ethanol correlated with both changes
resulting in subsequent increase in inflammatory cytokines
in pathology (r2 ⫽ 0.659) and free radical formation (r2 ⫽
such as TNF-␣ (Fig. 10).
Allopurinol Prevents Early Alcohol-Induced Liver Injury
attenuates hepatic necrosis and inflammation caused by chronic intragastric eth-
anol exposure in rats. Clin Nutr 17:127.
Adachi Y, Bradford BU, Gao W, Bojes HK and Thurman RG (1994) Inactivation of
Kono H, Rusyn I and Thurman RG (1999) Diphenyleneiodonium, an NADPH oxidase
Kupffer cells prevents early alcohol-induced liver injury. Hepatology 20:453– 460.
inhibitor, prevents early alcoholic liver injury by chronic intragastric ethanol
Adachi Y, Moore LE, Bradford BU, Gao W and Thurman RG (1995) Antibiotics
exposure in rats. Toxicologist 48:257.
prevent liver injury in rats following long-term exposure to ethanol. Gastroenter-
Marotto M, Thurman RG and Lemasters JJ (1988) Early midzonal cell death during
ology 108:218 –224.
low-flow hypoxia in the isolated, perfused rat liver: Protection by allopurinol.
Arteel GE, Iimuro Y, Yin M, Raleigh JA and Thurman RG (1997) Chronic enteral
ethanol treatment causes hypoxia in rat liver tissue in vivo. Hepatology 25:920 –
Morimoto M, Zern MA, Hagbjork AL, Ingelman-Sundberg M and French SW (1994)
Fish oil, alcohol, and liver pathology: Role of cytochrome P4502E1. Proc Soc Exp
Badger TM, Crouch J, Irby D and Shahare M (1993) Episodic excretion of ethanol
Biol Med 207:197–205.
during chronic intragastric ethanol infusion in the male rat: Continuous vs. cyclic
Nanji AA, Khettry U and Sadrzadeh SMH (1994) Lactobacillus feeding reduces
ethanol and nutrient infusions. J Pharmacol Exp Ther 264:938 –943.
endotoxemia and severity of experimental alcoholic liver disease. Proc Soc Exp
Baeuerle PA and Baltimore D (1989) A 65 KD subunit of active NF-B is required for
Biol Med 205:243–247.
inhibition of NF-B by IB. Genes Dev 3:1689 –1698.
Nanji AA, Mendenhall CL and French SW (1989) Beef fat prevents alcoholic liver
Baldwin AS (1996) The NF-B and IB proteins: New discoveries and insights. Annu
disease in the rat. Alcohol Clin Exp Res 13:15–19.
Rev Immunol 14:649 – 681.
Nanji AA, Rahemtulla A, Maio L, Khwaja S, Zhao S, Tahan SR and Thomas P (1997)
Bergmeyer HU (1988) Methods of Enzymatic Analysis. Academic Press, New York.
Alterations in thromboxane synthase and thromboxane A2 receptors in experi-
Bevilacqua MP, Pober JS, Mendrick DL, Cotran RS and Gimbrone MA (1987)
mental alcoholic liver disease. J Pharmacol Exp Ther 282:1037–1043.
Identification of an inducible endothelial-leukocyte adhesion molecule. Proc Natl
Acad Sci USA 84:9238 –9242.
Rivera CA, Bradford BU, Seabra V and Thurman RG (1998) Role of endotoxin in the
Bode JC, Bode C, Heidelbach R, Durr H-K and Martini GA (1984) Jejunal microflora
hypermetabolic state following acute ethanol exposure. Am J Physiol 275:G1252–
in patients with chronic alcohol abuse. Hepatogastroenterology 31:30 –34.
Bradford BU, Enomoto N, Ikejima K, Rose ML, Bojes HK, Forman DT and Thurman
Rose ML, Rivera CA, Bradford BU, Graves LM, Cattley RC, Schoonhoven R, Swen-
RG (1999) Peroxisomes are involved in the swift increase in alcohol metabolism.
berg JA and Thurman RG (1999) Kupffer cell oxidant production is central to the
J Pharmacol Exp Ther 288:254 –259.
mechanism of peroxisome proliferators. Carcinogenesis 20:27–33.
Bradford BU, Misra UK and Thurman RG (1993) Kupffer cells are required for the
Roy RS and McCord JM (1983) Superoxide and ischemia: Conversion of xanthine
swift increase in alcohol metabolism. Res Commun Subst Abuse 14:1– 6.
dehydrogenase to xanthine oxidase, in Oxy Radicals and Their Scavenger Systems.
Brass CA, Narciso J and Gollan JL (1991) Enhanced activity of the free radical
Vol. 2: Cellular and Medical Aspects (Greenwald RA and Cohen G eds) pp 145–153,
producing enzyme xanthine oxidase in hypoxic rat liver. J Clin Invest 87:424 – 431.
Elsevier Publishing Co., Alabama.
Cohen PJ (1992) Allopurinol administered prior to hepatic ischemia in the rat
Rusyn I, Tsukamoto H and Thurman RG (1998) WY-14,643 rapidly activates nuclear
prevents chemiluminescence following restoration of circulation. Can J Anaesth
factor B in Kupffer cells before hepatocytes. Carcinogenesis 19:1217–1222.
Thompson JA and Reitz RC (1978) Effects of ethanol ingestion and dietary fat levels
Decker T, Lohmann-Matthes ML, Karck U, Peters T and Decker K (1989) Compar-
on mitochondrial lipids in male and female rats. Lipid 13:540 –550.
ative study of cytotoxicity, tumor necrosis factor, and prostaglandin release after
Thurman RG (1998) Mechanisms of hepatic toxicity. II. Alcoholic liver injury in-
stimulation of rat Kupffer cells, murine Kupffer cells, and murine inflammatory
volves activation of Kupffer cells by endotoxin. Am J Physiol 275:G605–G611.
liver macrophages. J Leukoc Biol 45:139 –146.
Tsukamoto H, French SW, Reidelberger RD and Largman C (1985) Cyclical pattern
Duling DR (1994) Simulation on multiple isotropic spin-trap EPR spectra. J Magn
of blood alcohol levels during continuous intragastric ethanol infusion in rats.
Alcohol Clin Exp Res 9:31–37.
Dustin ML and Springer TA (1988) Lymphocyte function-associated antigen-1
Tsukamoto H, Gaal K and French SW (1990) Insights into the pathogenesis of
(LFA-1) interaction with intercellular adhesion molecule-1 (ICAM-1) is one of at
alcoholic liver necrosis and fibrosis: Status report. Hepatology 12:599 – 608.
least three mechanisms for lymphocyte adhesion to cultured endothelial cells.
Tsukamoto H, Reiderberger RD, French SW and Largman C (1984) Long-term
J Cell Biol 107:321–331.
cannulation model for blood sampling and intragastric infusion in the rat. Am J
French SW, Miyamoto K and Tsukamoto H (1986) Ethanol-induced hepatic fibrosis
in the rat: role of the amount of dietary fat. Alcohol Clin Exp Res 10:S13–S19.
Watanabe N, Suzuki J and Kobayashi Y (1996) Role of calcium in tumor necrosis
Fukui H, Brauner B, Bode JC and Bode C (1991) Plasma endotoxin concentrations in
factor-␣ produced by activated macrophages. J Biochem 120:1190 –1195.
patients with alcoholic and non-alcoholic liver disease: Reevaluation with an
Wiezorek JS, Brown DH, Kupperman DE and Brass CA (1994) Rapid conversion to
improved chromogenic assay. J Hepatol 12:162–169.
high xanthine oxidase activity in viable Kupffer cells during hypoxia. J Clin Invest
Glassman EB, McLaughlin GA, Forman DT, Felder MR and Thurman RG (1989)
Role of alcohol dehydrogenase in the swift increase in alcohol metabolism (SIAM):
Yin M, Wheeler MD, Kono H, Bradford BU, Gallucci RM, Luster MI and Thurman
Studies with deermice deficient in alcohol dehydrogenase. Biochem Pharmacol
RG (1999) Essential role of tumor necrosis factor ␣ in alcohol-induced liver injury.
Iimuro Y, Frankenberg Mv, Arteel GE, Bradford BU, Wall CA and Thurman RG
Yu K, Bayona W, Kallen CB, Harding HP, Ravera CP, McMahon G, Brown M and
(1997b) Female rats exhibit greater susceptibility to early alcohol-induced injury
Lazar MA (1995) Differential activation of peroxisome proliferator-activated re-
than males. Am J Physiol 272:G1186 –G1194.
ceptor by eicosanoids. J Biol Chem 270:23975–23983.
Iimuro Y, Gallucci RM, Luster MI, Kono H and Thurman RG (1997a) Antibodies to
Zabel U, Schreck R and Baeuerle PA (1991) DNA binding of purified transcription
tumor necrosis factor-␣ attenuate hepatic necrosis and inflammation due to
factor NF-B. Affinity, specificity, Zn2⫹ dependence, and differential half-site
chronic exposure to ethanol in the rat. Hepatology 26:1530 –1537.
recognition. J Biol Chem 266:252–260.
Karwinski W, Farstad M, Ulvik R and Soreide O (1991) Sixty-minute normothermic
Zhong Z, Lemasters JJ and Thurman RG (1989) Role of purines and xanthine
liver ischemia in rats. Evidence that allopurinol improves liver cell energy metab-
oxidase in reperfusion injury in perfused rat liver. J Pharmacol Exp Ther 250:
olism during reperfusion but that timing of drug administration is important.
470 – 475.
Knecht KT, Adachi Y, Bradford BU, Iimuro Y, Kadiiska M, Qun-hui X and Thurman
Send reprint requests to: Ivan Rusyn, Laboratory of Hepatobiology and
RG (1995) Free radical adducts in the bile of rats treated chronically with intra-
Toxicology, Department of Pharmacology, CB#7365, Mary Ellen Jones Bldg.,
gastric alcohol: Inhibition by destruction of Kupffer cells. Mol Pharmacol 47:1028 –
University of North Carolina at Chapel Hill, Chapel Hill, NC 27599-7365.
E-mail: [email protected]
Kono H, Rusyn I and Thurman RG (1998) Allopurinol, a xanthine oxidase inhibitor,
Source: http://www.rusynlab.org/publications/2000/Kono_JPET_2000.pdf
Painkillers can bring relief to people who are suffering from pain when taken as directed. However, the dangers of misuse must also be highlighted. Many painkillers are available over-the- effects such as drowsiness. This is why it counter (OTC) from pharmacies and is so important to read the information A huge market exists for painkillers and it shops without the need for a
Allergology International. 2007;56:37-43DOI: 10.2332! Awarded Article, Annual Meeting of JSA The Relationship between ExhaledNitric Oxide Measured with an Off-lineMethod and Airway ReversibleObstruction in Japanese Adults withAsthmaTakahiro Tsuburai1, Naomi Tsurikisawa1, Masami Taniguchi1, Sonoko Morita1, Emiko Ono1,Chiyako Oshikata1, Mamoru Ohtomo1, Yuji Maeda1, Kunihiko Ikehara2 and Kazuo Akiyama1


