Levitra enthält Vardenafil, das eine kürzere Wirkdauer als Tadalafil hat, dafür aber schnell einsetzt. Männer, die diskret bestellen möchten, suchen häufig nach levitra kaufen ohne rezept. Dabei spielt die rechtliche Lage in der Schweiz eine wichtige Rolle.
Sie, sies, gitmo revised guidelines for the management of follicular lymphoma
SIE, SIES, GITMO revised guidelines for the management of
follicular lymphoma
Pier Luigi Zinzani,1 Monia Marchetti,2 Atto Billio,3 Giovanni Barosi,4* Angelo Michele Carella,5
Mario Lazzarino,6 Maurizio Martelli,7 Alessandro Rambaldi,8 Luigi Rigacci,9 Corrado Tarella,10
Umberto Vitolo,11 and Sante Tura12
By using the GRADE system, we updated the guidelines for management of follicular cell lymphoma issuedin 2006 from SIE, SIES, and GITMO group. We confirmed our recommendation to frontline chemoimmuno-therapy in patients with Stage III–IV disease and/or high tumor burden. Maintenance rituximab was alsorecommended in responding patients. In patients relapsing after an interval longer than 12 months fromfrontline therapy, we recommended chemoimmunotherapy with non cross-resistant regimens followed byrituximab maintenance. High dose chemotherapy followed by hematopoietic stem cell transplant wasrecommended for young fit patients who achieve a response after salvage chemoimmunotherapy. Am. J.
Hematol. 88:185–192, 2013.
2012 Wiley Periodicals, Inc.
consecutive consensus conferences, the issues were analyzed and dis-
Follicular cell lymphoma (FL) is a frequent disorder for
cussed according to the nominal group technique, as previously
described [5].
proposed. In order to promote widespread adoption of
appropriate clinical practice, the Italian Society of Hematol-
Issue 1: Staging (consensus-based recommendations)
ogy (SIE), and the affiliate societies SIES (Societa Italiana
FL is a (18)F-FDG avid disease, since more than 90% of
di Ematologia Sperimentale) and GITMO (Gruppo Italiano
patients with FL show a PET positive disease and sensitiv-
Trapianto Midollo Osseo) established regular updating of
ity of staging PET is usually higher than 95% [6–9]. Pub-
published guidelines. Previous guidelines addressed indo-
lished literature includes 10 retrospective analyses [9–18]
lent non-Hodgkin's lymphomas (NHL) [1], but more recently,
and 1 prospective one [19]: pooled analysis of 356 patients
specific treatment options were devoted to FL; therefore,
revealed that 24% were upstaged from Stage I–II to Stage
current guidelines are specifically directed to the manage-
III–IV. Baseline PET also showed to have a high prognostic
ment of FL. In the 2008 WHO classification, grade 3b FL
were separated and are universally treated as diffuse large
reported the clinical outcomes in patients in which the ther-
B-cell lymphoma (DLBCL) [2]. Therefore, the present guide-
apy was adjusted according to a PET-staged disease. The
lines targeted grade 1-2-3a FL. We used the GRADE
assessment of bone marrow involvement by PET is not reli-
(grades of recommendation, assessment, development,
able due to low sensitivity [8,9,20]. Globally, the analyzed
and evaluation) system [3], which is based on a sequential
studies showed a moderate quality.
assessment of the quality of evidence followed by an analy-
The predictive role of pretreatment BCL2/IgH levels in
sis of the benefit-risk balance and subsequent judgment
bone marrow and peripheral blood is still controversial. In a
about the strength of recommendations.
seminal study, Rambaldi et al. [21] showed that the pres-
Guidelines development process.
The Advisory Council (AC), com-
posed of three members with expertise in clinical epidemiology, hema-
1Istituto di Ematologia ed Oncologia Medica ‘ Seragnoli,' Universita di Bolo-
tology, and critical appraisal, oversaw the process. An Expert Panel
gna, Bologna, Italy; 2Unita di Ematologia, Ospedale Cardinal Massaia, Asti,
(EP) was selected according to the conceptual framework elements of
Italy; 3Unita di Ematologia, Ospedale Civile di Bolzano, Bolzano, Italy; 4Lab-oratory of Clinical Epidemiology and Center of the Study of Myelofibrosis,
the NIH Consensus Development Program [4]. During a first meeting,
Fondazione IRCCS Policlinico San Matteo, Pavia, Italy; 5Divisione di Emato-
the EP decided which clinical issues needed an update and the AC
logia I, IRCCS Azienda Ospedaliera Universitaria San Martino, Genova,
checked which clinical queries might be addressed by a critical
Italy; 6Divisione di Ematologia, Fondazione IRCCS Policlinico San Matteo,
appraisal of evidence [3].
Pavia, Italy; 7Dipartimento di Biotecnologie Cellulari ed Ematologia, Cattedra
Producing and grading evidence-based recommendations.
di Ematologia, Universita La Sapienza, Roma; 8Divisione di Ematologia,
selected the clinical questions that needed to be addressed by a critical
Spedali Riuniti, Bergamo, Italy; 9Unit of Hematology, AOU Careggi, Univer-
appraisal of evidence. The EP chose the critical outcomes for each
sity of Florence, Firenze, Italy; 10Divisione di Ematologia, Ospedale Mauri-ziano, Torino, Italy; 11Divisione di Ematologia, Azienda Ospedaliera San Gio-
clinical query. Literature search was performed in July 2011 and limited
vanni Battista, Torino, Italy; 12Department of Hematology, University of Bolo-
to English-language publications edited after 2005. The search included
gna, Bologna, Italy
proceedings 2009 through 2010 of the American Society of Hematol-ogy, the European Hematology Association, and the 11-ICML (11th
Conflict of interest: Nothing to report
International Conference on Malignant Lymphoma). According to
*Correspondence to: Giovanni Barosi, Laboratorio di Epidemiologia Clinica-
GRADE methodology [3], the AC prepared ‘ evidence tables'' and
Centro per lo Studio della Mielofibrosi, Fondazione IRCCS Policlinico S.
‘ quality of evidence tables'' (available by the corresponding author on
Matteo, Viale Golgi 19, 27100 Pavia, Italy. E-mail:
[email protected]
request) for each critical appraisal. The EP received the critical apprais-
Contract grant sponsors: SIE, SIES, GITMO Societies, Mundipharma, Jans-
als and was asked to draft recommendations based on the benefit to
sen Cilag, Celgene, Roche (Italy).
risk profile of each compared intervention. Definite agreement of the
Received for publication 8 June 2012; Revised 15 November 2012; Accepted
recommendations and of their strength (weak or strong) was made
through subsequent face-to-face meetings.
Am. J. Hematol. 88:185–192, 2013.
Published online 10 December 2012 in Wiley Online Library (wileyonlinelibrary.
methodology was applied by the EP for all the issues worth to be
updated but not addressable by a critical appraisal. During three
DOI: 10.1002/ajh.23372
2012 Wiley Periodicals, Inc.
American Journal of Hematology
ence of less than 1/1,000 BCL2/IgH (1) cells in the bone
Issue 3: First line therapy (evidence–based recommen-
marrow was the best predictor of complete response after
first-line treatment and levels less than 1/100 a good pre-
In asymptomatic stage II–IV non bulky patients is rit-
dictor of five-year event-free survival (EFS). However, this
uximab alone better than watchful waiting? With the
result has not been validated, and in 238 patients with re-
advent of rituximab and its relatively favorable side effect
fractory-relapsed FL enrolled into the EORTC 20981 phase
profile, a randomized trial compared watchful waiting with
III trial, a BCL2 positive bone marrow was associated with
rituximab 375 mg/m2 weekly for 4 consecutive weeks fol-
a worse progression-free survival (PFS) but the BCL2/IgH
lowed by rituximab maintenance every 2 months for 2 years
levels were not correlated with the rates of post-induction
[27] in 462 asymptomatic patients. Time for initiation of
response [22]. However, recognizing that FLIPI was vali-
new therapy was significantly improved in the maintenance
dated in the prerituximab era [23], a FLIPI 2 score including
group in comparison with the watchful group: at 3 years 52
age, serum beta2 microglobulin, hemoglobin concentration,
versus 9% of patients required treatment (HR, 0.20; 95%
bone marrow involvement and tumor burden correlating
CI 0.13–0.29; P 5 0.001). PFS was also significantly
with the longest diameter of the largest involved lymph
improved (81 vs. 33% at 3 years) by rituximab maintenance
node was recently proposed [24]. Both indexes have not
(HR, 0.21; 95% CI 0.15–0.29; P 5 0.001). No statistically
been validated in prospective trials; therefore, they cannot
significant difference in OS was detected. Quality of life
be used to inform treatment decision in clinical trials.
appeared to be ameliorated in patients receiving rituximab
Recommendations Appropriate staging is a fundamental
[28]. The panel agreed that improvement in long-term sur-
step in the initial approach to patients with FL. Initial work-
vival was the critical endpoint to be considered in this set-
up should include a CT scan of the neck, thorax, abdomen
ting and that more evidence is needed before recommend-
and pelvis, and a bone marrow biopsy. FL is a FDG avid
ing rituximab in asymptomatic patients.
disease and PET allows the identification of a higher num-
Recommendations For asymptomatic Stage II–IV, non
ber of nodal and extranodal areas compared with CT scan.
bulky patients watchful waiting remains the standard of
PET scan should be included in staging of patients with lim-
care and rituximab cannot be recommended (quality of evi-
ited-stage disease at CT scan and possibly candidates to
dence, low; strength of recommendation, weak).
radiotherapy only. The panel agreed that PET upstaged
In patients with stage I–II disease, which dose of
patients should receive a therapy according to the new
radiotherapy is recommended? Involved field radiother-
stage, even though there is no evidence that this choice is
apy (IF-RT) remains the recommended treatment for
able to improve the outcome of the disease.
patients with limited stage disease, as detailed in our previ-
Pretreatment PET is also advisable to allow an optimal
ous guidelines [1]. A recent randomized trial compared 40–
assessment of response for those patients needing chemother-
45 Gy with 24 Gy radiotherapy in 661 sites in patients with
apy in which an early stage disease (i.e., Stage II) and therapy
indolent NHL, predominantly follicular, reporting no differ-
fitness make the probability of CR high. Staging should be
ence in the major outcomes, which is response rate, PFS
assigned according to the Ann Arbor system. Bone marrow bi-
and OS [29].
opsy histology should be performed with monolateral upper
Recommendations Patients with Stage I–II disease, a low
posterior iliac spine biopsy of at least 20 mm length and should
tumor burden, and with documented contiguity of involved
include appropriate immunohistochemistry for the lymphoma-
lymph-nodes treatable in the same radiotherapy field,
tous tissue. A complete blood count and routine blood chemis-
should receive external involved field radiotherapy, at the
try including LDH, beta2microglobulin and uric acid are
dose of 24 Gy (quality of evidence, low; strength of recom-
required. Like for all patients' candidate to receive cytotoxic
mendation, strong).
and/or immunomodulatory drugs, screening for main infectious
In patients with stage II–IV deserving treatment, is
diseases, including HIV and hepatitis B and C, is recom-
chemoimmunotherapy better than chemotherapy? Che-
mended. Bone marrow and peripheral blood tests with polymer-
moimmunotherapy with rituximab was recommended for
ase chain reaction for t(14;18) chromosomal translocation and/
patients candidates to chemotherapy in our previous guide-
or for immunoglobulin gene rearrangement (Ig CDR3) is not
lines [1]. Four randomized trials [30–33] comparing chemo-
recommended for routine assessment and outside clinical trials.
immunotherapy with rituximab to chemotherapy without rit-
A follicular lymphoma international prognostic index (FLIPI) (>4
uximab in naı¨ve patients with FL Stage III–IV were
nodal sites, elevated LDH, age >60 years, Stage III–IV, hemo-
selected. A pooled analysis was performed on the critical
globin <12 g/dl) should be determined in all the patients.
endpoints, that is, OS, failure free survival (FFS) andsevere infections (Fig. 1). OS (HR, 0.59; 95% CI 0.44–
Issue 2: When to start treatment (consensus-based
0.79) and FFS improved (HR, 0.54; 95% CI 0.44–0.66).
However, no relevant increase of severe infections was
Based to the lack of overall survival (OS) improvement
shown. Stage I–II patients with a high tumor burden were
after treatment of asymptomatic advanced-stage patients
not enrolled into the above trials, but in absence of new evi-
[25], and according to the previous SIE guideline edition [1]
dence, the panel maintained the recommendation of the
and to GELF data [26], the panel agreed on the following
previous version of the guidelines [1], and agreed that
patients with Stage II disease and high tumor burden, or
Recommendations Treatment can be started in patients
FLIPI >2 should receive frontline chemoimmunotherapy.
with Stage II–IV disease in case of one of the following fea-
Recommendations Patients with Stage III–IV should receive
tures occurs: systemic symptoms, high tumor burden (i.e.,
front-line chemoimmunotherapy. No evidence indicates che-
>3 lymph nodes measuring >3 cm or a single lymph node
moimmunotherapy in Stage II disease. However, the panel
>7 cm), extranodal disease, cytopenia due to marrow
agreed that these patients should receive chemoimmuno-
involvement, spleen involvement (516 cm by CT), leukemic
therapy when there is high tumor burden or high-risk scor-
phase, serous effusion, symptomatic or life endangering
ing system (quality of evidence, moderate; strength of
organ involvement, rapid lymphoma progression, consis-
tently increased LDH levels. A policy of watchful waiting is
In patients candidates to frontline chemoimmuno-
not recommended in patients with Stage I–II disease, with
the exception of patients with a short life expectancy due to
chosen? Two randomized trials comparing different chemo-
severe comorbidity or with contraindications to therapy.
therapy regimens associated with rituximab are available
American Journal of Hematology
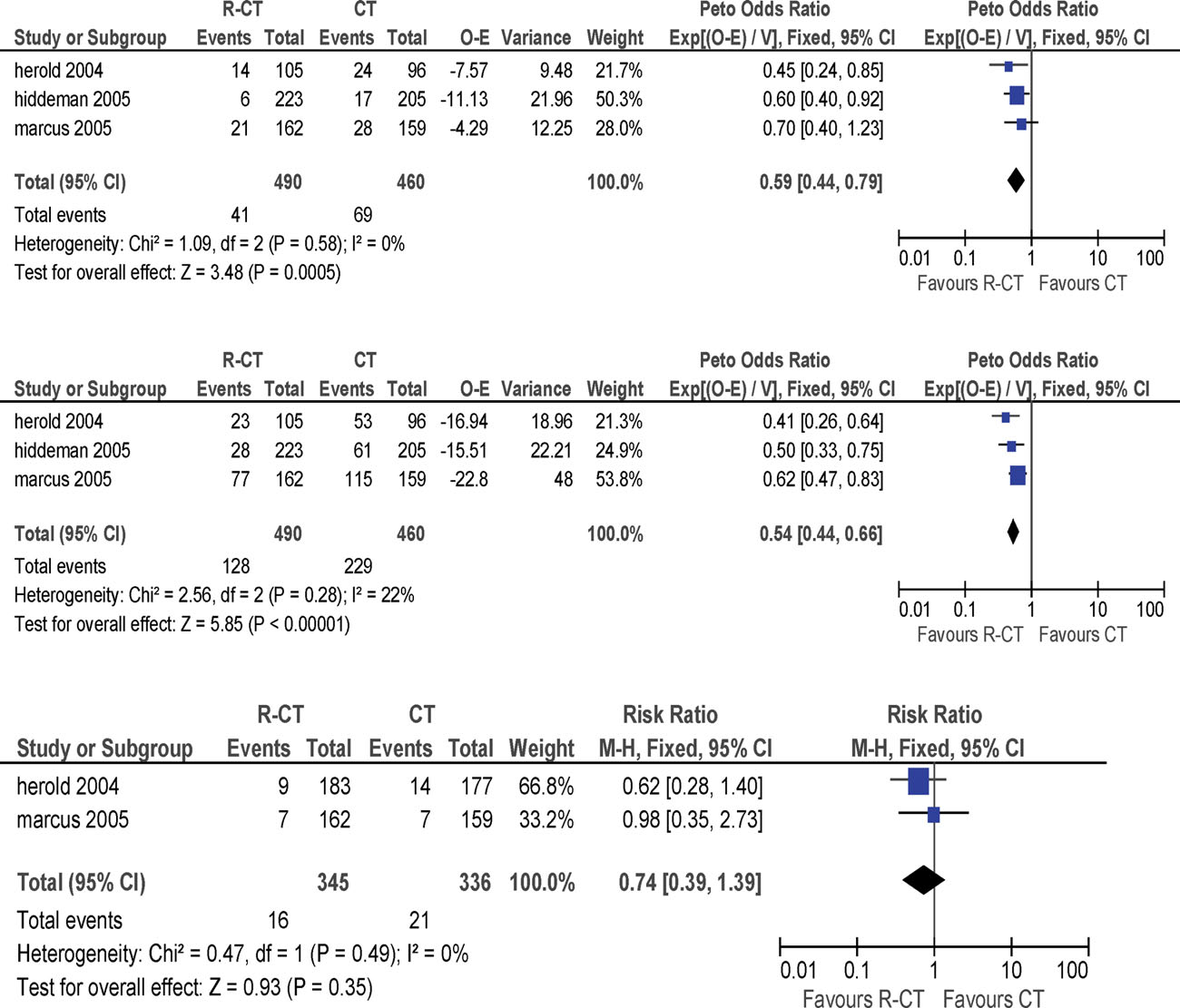
Result of the pooled analysis on the critical endpoints (overall survival, failure free survival, and severe infections) of the three published trials comparing che-
moimmunotherapy with rituximab the chemotherapy without rituximab in patients with follicular lymphoma Stage III–IV. [Color figure can be viewed in the online issue,which is available at wileyonlinelibrary.com.]
for analysis; however, both have been reported only in
Recommendations There is evidence that many frontline
abstract form. Federico et al. in the FOLL05 trial compared
chemotherapy regimens, whether antracycline-based poly-
R-CVP versus R-CHOP versus R-FM in a homogenous FL
chemotherapy (CHOP or CHOP like regimens) or fludara-
population of 534 patients [34]. A trend to longer 3 year
bine-based polychemotherapy, or CVP regimen or benda-
PFS and a significant amelioration of time to treatment fail-
mustine can be used in association with rituximab (quality
ure was noted in the patients treated with R-CHOP and R-
of evidence, low; strength of recommendation, weak).
FM. However, a higher rate of adverse events was shown
In patients candidates to frontline chemoimmuno-
with R-FM compared to the other two treatment schedules.
therapy, high-dose chemoimmunotherapy with autolo-
A preliminary GRADE table could be built based on the
data reported by the oral presentation. Rummel et al.
chemoimmunotherapy? The impact of high-dose therapy
tested R-bendamustine versus R-CHOP in a heterogene-
and autologous hematopoietic stem cell transplantation
ous population of 513 patients, half of which had a FL while
(HSCT) versuss conventional-dose chemotherapy in the
the remaining patients had a diagnosis of either mantle cell
management of FL has been faced by a systematic review
lymphoma or non-FL indolent lymphoma [35]. In the R-
of the randomized clinical trials addressing the question
bendamustine group PFS was prolonged from 35 to 55
[36]. Seven trials proved eligible, four of which provided
months compared to R-CHOP (HR, 0.57; 95% CI 0.43–
data from 941 patients that could be included in a meta-
0.77). Moreover, R-bendamustine had a lower rate of
analysis and three of which remain unpublished. The
Grade 3–4 adverse events compared to R-CHOP. In elderly
results suggest that high-dose therapy and autologous SCT
unfit patients several therapeutic options have been tested
as part of FL initial treatment does not improve OS.
in phase II or retrospective studies: rituximab monotherapy,
In the post-rituximab era, only one trial compared high-
chlorambucil and rituximab, abbreviated chemoimmunother-
dose therapy and autograft with R-CHOP: 136 patients with
apy. However, no randomized study is available to guide
untreated advanced FL Grades 1–3 were randomized
therapeutic decision. No GRADE table could be built based
either to six cycles of R-CHOP or to R-HDS schedule [37].
on the subset of data reported by the abstract.
The R-HDS consisted of two cycles of APO regimen (dox-
American Journal of Hematology
orubicin, vincristine, and prednisone totaling 75 mg/sqm of
patients. No significant difference in mortality was reported
doxorubicin administration); patients not achieving complete
in the two groups. Grades 3–4 adverse effects were 24% in
remission (CR) received two additional DHAP; the high-
the rituximab group and 17% in the observation group (RR,
dose phase consisted of 2 g/sqm etoposide followed by
1.46; 95% CI 1.14–1.87). Grades 3–4 neutropenia was 1%
two rituximab doses; afterwards 7 g/sqm of cyclophospha-
in the observation group and 4% in the maintenance group.
mide were delivered followed again by two rituximab doses;
Grades 3–4 infections were 1% in the observation group
eventually mitoxantrone 60 mg/sqm and melphalan 180
and 4% in the maintenance group. No statistical difference
mg/sqm followed by autologous HSCT were administered.
was documented in quality of life between the two groups.
CRs were 62% following R-CHOP and 85% following R-
Recommendations Maintenance therapy with rituximab is
HDS (P < 0.001). Four years projected values for EFS
recommended in patients who reach at least a partial
were 28% for R-CHOP and 61% for R-HDS (P < 0.001).
response at the end of first-line therapy (quality of evi-
Four years projected values for PFS were 31% for
dence, high; strength of recommendation, strong).
R-CHOP and 68% for R-HDS (P < 0.001). OS was similar
In patients who achieved partial response after
in the two arms (R-CHOP, 80%; R-HDS, 81%). The cumu-
first-line chemoimmunotherapy, is consolidation with
lative incidence of sMDS/AML at 4 years was 6.6% for
radioimmunoconjugates an option? Four hundred and
R-HDS and 1.7% for R-CHOP (P 5 0.111). A total of 26
fourteen patients were enrolled in a multicenter randomized
non-fatal grades III to IV extra-hematologic early toxicities
trial (FIT trial) testing ibritumomab tiuxetan consolidation
occurred in the R-HDS arm compared to a total of seven
after response (PR or CR) to first-line chemotherapy in FL
registered in the R-CHOP arm (P < 0.001).
Stage III–IV compared to observation [42]. Radioimmuno-
Recommendation Upfront high-dose chemoimmunotherapy
therapy (RIT) consolidation converted 77% of patients who
with autologous stem cell support cannot be recommended
were in PR after induction therapy to CR (P < 0.001). After a
(quality of evidence, low; strength of recommendation,
median follow-up of 3.5 years, median PFS was 36.5 months
in the RIT group and 13.3 months in the control group (HR,
Issue 4: Assessment of response (consensus-based
0.465; P < 0.0001). At follow-up, there was no difference in
OS between the two groups. Grades 3–4 infections were 8%
The independent prognostic significance of PET-CT per-
in the RIT arm compared to 2.4% in the control group. The
formed at the end of induction therapy has been recently
trial, however, enrolled only 15% of patients receiving rituxi-
confirmed [38]; irrespective of conventional response [39],
mab as part of the induction regimen. This conclusion is fur-
PET positivity was associated with a reduced PFS (33 vs.
ther supported by several phase II studies where ibritumo-
71%) and an increased risk of death (HR, 7.0; P 5 0.001).
mab tiuxetan was given to patients after remission induction
However, no study addressed the management of patients
with R-CHOP [43,44] or FNR [45,46].
with discordant CT and PET results. Therefore, therapeutic
Recommendations Ibritumomab tiuxetan proved to prolong
decisions cannot be actually based on PET results. The
progression free survival in patients achieving partial
prognostic role of molecular response is controversial, even
response after first-line chemoimmunotherapy. However,
though any residual positivity at the end of a rituximab con-
the lack of comparison between ibritumomab tiuxetan and
taining program is associated with a remarkably poor out-come [21]. The prognostic role of BCL2/IGH levels after
rituximab maintenance does not allow to produce recom-
induction treatment still needs to be assessed in patients
mendations on radioimmunotherapy in this setting (quality
receiving rituximab maintenance.
of evidence, low; strength of recommendation, weak). The
Recommendations Clinical response to first-line therapy
panel claimed to be important to have a randomized study
should be assessed according to revised IWG criteria [40].
evaluating the role of radioimmunotherapy versus rituximab
Even though there is preliminary evidence that the best
maintenance in patients who achieved a response after
response evaluation includes PET, the panel did not reach a
consensus on the extensive use of PET in response assess-
Issue 6: Relapsed/refractory patients (evidence-based
ment. The panel agreed that PET could be indicated in
patients in which the intention of therapy is achieving CR.
In patients relapsing after first line chemoimmuno-
There is no evidence to support interim PET for guiding
therapy and requiring treatment, is rituximab and
treatment decisions. PET is not recommended for routine
chemotherapy reinduction superior to chemotherapy
use in the follow-up setting. The assessment of molecular
alone? A trial randomized 465 FL patients relapsed after
response is not recommended for routine assessment.
first-line chemotherapy not including rituximab to eitherR-CHOP or CHOP [47]. R-CHOP induction yielded an
Issue 5: Post-induction therapy (evidence-based
increased CR rate compared to CHOP therapy (CHOP,
15.6%; R-CHOP, 29.5%; P > 0.001). At a median follow-up
In patients with at least partial response after first-
of 33 months, patients treated with R-CHOP had signifi-
line chemoimmunotherapy, is maintenance with rituxi-
cantly longer median PFS (33 vs. 20 months) from first ran-
mab better than watchful waiting? The panel agreed that
domization (HR, 0.65; P < 0.001). A slight non significant
improvement in PFS was the critical endpoint to be consid-
increase of Grade 3–4 neutropenia was reported in the
ered in this setting. The PRIMA study [41] randomized
R-CHOP arm (55 vs. 48%).
1019 FL patients responsive to R-CHOP or RCVP to obser-
Recommendations In fit patients relapsing after first-line
vation or rituximab maintenance (12 infusions of 375 mg/
chemoimmunotherapy and requiring treatment, rituximab
sqm given intravenously one every 8 weeks) starting 8
should be added to chemotherapy as reinduction, provided
weeks after the last induction treatment. Three-year PFS
there is no evidence of resistance to rituximab (quality of
was 74.9% (95% CI 70.9–78.9) in the rituximab mainte-
evidence, low; strength of recommendation, weak).
nance group and 57.6% (95% CI 53.2–62.2) in the obser-
In patients relapsing after first-line chemoimmuno-
vation group (stratified log rank, P 5 0.0001). The HR for
therapy and achieving a response to reinduction rituxi-
risk of progression was significantly in favor for the rituxi-
mab and chemotherapy, is rituximab maintenance
mab maintenance group (HR, 0.55; 95% CI 0.44–0.68).
better than observation? Van Oers et al. [47] randomly
Conversion from PR to CR was documented in 44% of the
assigned relapsing/resistant patients after R-CHOP or
American Journal of Hematology
CHOP to either observation or maintenance with single
(3–5) and rituximab (375 mg/m2 on day 13; 1,000 mg/m2 on
agent rituximab 375 mg/m2 once every 2 months for a max-
days –6, 11 and 18) (FCR ) regimen in 47 chemosensitive
imum of 2 years. When CHOP 1 rituximab was used for
FL patients treated with sibling donor (n 5 45) or matched
induction, the median PFS from second randomization for
unrelated donor (n 5 2). With a median follow-up of 60
patients who received rituximab maintenance therapy was
months PFS and OS were 83% and 85%, respectively. Non
4.4 years compared with 1.9 years (HR, 0.69; P 5 0.43).
relapse mortality (NRM) accounted for 15% of the patients.
When CHOP only was used for induction, these figures
Pinana et al. [62] described the long term outcome of 37 FL
were, respectively, 3.1 years versus 1 year in the observa-
patients with median age of 50 years (range 34–62 years) en-
tion arm (HR, 0.37; P < 0.001). For patients in CR, median
rolled in two prospective protocols between 1999 and 2007.
PFS was 4.4 years in the rituximab maintenance arm ver-
Patients with relapsed or refractory FL were treated with a
sus 1.2 years in the observation arm (HR, 0.48; P 5
conditioning regimen based on fludarabine (125–150 mg/m2)
0.003). The last median follow-up [21] of 6 years showed
combined with melphalan (80–140 mg/m2). With a median
that maintenance rituximab significantly improved median
follow-up of 52 months (range, 0.6–113 months), DFS at 4
PFS (3.7 vs. 1.3 years; HR, 0.55), but was associated with
years for patients with PD, PR or CR at transplantation were
a significantly higher rate of severe (Grades 3–4) infection
29, 48, and 64%, respectively. The 4-year cumulative inci-
(9.7 vs. 2.4%; P 5 0.01). There was a non significant trend
dence of nonrelapse mortality were 71, 33, and 26%, respec-
towards improved OS at 5 years with maintenance (74 vs.
tively. Thomson et al. [63] reported the results of 82 consecu-
64%; P < 0.07). Seven out of 167 patients withdrew from
tive patients with relapsed or refractory FL conditioned for
maintenance treatment because of toxicity: four had
allogeneic HSCT with alemtuzumab (20 mg on days –8 to –4)
recurrent infections, one had severe neutropenia, one had
combined with fludarabine (30 mg/m2 on days –7 to –3) and
ventricular arrythmia, and one had persistent general com-
melphalan (140 mg/m2 on day –2). With a median follow-up
plaints. There were no deaths related to maintenance treat-
of 43 months the NRM was 15% at 4 years. Risk of relapse
ment. Three other randomized trials assessed rituximabmaintenance in relapsed FL patients [48–50]. However, the
was 26%. At 4 years, the OS rate was 76%.
studies enrolled patients with refractory FL, mantle cell lym-
The EBMT Group reported a retrospective analysis on 44
phoma or other indolent NHL. Moreover, no chemotherapy
matched unrelated donor stem cell transplantation (MUD-
reinduction was applied in two trials [49,50]. All the above
SCT) for relapsed or refractory FL. Compared to myeloabla-
studies were reported by a Cochrane systematic review
tive conditioning regimens, RIC showed on multivariate anal-
[51]. Meta-analysis of the four available randomized trials
ysis reduced NRM and significantly longer PFS and OS [64].
reported that OS was significantly ameliorated (HR, 0.58;
Hari et al. [65] compared retrospectively the outcomes of
95% CI 0.42–0.79) while also infection and severe infec-
208 FL patients (27–70 years) treated either with myeloa-
tions were increased (HR, 1.99; 95% CI 1.24–6.76).
blative conditioning (n 5 120) or reduced intensity condi-tioning (n 5 88) before an HLA-identical sibling allogeneic
Recommendations In patients relapsing after first-line che-
moimmunotherapy and achieving a response to reinduction
HSCT. There were no significant difference in 3-year PFS
rituximab and chemotherapy, rituximab maintenance is
or OS between the two cohorts. On multivariate analysis,
recommended (quality of evidence, low; strength of recom-
an increased risk of disease progression after RIC was
mendation, weak).
observed (RR, 2.97, P 5 0.04).
Recommendations Young (<65-year old) fit patients who
relapsed after or were refractory to a previous therapy
Which role for autologous HSCT? Two retrospective
including autologous SCT are candidates to allogeneic SCT.
The availability of a compatible donor and the patient
studies [52,53] analyzed the outcomes of patients treated
preference should be considered in making this decision
with autologous HSCT or chemotherapy or chemoimmuno-
Which role for radioimmunoconjugates? The efficacy
therapy in patients progressed or relapsed FL. Other
and safety of radiolabelled ibritumomab tiuxetan (single
cohorts assessed the role of autologous HSCT in rituximab
dose of 14.8 MBq/Kg) and tositumomab in patients with re-
pretreated patients [37,54–59]. The efficacy of rituximab
fractory/relapsed indolent NHL were compared with rituxi-
prior to stem cell collection as in vivo purging has been
mab (375 mg/sqm once weekly for 4 weeks) and unlabelled
tested by a randomized trial [60]. A retrospective analysis
tositumomab, respectively, in two randomized trials [66,67].
conducted by the Gruppo Italiano Terapie Innovative nei
Response rates ranged from 55 to 86% and CRs were
Linfomi (GITIL) reported the results of addition of rituximab
achieved in more than 30% of the patients. Higher response
pre-HSCT [54]. Rituximab maintenance after autologous
rates, longer TTP and fewer adverse effects were observed
HSCT was also assessed [60]. Several design limitations
by retrospective analyses of patients receiving ibritumomab
restrict the applicability of the trial results to the indication
tiuxetan or tositumomab as a second line therapy versus
of autologous SCT in patients relapsed or refractory. So the
third or further lines [68,69].
panel provided consensus based-recommendations.
Recommendations The panel argued that for relapsed/re-
fractory patients, treatment with radioimmunoconjugates is
Recommendations Autologous HSCT is recommended in
a therapeutic option. This should apply for those patients
young (<65-year old) fit patients relapsing within 12 months
non eligible to high-dose chemotherapy and HSCT.
from the end of frontline chemoimmunotherapy and achiev-ing a response to chemoimmunotherapy reinduction. Auto-
logous HSCT is a therapeutic option in young (<65-year
At present, several treatments are available for FL but
old) fit patients relapsing after at least 12 months from the
the information derived from literature may not fit with
end of frontline chemoimmunotherapy and achieving a
relevant clinical questions, and the endpoints and/or the
response to chemoimmunotherapy reinduction. No suffi-
population of patients included in trials are not always those
cient evidence support universal rituximab maintenance in
relevant in the clinical practice. In this project, aimed at
patients achieving a response after autologous HSCT.
revising the guidelines for management of FL issued in
Which role for allogeneic HSCT? Khouri [61] recently
2005, we made specific evidence-based recommendations
reported the 8-year experience with the fludarabine (30 mg/
for the most relevant key issues according to the GRADE
m2 on days –5 –3), cyclophosphamide (750 mg/m2 on days
methodology, which imposes a preliminary judgment of the
American Journal of Hematology
American Journal of Hematology
quality of evidence and a subsequent assessment of the
9. Wo¨hrer S, Jaeger U, Kletter K, et al. 18F-fluoro-deoxy-glucose positron emis-
strength of the recommendation based on a qualitative risk-
sion tomography (18FFDG-PET) visualizes follicular lymphoma irrespective ofgrading. Ann Oncol 2006;17:780–784.
benefit analysis. Also other institutions recently produced or
10. Janikova A, Bolcak K, Pavlik T, et al. Value of [18F]fluorodeoxyglucose posi-
updated evidence-based guidelines for the management of
tron emission tomography in the management of follicular lymphoma: The
FL (NCCN, BCSH, ESMO) [70–72] (Table I). Systematic
end of a dilemma? Clin Lymphoma Myeloma 2008;8:287–293.
reviews and consensus conferences addressed to specific
11. Le Dortz L, De Guibert S, Bayat S, et al. Diagnostic and prognostic impact of
18F-FDG PET/CT in follicular lymphoma. Eur J Nucl Med Mol Imaging
therapeutic issues, such as HSCT [73] and radioimmuno-
therapy [74] have also been published. The majority of pro-
12. Bishu S, Quigley JM, Bishu SR, et al. Predictive value and diagnostic accu-
duced recommendations in our project are common to the
racy of F-18-fluoro-deoxyglucose positron emission tomography treated grade
above guidelines: in particular, several chemotherapy regi-
1 and 2 follicular lymphoma. Leuk Lymphoma 2007;48:1548–1555.
mens are accepted for association with rituximab in front-
13. Jerusalem G, Beguin Y, Naijar F, et al. Positron emission tomography (PET)
with 18Ffluorodeoxyglucose (18F-FDG) for the staging of low-grade non-
line therapy of symptomatic advanced stage disease. In
Hodgkin's lymphoma (NHL). Ann Oncol 2011;12:825–830.
deciding the best frontline therapy, we grounded our deci-
14. Imataki O, Tamai Y, Yokoe K, et al. The utility of FDG-PET for managing
sion on the resulting efficacy and safety evidence. However,
patients with malignant lymphoma: Analysis of data from a single cancer
the economic impact of frontline chemoimmunotherapy was
center. Intern Med 2009;48:1509–1513.
15. Wirth A, Foo M, Seymour JF, et al. Impact of [18f] fluorodeoxyglucose posi-
also assessed in several studies, and R-CVP resulted cost-
tron emission tomography on staging and management of early-stage
effective versus CVP [75–77], and R-CHOP versus CHOP
follicular non-hodgkin lymphoma. Int J Radiat Oncol Biol Phys 2008;71:213–
[77]. Moreover, rituximab maintenance after chemoimmuno-
therapy was associated with an incremental cost per QALY
16. Karam M, Novak L, Cyriac J, et al. Role of fluorine-18 fluoro-deoxyglucose
positron emission tomography scan in the evaluation and follow-up of patients
€12,600 to €18,147 versus R-CHOP followed by
with low-grade lymphomas. Cancer 2006;107:175–183.
observation [78,79]. The guidelines issued in the last year,
17. Hofman MS, Hicks RJ. Imaging in follicular NHL. Best Pract Res Clin Haema-
however, showed discrepancies about recommendations on
radioimmunoconjugates and HSCT. Indeed, the available
18. Blum RH, Seymour JF, Wirth A, et al. Frequent impact of [18F]fluorodeoxyglu-
evidence for such therapies is low-level due to indirectness
cose positron emission tomography on the staging and management ofpatients with indolent non-Hodgkin's lymphoma. Clin Lymphoma 2003;4:43–
in available randomized studies and to lack of randomized
studies. The results of ongoing trials investigating new ther-
19. Scott AM, Gunawardana DH, Wong J, et al. Positron emission tomography
apeutic modalities and novel agents will probably modify
changes management, improves prognostic stratification and is superior to
the treatment management of FL in the next years. Thus,
gallium scintigraphy in patients with low-grade lymphoma: Results of a multi-center prospective study. Eur J Nucl Med Mol Imaging 2009;36:347–353.
we have planned to update the present guidelines by the
20. Pakos EE, Fotopoulos AD, Ioannidis JP. 18F-FDG PET for evaluation of bone
end of 2015.
marrow infiltration in staging of lymphoma: A meta-analysis. J Nucl Med
Author Contributions
21. Rambaldi A, Carlotti E, Oldani E, et al. Quantitative PCR of bone marrow
GB, ST, MM, and AB designed the research; MM and AB
BCL2/IgH1 cells at diagnosis predicts treatment response and long-term out-
performed the systematic review of literature, graded the
come in follicular non-Hodgkin lymphoma. Blood 2005;105:3428–3433.
evidence, and prepared the summary tables of evidence.
22. van Oers MH, To¨nnissen E, Van Glabbeke M, et al. BCL-2/IgH polymerase
PLZ, AMC, ML, MM, AR, LR, CT, UV, and ST formed the
chain reaction status at the end of induction treatment is not predictive for
panel of experts who discussed the summaries of evidence
progression-free survival in relapsed/resistant follicular lymphoma: Results ofa prospective randomized EORTC 20981 phase III intergroup study. J Clin
and produced recommendations. MM wrote the preliminary
version of the paper. All authors participated in writing sig-
23. Solal-Celigny P, Roy P, Colombat P, et al. Follicular Lymphoma international
nificant sections of the paper.
prognostic index. Blood 2004;104:1258–1265.
24. Federico M, Bellei M, Marcheselli L, et al. Follicular lymphoma international
prognostic index 2: A new prognostic index for follicular lymphoma developed
The SIE administered all aspects of the meetings. The
by the international follicular lymphoma prognostic factor project. J Clin Oncol
funding sources had no role in identifying statements,
abstracting data, synthesizing results, grading evidence, or
25. Ardeshna KM, Smith P, Norton A, et al. Long-term effect of a watch and wait
policy versus immediate systemic treatment for asymptomatic advanced-stage
preparing the manuscript or in the decision to submit the
non-Hodgkin lymphoma: A randomized controlled trial. Lancet 2003;362:516–
manuscript for publication.
26. Solal-Celigny P, Lepage E, Brousse N, et al. Doxorubicin containing regimen
with or without interferon alfa 2b for advanced follicular lymphomas: Final
1. Barosi G, Carella A, Lazzarino M, et al. Management of nodal indolent (non
analysis of survival and toxicity in the Groupe d'Etude des Lymphomes Folli-
marginal-zone) nonHodgkin's lymphoma: Practice guidelines from the Italian
culaire 86 trial. J Clin Oncol 1998;16:2332–2338.
Society of Haematology, Italian Society of Experimental Hematology and Ital-
27. Ardeshna K, Qian W, Smith P, et al. An intergroup randomized trial of rituxi-
ian Group for Bone Marrow Transplantation. Haematologica 2005;90:1237–
mab versus a watch and wait strategy in patients with stage II, III, IV, asymp-
tomatic, non-bulky follicular lymphoma (grades 1, 2 and 3a). A preliminary
2. Bosga-Bouer AG, van den Berg A, Haralambieva E, et al. Molecular, cytoge-
analysis. Blood (ASH Annual Meeting Abstracts) 2010;116:6.
netic, and immunophenotypic characterization of follicular lymphoma grade
28. Ardeshna KM, Quian W, Stephens R, et al. Preliminary results of quality of
3b; a separate entity or part of the spectrum of diffuse large B-cell lymphoma
life (QOL) analyses from the intergroup phase III randomized trial of
or follicular lymphoma? Hum Pathol 2006;37:528–533.
Rituximab vs. a watch and wait approach in patients with advanced stage,
3. Guyatt GH, Oxman AD, Vist GE, et al. GRADE: An emerging consensus on
asymptomatic, non-bulky follicular lymphoma (FL). Ann Oncol 2011;22:19.
rating quality of evidence and strength of recommendations. Br J Med
29. Lowry L, Smith P, Qian W, et al. Reduced dose radiotherapy for local control
in non-Hodgkin lymphoma: A randomized phase III trial. Radiother Oncol
4. Ferguson JH. The NIH consensus development program. The evolution of
guidelines. Int J Technol Assess Health Care 1996;12:460–474.
30. Herold M, Pasold R, Srock S, et al. Results of a prospective randomized
5. Mauro FR, Bandini G, Barosi G, et al. SIE, SIES, GITMO updated clinical
open label phase III study comparing rituximab plus mitoxantrone, chlor-
recommendations for the management of chronic lymphocytic leukemia. Leuk
ambucile, prednisolone chemotherapy (R-MCP) versus MCP alone in
untreated advanced indolent non-Hodgkin's lymphoma (NHL) and Mantle-
6. Meignan M, Gallamini A, Haioun C, et al. Report on the Second International
Cell-lymphoma (MCL). ASH Annual Meeting Abstracts 2004;104:584.
Workshop on interim positron emission tomography in lymphoma held in Men-
31. Hiddman W, Kneba M, Dreyling M, et al. Frontline therapy with Rituximab
ton, France, 8-9 April 2010. Leuk Lymphoma 2010;51:2171–2180.
added to the combination of cyclophosphamide, doxorubicin, vincristine, and
7. Tsukamoto N, Kojima M, Hasegawa M, et al. The usefulness of (18)F-fluoro-
prednisone (CHOP) significantly improves the outcome for patients with
deoxyglucose positron emission tomography ((18)F-FDG-PET) and a compar-
advanced-stage follicular lymphoma compared with therapy with CHOP alone:
ison of (18)F-FDG-pet with (67)gallium scintigraphy in the evaluation of lym-
Results of a prospective randomized study of the German Low-Grade
phoma: Relation to histologic subtypes based on the World Health Organiza-
Lymphoma Study Group. Blood 2005;106:3725–3732.
tion classification. Cancer 2007;110:652–659.
32. Buske C, Hoster E, Dreyling M, et al. Rituximab in combination with CHOP in
8. Elstrom R, Guan L, Baker G, et al. Utility of FDG-PET scanning in lymphoma
patients with follicular lymphoma: Analysis of treatment outcome of 552
by WHO classification. Blood 2003;101:3875–3876.
patients treated in a randomized trial of the German Low Grade Lymphoma
American Journal of Hematology
Study Group (GLSG) after a follow up of 58 months. Blood (ASH Annual
in advanced relapsed and refractory follicular lymphoma. Ann Oncol
Meeting Abstracts) 2008;112:2599.
33. Marcus R, Imrie K, Belch A, et al. CVP chemotherapy plus rituximab com-
56. Peters AC, Duan Q, Russell JA, et al. Durable event-free survival following au-
pared with CVP as first-line treatment for advanced follicular lymphoma.
tologous stem cell transplant for relapsed or refractory follicular lymphoma:
Positive impact of recent rituximab exposure and low-risk follicular lymphoma
34. Federico M, Luminari S, Dondi A, et al. R-CVP vs. R-CHOP vs. R-FM for the
international prognostic index score. Leuk Lymphoma 2011;52:2124–2129.
initial treatment of patients with advanced stage follicular lymphoma.
57. Le Gouill S, De Guibert S, Planche L, et al. Impact of the use of autologous
Preliminary results of FOLL05 trial. Ann Oncol 2011;22:135.
stem cell transplantation at first relapse both in naive and previously rituximab
35. Rummel M, Niederle N, Maschmeyer G, et al. Bendamustine plus rituximab in
exposed follicular lymphoma patients treated in the GELA/GOELAMS FL2000
respect of progression free survival and CR rate when compared to CHOP plus
study. GELA and GOELAMS. Haematologica 2011;96:1128–1135.
rituximab as first-line treatment of patients with advanced follicular, indolent, and
58. Hoerr AL, Gao F, Hidalgo J, et al. Effects of pretransplantation treatment with
mantle cell lymphomas: Final results of a randomized phase III study of the AtiL
rituximab on outcomes of autologous stem-cell transplantation for non-Hodg-
(Study Group Indolent Lymphomas, Germany). Blood 2009;114:404.
kin's lymphoma. J Clin Oncol 2004;22:45614566.
36. Al Khabori M, de Almeida JR, Guyatt GH, et al. Autologous stem cell trans-
59. Kang TY, Rybicki LA, Bolwell BJ, et al. Effect of prior rituximab on high-dose
plantation in follicular lymphoma: A systematic review and meta-analysis. J
therapy and autologous stem cell transplantation in follicular lymphoma. Bone
Natl Cancer Inst 2012;104:18–28.
Marrow Transplant 2007;40:973978.
37. Ladetto M, De Marco F, Benedetti F, et al. Gruppo Italiano Trapianto di Mid-
60. Pettengell R, Schmitz N, Gisselbrecht C, et al. Randomized study of
ollo Osseo (GITMO); Intergruppo Italiano Linfomi (IIL). Prospective, multicen-
rituximab in patients with relapsed or resistant follicular lymphoma prior to
ter randomized GITMO/IIL trial comparing intensive (R-HDS) versus conven-
high-dose therapy as in vivo purging and to maintain remission following high-
tional (CHOP-R) chemoimmunotherapy in high-risk follicular lymphoma at di-
dose therapy. ASCO Annual Meeting 2010; Chicago, Abstract 8005.
agnosis: The superior disease control of R-HDS does not translate into an
61. Khouri IF, McLaughlin P, Saliba RM, et al. Eight-year experience with
overall survival advantage. Blood 2008;111:4004–4013.
allogeneic stem cell transplantation for relapsed follicular lymphoma after
38. Trotman I, Fournier M, Lam T, et al. Positron emission tomography—Com-
nonmyeloablative conditioning with fludarabine, cyclophosphamide, and rituxi-
puted tomography (PETCT) after induction therapy is highly predictive of
mab. Blood 2008;111:5530–5536.
patient outcome in follicular lymphoma: Analysis of PET-CT in a subset of
62. Pin˜ana JL, Martino R, Gayoso J, et al. GELTAMO Group. Reduced intensity
PRIMA trial participants. J Clin Oncol 2011;29:3194–3200.
conditioning HLA identical sibling donor allogeneic stem cell transplantation
39. Cheson BD, Horning SJ, Coiffier B, et al. Report of an international workshop
for patients with follicular lymphoma: Long term follow-up from two prospec-
to standardize response criteria for non-Hodgkin's lymphomas. NCI Spon-
tive multicenter trials. Haematologica 2010;95:1176–1182.
sored International Working Group. J Clin Oncol 1999;17:1244.
63. Thomson KJ, Morris EC, Milligan D, et al. T-cell depleted reduced-intensity
40. Cheson BD, Pfistner B, Juweid ME, et al. Revised response criteria for malig-
transplantation followed by donor leukocyte infusions to promote graft-versus
nant lymphoma. J Clin Oncol 2007;25:579–586.
lymphoma activity results in excellent long-term survival in patients with multi-
41. Salles G, Seymour JF, Offner F, et al. Rituximab maintenance for 2 years in
ply relapsed follicular lymphoma J Clin Oncol 2010;28:3695–3700.
patients with high tumor burden follicular lymphoma responding to rituximab
64. Avivi I, Montoto S, Canals C, et al. Matched unrelated donor stem cell trans-
plus chemotherapy (PRIMA): A phase 3, randomized controlled trial. Lancet
plant in 131 patients with follicular lymphoma: An analysis from the Lym-
phoma Working Party of the European Group for Blood and Marrow Trans-
42. Morschhauser F, Radford J, van Hoof A, et al. Phase III trial of consolidation
plantation. Br J Haematol 2009;147:719–728.
therapy with yttrium-90Ibritumomab tiuxetan compared with no additional ther-
65. Hari P, Carreras J, Zhang MJ, et al. Allogeneic transplants in follicular lym-
apy after first remission in advanced follicular lymphoma J Clin Oncol
phoma: Higher risk of disease progression after reduced-intensity compared to
myeloablative conditioning. Biol Blood Marrow Transplant 2008;14:236–245.
43. Jacobs SA, Swerdlow SH, Kant J, et al. Phase III trial of short course CHOP-
66. Witzig TE, Gordon LI, Cabanillas F, et al. Randomized controlled trial of yttrium-
R followed by 90 yibritumomab tiuxetan and extended rituximab in previously
90-labelled ibritumomab tiuxetan radioimmunotherapy versus rituximab immuno-
untreated follicular lymphoma. Clin Cancer Res 2008;14:7088–7094.
therapy for patients with relapsed or refractory low-grade, follicular, or trans-
44. McLaughlin P, Neelapu S, Fanale M, et al. R-FND followed by radioimmuno-
formed B-cell non-Hodgkin's lymphoma. J Clin Oncol 2002;20:2453–2463.
therapy for high-risk follicular lymphoma. Blood 2008;112:3056a (abstract).
67. Davies AJ, Rohatiner AZ, Howell S, et al. Tositumomab and iodine I 131 tosi-
45. Hainsworth JD, Spiegel DR, Markus TM, et al. Rituximab plus short-duration
tumomab for recurrent indolent and transformed B-cell non-Hodgkin's lym-
chemotherapy followed by yttrium-90 ibritumomab tiuxetan as first line treat-
phoma. J Clin Oncol 2004;22:1469–1479.
ment for patients with follicular non-Hodgkin lymphoma: A phase II trial of the
68. Gordon LI, Molina A, Witzig T, et al. Durable responses after ibritumomab
Sarah Cannon Oncology Research Consortium. Clin Lymphoma Myeloma
tiuxetan radioimmunotherapy for CD20Þ B-cell lymphoma: Long-term follow-
up of a phase 1/2 study. Blood 2004;103:4429–4431.
46. Zinzani PL, Tani M, Pulsoni A, et al. A phase II trial of short course fludara-
69. Gregory SA, Leonard JP, Knox SJ, et al. The iodine 1–131 tositumab thera-
bine, mitoxantrone, rituximab followed by 90Y-ibritumomab tiuxetan in
peutic regimen: Summary of safety in 995 patients with relapsed/refractory
untreated intermediate/high-risk follicular lymphoma. Ann Oncol 2012;23:415–
low grade (LG) and transformed LG non-Hodgkin's lymphoma. (abs.6732). J
Clin Oncol 2004;22:615s.
47. Van Oers M, Klasa R, Marcus RE, et al. Rituximab maintenance improves clini-
cal outcome of relapsed/resistant follicular non Hodgkin lymphoma in patients
70. NCCN Clinical Practice Guidelines in Oncology (NCCNTM). Non-Hodgkin's
both with and without rituximab during induction: Results of a prospective
Lymphomas. Ves 4.2011. Available at: www.nccn.org. Accessed on November
randomized phase 3 intergroup trial. Blood 2006;108:32953301.
48. Forstpointer R, Unterhalt M, Dreyling M, et al. Maintenance therapy leads to
71. Mc Namara C, Davies J, Dyer M, et al. On behalf of the British Committee for
a significant prolongation of response duration after salvage therapy with a
Standards in Haematology. Guidelines on the investigation and management
combination of rituximab, fludarabine, cyclophoshamide, and mitoxantrone (R-
of follicular lymphoma. Br J Haematol 2012;156:446–467.
FCM) in patients with recurring and refractory follicular and mantle cell lym-
72. Dreyling M, Ghielmini M, Marcus R, on behalf of the ESMO Guidelines Work-
phomas: Results of a prospective randomized study of the German low Grade
ing Group. Newly diagnosed and relapsed follicular lymphomas: ESMO clini-
Lymphoma Study Group (GLSG). Blood 2006;108:4003–4008.
cal practice guidelines for diagnosis, treatment and follow-up. Ann Oncol
49. Hainsworth J, Litchy S, Shaffer D, et al. Maximizing therapeutic benefit of rit-
2011;22 (Suppl 6):59–63.
uximab: Maintenance therapy versus re-treatment in patients with indolent
73. Oliansky DM, Gordon L, King J, et al. The role of cytotoxic therapy with hema-
non Hodgkin's lymphoma-A randomized phase II trial of the Minnie Pearl Can-
topoietic stem cell transplantation in the treatment of follicular lymphoma: An
cer Research Network. J Clin Oncol 2005;23:1088–1095.
evidence base review. Biol Blood Marrow Transplant 2010;16:443–468.
50. Ghielmini M, Schmitz S, Cogliatti S, et al. Prolonged treatment with rituximab
74. Vitolo U, Barosi G, Fanti S, et al. Consensus conference on the use of 90-yt-
in patients with follicular lymphoma significantly increases event-free survival
trium-ibritumumab tiuxetan therapy in clinical practice. A project of the Italian
and response duration compared with the standard weekly x 4 schedule.
Society of Hematology. Am J Hematol 2009;85:147–154.
75. Hornberger J, Reyes C, Lubeck D, et al. Economic evaluation of rituximab
51. Vidal L, Gafter-Gvili A, Salles G, et al. Rituximab maintenance for the treatment
plus cyclophosphamide, vincristine and prednisolone for advanced follicular
of patients with follicular lymphoma: An updated systematic review and meta-
lymphoma. Leuk Lymphoma 2008;49:227–236.
analysis of randomized trials. J Natl Cancer Inst 2011;103:1799–1806.
76. Braga P, Carvalho S, Gomes M, et al. Economic analysis of rituximab in com-
52. Sebban C, Brice P, Delarue R, et al. Impact of rituximab and/or high-dose
bination with cyclophosphamide, vincristine and prednisolone in the treatment
therapy with autotransplant at time of relapse in patients with follicular lym-
of patients with advanced follicular lymphoma in Portugal. Acta Med Port
phoma: A GELA study. J Clin Oncol 2008;26:3614–3620.
53. Rohatiner A, Nadler L, Davies A, et al. Myeloablative therapy with autologous
77. Dundar Y, Bagust A, Hounsome J, et al. Rituximab for the first-line treatment
bone marrow transplantation for follicular lymphoma at the time of second or
of stage III/IV follicular non-Hodgkin's lymphoma. Health Technol Assess
subsequent remission: Long term follow-up. J Clin Oncol 2007;25:2554–2559.
2009;13 (Suppl 1):23–28.
54. Tarella C, Zanni M, Magni M, et al. Rituximab improves the efficacy of high-
78. Soini EJ, Martikainen JA, Nousiainen T. Treatment of follicular non-Hodgkin's
dose chemotherapy with autograft for high-risk follicular and diffuse large B-
lymphoma with or without rituximab: Cost-effectiveness and value of informa-
cell lymphoma: A multicenter Gruppo Italiano Terapie innovative nei linfomi
tion based on a 5-year follow-up. Ann Oncol 2011;22:1189–1197.
survey. J Clin Oncol 2008;26:3166–3175.
79. Kasteng F, Erlanson M, Hagberg H, et al. Cost-effectiveness of maintenance
55. Arcaini L, Montanari F, Alessandrino EP, et al. Immunochemotherapy with in
rituximab treatment after second line therapy in patients with follicular lym-
vivo purging and autotransplant induces long clinical and molecular remission
phoma in Sweden. Acta Oncol 2008;47:1029–1036.
American Journal of Hematology
Source: http://www.siesonline.it/sieswp/wp-content/uploads/2013/03/16-Linee-Guida-Linfomi-Follicolari.pdf
Us er information, pleas e read carefully!G . P OHL -B OS K A MP G mbH & C o.,K ieler S traß e 1 1, D-25551 Hohenlockstedt, G ermany S pray 0.4 mg /dos e Active ingredient: glyceryl trinitrate C ompos ition: 1 puff of the s pray contains 0.4 mg glyceryl trinitrate. Other cons tituents : medium-chaintriglycerides, medium-chain partial glycerides, absolute ethanol, peppermint oil.
HBV treatment and pregnancy Jörg Petersen⇑ Liver Unit, IFI Institute for Interdisciplinary Medicine, Asklepios Klinik St. Georg Hamburg, Germany See Article, pages MS The management of hepatitis B virus (HBV) infection in preg- often results in clinical remission This scenario is in contrast nancy is complex. Because infection with HBV in infancy often to the oral antiviral agents that generally require long-term ther-

