Levitra enthält Vardenafil, das eine kürzere Wirkdauer als Tadalafil hat, dafür aber schnell einsetzt. Männer, die diskret bestellen möchten, suchen häufig nach levitra kaufen ohne rezept. Dabei spielt die rechtliche Lage in der Schweiz eine wichtige Rolle.
Thermal.uow.edu.au
Thermoregulation: some concepts have changed.
Functional architecture of the thermoregulatory
system
Andrej A. Romanovsky
292:37-46, 2007. First published Sep 28, 2006;
Am J Physiol Regulatory Integrative Comp Physiol doi:10.1152/ajpregu.00668.2006
You might find this additional information useful.
This article cites 123 articles, 86 of which you can access free at:
including high-resolution figures, can be found at:
Updated information and services
American Journal of Physiology - Regulatory, Integrative
Additional material and information
and Comparative Physiology can be found at:
This information is current as of January 4, 2007 .
publishes original investigations that
The American Journal of Physiology - Regulatory, Integrative and Comparative Physiology
illuminate normal or abnormal regulation and integration of physiological mechanisms at all levels of biological organization,
ranging from molecules to humans, including clinical investigations. It is published 12 times a year (monthly) by the American
Physiological Society, 9650 Rockville Pike, Bethesda MD 20814-3991. Copyright 2005 by the American Physiological Society.
Am J Physiol Regul Integr Comp Physiol 292: R37–R46, 2007.
First published September 28, 2006; doi:10.1152/ajpregu.00668.2006.
CALL FOR PAPERS Physiology and Pharmacology of Temperature Regulation
Thermoregulation: some concepts have changed.
Functional architecture of the thermoregulatory system
Andrej A. Romanovsky
Systemic Inflammation Laboratory, Trauma Research, St. Joseph's Hospital and Medical Center, Phoenix, Arizona
Submitted 21 September 2006; accepted in final form 23 September 2006
99 –101, 104, 106, 107, 110, 111, 115, 118, 120, 123–127),
changed. Functional architecture of the thermoregulatory system.
Am
four reviews (20, 31, 32, 108), ten editorials (33, 68a, 70, 89,
J Physiol Regul Integr Comp Physiol 292: R37–R46, 2007. First
90, 95, 109, 112, 113, 121) and one point (57)-counterpoint
published September 28, 2006; doi:10.1152/ajpregu.00668.2006.—
(11) exchange within the Special Call for Papers on Physiology
While summarizing the current understanding of how body tempera-
and Pharmacology of Temperature Regulation published in
ture (Tb) is regulated, this review discusses the recent progress in the
several issues of the
American Journal of Physiology—Regu-
following areas: central and peripheral thermosensitivity and temper-
latory, Integrative and Comparative Physiology over the past
ature-activated transient receptor potential (TRP) channels; afferentneuronal pathways from peripheral thermosensors; and efferent ther-
two years. While closing this Call, the present review summa-
moeffector pathways. It is proposed that activation of temperature-
rizes the recent progress in our understanding of how body
sensitive TRP channels is a mechanism of peripheral thermosensitiv-
temperature is regulated. To give readers an idea of how
ity. Special attention is paid to the functional architecture of the
remarkable this progress is, the new understanding (based on
thermoregulatory system. The notion that deep Tb is regulated by a
the latest developments) is compared with the information
unified system with a single controller is rejected. It is proposed that
provided by a typical textbook chapter on thermoregulation. It
Tb is regulated by independent thermoeffector loops, each having its
seems that this chapter may need some updates, especially in
own afferent and efferent branches. The activity of each thermoeffec-
its coverage of thermosensors, thermoeffectors, and the func-
tor is triggered by a unique combination of shell and core Tbs.
tional architecture of the thermoregulatory system.
Temperature-dependent phase transitions in thermosensory neuronscause sequential activation of all neurons of the corresponding ther-
moeffector loop and eventually a thermoeffector response. No com-putation of an integrated Tb or its comparison with an obvious or
hidden set point of a unified system is necessary. Coordination
between thermoeffectors is achieved through their common controlled
A typical textbook chapter would say that brain temperature
is detected by central thermosensory neurons (central "thermo-
b. The described model incorporates Kobayashi's views,
but Kobayashi's proposal to eliminate the term sensor is rejected. A
receptors"). Most of them are warm-sensitive, that is, they
case against the term set point is also made. Because this term is
increase their activity with an increase in brain temperature.
historically associated with a unified control system, it is more
The abundance of warm-sensitive central sensors is consistent
misleading than informative. The term balance point is proposed to
with the following two facts. First, our thermal physiology is
designate the regulated level of Tb and to attract attention to the
"asymmetrical:" Tb is positioned very closely, within just a few
multiple feedback, feedforward, and open-loop components that con-
degrees Celsius, to the upper survival limit (which is possibly
tribute to thermal balance.
determined by the denaturation of regulatory proteins) butrelatively far, a few tens of degrees, from the lower limit(which is likely determined by the freezing of water). There-
BY USING POWERFUL AUTONOMIC and even more powerful behav-
fore, core overheating is much more dangerous than overcool-
ioral means, our species survives while being exposed to a
ing. Second, humans are endothermic animals (meaning that
wide range of ambient temperatures: from ⫺110°C (the sur-
their principal source of heat is their own body), as opposed to
face of the Moon) to 2,000°C (the air around a space shuttle as
ectothermic animals (that receive heat primarily from the
it reenters the atmosphere). Even in these diversified thermal
environment). Hence, sensors for limiting heat gain have to be
environments, we usually manage to maintain our deep (core)
located inside the body. Although much less common, cold-
body temperature (Tb) within a few tenths of a degree Celsius.
sensitive neurons (i.e., those that increase their activity with a
In fact, an exodus of Tb from its usual range is so suggestive of
decrease in brain temperature) also exist. However, the cold
a pathological condition, that Tb is monitored regularly in all
sensitivity of most of them seems to be due to inhibitory
hospitalized patients and reported in every medical history.
synaptic input from nearby warm-sensitive neurons.
Various aspects of Tb regulation have been discussed in fifty-
Thermoregulatory responses in a variety of animal species
one original articles (4, 7, 13, 22, 23, 29, 35–38, 40 – 42, 45, 46,
can be elicited by local thermal stimulation of various areas in
48 –51, 54, 55, 59, 61, 62, 64 – 66, 71, 73, 76, 77, 79, 80, 85, 88,
the central nervous system, including several brain stem neu-
Address for reprint requests and other correspondence: A. A. Romanovsky,
The costs of publication of this article were defrayed in part by the payment
Trauma Research, St. Joseph's Hospital, 350 W. Thomas Rd., Phoenix, AZ
of page charges. The article must therefore be hereby marked "
advertisement"
85013 (E-mail:
[email protected]).
in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
0363-6119/07 $8.00 Copyright 2007 the American Physiological Society
IN FOCUS: THERMOREGULATION 2007
ronal groups [that used to be referred to as the reticular
shows a powerful dynamic (phasic) component: these cells are
formation(s) of the medulla oblongata, pons, and midbrain; see
very active when the temperature is changing, but quickly
Ref. 9] and the spinal cord, but thermosensitive neurons of the
adapt to a stable temperature. Such a response enables the
preoptic anterior hypothalamus (POA) are considered to be the
organism to rapidly react to environmental changes. In addition
most important for triggering autonomic thermoeffector re-
to superficial cold- and warm-sensitive neurons, there are
sponses. It bears mentioning that the locations of thermosen-
peripheral deep-body sensors, which respond to the core body
sitive neurons triggering various thermoregulatory behaviors
temperature. They are located in the esophagus, stomach, large
are largely unknown (see
Behavioral effectors).
intra-abdominal veins, and other organs.
For a long time, it was assumed that the roles of cold- and
This basic information about peripheral thermosensors has
warm-sensitive POA neurons are reciprocal and "symmetri-
been recently updated with three developments:
1) the discov-
cal," that is, that all thermoregulatory responses could be
ery of a subclass of transient receptor potential (TRP) ion
triggered by either activation of one class of the temperature-
channels known as thermoTRP channels;
2) the progress in
sensitive neurons or inhibition of the other (10); your textbook
identifying thermoafferent pathways and their differential in-
is likely to share this assumption. During the past decade,
volvement in thermosensation and Tb regulation; and
3) the
however, the idea of equally important roles of warm- and
challenge to the old idea that a separate neuronal network
cold-sensitive neurons was put to rest. Elegant studies by
computes some integrated Tb (from codes of local Tbs) and
Kanosue and colleagues (21, 128), involving thermal and
compares it to a set point Tb to form a thermal sensation and to
chemical stimulation of POA cells, showed that both cold-
send "orders" to thermoeffectors.
defense and heat-defense autonomic responses are initiated bythe corresponding changes in the activity of warm-sensitive
neurons; increased activity of warm-sensitive POA neuronstriggers heat-defense responses, while decreased activity trig-
The mammalian TRP superfamily consists of ⬃30 channels
gers cold-defense responses.
divided in six subfamilies known as the TRPC (canonical),
Morphological identification of thermosensitive neurons has
TRPV (vanilloid), TRPM (melastatin), TRPML (mucolipin),
been another major achievement. Griffin et al. (43) showed that
TRPP (polycystin), and TRPA (ankyrin). Of these, the heat-
these cells are characterized by the horizontal orientation of
activated TRPV1-V4, M2, M4, and M5 and the cold-activated
their dendrites: toward the third ventricle medially and the
TRPM8 and A1 are often referred to as the thermoTRP
medial forebrain bundle laterally. Because neurons conveying
channels. Involvement of these recently cloned and character-
temperature signals from the body surface and viscera enter the
ized channels in thermoregulation has been studied intensively.
hypothalamus via the periventricular stratum and medial fore-
In the present Call for Papers, these studies are represented by
brain bundle (24), such an orientation seems ideal for receiving
the original articles by Ni et al. (80) and Wechselberger et al.
input through both projections. The present Call for Papers has
(123), the invited review by Caterina (20), and, to a certain
contributed to our understanding of the functional properties of
extent, the point-counterpoint exchange between Kobayashi et
thermosensitive neurons (11, 57, 123). Because warm-sensitive
al. (57) and Boulant (11). While referring the reader to the
POA neurons display spontaneous membrane depolarization,
review by Caterina (20) and several other recent reviews (30,
as shown by Boulant and colleagues (123, 129), these cells are
87, 102) for more detailed information, I would like to empha-
considered pacemakers; their thermosensitivity is due to cur-
size a few points.
rents that determine the rate of spontaneous depolarization
First, activation of all thermoTRP channels results in an
between successive action potentials. It is true, however, that
inward nonselective cationic current and, consequently, in an
mechanisms of hypothalamic thermosensitivity continue to be
increase in the resting membrane potential. This mechanism
disputed (57). The changes in ion currents that underlie both
agrees more readily with a role for these channels in peripheral
central (
vide supra) and peripheral (
vide infra) thermosensitiv-
thermosensitivity (83) rather than hypothalamic thermosensi-
ity are likely to involve temperature-dependent phase transi-
tivity (11, 129). Furthermore, the TRPV4 channel (which is
likely to play a physiological role in thermoregulation;
videinfra) does not seem to be expressed in the bodies of POAneurons (39), but it is expressed by the neuronal bodies of
dorsal root ganglia (44).
There are also peripheral thermosensory neurons (peripheral
Second, although each thermoTRP channel is activated
"thermoreceptors") that detect shell temperatures in the skin
within a relatively narrow temperature range, the range that
and in the oral and urogenital mucosa. A typical textbook
they cover cumulatively is very wide: from noxious cold to
chapter would state that most superficial sensors are cold-
noxious heat (Fig. 1). Furthermore, they cover this temperature
sensitive. Because central thermosensors are concerned mainly
range in an overlapping fashion, and their activities have
with warmth, specialization of peripheral sensors in cold sen-
different sensitivities to temperature. These features make the
sitivity is not that surprising. Skin cold sensors are located in or
thermoTRP channels well suited to the job of peripheral
immediately beneath the epidermis. Their signals are conveyed
thermosensors. It is important to note, however, that the tem-
by thin myelinated A␦ fibers. The less common warm sensors
perature ranges shown in Fig. 1 were obtained in vitro. In vivo,
are located slightly deeper in the dermis; their signals travel via
a specific cell type on which a thermoTRP channel is expressed
unmyelinated C fibers. Peripheral thermosensors are not pace-
and concomitant activation with chemical ligands affect the
makers, and mechanisms of peripheral thermosensitivity are
temperature range in which the channel is activated and, in
thought to involve changes in the resting membrane potential.
some cases, bring it closer to physiological values of deep Tb.
Importantly, the response of most peripheral thermosensors
Indeed, although the TRPV1 channel is widely thought to be
AJP-Regul Integr Comp Physiol • VOL 292 • JANUARY 2007 • www.ajpregu.org
IN FOCUS: THERMOREGULATION 2007
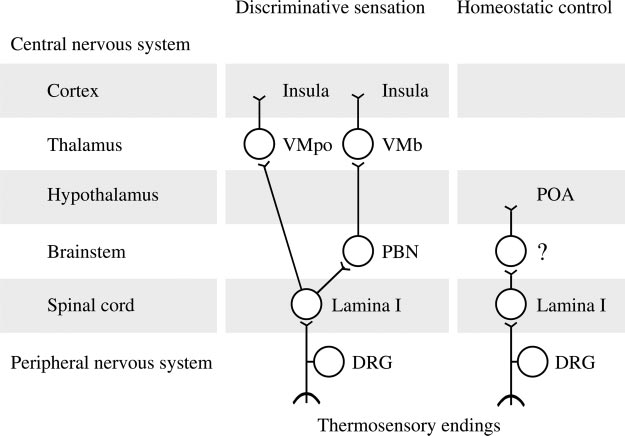
ies of these bipolar cells are located in the dorsal root ganglia,and the central axons project to the dorsal horn of the spinalcord (mostly lamina I), where they synapse with secondarymonopolar neurons. Axons of these secondary neurons crossthe midline and ascend in the lateral funiculus of the spinalcord. It was believed for a long time that the secondary neuronsproject directly to the ipsilateral ventrobasal complex of thethalamus, from where their signals are conveyed to the ipsilat-eral somatosensory cortex (postcentral gyrus) by tertiary neu-rons, and your textbook may hold this to be true. According toCraig (27, 28), however, this pathway, which is involved intactile sensation, is uninvolved in temperature sensation. In-stead, the lamina-I neurons carry temperature signals to theinsular cortex (the island of Reil) with a relay in the postero-lateral thalamus (specifically, the posterior part of the ventro-medial nucleus) or with two relays (in the parabrachial nucleus
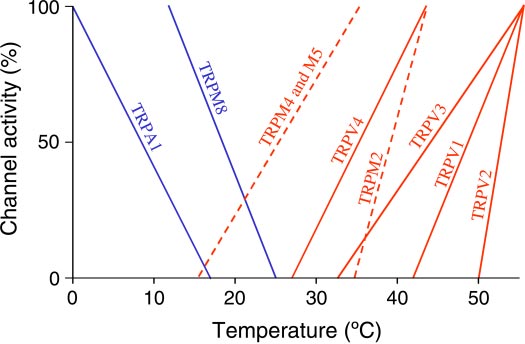
Fig. 1. Schematic representation of the dependence of the activity of cold-activated (blue) and heat-activated (red) thermoTRP channels on temperature.
and the basal part of the ventromedial nucleus of the thalamus)
The thresholds of activation and temperatures of maximal activation are based
(Fig. 2). These two branches of the spino-thalamo-cortical
on the activity of the channels in heterologous systems; some of the thresholds
pathway are involved in discriminative temperature sensation.
are means of values obtained in several studies. The figure is adapted from
A functional magnetic resonance imaging study in humans by
Patapoutian et al. (87). Information on the TRPM2 is added based on Togashi
Hua et al. (48) published in this Call for Papers shows that this
et al. (119); information on the TRPM4 and M5 is added based on Talavera etal. (114); the added lines are dashed. Please note that quantitative aspects of the
pathway is organized topically, as evident from the topical
relationships shown should be looked upon with great skepticism, as the figure
representation of skin temperature in the insula. This pathway
does not account for several important factors (81). Nevertheless, this figure
allows for sensing temperature at a high spatial resolution (e.g.,
illustrates how well thermoTRP channels are suited for detecting physiologi-
temperature of the surface under the tip of an individual finger
cally relevant temperatures both in the shell and core.
can be assessed). Therefore, this pathway is important formaking decisions about a wide range of issues related to
activated at pain-inducing temperatures of ⬎42°C (20, 30), Ni
interactions with the environment, but it appears to have little
et al. (80) showed that increasing temperature within the
to do with Tb regulation, that is, with triggering thermoeffector
normal physiological range (perhaps as low as ⬃34.5°C) can
responses to defend deep Tb. It should also be noted that the
exert a direct stimulatory effect on pulmonary sensory neurons,
spino-thalamo-cortical pathway (as described here for humans)
and this effect is likely mediated through the activation of the
is not the same in other vertebrates, as several interspecies
TRPV1 channel and other subtypes of TRPV channels. For
differences have been noticed (27).
some TRPV channels (namely, the V3 and V4), likely physi-
Homeostatic control. Thermoeffector responses are trig-
ological roles have been established (63, 74). Other thermo-
gered by thermal exposures massive enough to affect heat
TRP channels are currently under investigation.
exchange between the body and the environment. Tempera-
Afferent Pathways
ture-generated signals from large areas of the shell are sent tothe brain through the spino-reticulo-hypothalamic pathway
Discriminative sensation. There is little doubt that afferent
(Fig. 2), in which the secondary lamina-I neurons project to the
pathways start with primary thermosensory neurons. The bod-
reticular formation (medullar, pontine, and midbrain neuronal
Fig. 2. Afferent neuronal pathways for discriminative sensa-tion/localization of a thermal stimulus and for homeostaticcontrol of body temperature. DRG, dorsal root ganglion; PBN,parabrachial nucleus; POA, preoptic anterior hypothalamus;VMb, basal part of the ventromedial nucleus of the thalamus(formerly known as the parvicellular part of the ventroposteriormedial nucleus); VMpo, posterior part of the ventromedialnucleus of the thalamus; ?, unknown location(s) within themedulla, pons, and midbrain.
AJP-Regul Integr Comp Physiol • VOL 292 • JANUARY 2007 • www.ajpregu.org
IN FOCUS: THERMOREGULATION 2007
groups). In this pathway, thermosensory "lines" converge at
fector determines which combinations of core and shell Tbs
the level of lamina-I neurons (i.e., a single lamina-I cell receive
activate this effector (see Thermoeffector Loops).
inputs from multiple thermosensory neurons; see Ref. 82) and
Despite its conceptual strength, Kobayashi's model has not
possibly at other levels, so that downstream neurons (e.g., brain
become widely accepted yet, perhaps because the author favors
stem neurons in thermoeffector pathways) can collect signals
rather drastic terminological changes: he proposes to call
from large thermoreceptive fields (5, 6). The tertiary neurons of
sensors thermostats (or comparators). Kobayashi is certainly
the afferent spino-reticulo-hypothalamic pathway project to
right that, from the engineering point of view, the role of
hypothalamic structures (including the POA) either via the
thermoreceptors in his model is that of thermostats, and not of
periventricular stratum passing along the wall of the third
sensors. However, in the minds of the majority of medical and
ventricle or via the medial forebrain bundle, which passes more
biological scientists and students, the current terms (sensor,
laterally. As described above, warm-sensitive POA neurons
thermosensor, sensory, somatosensory, etc.) are associated
have a dendrite orientation ideal for collecting information
with no particular engineering analog. Hence, biologists feel no
from both the periventricular stratum and the medial forebrain
urge whatsoever to get rid of the entire family of widely used
bundle. More precise delineation of this homeostatic, spino-
biological terms or to start translating them to the engineering
reticulo-hypothalamic pathway, especially of the "reticulo"
language. (Later in this review, I will discuss a different term,
part of it, requires further study.
set point, which is associated with a false idea in the minds ofmost biologists and physicians and, therefore, must be re-
Thermosensors or Thermostats?
placed.) In the case of thermosensors, a more productive
approach might be to save the term, but to provide it with a
Our views on how temperature is sensed have been chal-
different meaning.
lenged recently by Kobayashi and colleagues (56, 58, 83). Thepre-Kobayashi models of Tb regulation assumed that thermo-
receptors code temperatures of different parts of the body (intoneuronal activity codes) and that these codes of local temper-
atures are then integrated by a separate network (that consists
Your textbook is likely to name various autonomic ther-
of several neurons with different roles) into some mean tem-
moeffectors and possibly some behavioral ones, to discuss
perature. The location of the integrating network was unclear
their anatomy, mechanisms of physiological control, and ef-
(8a). Nevertheless, it was often assumed that this integrating
fects on heat balance. Various aspects of thermoregulatory skin
network also compares the integrated temperature with an
vasoconstriction (29, 118) and vasodilation (4, 55, 71, 127),
external or internal reference signal and, based on such a
thermogenesis in brown adipose tissue (BAT) (79), shivering
comparison, somehow generates individual orders to thermoef-
(62), and thermoregulatory behavior (41) have been addressed
in the present Call for Papers. Some interactions between
Kobayashi and colleagues proposed a different scenario (56,
thermogenesis, energy metabolism, and body temperature reg-
58, 83). According to them, a sensory neuron is wired through
ulation have been analyzed in several original articles (37, 45,
a number of neurons to an effector cell. When the temperature
49, 124), two editorials (112, 113), and the invited review by
to which the temperature-sensitive part of a sensory neuron is
Diepvens et al. (31). I would also like to refer the reader to the
exposed reaches the activation threshold of this neuron, the
fundamental review on BAT by Cannon and Nedergaard (17)
neuron fires and, through its connections, sends a signal to the
published in Physiological Reviews. What your textbook is
effector cell. If a large number of sensory neurons sends
unlikely to cover is the efferent neuronal pathways to ther-
signals to their effector cells, a thermoeffector response occurs.
In this model, a decision to trigger an effector is made "auto-matically" (principally, by sensory neurons) and involves nei-
Efferent Pathways
ther a separate decision-making network nor operations withtemperature codes (computation of a mean Tb). According to
Autonomic effectors. Efferent pathways to thermoeffectors
this model, a sensation is a "side product" of the activation of
have not been well characterized in humans, although this topic
those neurons that are wired to cause certain effector responses
has recently started receiving attention (34). Even in the rat, the
(i.e., feeling cold means having activated those neurons that
most studied laboratory species, not all thermoeffector path-
trigger cold-defense responses). The same principle is used by
ways have been mapped. Some of the neuronal circuitries
Kobayashi and colleagues (58) to explain how we sense other
connecting the warm-sensitive hypothalamic neurons to auto-
modalities as well.
nomic thermoeffectors in the rat, particularly the BAT and
Kobayashi's model also explains how deep Tbs and periph-
skeletal muscles (heat-production effectors) and the vascula-
eral (e.g., skin) Tbs contribute to thermoregulation. Deep Tbs
ture of the tail (a specialized heat-exchange organ), have been
are regulated variables, and they are very stable. From the point
characterized during the past decade (Fig. 3). Studies of these
of view of the control theory, they serve as feedback signals.
pathways have been propelled by the development of transsyn-
Peripheral Tbs, on the other hand, are not regulated; they are
aptic retrograde tracing techniques using pseudorabies virus,
highly variable (97). They are feedforward signals that, accord-
along with the refinement of techniques for discrete lesioning
ing to the control theory, allow the body to respond to a
and pharmacological stimulation and inhibition of neural struc-
thermal load "in advance," that is, before deep Tbs start
tures. The revealed pathways appeared complex, and their
changing. In Kobayashi's model, both deep and peripheral Tbs
detailed description is beyond the scope of the present paper;
drive effector responses in a similar way. Which central and
see the invited review by DiMicco and Zaretsky (32) in the
peripheral sensory neurons are wired to a particular thermoef-
present issue, as well as the reviews by Nagashima et al. (78)
AJP-Regul Integr Comp Physiol • VOL 292 • JANUARY 2007 • www.ajpregu.org
IN FOCUS: THERMOREGULATION 2007
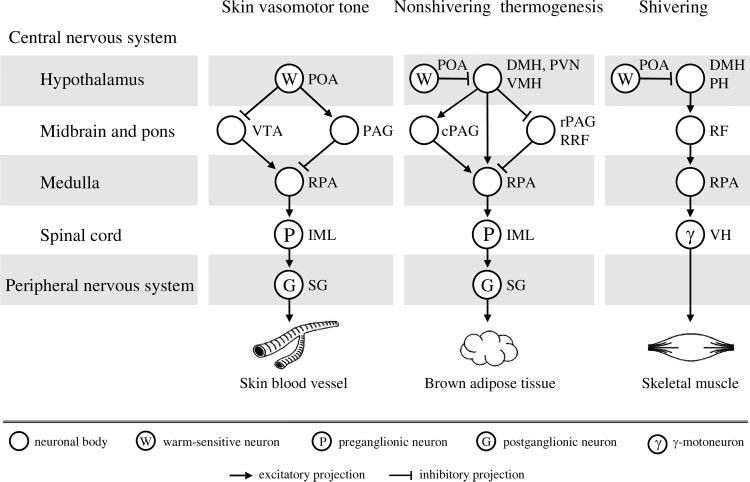
Fig. 3. Efferent neuronal pathways for con-trol of skin vasomotor tone, nonshiveringthermogenesis in brown adipose tissue, andshivering in the rat. The concept was takenfrom Nagashima et al. (78); the figure wassubstantially modified and published in Ro-manovsky (93) by permission from Elsevier.
The Romanovsky (93) version is reproducedhere with a minor modification and by per-mission from both Elsevier and BlackwellPublishing. DMH, dorsomedial hypothala-mus; IML, intermediolateral column; PAG,periaqueductal gray matter; cPAG, caudalPAG; rPAG, rostral PAG; PH, posterior hy-pothalamus; PVN, paraventricular nucleus;RF, reticular formation; RPA, raphe´/peripy-ramidal area; RRF, retrorubral field; SG,sympathetic ganglia; VH, ventral horn;VMH, ventromedial hypothalamus; VTA,
ventral tegmental area.
and Morrison (75) published elsewhere. However, a few points
and editorial foci by DiMicco and Zaretsky (33) and McAllen
As shown in Fig. 3, both the BAT and skin vasculature are
Behavioral effectors. Evidence (mostly from stimulation
controlled by sympathetic ganglia, with the bodies of pregan-
experiments) suggests that different thermoregulatory behav-
glionic neurons located in the intermediolateral column of the
iors in the rat (e.g., relaxed postural extension, thermoregula-
spinal cord. These spinal neurons receive direct input from
tory grooming, and locomotion) use distinct neural circuitries
cells located primarily in the raphe´/peripyramidal area of the
(92). However, the neuroanatomic substrate of no thermoreg-
medulla. These medullary cells are under the control of hypo-
ulatory behavior has been studied extensively, and little is
thalamic (dorsomedial and paraventricular nuclei), midbrain
known about the neuroanatomy of behavioral thermoregulation
(periaqueductal gray matter, retrorubral field, and ventral teg-
(78). This situation is likely to change, as behavioral thermo-
mental area), and possibly pontine (locus coeruleus) neurons
regulation is becoming a subject of keen attention (1, 2, 59, 60,
that receive input from warm-sensitive POA cells (8, 18, 84).
68). In the present issue, Konishi et al. (59) report that neurons
Although the efferent pathways for skin vasomotor tone and
in the median preoptic nucleus are involved in the intensifica-
nonshivering thermogenesis have some similarities, they are
tion of an operant thermoregulatory behavior (moving to a
not identical, and both differ substantially from the pathway
reward zone during heat exposure to trigger a breeze of cold
controlling shivering. Within the shivering pathway, ␥ and ␣
air) caused by hypertonic saline. For a different behavioral
motoneurons of the ventral horn receive direct and indirect
response (moving to a cold environment during bacterial li-
inputs from the midbrain and brain stem, including the raphe´/peripyramidal area of the medulla (116). Axons of the mid-
popolysaccharide-induced shock), two other substrates have
brain neurons descend the spinal cord with the reticulospinal
been recently identified by Almeida et al. (2): neurons of the
and rubrospinal tracts. These midbrain neurons are under
dorsomedial hypothalamic nucleus and fibers passing through
control of posterior hypothalamic neurons, which, in turn,
the paraventricular hypothalamic nucleus. By studying
receive inhibitory input from warm-sensitive POA cells.
warmth- and cold-seeking behaviors of rats in six different
The efferent pathways described are to a large extent inhib-
tests, Almeida et al. (2) also showed that these behaviors do not
itory. Consequently, thermoeffector activation involves disin-
require an intact POA, whereas autonomic thermoregulatory
hibition of tonically inhibited neurons. Importantly, thermoef-
responses do.
fectors are controlled relatively independently of each other(69, 78), and certain portions of the pathways may be recruited
FUNCTIONAL ARCHITECTURE OF THE
in a thermoregulatory response in a stimulus-specific fashion.
The latter speculation is supported by findings that the para-ventricular nucleus (16, 47, 67) and locus coeruleus (3) seem to
"Think simple" as my old master used to say—meaning
mediate a thermogenic response to bacterial lipopolysaccharide
reduce the whole of its parts into the simplest terms, getting
or prostaglandin E
back to first principles.
2 but not cold-induced thermogenesis or a
—Frank Lloyd Wright (1868 –1959)
b rise in rodents. In the present Call for Papers,
neural circuitries of autonomic thermoeffector responses have
"The ability to simplify means to eliminate the unnecessary
been subjects of original articles by Nakamura and Morrison
so that the necessary may speak."
(79), Ootsuka and McAllen (85), Tanaka and McAllen (115)
—Hans Hofmann (1880 –1966)
AJP-Regul Integr Comp Physiol • VOL 292 • JANUARY 2007 • www.ajpregu.org
IN FOCUS: THERMOREGULATION 2007
controlled by a distinct group of neurons (see ThermoeffectorLoops).
Thermoregulatory pathways form distinct thermoeffec-
It is also accepted now that the anatomically distinct ther-
tor loops. The efferent parts of the loops clearly differ, because
moeffectors function largely independently (105), and a body
each effector has its own efferent pathway (Fig. 3); the article
of experimental data has been accumulated showing that dif-
by Ootsuka and McAllen (85) in this Call for Papers provides
ferent effectors sometimes defend drastically different levels of
further support for this thesis. The afferent parts are also not
Tb (for a review, see Ref. 94). An example of differential
identical, as each effector receives a unique combination of
responses of thermoeffectors is endotoxin shock: it is accom-
signals from peripheral and central thermosensors. The ques-
panied by a large (2°C) decrease in the threshold hypothalamic
tion as to which temperatures (thermosensors in which parts of
temperature for activation of cold-induced thermogenesis, but
the body) trigger which effectors has been recently revisited in
a small (a few tenths of a degree) or no decrease in the
several studies (14, 116), including the one by Nakamura and
threshold hypothalamic temperature for triggering tail skin
Morrison (79) in the present issue.
vasodilation (98). Data showing independent effector re-
In general (although fully realizing that generalization may
sponses are often questioned (e.g., Refs. 15 and 19) based on
not be the best approach in this particular case), behavioral
the fact that a thermoeffector can be recruited by another
responses depend more on signals from peripheral thermosen-
homeostatic system to meet a competing demand and, hence,
sors (shell temperatures) than central thermosensors (core tem-
can become unavailable for thermoregulation. In the example
perature) (92), whereas deep Tb is relatively more important
with endotoxin shock (98), skin vasoconstriction is needed to
for triggering autonomic responses (52, 103). Such an organi-
maintain blood pressure, thus preventing thermoregulatory
zation reflects the fact that behavioral responses are often
skin vasodilation. The real question, however, is not whether
aimed at escaping the forthcoming thermal insult. In contrast,
an "unusual" thermoeffector response has a compelling teleo-
autonomic cold-defense responses (energetically expensive)
logical explanation, but whether the thresholds of different
and heat-defense responses (water-consuming) are often re-
thermoeffectors can change independently of each other. The
cruited only when deep Tb starts changing because behavioral
data accumulated (reviewed in Ref. 94) clearly show that
mechanisms were ineffective or could not be used (e.g., due to
thermoeffector thresholds often change independently, and this
competing behavioral demands). Even within the autonomic
is a rather strong argument against a unified model of the
responses, different thermoeffector responses are triggered by
thermoregulatory system with a single controller. Models of
different combinations of peripheral and central Tbs. Because
the thermoregulation system with multiple controllers (rela-
peripheral thermosensors are mostly cold sensors, information
tively independent thermoeffector loops) to replace the unified
from peripheral sensors is relatively more important for trig-
system have been proposed (e.g., Ref. 53).
gering cold-defense effectors (14, 79, 103, 116) than heat-
Furthermore, it has been realized that any regulatory system
defense ones (103). Because central thermosensors are mostly
can exist, using the words of Partridge (86), as a group of
warm sensors, information from central sensors is relatively
relatively autonomous controllers, acting in a common envi-
more important for triggering heat-defense responses (103).
ronment with generally compatible rules, but at any particular
Combinations of shell and core Tbs that trigger the same
time, operating with only limited active coordinating linkages,
thermoeffector response in different species are also likely to
and at no time acting as a unified system with a single
differ. The great thermal inertia of large animals makes tran-
controller. In fact, almost any regulated variable in the body,
sient thermal exposures less threatening, which decreases the
for example, arterial blood pressure (39), is likely to be an
importance of feedforward regulation. Indeed, peripheral ther-
emergent product of a decentralized control system.
mosensitivity is relatively more important in smaller animals,
Coming back to the thermoregulatory system, basic coordi-
whereas central thermosensitivity is relatively more important
nation between thermoeffectors is likely to be achieved
in larger animals (72). Therefore, caution should be exercised
through their dependence on a common variable: Tb. Such
while extrapolating results obtained in rats (this section of the
coordination can be explained, in a simplified way, as follows.
present review is based primarily on such results) to human
When one thermoeffector is activated, its activity changes Tb,
which changes the position of Tb relative to the thresholds ofother thermoeffectors, which, in turn, triggers activation or
cessation of other thermoeffector mechanisms (for a moredetailed description, see Ref. 93).
Usually, the recruitment of thermoeffectors into a thermo-
regulatory response looks like a highly coordinated event.
What To Do with the Term Set Point?
Those effectors that affect heat balance in the opposite direc-tions are typically not activated simultaneously. Energetically
On a related issue, there has been a recent upsurge of interest
expensive and water-consuming responses are typically trig-
in the term set point (12, 15, 19, 93, 94, 96). The original
gered after those that do not consume a lot of energy or water.
meaning of the term—a physical (thermal, electric, etc.), ex-
For a long time, such coordination between thermoeffectors
ternally originated reference signal in a unified control sys-
was explained with the help of a complex neuronal network
tem—is now considered invalid almost unanimously. How-
(coordinator) within a single integrated system, the same (or a
ever, the term is still used widely, mostly in the following three
similar) network that was thought to be responsible for the
meanings. First, it is used to designate some internal property
formation of thermal sensations (see Thermosensors or Ther-
of the unified thermoregulatory system that substitutes for an
mostats?). However, the coordinator has never been found
external reference signal. Needless to say, such a usage is
experimentally. Furthermore, each effector was found to be
obviously wrong as it refers to the same unified system.
AJP-Regul Integr Comp Physiol • VOL 292 • JANUARY 2007 • www.ajpregu.org
IN FOCUS: THERMOREGULATION 2007
Second, it is used to designate some internal characteristics
unified control system. To understand how thermoeffector
(usually thresholds) of individual thermoeffector loops or their
loops work, we need to study, among other things, thermosen-
subcomponents (e.g., Kobayashi's "thermostats" or individual
sors (including the thermoTRP channels; Fig. 1), as well as the
thermoTRP channels). There is nothing wrong with such a
afferent (Fig. 2) and efferent (Fig. 3) portions of thermoeffector
usage of the term, except that it creates confusion: this usage
loops. Figures 1–3 represent an attempt to summarize the
refers to a set point as a property of an individual component,
recent progress achieved in these three areas. I invite the
whereas all definitions of thermoregulatory responses (see next
readers to use these figures in the classroom to complement the
paragraph) use the term set point while referring to the entire
thermoregulation chapter they are currently using. I encourage
viewing these figures as drafts and asking your students to
Third, many colleagues use this term to designate a regu-
correct and update them. A major shortcoming of these figures
lated level of Tb (e.g., Refs. 12, 15, 19). Such a usage is in
is that they follow the organization of the same unified ther-
accordance with the most recent thermophysiological glossary
moregulatory system that is extensively criticized in this re-
(25), and many definitions of thermoregulatory responses (fe-
view. Although the central controller is eliminated, different
ver, anapyrexia, hypothermia, hyperthermia) are built upon this
thermoeffectors loops are cut across to represent the same level
definition of the set point. However, in the minds of biologists,
of all loops in each cross section: sensors, afferent pathways,
physicians, and students, the term set point is strongly, perhaps
and efferent pathways. To correct this shortcoming would
inseparably, associated with the reference signal of a unified
require constructing a figure for each thermoeffector that
thermoregulatory system. Even today, it is not unusual for
would represent the entire loop. Inspirational examples of such
scientists outside the thermoregulation field to talk about the
constructs can be found in the article of Nakamura and Mor-
set point temperature (117) or about neurons that detect the
rison (79) in the present issue. Investigations of the roles of
error signal (26). Even for scientists within the thermoregula-
individual thermoTRP channels in the control of different
tion field, it is rather typical to seemingly accept the relative
thermoeffectors have just been started, for example, by
mutual independence of individual thermoeffector loops, but to
Almeida et al. (1, 2), who used TRPV1 and TRPM8 agonists to
use (whether intentionally or not) the unified model with a
cause thermoregulatory locomotion.
single controller while describing how Tb is regulated (12, 15),
to still conclude that all thermoeffectors operate according to a
common plan (15), and to propose a neuronal model of a set
I thank all of the past and present members of my laboratory, especially Drs.
point of a unified thermoregulation system (12).
Alexandre A. Steiner, Victoria F. Turek, and M. Camila Almeida, for helping
In other words, the intrinsic association of the term set point
me with the work on which the current review was partially built, for educating
with nonexistent physical signals (a computed mean T
me on various aspects of thermoregulation, and for discussing with me early
drafts of this manuscript and the figures. My views on how T
b is regulated have
b) within the nonexistent unified thermoregulatory
been influenced by enlightening discussions with Drs. Kazuyuki Kanosue and
system complicates the usage of this term. It provides reference
the late Lloyd D. Partridge. A draft of Fig. 3 was discussed with Drs. Shaun F.
to the engineering analogies that are more misleading than
Morrison and Christopher J. Madden. Dr. Thomas M. Hamm and F. E. Farmer
informative. To eliminate this often unintended reference, I
read an early version of this review and provided important feedback.
have proposed to use the term balance point when talking about
This review closes the Special Call for Papers on Physiology and Pharma-
cology of Temperature Regulation of the American Journal of Physiology—
the regulated level of Tb (93, 94, 96). The balance point-based
Regulatory, Integrative and Comparative Physiology (volumes 288 –292,
definitions work for all cases where the set point-based defi-
2005–2007). As Guest Editor for this Call, I thank Dr. Pontus B. Persson,
nitions of thermoregulatory responses work (25). More impor-
Editor-in-Chief, for accepting the proposal for this project. I also thank the
tantly, they also work for all cases where the set point-based
authors of the more than 100 manuscripts submitted in response to this Call, aswell as the many colleagues in the field who expertly reviewed these submis-
definitions may not work (94). As an added benefit of such a
sions. Special thanks go to Olivia Kaferly, Assistant to the Editor-in-Chief.
substitution, the term balance point redirects the scientific
Like a good mother takes care of her children, Olivia took an excellent care of
search from looking for the location of the set point (or
the editors, authors, and referees involved. She successfully led us through the
building a new model of it) to studying the multiple feedback,
project and made sure we would not fall in various organizational, technical,
feedforward, and open-loop components that contribute to
ethical, and other potholes.
thermal balance in the thermoregulatory system operating as afederation of independent thermoeffector loops. Interestingly,
many scientists in the field are already avoiding using the term
The author's research reviewed in this paper has been supported by grants
set point altogether or replacing it with different terms (e.g.,
from the National Institute of Neurological Disorders and Stroke (NS41233),Arizona Biomedical Research Commission (8016), and St. Joseph's Founda-
Ref. 10). In his editorial about the regulation of body fat
content, Wade (122) suggests to put the notions of lipostats andset points behind us and to move on. Applying Wade's sug-
gestion to Tb regulation, it is time to free the thermophysi-ological terminology of remnants of the unified control system
1. Almeida MC, Steiner AA, Branco LGS, and Romanovsky AA.
Cold-seeking behavior as a thermoregulatory strategy in systemic inflam-
and to focus our research on studying independent thermoef-
mation. Eur J Neurosci 23: 3359 –3367, 2006.
fector loops.
2. Almeida MC, Steiner AA, Branco LGS, and Romanovsky AA. Neural
substrate of cold-seeking behavior in endotoxin shock. PLoS one 1: e1,2006.
INSTEAD OF CONCLUSIONS
3. Almeida MC, Steiner AA, Coimbra NC, and Branco LGS. Thermoef-
In this review, I have tried to make a point that thermoef-
fector neuronal pathways in fever: a study in rats showing a new role ofthe locus coeruleus. J Physiol 558: 283–294, 2004.
fectors can coordinate their activities and regulate Tb while
4. Aoki K, Stephens DP, Zhao K, Kosiba WA, and Johnson JM.
functioning within relatively independent loops, without a
Modification of cutaneous vasodilator response to heat stress by daytime
AJP-Regul Integr Comp Physiol • VOL 292 • JANUARY 2007 • www.ajpregu.org
IN FOCUS: THERMOREGULATION 2007
exogenous melatonin administration. Am J Physiol Regul Integr Comp
28. Craig AD. Interoception: the sense of the physiological condition of the
Physiol 291: R619 –R624, 2006.
body. Curr Opin Neurobiol 13: 500 –505, 2003.
5. Asami A, Asami T, Hori T, Kiyohara T, and Nakashima T. Ther-
29. DeGroot DW and Kenney WL. Impaired defense of core temperature in
mally-induced activities of the mesencephalic reticulospinal and rubro-
aged humans during mild cold stress. Am J Physiol Regul Integr Comp
spinal neurons in the rat. Brain Res Bull 20: 387–398, 1988.
Physiol 292: R103–R108, 2007.
6. Asami T, Hori T, Kiyohara T, and Nakashima T. Convergence of
30. Dhaka A, Viswanath V, and Patapoutian A. TRP ion channels and
thermal signals on the reticulospinal neurons in the midbrain, pons and
temperature sensation. Annu Rev Neurosci 29: 135–161, 2006.
medulla oblongata. Brain Res Bull 20: 581–596, 1988.
31. Diepvens K, Westerterp KR, and Westerterp-Plantenga MS. Obesity
7. Atanackovic D, Pollok K, Faltz C, Boeters I, Jung R, Nierhaus A,
and thermogenesis related to the consumption of caffeine, ephedrine,
Braumann K-M, Hossfeld DK, and Hegewisch-Becker S. Patients
capsaicin, and green tea. Am J Physiol Regul Integr Comp Physiol 292:
with solid tumors treated with high-temperature whole body hyperther-
R77–R85, 2007.
mia show a redistribution of naive/memory T-cell subtypes. Am J Physiol
32. DiMicco JA and Zaretsky DV. The dorsomedial hypothalamus: a new
Regul Integr Comp Physiol 290: R585–R594, 2006.
player in thermoregulation. Am J Physiol Regul Integr Comp Physiol
8. Bamshad M, Song CK, and Bartness TJ. CNS origins of the sympa-
292: R47–R63, 2007.
thetic nervous system outflow to brown adipose tissue. Am J Physiol
33. DiMicco JA and Zaretsky DV. The mysterious role of prostaglandin E2
Regul Integr Comp Physiol 276: R1569 –R1578, 1999.
in the medullary raphe´: a hot topic or not? Am J Physiol Regul Integr
8a.Berner NJ and Heller HC. Does the preoptic anterior hypothalamus
Comp Physiol 289: R1589 –R1591, 2005.
receive thermoafferent information? Am J Physiol Regul Integr Comp
34. Egan GF, Johnson J, Farrell M, McAllen R, Zamarripa F, McKinley
Physiol 274: R9 –R18, 1998.
MJ, Lancaster J, Denton D, and Fox PT. Cortical, thalamic, and
9. Blessing WW. Inadequate frameworks for understanding bodily ho-
hypothalamic responses to cooling and warming the skin in awake
meostasis. Trends Neurosci 20: 235–239, 1997.
humans: a positron-emission tomography study. Proc Natl Acad Sci USA
10. Bligh J. A theoretical consideration of the means whereby the mamma-
102: 5262–5267, 2005.
lian core temperature is defended at a null zone. J Appl Physiol 100:
35. Fabricio ASC, Rae GA, Zampronio AR, D'Orle´ans-Juste P, and
1332–1337, 2006.
Souza GEP. Central endothelin ETB receptors mediate IL-1-dependent
11. Boulant JA. Counterpoint: heat-induced membrane depolarization of
fever induced by preformed pyrogenic factor and corticotropin-releasing
hypothalamic neurons: an unlikely mechanism of central thermosensi-
factor in the rat. Am J Physiol Regul Integr Comp Physiol 290: R164 –
tivity. Am J Physiol Regul Integr Comp Physiol 290: R1481–R1484,
2006; Discussion R1484, 2006.
36. Fabricio ASC, Tringali G, Pozzoli G, Melo MC, Vercesi JA, Souza
12. Boulant JA. Neuronal basis of Hammel's model for set-point thermo-
GEP, and Navarra P. Interleukin-1 mediates endothelin-1-induced
regulation. J Appl Physiol 100: 1347–1354, 2006.
fever and prostaglandin production in the preoptic area of rats. Am J
13. Bradford CD, Cotter JD, Thorburn MS, Walker RJ, and Gerrard
Physiol Regul Integr Comp Physiol 290: R1515–R1523, 2006.
DF. Exercise can be pyrogenic in humans. Am J Physiol Regul Integr
37. Fahlman A, Schmidt A, Handrich Y, Woakes AJ, and Butler PJ.
Comp Physiol 292: R143–R149, 2007.
Metabolism and thermoregulation during fasting in king penguins,
14. Bratincsak A and Palkovits M. Evidence that peripheral rather than
Aptenodytes patagonicus, in air and water. Am J Physiol Regul Integr
intracranial thermal signals induce thermoregulation. Neuroscience 135:
Comp Physiol 289: R670 –R679, 2005.
525–532, 2005.
38. Feleder C, Perlik V, Tang Y, and Blatteis CM. Putative antihyperpy-
15. Cabanac M. Adjustable set point: to honor Harold T. Hammel. J Appl
retic factor induced by LPS in spleen of guinea pigs. Am J Physiol Regul
Physiol 100: 1338 –1346, 2006.
Integr Comp Physiol 289: R680 –R687, 2005.
16. Caldeira JC, Franci CR, and Pela IR. Bilateral lesion of hypothalamic
39. Fink GD. Hypothesis: the systemic circulation as a regulated free-market
paraventricular nucleus abolishes fever induced by endotoxin and bra-
economy. A new approach for understanding the long-term control of
dykinin in rats. Ann NY Acad Sci 856: 294 –297, 1998.
blood pressure. Clin Exp Pharmacol Physiol 32: 377–383, 2005.
17. Cannon B and Nedergaard J. Brown adipose tissue: function and
40. Ganta CK, Helwig BG, Blecha F, Ganta RR, Cober R, Parimi S,
physiological significance. Physiol Rev 84: 277–359, 2004.
Musch TI, Fels RJ, and Kenney MJ. Hypothermia-enhanced splenic
18. Cano G, Passerin AM, Schiltz JC, Card JP, Morrison SF, and Sved
AF. Anatomical substrates for the central control of sympathetic outflow
cytokine gene expression is independent of the sympathetic nervous
to interscapular adipose tissue during cold exposure. J Comp Neurol 460:
system. Am J Physiol Regul Integr Comp Physiol 291: R558 –R565,
303–326, 2003.
19. Caputa M. Comments on "Do fever and anapyrexia exist? Analysis of
41. Gilbert C, Le Maho YL, Perret M, and Ancel A. Body temperature
set point-based definitions". Am J Physiol Regul Integr Comp Physiol
changes induced by huddling in breeding male emperor penguins. Am J
289: R281, 2005; Reply R281–R282, 2005.
Physiol Regul Integr Comp Physiol 292: R176 –R185, 2007.
20. Caterina MJ. Transient receptor potential ion channels as participants in
42. Gray DA, Maloney SK, and Kamerman PR. Lipopolysaccharide-
thermosensation and thermoregulation. Am J Physiol Regul Integr Comp
induced fever in Pekin ducks is mediated by prostaglandins and nitric
Physiol 292: R64 –R76, 2007.
oxide and modulated by adrenocortical hormones. Am J Physiol Regul
21. Chen XM, Hosono T, Yoda T, Fukuda Y, and Kanosue K. Efferent
Integr Comp Physiol 289: R1258 –R1264, 2005.
projection from the preoptic area for the control of non-shivering ther-
43. Griffin JD, Saper CB, and Boulant JA. Synaptic and morphological
mogenesis in rats. J Physiol 512: 883– 892, 1998.
characteristics of temperature-sensitive and -insensitive rat hypothalamic
22. Chevrier C, Bourdon L, and Canini F. Cosignaling of adenosine and
neurones. J Physiol 537: 521–535, 2001.
adenosine triphosphate in hypobaric hypoxia-induced hypothermia. Am J
44. Guler AD, Lee H, Iida T, Shimizu I, Tominaga M, and Caterina M.
Physiol Regul Integr Comp Physiol 290: R595–R600, 2006.
Heat-evoked activation of the ion channel, TRPV4. J Neurosci 22:
23. Chu AL, Jay O, and White MD. The effects of hyperthermia and
6408 – 6414, 2002.
hypoxia on ventilation during low-intensity steady-state exercise. Am J
45. Gutman R, Choshniak I, and Kronfeld-Schor N. Defending body
Physiol Regul Integr Comp Physiol 292: R195–R203, 2007.
mass during food restriction in Acomys russatus: a desert rodent that does
24. Cliffer KD, Burstein R, and Giesler GJ Jr. Distributions of spinotha-
not store food. Am J Physiol Regul Integr Comp Physiol 290: R881–
lamic, spinohypothalamic, and spinotelencephalic fibers revealed by
anterograde transport of PHA-L in rats. J Neurosci 11: 852– 868, 1991.
46. Helwig BG, Parimi S, Ganta CK, Cober R, Fels RJ, and Kenney MJ.
25. Commission for Thermal Physiology of the International Union of
Aging alters regulation of visceral sympathetic nerve responses to acute
Physiological Sciences. Glossary of terms for thermal physiology. Jpn
hypothermia. Am J Physiol Regul Integr Comp Physiol 291: R573–R579,
J Physiol 51: 245–280, 2001.
26. Costa AC, Stasko MR, Stoffel M, and Scott-McKean JJ. G-protein-
47. Horn T, Wilkinson MF, Landgraf R, and Pittman QJ. Reduced
gated potassium (GIRK) channels containing the GIRK2 subunit are
febrile responses to pyrogens after lesions of the hypothalamic paraven-
control hubs for pharmacologically induced hypothermic responses.
tricular nucleus. Am J Physiol Regul Integr Comp Physiol 267: R323–
J Neurosci 25: 7801–7804, 2005.
27. Craig AD. How do you feel? Interoception: the sense of the physiolog-
48. Hua LH, Strigo IA, Baxter LC, Johnson SC, and Craig AD. Antero-
ical condition of the body. Nat Rev Neurosci 3: 655– 666, 2002.
posterior somatotopy of innocuous cooling activation focus in human
AJP-Regul Integr Comp Physiol • VOL 292 • JANUARY 2007 • www.ajpregu.org
IN FOCUS: THERMOREGULATION 2007
dorsal posterior insular cortex. Am J Physiol Regul Integr Comp Physiol
68. Maruyama M, Nishi M, Konishi M, Takashige Y, Nagashima K,
289: R319 –R325, 2005.
Kiyohara T, and Kanosue K. Brain regions expressing Fos during
49. Hu¨bschle T, Mu¨tze J, Mu¨hlradt PF, Korte S, Gerstberger R, and
thermoregulatory behavior in rats. Am J Physiol Regul Integr Comp
Roth J. Pyrexia, anorexia, adipsia, and depressed motor activity in rats
Physiol 285: R1116 –R1123, 2003.
during systemic inflammation induced by the Toll-like receptors-2 and -6
68a.McAllen RM. The cold path to BAT. Am J Physiol Regul Integr Comp
agonists MALP-2 and FSL-1. Am J Physiol Regul Integr Comp Physiol
Physiol 292: R124 –R126, 2007.
290: R180 –R187, 2006.
69. McAllen RM. Preoptic thermoregulatory mechanisms in detail. Am J
50. Ivanov AI, Steiner AA, Patel S, Rudaya AY, and Romanovsky AA.
Physiol Regul Integr Comp Physiol 287: R272–R273, 2004.
Albumin is not an irreplaceable carrier for amphipathic mediators of
70. McAllen RM and McKinley MJ. Personal body maps. Am J Physiol
thermoregulatory responses to LPS: compensatory role of ␣1-acid gly-
Regul Integr Comp Physiol 289: R317–R318, 2005.
coprotein. Am J Physiol Regul Integr Comp Physiol 288: R872–R878,
71. McCord GR, Cracowski J-L, and Minson CT. Prostanoids contribute
to cutaneous active vasodilation in humans. Am J Physiol Regul Integr
51. Jay O, Garie´py LM, Reardon FD, Webb P, Ducharme MB, Ramsay
Comp Physiol 291: R596 –R602, 2006.
T, and Kenny GP. A three-compartment thermometry model for the
72. Mercer JB and Simon E. A comparison between total body thermo-
improved estimation of changes in body heat content. Am J Physiol Regul
sensitivity and local thermosensitivity in mammals and birds. Pflu¨gers
Integr Comp Physiol 292: R167–R175, 2007.
Arch 400: 228 –234, 1984.
52. Jessen C. Independent clamps of peripheral and central temperatures and
73. Mochizuki T, Klerman EB, Sakurai T, and Scammell TE. Elevated
their effects on heat production in the goat. J Physiol 311: 11–22, 1981.
body temperature during sleep in orexin knockout mice. Am J Physiol
53. Kanosue K, Romanovsky AA, Hosono T, Chen XM, and Yoda T.
Regul Integr Comp Physiol 291: R533–R540, 2006.
"Set point" revisited In: Thermal Physiology 1997, edited by Nielsen
74. Moqrich A, Hwang SW, Earley TJ, Petrus MJ, Murray AN, Spencer
Johannsen B and Nielsen R. Copenhagen: The August Krogh Institute,
KS, Andahazy M, Story GM, and Patapoutian A. Impaired ther-
1997, p. 39 – 43.
mosensation in mice lacking TRPV3, a heat and camphor sensor in the
54. Kenny GP, Jay O, Zaleski WM, Reardon ML, Sigal RJ, Journeay
skin. Science 307: 1468 –1472, 2005.
WS, and Reardon FD. Postexercise hypotension causes a prolonged
75. Morrison SF. Central pathways controlling brown adipose tissue ther-
perturbation in esophageal and active muscle temperature recovery. Am J
mogenesis. News Physiol Sci 19: 67–74, 2004.
Physiol Regul Integr Comp Physiol 291: R580 –R588, 2006.
76. Mouihate A, Ellis S, Harre E-M, and Pittman QJ. Fever suppression
55. Kenny GP, Murrin JE, Journeay WS, and Reardon FD. Differences
in near-term pregnant rats is dissociated from LPS-activated signaling
in the postexercise threshold for cutaneous active vasodilation between
pathways. Am J Physiol Regul Integr Comp Physiol 289: R1265–R1272,
men and women. Am J Physiol Regul Integr Comp Physiol 290: R172–
77. Mouihate A, Horn TF, and Pittman QJ. Oxyresveratrol dampens
56. Kobayashi S. Temperature-sensitive neurons in the hypothalamus: a new
neuroimmune responses in vivo: a selective effect on TNF-␣. Am J
hypothesis that they act as thermostats, not as transducers. Prog Neuro-
Physiol Regul Integr Comp Physiol 291: R1215–R1221, 2006.
biol 32: 103–135, 1989.
78. Nagashima K, Nakai S, Tanaka M, and Kanosue K. Neuronal circuit-
57. Kobayashi S, Hori A, Matsumura K, and Hosokawa H. Point:
ries involved in thermoregulation. Auton Neurosci 85: 18 –25, 2000.
heat-induced membrane depolarization of hypothalamic neurons: a pu-
79. Nakamura K and Morrison SF. Central efferent pathways mediating
tative mechanism of central thermosensitivity. Am J Physiol Regul Integr
skin cooling-evoked sympathetic thermogenesis in brown adipose tissue.
Comp Physiol 290: R1479 –R1480, 2006. Discussion R1484, 2006.
Am J Physiol Regul Integr Comp Physiol 292: R127–R136, 2007.
58. Kobayashi S, Okazawa M, Hori A, Matsumura K, and Hosokawa H.
80. Ni D, Gu Q, Hu H-Z, Gao N, Zhu MX, and Lee L-Y. Thermal
Paradigm shift in sensory system⫺animals do not have sensors. J Therm
sensitivity of isolated vagal pulmonary sensory neurons: role of transient
Biol 31: 19 –23, 2006.
receptor potential vanilloid receptors. Am J Physiol Regul Integr Comp
59. Konishi M, Kanosue K, Kano M, Kobayashi A, and Nagashima K.
Physiol 291: R541–R550, 2006.
The median preoptic nucleus is involved in the facilitation of heat-
81. Nilius B, Talavera K, Owsianik G, Prenen J, Droogmans G, and
escape/cold-seeking behaviour during systemic salt loading in rats. Am J
Voets T. Gating of TRP channels: a voltage connection? J Physiol 567:
Physiol Regul Integr Comp Physiol 292: R150 –R159, 2007.
35– 44, 2005.
60. Konishi M, Nagashima K, Asano K, and Kanosue K. Attenuation of
82. Nomoto S, Shibata M, Iriki M, and Riedel W. Role of afferent
metabolic heat production and cold-escape/warm-seeking behaviour dur-
pathways of heat and cold in body temperature regulation. Int J Bio-
ing a cold exposure following systemic salt loading in rats. J Physiol 551:
meteorol 49: 67– 85, 2004.
713–720, 2003.
83. Okazawa M, Takao K, Hori A, Shiraki T, Matsumura K, and
61. Kozak W, Wrotek S, and Kozak A. Pyrogenicity of CpG-DNA in mice:
Kobayashi S. Ionic basis of cold receptors acting as thermostats. J Neu-
role of interleukin-6, cyclooxygenases, and nuclear factor-B. Am J
rosci 22: 3994 – 4001, 2002.
Physiol Regul Integr Comp Physiol 290: R871–R880, 2006.
84. Oldfield BJ, Giles ME, Watson A, Anderson C, Colvill LM, and
62. Kvadsheim PH, Folkow LP, and Blix AS. Inhibition of shivering in
McKinley MJ. The neurochemical characterisation of hypothalamic
hypothermic seals during diving. Am J Physiol Regul Integr Comp
pathways projecting polysynaptically to brown adipose tissue in the rat.
Physiol 289: R326 –R331, 2005.
Neuroscience 110: 515–526, 2002.
63. Lee H, Iida T, Mizuno A, Suzuki M, and Caterina MJ. Altered
85. Ootsuka Y and McAllen RM. Comparison between two rat sympathetic
thermal selection behavior in mice lacking transient receptor potential
pathways activated in cold defense. Am J Physiol Regul Integr Comp
vanilloid 4. J Neurosci 25: 1304 –1310, 2005.
Physiol 291: R589 –R595, 2006.
64. Leite LH, Lacerda ACR, Marubayashi U, and Coimbra CC. Central
86. Partridge LD. The good enough calculi of evolving control systems:
angiotensin AT1-receptor blockade affects thermoregulation and running
evolution is not engineering. Am J Physiol Regul Integr Comp Physiol
performance in rats. Am J Physiol Regul Integr Comp Physiol 291:
242: R173–R177, 1982.
R603–R607, 2006.
87. Patapoutian A, Peier AM, Story GM, and Viswanath V. ThermoTRP
65. Li Z, Perlik V, Feleder C, Tang Y, and Blatteis CM. Kupffer
channels and beyond: mechanisms of temperature sensation. Nat Rev
cell-generated PGE2 triggers the febrile response of guinea pigs to
Neurosci 4: 529 –539, 2003.
intravenously injected LPS. Am J Physiol Regul Integr Comp Physiol
88. Perlik V, Li Z, Goorha S, Ballou LR, and Blatteis CM. LPS-activated
290: R1262–R1270, 2006.
complement, not LPS per se, triggers the early release of PGE2 by
66. Lim CL, Wilson G, Brown L, Coombes JS, and Mackinnon LT.
Kupffer cells. Am J Physiol Regul Integr Comp Physiol 289: R332–R339,
Preexisting inflammatory state compromises heat tolerance in rats ex-
posed to heat stress. Am J Physiol Regul Integr Comp Physiol 292:
89. Persson PB. Temperature control: from molecular insights, regulation in
R186 –R194, 2007.
king penguins and diving seals, to studies in humans. Am J Physiol Regul
67. Lu J, Zhang YH, Chou TC, Gaus SE, Elmquist JK, Shiromani P, and
Integr Comp Physiol 291: R512–R514, 2006.
Saper CB. Contrasting effects of ibotenate lesions of the paraventricular
90. Pittman QJ. Endothelin—an emerging role in proinflammatory path-
nucleus and subparaventricular zone on sleep-wake cycle and tempera-
ways in brain. Am J Physiol Regul Integr Comp Physiol 290: R162–
ture regulation. J Neurosci 21: 4864 – 4874, 2001.
AJP-Regul Integr Comp Physiol • VOL 292 • JANUARY 2007 • www.ajpregu.org
IN FOCUS: THERMOREGULATION 2007
91. Pollack GH. Cells, Gels and the Engines of Life: A New, Unifying
saccharide per se —not by lipoprotein contaminants. Am J Physiol Regul
Approach to Cell Function. Seattle: Ebner, 2001.
Integr Comp Physiol 289: R348 –R352, 2005.
92. Roberts WW. Differential thermosensor control of thermoregulatory
111. Steiner AA, Rudaya AY, Robbins JR, Dragic AS, Langenbach R,
grooming, locomotion, and relaxed postural extension. Ann NY Acad Sci
and Romanovsky AA. Expanding the febrigenic role of cyclooxygen-
525: 363–374, 1988.
ase-2 to the previously overlooked responses. Am J Physiol Regul Integr
93. Romanovsky AA. Temperature regulation. In: Lecture Notes on Human
Comp Physiol 289: R1253–R1257, 2005.
Physiology, edited by Petersen O. Oxford: Blackwell, 2006, chap. 23, p.
112. Sze´kely M. Orexins, energy balance, temperature, sleep-wake cycle.
Am J Physiol Regul Integr Comp Physiol 291: R530 –R532, 2006.
94. Romanovsky AA. Do fever and anapyrexia exist? Analysis of set
113. Szele´nyi Z. Neuronal CCK and thermoregulation: two receptors with
point-based definitions. Am J Physiol Regul Integr Comp Physiol 287:
different functions. Am J Physiol Regul Integr Comp Physiol 292:
R992–R995, 2004.
R109 –R111, 2007.
95. Romanovsky AA. Vioxx, Celebrex, Bextra . . do we have a new target
114. Talavera K, Yasumatsu K, Voets T, Droogmans G, Shigemura N,
for anti-inflammatory and antipyretic therapy? Am J Physiol Regul Integr
Ninomiya Y, Margolskee RF, and Nilius B. Heat activation of TRPM5
Comp Physiol 288: R1098 –R1099, 2005.
underlies thermal sensitivity of sweet taste. Nature 438: 1022–1025,
96. Romanovsky AA, Almeida MC, Aronoff DM, Ivanov AI, Konsman
JP, Steiner AA, and Turek VF. Fever and hypothermia in systemic
115. Tanaka M and McAllen RM. A subsidiary fever center in the medullary
inflammation: recent discoveries and revisions. Front Biosci 10: 2193–
raphe´? Am J Physiol Regul Integr Comp Physiol 289: R1592–R1598,
97. Romanovsky AA, Ivanov AI, and Shimansky YP. Selected contribu-
116. Tanaka M, Owens NC, Nagashima K, Kanosue K, and McAllen RM.
tion: ambient temperature for experiments in rats: a new method for
Reflex activation of rat fusimotor neurons by body surface cooling, and
determining the zone of thermal neutrality. J Appl Physiol 92: 2667–
its dependence on the medullary raphe. J Physiol 572: 569 –583, 2006.
117. Tankersley CG, Irizarry R, Flanders SE, Rabold R, and Frank R.
98. Romanovsky AA, Shido O, Sakurada S, Sugimoto N, and Nagasaka
Unstable heart rate and temperature regulation predict mortality in
T. Endotoxin shock: thermoregulatory mechanisms. Am J Physiol Regul
AKR/J mice. Am J Physiol Regul Integr Comp Physiol 284: R742–R750,
Integr Comp Physiol 270: R693–R703, 1996.
99. Rudaya AY, Steiner AA, Robbins JR, Dragic AS, and Romanovsky
118. Thompson CS, Holowatz LA, and Kenney WL. Cutaneous vasocon-
AA. Thermoregulatory responses to lipopolysaccharide in the mouse:
strictor responses to norepinephrine are attenuated in older humans. Am J
dependence on the dose and ambient temperature. Am J Physiol Regul
Physiol Regul Integr Comp Physiol 288: R1108 –R1113, 2005.
Integr Comp Physiol 289: R1244 –R1252, 2005.
119. Togashi K, Hara Y, Tominaga T, Higashi T, Konishi Y, Mori Y, and
100. Rummel C, Barth SW, Voss T, Korte S, Gerstberger R, Hu¨bschle T,
Tominaga M. TRPM2 activation by cyclic ADP-ribose at body temper-
and Roth J. Localized vs. systemic inflammation in guinea pigs: a role
ature is involved in insulin secretion. EMBO J 25: 1804 –1815, 2006.
for prostaglandins at distinct points of the fever induction pathways?
120. Vallone D, Frigato E, Vernesi C, Foa´ A, Foulkes NS, and Bertolucci
Am J Physiol Regul Integr Comp Physiol 289: R340 –R347, 2005.
C. Hypothermia modulates circadian clock gene expression in lizard
101. Saha S, Engstrom L, Mackerlova L, Jakobsson P-J, and Blomqvist
peripheral tissues. Am J Physiol Regul Integr Comp Physiol 292: R160 –
A. Impaired febrile responses to immune challenge in mice deficient in
microsomal prostaglandin E synthase-1. Am J Physiol Regul Integr Comp
121. Van Someren EJW. Thermoregulation and aging. Am J Physiol Regul
Physiol 288: R1100 –R1107, 2005.
Integr Comp Physiol 292: R99 –R102, 2007.
102. Saito S and Shingai R. Evolution of thermoTRP ion channel homologs
122. Wade GN. Regulation of body fat content? Am J Physiol Regul Integr
in vertebrates. Physiol Genomics In press.
Comp Physiol 286: R14 –R15, 2004.
103. Sakurada S, Shido O, Fujikake K, and Nagasaka T. Relationship
123. Wechselberger M, Wright CL, Bishop GA, and Boulant JA. Ionic
between body core and peripheral temperatures at the onset of thermo-
channels and conductance-based models for hypothalamic neuronal ther-
regulatory responses in rats. Jpn J Physiol 43: 659 – 667, 1993.
mosensitivity. Am J Physiol Regul Integr Comp Physiol 291: R518 –
104. Sasaki K, Taniguchi M, Miyoshi M, Goto O, Sato K, and Watanabe
T. Are transcription factors NF-B and AP-1 involved in the ANG
124. Weiland TJ, Voudouris NJ, and Kent S. CCK2 receptor nullification
II-stimulated production of proinflammatory cytokines induced by LPS
attenuates lipopolysaccharide-induced sickness behavior. Am J Physiol
in dehydrated rats? Am J Physiol Regul Integr Comp Physiol 289:
Regul Integr Comp Physiol 292: R112–R123, 2007.
R1599 –R1608, 2005.
125. Wernstedt I, Edgley A, Berndtsson A, Fa¨ldt J, Bergstro¨m G, Wal-
105. Satinoff E. Neural organization and evolution of thermal regulation in
lenius V, and Jansson J-O. Reduced stress- and cold-induced increase
mammals. Science 201: 16 –22, 1978.
in energy expenditure in interleukin-6-deficient mice. Am J Physiol Regul
106. Schmidt A, Alard F, and Handrich Y. Changes in body temperature in
Integr Comp Physiol 291: R551–R557, 2006.
king penguins at sea: the result of fine adjustments in peripheral heat
126. Whyte DG and Johnson AK. Lesions of the anteroventral third ventri-
loss? Am J Physiol Regul Integr Comp Physiol 291: R608 –R618, 2006.
cle region exaggerate neuroendocrine and thermogenic but not behavioral
107. Shabtay A and Arad Z. Reciprocal activation of HSF1 and HSF3 in
responses to a novel environment. Am J Physiol Regul Integr Comp
brain and blood tissues: is redundancy developmentally related? Am J
Physiol 292: R137–R142, 2007.
Physiol Regul Integr Comp Physiol 291: R566 –R572, 2006.
127. Widmer RJ, Laurinec JE, Young MF, Laine GA, and Quick CM.
108. Simon A and van der Meer JWM. Pathogenesis of familial periodic
Local heat produces a shear-mediated biphasic response in the thermo-
fever syndromes or hereditary autoinflammatory syndromes. Am J
regulatory microcirculation of the Pallid bat wing. Am J Physiol Regul
Physiol Regul Integr Comp Physiol 292: R86 –R98, 2007.
Integr Comp Physiol 291: R625–R632, 2006.
109. Simon E. Ion channel proteins in neuronal temperature transduction:
128. Zhang YH, Yanase-Fujiwara M, Hosono T, and Kanosue K. Warm
from inferences to testable theories of deep-body thermosensitivity. Am J
and cold signals from the preoptic area: which contribute more to the
Physiol Regul Integr Comp Physiol 291: R515–R517, 2006.
control of shivering in rats? J Physiol 485: 195–202, 1995.
110. Steiner AA, Chakravarty S, Robbins JR, Dragic AS, Pan J, Herken-
129. Zhao Y and Boulant JA. Temperature effects on neuronal membrane
ham M, and Romanovsky AA. Thermoregulatory responses of rats to
potentials and inward currents in rat hypothalamic tissue slices. J Physiol
conventional preparations of lipopolysaccharide are caused by lipopoly-
564: 245–257, 2005.
AJP-Regul Integr Comp Physiol • VOL 292 • JANUARY 2007 • www.ajpregu.org
Source: http://thermal.uow.edu.au/Thermoregulation-2007-AmJPhysiol.pdf
Informe Sobre El Mercado Energético Global Al 3 de diciembre de 2010 Por Hernán F. Pacheco Índice: Análisis I: El reload de la política energética estadounidense y el tema del cambio climático El perjuicio de la quita de los subsidios en la energía solar Los republicanos afines a la energía nuclear
ARTIGO DE REVISÃO / REVIEW ARTICLE Data de recepção / Received in: 17/09/2010 Data de aceitação / Accepted for publication in: 10/11/2010 Marta Salgado, Rute Reis, António Vinhas de Sousa, Elza Tomaz, Irina Dydenko, Andreia Ferrão, Fátima Ferreira, Filipe Inácio Serviço de Imunoalergologia / Immunoallergology Department – Hospital de São Bernardo, Setúbal



