Chemshow.cn

Supelco Ionic LiquidsThe Dawning of a New Era in GC Phase Technology
• Liquid Chromatography
• Sample Handling
• Gas Chromatography
The evolution of GC Phase Technology
from Substituted Polysiloxane Polymers
and Polyethylene Glycols in the 1950's,
to Bonded Phases in the 1980's, and on
to our new Ionic Liquid phases today.


Reporter
See you at the Conference!
Visit us on the web at sigma-aldrich.com/thereporter
Market Segment Manager
Dear Col eague,
Conferences and exhibitions are an excel ent opportunity for vendors and conferees to share information on their
latest developments and research. It also gives vendors an opportunity to get feedback from customers on new
"Contributed Articles" are submitted
product needs and requirements. Every year Supelco attends over 30 tradeshows worldwide, some very small
by our customers based upon their
and focused in very specific areas of analytical chemistry like the ASMS Conference and others very large and
work with Sigma-Aldrich products.
diverse like PITTCON.
We encourage you to submit articles
Just like you, we can't attend every tradeshow, but we are trying to make it easier for you to stay current with
describing your work for consideration in future publications.
our new developments and to get information on what we present at many of the tradeshows that we attend. Below are a few of the ways we can help you keep up with the most recent information we are presenting at the upcoming conferences. We would like for you to attend the tradeshows and stop by our booth, but if you can't attend then you can:
Table of Contents
l Request a CD containing oral and poster presentations from PITTCON® 2008.
Liquid Chromatography
l Visit our website (sigma-aldrich.com/analytical-events) to see
the most up-to-date list of tradeshows and conferences that we
are attending and find recently presented materials.
l Contact Technical Service or your local sales specialist to get
more information on new products and recent publications.
Upcoming major tradeshows where you can visit us:
HPLC 2008 International Symposium
Int'l Symposium on Capil ary Chromatography Riva Del Garda, Italy
American Chemical Society Mtg
Gulf Coast Conference
Gas Chromatography
American Association of Pharma Scientists
Eastern Analytical Symposium
We wil also be at other smal er, more focused shows. For more information on these shows, visit our website at
sigma-aldrich.com/analytical-events. We hope to see you at one of the upcoming tradeshows! You can also find information on upcoming local technical seminars on the events page.
Market Segment Manager
Reporter is published 5 times a year by Supelco Marketing, 595 North Harrison Road, Bel efonte, PA 16823-0048.
Accelerating Customers' Success through Leadership in Life Science, High Technology and Service

Supelco Patented Ionic Liquid GC Phase Technology
Leonard M. Sidisky and Michael D. Buchanan
Figure 1. Modifiable Components of a Dicationic Phase
With a few exceptions, just two types of columns are
typical y used in gas chromatography (GC). The first type includes columns that contain a substituted polysiloxane polymer phase, the origin of which can be traced back to the 1950's and the very birth of the GC technique. The
second type is comprised of columns coated with a
[1,9-di(3-vinyl-imidazolium) nonane bis(trifluoromethyl) sulfonyl imidate]
G004214, G004216, G004215
polyethylene glycol phase, a phase that has remained virtual y unchanged nearly as long as GC has been
of ionic liquids permits numerous opportunities for
practiced. With the successful use of ionic liquids as viable
modification. As shown in Figure 1, the components that
Gas Chromatography
GC stationary phases, analysts will be able to perform
can be modified include the cation, linkage and anion.
previously unthought of separations.
l Both dicationic (shown) and polycationic ionic liquids
have been shown to make suitable GC phases.
The Revolution: Ionic Liquid GC Phases
l The choice of cations evaluated thus far includes
Chromatographic characteristics: Ionic liquids are a
imidazolium (shown), phosphonium, and pyrrolidini-
um. Plans exist for evaluating others. The cations
class of non-molecular ionic solvents with low melting
may be the same, but do not necessarily need to be.
points. These liquids are unique combinations of cations
l Modifications to the linkage include changing type
and anions. The practical use of ionic liquids as GC
and/or length. For example, an alkane (shown) or
stationary phases has long been desired because of
polyethylene glycol, or some other type, of various
lengths might be used. If the ionic liquid is polyca-
tionic, the types and lengths of the linkages may be
1. The ability to remain liquids over wide temperature
the same, but they can be different.
ranges, expanding the GC column operating
l The number of anion candidates is also large. The
temperature range compared to traditional
initial work has included the use of bis(trifluoromethyl)
(US and Canada only)
stationary phases (substituted polysiloxane
sulfonyl imidate [nTf2-] (shown) and trifluoromethyl
polymers or polyethylene glycols).
sulfonate [triflate], which has shown promise in
2. Very low volatility, providing low column
improving peak symmetry.
bleed, stable retention times, long column
l Additional y, further modifications of the cation or
life, and increased maximum temperatures.
linkage, (such as the addition of pendant groups,
3. Highly polar nature, expanding the polarity
derivatization, or chiral characteristics) can be
scale upward.
explored. Short alkanes, vinyl groups (shown), and
4. Novel selectivity, al owing application-specific use.
hydroxyl groups are a few choices that have been
successful y used for cation modification.
Previous work with ionic liquids as GC phases focused
l Other possibilities exist, such as bonding, crosslinking,
on monocationic ionic liquids, which did not exhibit the
blending ionic liquids, or doping into existing non-
desired chromatographic characteristics specified above.
ionic GC phases.
Prof. Daniel Armstrong (University of Texas at Arlington)
Experimentation has shown that modification to any
has expanded on this work, showing that dicationic and
single component, even a slight modification in linkage
polycationic ionic liquids as GC phases exhibit desired
length, can be used to achieve a desired chromatographic
benefit. Through further characterization, it is hoped that the relationships between the effects on phase character-istics / chromatographic performance caused by modifica-
With the successful use of ionic liquids
/ 814-359-3441 technical service: 800-359-3041
tions of each of these components can be predicted,
as viable GC stationary phases,
leading to the rapid development of phases/columns with
analysts wil be able to perform
targeted selectivities. Supelco R&D chemists are actively
previously unthought of separations.
involved in discovering these cause and effect relationships.
Sigma-Aldrich/Supelco is the first-to-market with this
Phase modification: Whereas the chemical structures
new, innovative, and patented (US 2008/0027231 A1; other
of existing GC phases al ow limited modification (changing
patents pending) technology, developed in conjunction with
the pendant group on polysiloxane polymers or adjusting
Prof. Daniel Armstrong (University of Texas at Arlington).
the length of polyethylene glycols), the chemical structure
(continued on page 4)
dering: 800-247-6628 or

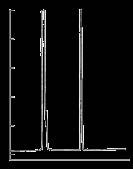
Figure 2. BTEX and n-Alkanes on the SLB-IL100
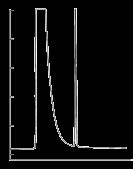
Figure 3. Rapeseed Oil FAMEs on the SLB-IL100
column: SLB-IL100, 30 m x 0.25 mm I.D., 0.20 µm (28884-U)
column: SLB-IL100, 30 m x 0.25 mm I.D., 0.20 µm (28884-U)
1. Myristic (C14:0)
det.: FID, 250 °C
det.: FID, 250 °C
2. Palmitic (C16:0)
carrier gas: helium, 26 cm/sec @ 110 °C
carrier gas: helium, 30 cm/sec @ 180 °C
3. Stearic (C18:0)
injection: 0.1 µL, 300:1 split
injection: 1 µL, 100:1 split
4. Oleic (C18:1n9c)
liner: 4 mm I.D., split, cup
liner: 4 mm I.D., split, cup
5. Linoleic (C18:2)
sample: NEAT mixture containing v
sample: Rapeseed oil FAME mix,
6. Linolenic (C18:3)
percentages of each component
5 mg/mL total FAMEs in
7. Arachidic (C20:0)
methylene chloride
8. cis-11-Eicosenoic
9. Behenic (C22:0)
10. Erucic (C22:1)
11. Lignoceric (C24:0)
Gas Chromatography
(continued from page 3)
di-, and tri-unsaturated fatty acids ranging in carbon length
The SLB™-IL100: The First Phase in the Line
from C14 to C24. The elution of C18:3 after C20:0 and
The SLB-IL100 is the first commercial offering in this new
C20:1 is typical y observed with highly polar phases.
line of novel columns, those that utilize ionic liquid phase
Based on the rapeseed oil elution pattern and other
chemistry. The SLB-IL100 column has a polarity/selectivity
characterizations, it has been determined that the SLB-
roughly equivalent to that of the traditional TCEP phase,
IL100 phase is virtual y equivalent in polarity/selectivity to
and exemplifies some of the desired characteristics that
the TCEP phase, currently one of the highest polarity/
ionic liquids are predicted to possess. Namely, a higher
selectivity GC phases.
maximum temperature compared to non-ionic liquid columns with similar polarity/selectivity. Specifical y, the
Outlook for Ionic Liquid GC Phase Technology
SLB-IL100 has a 230 °C maximum temperature, resulting
The patented and successful use of ionic liquids as viable
from the robustness and low volatility of the phase,
GC stationary phases heralds in a new and exiting chapter
whereas traditional TCEP columns with equivalent polarity/
in GC phase technology. Now analysts wil be able to
selectivity have a 140 °C maximum temperature. The
perform previously unthought of separations with the
SLB-IL100 column is expected to broaden the range of
potential to go way beyond applications possible using
applications that can be performed on highly polar columns.
traditional phases. For example, an ionic liquid phase with a polarity/selectivity similar to that of Carbowax® 20M, but
Example Applications on the SLB-IL100
with a maximum temperature over 300 °C, is just one of
BTEX and n-Alkanes
many possibilities currently being investigated. Look for
Figure 2 il ustrates the separation of benzene, toluene,
additional Supelco ionic liquid phases to be introduced in
ethyl benzene, and the xylene isomers (BTEX) in the
the coming months. This is truly an exciting time in GC
presence of C11 and C13 n-alkanes. The high polarity/
phase development!
selectivity of the ionic liquid phase results in the elution of toluene after C13 at 110 °C. This is desirable because the
SLB-IL100 Specifications
aliphatic fraction of gasoline consists of n-alkanes up to
Phase: non-bonded; 1,9-di(3-vinyl-imidazolium) nonane
C13. Therefore, the quantitation of aromatics in products
bis(trifluoromethyl) sulfonyl imidate
Temp. Limits: Subambient to 230 °C
based on gasoline (such as mineral spirits) requires a column with a polarity/selectivity able to separate the aromatic fraction from the aliphatic fraction. As shown,
+ Featured Products
the SLB-IL100 has this necessary polarity/selectivity.
Description
Rapeseed Oil FAMEs
SLB-IL100 Fused Silica Capillary Column
Figure 3 il ustrates the separation of the fatty acids
30 m x 0.25 mm I.D., 0.20 µm
(analyzed as FAMEs) found in a rapeseed oil sample.
Rapeseed oil contains a variety of saturated and mono-,

Improve GC Reproducibility by Using
FocusLiner™ Inlet Liners
Robert F. Wallace
The wool plug can be
easily dislodged without
Figure 2. Tailing Solvent Peak – Wool Plug in
the chromatographer's
Incorrect Position
knowledge. As shown in
Poor sample reproducibility observed by chromatogra-
Figure 1, a common cause
phers from one consecutive injection to another may be
of wool plug displacement
an indication that small variations in the injection volume
within the liner is that
have occurred. Placing a small plug of either glass or
repeated injections
quartz wool inside an inlet liner has historical y been used
progressively move the
to overcome this. However, this procedure does have
Gas Chromatography
wool plug until no further
distinct drawbacks. FocusLiner inlet liners are specifical y
contact with the needle is
designed to exhibit the benefits of using a wool plug
made. A sudden change in
without the drawbacks.
the inlet pressure, like changing the septum, can
With a FocusLiner inlet liner, the
also result in the move-
chromatographer can be assured that the
Figure 3. Sharp Solvent
ment of the wool plug.
Peak – Wool Plug in
Relocation of the wool
wool plug is always in the correct position
plug from the correct
position can be character-
The Problem with Wool Plugs in Traditional Liners
ized by excessive tailing of
In addition to preventing non-volatile material from
the solvent peak, as shown
(US and Canada only)
entering the column, a wool plug exhibits two benefits
in Figure 2. As shown in
that assist in reducing injection volume variability. 1) The
Figure 3, sharp solvent
increased surface area facilitates the maximum vaporiza-
peaks are only observed
tion of the sample. 2) Any droplets formed on the outside
when the wool plug is in
of the needle are wiped off. Both of these benefits require
the correct position to
that the needle tip penetrate the wool plug. Therefore,
wipe the needle tip.
the position of the wool plug in the injection liner is critical. Unfortunately, there is no guarantee that once a
Overcoming the Drawbacks
liner is instal ed in the injector that the wool plug will stay
FocusLiner inlet liners utilize an innovative design that
in the correct position.
overcomes the drawbacks observed with the use of wool plugs in traditional inlet liners. With FocusLiner inlet liners,
Figure 1. The Problem – Wool Plug in Traditional Inlet Liner
the wool plug is held in position by two tapered sections. As shown in Figure 4 (page 6), these tapered sections
secure the wool plug in the correct position, even after repeated injections and exposure to sudden pressure changes. With a FocusLiner inlet liner, the chromatogra-
/ 814-359-3441 technical service: 800-359-3041
pher can be assured that the wool plug is always in the
THE PROBLEM:
correct position. This will ensure that the needle tip
penetrates the wool plug, wiping any residual liquid
preventing needle
wiping or sample
sample from the needle tip while providing sufficient
surface area for maximum volatilization of the sample.
The effect on sample precision (measured as %RSD)
Inlet Liner
caused by the position of the wool plug in the liner was
(continued on page 6)
dering: 800-247-6628 or
withdrawal. As shown in Figure 2, a tailing solvent peak
Figure 4. The Solution – FocusLiner Inlet Liner
may interfere with the quantitation of peaks that elude shortly after the solvent peak. As shown in Figure 3, the correct position of the wool plug results in sharp solvent
peaks and more accurate quantitation of peaks that elude
Two tapered sections (A) secure
shortly after the solvent peak.
the wool plug (B) in the correct
position to ensure improved
Poor reproducibility and severe tailing may be observed
if the needle tip is not wiped during injections. As the
(continued from page 5)
sample is being delivered from the syringe, droplets will
measured. A 4 mm I.D. traditional inlet liner with the wool
form that wet the syringe needle tip. The volume of
plug moved to the center was evaluated against a 4 mm
sample that remains on the needle tip varies from
I.D. FocusLiner inlet liner. Another frequently used split
Gas Chromatography
injection to injection. The key to the improved reproduc-
liner was also evaluated. This liner design substitutes the
ibility provided by the FocusLiner inlet liner is the proper
wool plug with a sintered glass frit, which can be either
positioning of the wool plug in the liner, al owing the
fixed or removable. In this experiment a 4 mm I.D. fixed
needle tip to be wiped. The use of a FocusLiner inlet liner
frit liner was used.
provides precise, accurate, and reliable sample injections,
As shown in Figure 5, when the wool plug is moved to
resulting in improved reproducibility.
the center of the traditional inlet liner, %RSD values are in
the 8-10% range.
1. Technical Article TA-0043-A, FocusLiner: Improve GC Accuracy and Reproducibility
Figure 5. %RSD of Different Impressively, the Focus-
10 Fold, SGE (www.sge.com)
2. Technical Article TA-0004-A, 0.2% RSD's? It's Now a Reality with SGE's FocusLiner,
Wool Plug Positions
Liner inlet liner was able
SGE (www.sge.com)
to achieve %RSD values
3. R.F. Wal ace, Supelco, TheReporter, August 2006; Volume 24.4: 11
w/wool in center of liner
for the same probe
w/fixed sintered glass frit
compounds in the 0.2%
FocusLiner
range! This is up to 50
Description
times lower than those
measured with the
Split/Splitless, 78.5 x 6.3 x 4.0 mm
traditional inlet liner.
Split/Splitless, 78.5 x 6.3 x 4.0 mm, single taper
PerkinElmer® AutoSystem™ and Clarus
The fixed sintered glass
Split/Splitless, 92 x 6.2 x 4.0 mm
frit liner is also unable to
Split/Splitless, 92 x 6.2 x 4.0 mm, single taper
Shimadzu®14/15A/16 with SPL-14 Injector
match the precision
Split/Splitless, 99 x 5.0 x 3.4 mm
provided by the FocusLin-
Split/Splitless, 99 x 5.0 x 3.4 mm, single taper
Shimadzu 17A with SPL-17 Injector
er inlet liner. This result is
Split/Splitless, 95 x 5.0 x 3.4 mm
not surprising as a key
Split/Splitless, 95 x 5.0 x 3.4 mm, single taper
element to achieving
Varian® 1075/1077 Injector
Split, 72 x 6.3 x 4.0 mm
good sample reproducibil-
Split, 72 x 6.3 x 4.0 mm, single taper
ity is the needle tip being
Varian 1078/1079 Injector
Split/Splitless, 54 x 5.0 x 3.4 mm, single taper
wiped during injection. Therefore, liners with fixed or
Varian CP-1177 Injector
removable frits can only be used with limited success.
Split/Splitless, 78.5 x 6.3 x 4.0 mm
Split/Splitless, 78.5 x 6.3 x 4.0 mm, single taper
1. Al of these FocusLiner inlet liners are packs of 5 and are packed
with quartz wool. Additional pack sizes can be viewed at our website
Sample accuracy is also a critical factor in providing
confidence in sample quantitation. Peak areas for probe compounds using the FocusLiner inlet liner were found to
! Related Information
be, on average, 25% higher than a liner where the wool plug is positioned incorrectly.
Our full line of FocusLiner inlet liners can be viewed online at sigma-aldrich.com/focusliner. For more information on inlet
Solvent peak tailing is also observed if the wool plug is
liners, request the Capil ary GC Inlet Liner Selection Guide,
incorrectly positioned, caused by slow vaporization near
T196899 (BBB) and the poster Selecting the Appropriate Inlet
the cool septum cap as the needle is wiped during
Liner, T404081 (HCH).
Improving the Chiral Separation of Dorzolamide
A Case Study of Chiral Analytical Services
they are remarkably stable and effective in reversed phase,
normal phase, polar organic, and polar ionic chromato-
In the mid 1980s and early 1990s, several pharmaceuti-
graphic modes without memory effects. The CHIROBIOTIC
cal companies began to explore the development of
phases mainly rely on strong anionic or cationic binding,
carbonic anhydrase inhibitors for the topical treatment of
hydrogen bonding, and p-p complexation to achieve
glaucoma. Dorzolamide, a water soluble sulfonamide
separation of various enantiomers (3). Conversely, the
possessing two chiral centers, emerged as a product of
CYCLOBOND phases rely on inclusion as the retention
these research efforts (1).
mechanism. Inclusion complexing arises due to apolar segments of chiral molecules becoming attracted to the
Figure 1. Structures of Dorzolamide and its Enantiomer
apolar cyclodextrin cavity. While apolar segments may occupy the inside of the cavity, more polar segments of the analyte may interact through dipole-dipole interactions, hydrogen bonding, and steric interactions at the mouth of the cavity, al owing the cyclodextrin phases to distinguish
Liquid Chromatography
between isomers differing in stereochemistry. The CYCLO-BOND phases thrive in reversed phase and polar organic
modes, and some (CYCLOBOND I 2000 RN, SN, DMP, and
DNP) are also compatible with normal phase mode (4).
A sample consisting of a 1:1 mixture of dorzolamide
Although the development of enantiomerical y pure
hydrochloride and its enantiomer [(4R, 6R)-4-(Ethylamino-
chiral drugs such as dorzolamide has recently proven
extremely beneficial to the treatment of various ailments,
sulfonamide 7,7-dioxide, monohydrochloride)] (dorzolamide
(US and Canada only)
manufacturers face the obstacle of chiral separation.
hydrochloride Related Compound A (2)) was tested in a
Previous efforts focusing on the chiral separation of
chiral screening protocol employing six of the Astec CHIRO-
dorzolamide from its undesired chiral enantiomer [(4R, 6R)-
BIOTIC and CYCLOBOND phases most likely to give positive
results: CHIROBIOTIC V2 (Vancomycin), CHIROBIOTIC T
(Teicoplanin), CHIROBIOTIC TAG (Teicoplanin Aglycone),
ride)] have been complicated. The chromatographic mode
CYCLOBOND I 2000 (b-cyclodextrin), CYCLOBOND I 2000
of separation described in the 2006 United States Pharma-
DNP (b-cyclodextrin, 3,5-Dinitrophenyl carbamate) CYCLO-
copeia, for instance, involves a derivatization of racemic
BOND I 2000 HP-RSP (b-cyclodextrin, High Performance
dorzolamide with chiral reagent (S)-(-)-a-methylbenzyl isocyanate prior to separation on a non-chiral silica phase
Although the development of enantiomerical y
(2). Elimination of this derivatization step would both
pure chiral drugs has proven extremely beneficial to
conserve time and decrease expenses associated with the
the treatment of various ailments, manufacturers
synthesis and analysis of dorzolamide.
face the obstacle of chiral separation.
In an effort to simplify the chiral separation of dorzol-
R,S-hydroxypropyl ether) (25 cm x 4.6 mm I.D., 5 µm particle
amide from its undesired enantiomer without prior deriva-
size). Mobile phases encompassing reversed-phase (70:30,
/ 814-359-3441 technical service: 800-359-3041
tization, a recent study employed Astec CHIROBIOTIC™
20 mM ammonium acetate, pH 4.0:acetonitrile), and polar
and CYCLOBOND™ columns in a chiral screen of the two
ionic (100:0.1:0.1, methanol:acetic acid:triethylamine)
dorzolamide enantiomers.
chromatographic modes of operation were applied to the
The Astec CHIROBIOTIC phases consist of macrocyclic
CHIROBIOTIC phases. The CYCLOBOND phases were
glycopeptides linked covalently to a silica surface by five
screened in the aforementioned reversed-phase mobile
covalent bonds. These phases possess broad selectivity and
phase as wel as a mobile phase referred to as polar organic
can differentiate between smal variability in chemical
mode (95:5:0.3:0.2, acetonitrile:methanol:acetic acid:
structure, making them valuable in the separation of a wide
triethylamine). Screening was executed on the Waters 2690
array of chiral molecules. Unlike most other chiral phases,
Separations Module utilizing a Waters 996 Photodiode Array
dering: 800-247-6628
(continued on page 8)
(continued from page 7)
TAG, the CHIROBIOTIC T showed no separation in both
Detector (UV at 220 and 254) and Waters Empower
reversed-phase and polar ionic modes of operation.
Acquisition Software (2002 Version).
Unlike the CHIROBIOTIC phases, the CYCLOBOND
Subsequent to initial screening, positive results were
columns in the screen showed no positive results. The
confirmed and optimized on the Agilent 1100 series HPLC
dorzolamide enantiomers were unretained on both
utilizing a VWD detector with a UV wavelength of 254 nm.
CYCLOBOND I 2000 and CYCLOBOND I 2000 HP-RSP and
Table 1 summarizes the results of the primary screen.
unresolved on the CYCLOBOND I 2000 DNP in reversed-
The summary table shows evidence of enantiomeric
phase. Polar organic mode produced no discernible peaks
selectivity observed on both the CHIROBIOTIC V2 (V2)
on the CYCLOBOND phases.
and CHIROBIOTIC TAG (TAG) in polar ionic mode and on
Because the V2, under polar ionic conditions, produced
the V2 in reversed-phase. Near baseline resolution was
the best resolution of the dorzolamide enantiomers in the
revealed on the V2 in polar ionic mode, while only partial
primary screen, it was selected for optimization purposes.
separation was observed on both the TAG in polar ionic
Consecutive attempts at optimization, including a decrease
mode and the V2 in reversed phase. Unlike the V2 and
in flow rate from 1.0 mL/min down to 0.25 mL/min and a
Table 1. Primary Screen Summary of Dorzolamide and its Enantiomer on Astec CHIROBIOTIC and CYCLOBOND phases
Mobile Phase
Liquid Chromatography
70:30, 20 mM NH OAc (pH 4.0):ACN
100:0.1:0.1, MeOH:HOAc:TEA
Partial Separation
70:30, 20 mM NH OAc (pH 4.0):ACN
Partial Separation
100:0.1:0.1, MeOH:HOAc:TEA
70:30, 20 mM NH OAc (pH 4.0):ACN
100:0.1:0.1, MeOH:HOAc:TEA
CYCLOBOND I 2000
70:30, 20 mM NH OAc (pH 4.0):ACN
CYCLOBOND I 2000
95:5:0.3:0.2, ACN:MeOH:HOAc:TEA
CYCLOBOND I 2000 HP-RSP
70:30, 20 mM NH OAc (pH 4.0):ACN
CYCLOBOND I 2000 HP-RSP
95:5:0.3:0.2, ACN:MeOH:HOAc:TEA
CYCLOBOND I 2000 DNP
70:30, 20 mM NH OAc (pH 4.0):ACN
CYCLOBOND I 2000 DNP
95:5:0.3:0.2, ACN:MeOH:HOAc:TEA
decrease in temperature from 25 °C down to 10 °C, succeeded in increasing resolution; however baseline
Figure 3. Analysis of Dorzolamide and its Enantiomer Using pH Adjusted Buffered Mobile Phase 5:95,
resolution was not quite achieved. The buffer salt
20 Ammonium Formate (pH 4.0):Methanol
ammonium formate was employed to sharpen the peaks
column: CHIROBIOTIC V2, 25 cm x 4.6 mm I.D., 5 µm particles
and enhance resolution. As seen in Figure 2, when
mobile phase: 5:95, 20 mM ammonium formate (pH 4.0):methanol
coupled with the mobile phase 0.05 w% ammonium
flow rate: 1.0 mL/min
formate in methanol, the aforementioned temperature
det.: UV at 254 nm
and flow rate changes produced near baseline resolution.
sample: 1.0 mg/mL in methanol
Ultimately, Figure 3 depicts that slight modification of the
2. [(4R, 6R)-4-(Ethylamino-5,6-dihydro-6-methyl-
ammonium formate mobile phase to include the addition
4H-thieno[2,3-b]thiopyran-2-sulfonamide 7,7-dioxide,
monohydrochloride)]
of water and pH adjustment (5:95, 20 mM ammonium
formate, pH 4.0:methanol) gave baseline resolution of the dorzolamide enantiomers with the original temperature
and flow rate (25 °C, 1.0 mL/min).
Figure 2. Analysis of Dorzolamide and its Enantiomer Using Buffered Mobile Phase 0.05 w% Ammonium Formate in Methanol on the CHIROBIOTIC V2
Liquid Chromatography
column: CHIROBIOTIC V2, 25 cm x 4.6 mm I.D., 5 µm particles
mobile phase: 0.05 w% ammonium formate in methanol
flow rate: 0.25 mL/min
efficiency. For this reason, procedures involving chiral
det.: UV at 254 nm
separation without prior derivatization have become much
sample: 1.0 mg/mL in methanol
more attractive to the scientific community than lengthy
and dated derivatization procedures.
2. [(4R, 6R)-4-(Ethylamino-5,6-dihydro-6-methyl-
4H-thieno[2,3-b]thiopyran-2-sulfonamide 7,7-dioxide,
monohydrochloride)]
1. J. Borras et. al. Bioorg. Med. Chem. 1999, 7, 2397-2406.
(US and Canada only)
2. United States Pharmacopeia, 29th rev,; United States Pharmacopeial Convention:
Washington, DC. 2005; pp 756-757.
3. CHIROBIOTIC Handbook, 5th ed.; T406120, JEV, Supelco, 595 North Harrison Road,
Bel efonte, PA 16823.
4. CYCLOBOND Handbook, 7th ed.; T406119, JEU, Supelco, 595 North Harrison Road,
Bel efonte, PA 16823.
+ Featured Products
Description
Of the six columns screened, the best resolution of the
Astec CHIROBIOTIC V2 Chiral HPLC Column
dorzolamide chiral enantiomers, conclusively, was observed
25 cm × 4.6 mm I.D., 5 µm particles
on the V2 stationary phase under polar ionic mode condi-tions. The vancomycin phase contains two ionic sites, making
! Related Information
it especial y good for the separation of both acidic and basic
For more information on custom chiral screening, method
molecules. Decreasing the pH to 4.0 enhanced the ionic
development, or chiral purification, please visit our website at
interactions between the secondary nitrogen attached to one
sigma-aldrich.com/astec, or contact Supelco Technical Service
chiral center of the analyte and the carboxylic acid groups of
at 800-��9-�0�1 (US and Canada only), 81�-��9-�0�1, or email
/ 814-359-3441 technical service: 800-359-3041
the V2 stationary phase. Conformational differences cause
one enantiomer to have a slightly stronger affinity to the
stationary phase than the other, thus, enhancing chiral
Did you know.?
separation of the dorzolamide enantiomers.
Supelco now offers Chiral Screening Services to assist
Like the aforementioned separation of the dorzolamide
customers in analytical method development and purifica-
enantiomers, chiral separations that may be achieved
tion. The screening service consists of an initial screen of
without extra derivatization steps conserve considerable
Astec chiral columns, method optimization, and purification
amounts of time. Since decreasing time ultimately conserves
of enantiomers. Enantiomers are identified as (+) or (-)
money, modern industries constantly focus efforts on
using the Chiralyser optical rotation detection system.
dering: 800-247-6628
seeking abbreviated procedures aimed at increasing their
Rapid, Sensitive, General-Purpose Cleaning Validation
Using Ascentis® Express HPLC Columns
related analytes comes at the expense of run time and is
The fol owing was generated by an outside source using
not needed in cleaning validation.
Sigma-Aldrich products. Technical content provided by:
This work was undertaken to investigate the use of
S. Bannister, M. Talbott, F. Hanciles
rapid gradients using recently introduced FCP columns on
Xcelience LLC, Tampa, FL
conventional instrumentation in the development of
general-purpose methods for cleaning validation. The
Verification of the removal of drug residue from multi-
benefits include high sensitivity and reductions in the time
product manufacturing equipment is required by GMP
needed to set up and run the method.
regulations and the suitability of applied analytical
Resolution, limits of detection and quantitation, and run
methods is judged with a combination of sensitivity,
time in HPLC analyses are improved by reducing the width
selectivity, and because the release of equipment is
of eluted bands. Contributions to bandwidth include both
Liquid Chromatography
dependent - speed. The FDA does not set quantitative
column (particle size, packing structure and resistance to
acceptance specifications, but the commonly used limit is
mass transfer in the stationary and mobile phases) and
based on not more than 0.1% of a dose carried over into
extracolumn volumes (injection, unswept and tubing).
a single dose of the next product. Translation of this into
Columns packed with 5 µm ful y porous particles have
an analytical limit combines the total product contact
been the standard for conventional HPLC for twenty-five
area, the mass (or volume) of product contacting the
years. Smal er-particle packings (3 µm) have been
surface, the mass (or volume) of each dose unit, the
available almost as long and offer higher efficiency (lower
sampled area, the rinse volume and the fraction of the
band dispersion) on conventional instrumentation, but
rinse sample used for analysis. The requisite limits are
require higher pumping pressures due to lower bed
commonly measured in ng/mL of injected sample.
permeability. Efficiency can be further increased by the
The ubiquity of HPLC in drug analysis makes it an
use of particles smal er than 3 µm but only with the use of
attractive choice for cleaning validation. Methods
instrumentation optimized with respect to both pressure
qualified for cleaning validation are often adaptations of
and extra-column effects.
drug-substance methods. The original methods are
Supelco has recently introduced reverse-phase packings
capable of determining the drug and its related impurities,
based on 2.7 µm silica particles in which a 0.5 µm layer of
but the ability to simultaneously measure multiple closely
90-Å porous silica has been deposited onto a 1.7 µm solid
Figure 1. Fused-Core Structure of Ascentis Express Compared to Total y Porous Particles
Ascentis Express Particle
Totally Porous Particle
spherical core (Figure 1). Advantages of columns packed
The high resolving power of gradient elution in the
with these particles include high efficiency, lower
analysis of closely related substances is the result of the
backpressure due to a very narrow particle size distribu-
reduction of peak width as a band moves through the
tion, and smal er efficiency losses with increasing velocity
column. The back of the band is accelerated by the
due to improved mass-transfer kinetics in the shal ow
stronger solvent. A broad gradient will elute a wide range
porous layer. The narrow particle size distribution al ows
of substances and a steep gradient will elute them quickly.
the use of larger pore column frits, which combined with
the greater stability of the packed bed should produce longer column lifetimes in routine use.
To judge the utility of Ascentis Express columns in
cleaning validation, an Agilent 1100 component system
Figure 2. Acidic and Neutral Drug Panel
with standard components (including a 10 mm/13 µL flow cel ) was used to develop a short gradient separation using
column: Ascentis Express C18, 10 cm x 4.6 mm I.D. (53827-U)
mobile phase A: water with 0.1% phosphoric acid
Ascentis Express C18, 10 cm x 4.6 mm for each of two
mobile phase B: acetonitrile with 0.1% phosphoric acid
panels: eight acidic or neutral drugs (AN) and six basic drugs
flow rate: 1.76 mL/min
1. Hydrochlorothiazide (9)
(B). For each separation, the flow rate was 1.76 mL/min,
det.: UV at 215 nm
2. Chlorthalidone (2)
3. Prednisolone (2)
detection was at 215 nm, and 100 µL injections were made
gradient: Min %A %B
4. Pravastatin (4)
5. Carbamazepine (2)
of aqueous solutions representing the final equipment
6. Diclofenac (14)
rinse. The separations are shown in Figures 2 & 3. Limits of
Liquid Chromatography
7. Ibuprofen (15)
8. Progesterone (2)
detection (ng/mL) are listed next to each analyte in Figures
These separations demonstrate the capabilities of
Ascentis Express columns on conventional, robust, instru-
mentation in rapid analyses of multiple drugs at low ppb levels suitable for development as methods for cleaning validations in multiproduct manufacturing facilities.
(US and Canada only)
+ Featured Products
Ascentis
Ascentis
Express C18
Express C8
Ascentis Express Columns
Figure 3. Basic Drug Panel
column: Ascentis Express C18, 10 cm x 4.6 mm I.D. (53827-U)
mobile phase A: water with 0.05M potassium phosphate and 0.1% TEA
and 0.6% OSA-Na at pH = 2.9
mobile phase B: acetonitrile
flow rate: 1.76 mL/min
2. Dipyridamole (29)
det.: UV at 215 nm
3. Propranolol (12)
4. Haloperidol (8)
gradient: Min %A %B
5. Amlodipine (29)
6. Fluoxetine (3)
! Related Information
/ 814-359-3441 technical service: 800-359-3041
For more information on Ascentis Express columns, request
T407044 (JHD) or visit sigma-aldrich.com/express
dering: 800-247-6628 or
Ascentis® Express HILIC HPLC Columns
A breakthrough in HPLC
Analysis of Polar Molecules on Ascentis Express HILIC and C18
columns: Ascentis Express HILIC, 10 cm x 2.1 mm I.D., 2.7 µm particles (53939-U)
column performance.
Ascentis Express C18, 10 cm x 2.1 mm I.D., 2.7 µm particles (53823-U)
mobile phase: 10:90; 100 mM ammonium formate, pH 3.0 with concentrated formic acid:acetonitrile
flow rate: 0.4 mL/min
Key Benefits of HILIC
1. Acenaphthene, 80 µg/mL in mobile phase
l Retention of highly polar
det.: UV at 254 nm
2. Adenosine, 35 µg/mL in mobile phase
Liquid Chromatography
analytes like metabolites
injection volume: 1 µL
3. Cytosine, 75 µg/mL in mobile phase
l Increased MS sensitivity
l Orthogonal selectivity to C18
Ascentis Express HILIC
Ascentis Express C18
Author Visits
Supelco Booth
at PITTCON
Professor Eugene F. Barry
(University of Massachusetts at Lowel ), co-author of Columns for Gas Chromatography: Performance and Selection, stopped by the Supelco booth at PITTCON. This 2007 Wiley book, ISBN 978-0-471-74043-8, should prove to be a great educational tool for those new to GC as well as an invaluable resource for those experienced in GC.
Dr. Eugene Barry with Product Manager Mike Buchanan (left) and
V.P. of Research & Development Mark Robil ard (right) at PITTCON® 2008.
LC-MS Mobile Phase Additives - Tips & Tricks
Shyam Verma
pH provided by the salt that permits both positive and
negative ion mode detection are issues of concern (6).
LC-MS is becoming a routine analytical tool in
Sodium Adduct Formation
research and industrial laboratories. The demand on sensitivity, specificity and speed of analysis requires use
Formation of alkali adduct is associated with decrease in
of high purity chemicals for sample preparation, mobile
sensitivity. When adduct formation tendency is strong,
phase and post-column additives. Additives are used to
addition of smal and defined amounts of sodium ions
suppress unwanted signals to selectively enhance the
(mostly pre-column) can help to obtain uniform and stable
signal of particular compounds in a mixture, for example,
molecular ions for detection in LC-MS (7). In addition to
glycosidic species in a mixture of peptides. Salts can
sensitivity, stability and perhaps specificity, of the molecular
suppress ionization in ESI sources.
ion are also important. The ability to form alkali adducts is useful for quantifying certain classes of molecules and for
Acids – The Most Common Additives
selectively enhancing the LC-MS signals. However, their true
Volatile, low molecular weight organic acids like formic
benefit, particularly that of sodium ion needs further studies.
and acetic acid are commonly used as additives in LC-MS
Sigma-Aldrich offers a wide range of high purity
mobile phase. Their primary advantage is that they improve
additives for LC-MS applications in addition to pure
ionization and resolution of a wide range of molecules (1).
CHROMASOLV solvents and ready-to-use blends. Our
Overcoming the TFA Suppression Effects
offering includes the most commonly used acids, bases, volatile salts and a sodium source (see Featured Products
The ionization-supressing effects of trifluoroacetic acid
below). All products are of high purity, usual y puriss p.a.,
(TFA) can be partly overcome by addition of other LC-MS
and are tested for LC-MS applications.
compatible organic acids, like formic or propionic acid (2).
Mobile phases for HPLC of proteins and peptides usual y
contain TFA to control the pH and improve peak shape and
1. Emmert J., Analytix, 2006, no.2, 8.
(US and Canada only)
2. Emmert J. and Rueck A, Analytix, 2006, No. 3, 16.
resolution. TFA enhances retention by ion pairing with the
3. "Eliminate TFA and Improve Sensitivity of Peptide Analysis by LC-MS" Supelco Ap-
peptide and improves peak shape by reducing silanol
plication Note 168 (T302168).
4. Apffel A., Fisher S., Goldberg G, Goodley P.C., Kuhlmann F.E., J. Chromatography,
interactions (3). However, TFA has adverse effects on MS
A, 1995, 712, 177-190.
detection. Its high surface tension prevents efficient spray
5. Wang G., Cole R.B., J. Am. Soc. Mass Spectrom., 1996, 7(10), 1050-1058.
formation and TFA ions in the gas phase ion-pair with the
6. Emmert J. and Leitner A, Analytix, 2006, No. 4, 9.
7. Emmert J. and Waelti T, Analytix, 2006, No. 5, 6.
peptide basic group suppressing their ionization and reducing the MS signal (4,5).
+ Featured Products
The Neutral Salts
Description
Pkg. Size Cat. No.
The neutral volatile salts, ammonium acetate and
Eluent Additives for LC-MS
ammonium formate, offer a much broader influence on
Trifluoroacetic acid, puriss* p.a.
analyte separation and ionization than do acids (6). Their
Trifluoroacetic acid, puriss p.a.
Formic acid, puriss p.a.
use, of course, is dictated by the particular LC-MS
Acetic acid, puriss p.a.
Propionic acid, puriss p.a.
Ammonium formate, puriss p.a.
Ammonium acetate, puriss p.a.
It may be necessary under certain circumstances to use
Sodium citrate tribasic dihydrate, puriss p.a.
Ammonium bicarbonate, puriss p.a.
more neutral conditions, either because the analytes are
Ammonium hydroxide solution 25%, puriss p.a.
/ 814-359-3441 technical service: 800-359-3041
Triethylamine, puriss p.a.
sensitive to acids or do not exhibit optimal resolution at low
LC-MS CHROMASOLV® Blends
pH. When acids are not suitable, volatile salts like ammo-
Water with 0.1% ammonium acetate
nium formate or acetate may be the additives of choice.
Methanol with 0.1% ammonium acetate
Acetonitrile with 0.1% ammonium acetate
However, limited solubility of the salt in organic solvents,
Acetonitrile with 0.1% formic acid
Selection of LC-MS Solvents and Blends
changing pH value during a gradient and the mildly acidic
2-Propanol CHROMASOLV LC-MS
Water with 0.1% formic acid and 0.01% TFA
Acetonitrile with 0.1% formic acid and 0.01% TFA
* "puriss" quality grade is defined as >98.5% assay, <0.1% ash, and specifica-
tion n + 0.001, d + 0.001 with no extraneous color and a homogeneous
For more information, request KCT on the attached postcard
appearance. "p.a." or pro analysis denotes a product with guaranteed trace impurity levels and/or suitability for the indicated analytical application.
dering: 800-247-6628
and visit our website: sigma-aldrich.com/chromasolv
Blood Fatty Acid Assessment
Kits for Sample Collection and
on the fatty acid content in the blood samples. Subse-
Derivatization for GC Analysis
quently, it helps the care providers in development and
Monitoring fatty acid profile in blood is important for
application of adequate preventive dietary strategies for
optimizing fat intake and managing dietary plans for
patients. Blood samples col ected as a small drop from the
We offer a complete line of products special y designed
fingertip can be analyzed to provide sufficient data for
for analysis of fatty acids. For more information, please
such an assessment (1).
Sigma-Aldrich offers kits for convenient col ection of
blood drops, their storage, shipment, and processing the
1. Marangoni F., Colombo C., Gal i C., Anal. Biochem, 2004, 326, 267-272.
samples for fatty acid analysis via gas chromatography.
A processing kit contains the derivatization reagent:
hydrogen chloride-methanol 1.25 M solution. This reagent
is used to derivatize the blood sample for an accurate and
Description
Pkg. Size
effective GC analysis. The treatment al ows efficient
Blood Col ection Kit
evaluation of the fatty acid status (3-n and 6-n polyun-
Includes blood col ection dipsticks, desiccant packs, foil-barrier
ziplock bags, 50 mL BHT solution and complete instructions.
saturated fatty acids).
Enough for 100 tests.
Derivatization Kit
These kits al ow efficient sample col ection and
Includes methanolic HCl solution (1.25M), saturated KCl solution,
distil ed water and working instruction sheet. Enough for 100 tests.
processing for quick col ection of analytical information
Analyses of Fatty Acids
A specially designed complete product line from Supelco
Solvents & Reagents l Capillary GC Columns l Chemical Standards
Vials, Syringes & Labware l Solid Phase Extraction l Technical Information
For al your FAME analytical needs visit sigma-aldrich.com/fame
For additional information, cal technical service at 800-359-3041 / 814-359-3041
l Products sorted by GC, HPLC,
Chiral and TLC techniques
l Reagents also listed by "Application"
l Vials, syringes and other useful items
for derivatization reactions
l Up-to-date application information
New! Derivatization Brochure
Listing over 400 Derivatization Reagents
To order your free copy either go to sigma-aldrich.com/derivatization, cal 800-359-3041 (US and Canada)
or 814-359-3041, email [email protected] or request KDI on the attached card.
(US and Canada only)
SPE Phases Catered to Your Compounds
SupelMIP™ SPE consists of
MIP Phases & Applications
molecularly imprinted polymers
for extraction of trace analytes
l Clenbuterol in urine
from complex matrixes.
l Triazines in water
l b-agonists and b-blockers in tissue,
urine and wastewater
l Chloramphenicol in milk, plasma,
l NNAL and TSNAs in urine
l Riboflavin in milk
/ 814-359-3441 technical service: 800-359-3041
Reduce Ion-suppression l Achieve Lower Detection Limits
Superior Selectivity l Minimal Method Development
To learn more about SupelMIP SPE, or to request a sample pack, please visit sigma-aldrich.com/supelmip
dering: 800-247-6628 or
Supelclean™ Sulfoxide SPE for the Extraction of
PCBs and other Aromatic Compounds in Oil
Researchers have found that dimethylsulfoxide (DMSO)
liquid-liquid extraction (LLE) is an effective means of
The fol owing was generated by an outside source using
separating PCBs (aromatic hydrocarbons) from aliphatic
Sigma-Aldrich products. Technical content provided by:
hydrocarbons (transformer oil) prior to GC-MS analysis (4).
Although effective, LLE is often tedious, time consuming
Toshiro Kaneko, Charles Mi, Michael Ye, An Trinh
and not greatly amenable to higher throughput applica-
1. AIST, National Metrology Institute of Japan, Tsukuba, 305-8563, Japan
tions. Based on the same extraction principles behind
2. Supelco, 595 N. Harrison Rd., Bel efonte, PA, 16823, USA
the DMSO LLE approach, we discuss the utility of a
sulfoxide-bonded SPE stationary phase towards the
extraction of PCBs from transformer oil. Using this new SPE phase, we are able to achieve quantitation levels
Polychlorobiphenyls (PCBs) were once heavily used as an
below 0.5 ppm (mg/kg).
indestructible coolant and insulating fluid in transformer and capacitor oils, and also as a stabilizing additive for a
Supelclean Sulfoxide SPE – How it Works
variety of products such as lubricating oils, hydraulic fluids,
Supelclean Sulfoxide SPE consists of a patent pending
flame retardants, paints and adhesives. However, because
silica-bonded sulfoxide (-SO) phase (Figure 1). The
of their high toxicity and resistance to environmental
technology was specifical y developed for the extraction
Solid Phase Extraction
degradation (persistent organic pol utant), production and
of polychlorinated biphenyls (PCBs) and related aromatic
distribution of PCBs have been banned since the 1970's.
compounds from transformer, waste and mineral oil.
Because of their stability and persistence in the
Under normal-phase conditions, PCB retention is facili-
environment, PCBs are still monitored routinely and
tated via interaction between the SPE phase's electrophilic
heavily regulated. A common sample matrix encountered
sulfur atom and the pi-electron cloud formed from
in PCB analyses is oil used in dielectric, hydraulic, and heat
aromatic rings inherent with PCBs.
transfer systems. There are numerous sample prep
The phase is first conditioned with acetone to remove
techniques currently available for PCB analysis in trans-
residual moisture from the phase. This is a critical step.
former oil ranging from sulfuric acid extraction (1) to SPE
Any residual moisture on the phase wil negatively affect
cleanup using silica gel, Florisil® (2), and/or Alumina (3).
resolution and selectivity during extraction. The sulfoxide
Most of these techniques are able to achieve lower limits
phase is then equilibrated with hexane and a diluted oil
of detection in the range of 5-10 ppm. However, as more
sample (1:1 v/v with hexane) is loaded onto the packed
transformers are decontaminated and waste sites undergo
tube. Increasing volumes of hexane are then applied.
treatment/remediation, lower limits of quantitation will be
As the hexane wash solvent passes through the car-
required to accurately determine PCB levels. This is a
tridge, PCBs are preferential y retained/retarded on the
chal enge because endogenous hydrocarbons found in
SPE phase whereas endogenous sample interferences (e.
transformer oil behave similarly to PCBs during sample
g., long chain hydrocarbons) are eluted from the phase
preparation. As a result, they are often co-extracted with
in the early fractions. Subsequent fractions are then
PCBs and can interfere with subsequent GC-MS analyses
eluted and col ected in later fractions for subsequent
and possibly damage the GC instrument.
GC-QMS or GC-HRMS analysis.
Figure 1. Supelclean Sulfoxide SPE
Separation of PCBs and Aliphatic Hydrocarbon
Figure 2. Supelclean Sulfoxide SPE Tube
Interferences Prior to GC Analysis
Glass, 6 g/20 mL (55252-U)
PCBs were extracted from oil and analyzed via GC-QMS
using procedure described in Table 2. Figure 3 describes
elution profile of PCBs vs. transformer oil (aliphatic hydrocarbons). As described in Figure 3, aliphatic hydro-carbons (oil interferences) are poorly retained on the
Table 1. Supelclean Sulfoxide SPE Extraction Method
sulfoxide SPE phase and elute off the packed bed within
for PCBs in Transformer Oil
the first 10-12 mL elution fraction. The retained chlorobi-
Supelclean Sulfoxide SPE Tube, Glass 6 g/20 mL (55252-U)
phenyl congeners (CBs) are more strongly retained and
1. Condition the SPE phase with 20 mL acetone
elute in the second 25 mL fraction.
(removes residual moisture from the phase).
2. Equilibrate the SPE phase with 40 mL of hexane.
Excellent Recovery and Lower Quantation
3. Load 0.4 mL diluted oil sample.
Levels Achieved
4. Elute aliphatic hydrocarbons (oil interferences)
Insulation oil was spiked with PCBs at the total level of
with 12 mL hexane
5. Elute PCBs with 25 mL hexane
3.7 mg/kg, extracted using Supelclean Sulfoxide SPE and
6. Col ect PCB fraction and concentrate under nitrogen
analyzed via GC-QMS using the procedure described in
for subsequent GC-QMS analysis (5)
Table 2. Recovery was determined against 13C-label ed PCB internal standards. An average Recovery ± RSD of 98.5 ±
Extraction and Analysis of PCBs in Transformer Oil
4.2 % was achieved for mono- to octa-chlorobiphenyls.
6 g of Supelclean Sulfoxide SPE was packed into a glass
Concentrations of nona- to deca-chlorobiphenyls in the
20 mL SPE cartridge (17 mm I.D. x 137 mm L) (Figure 2).
sample were lower than detection limits of the GC-QMS
Solid Phase Extraction
Commercial insulation oil (Japan Industrial Standard JIS
system. Mono- and di-chlorobiphenyls in other samples
C2320-1999, insulating oil, Class 1-2/4, paraffin oil) was
having lower PCB concentrations were not determined
spiked with a Kanechlor PCB mix at the total levels of 3.7
due to elution overlap with the tail-end of oil interfer-ences during sulfoxide SPE processing. This is of minor
(US and Canada only)
ppm (mg/kg) and diluted with hexane (1:1 v/v). The oil samples were extracted using the procedure described in
concern because the primary PCB homologues of concern
Table 1, and analyzed via GC-QMS using a 5% phenyl/ 95%
in transformer oil samples consist of the tri- to heptachlo-
methylpolysiloxane column and Agilent 5973N MSD (5).
(continued on page 18)
Figure 3. Elution Profile of Oil Interferences and PCB Congeners from Sulfoxide SPE
/ 814-359-3441 technical service: 800-359-3041
Elution Volume (mL, hexane)
dering: 800-247-6628 or
Table 2. Observed Concentrations of PCB Homologues of a PCB-fortified Insulation Oil Sample (n = 3).
di-
tri-
tetra- penta- hexa- hepta- octa- nona- deca-
CBs
CBs
CBs
CBs
CBs
CBs
CBs
CBs
CBs
CBs
(continued from page 17)
robiphenyls. Note that using the assay described in this
+ Featured Products
report, spike levels at the range of 0.045 - 0.9 mg/kg
Description
(ppm) for the individual PCBs were able to be determined.
Supelclean Sulfoxide SPE
Glass SPE Tube, 6 g/20 mL (17 mm I.D. x 137 mm L), pk 5
Polypropylene SPE Tube, 3 g/6 mL, pk 30
In this report, we demonstrated the utility of a new
silica-bonded sulfoxide SPE phase for the normal-phase
extraction of PCBs (and possible related aromatic com-
Related Products
Solid Phase Extraction
pounds) from difficult sample matrices such as transform-
Description
er oils. Because aliphatic hydrocarbons are often co-
Empty Glass SPE Tube (17 mm I.D. x 137 mm L) with PE frit,
extracted with PCBs using conventional SPE methods,
20 mL, with PE frit, luer cap, and screw-top cap, pk 5
Frit Insertion Tool for 20 mL Glass SPE tube
lower limits of detection (< 5 ppm) are often difficult to
Large Volume Reservoir (25 mL) for 6 mL SPE tubes,
achieve. Sulfoxide SPE al ows for the user to separate
Large Volume Reservoir (25 mL) for 6 mL SPE tubes,
aliphatic hydrocarbon interferences from PCBs prior to GC
SLB-5ms Capillary GC Columns
analysis using a generic/simple method. By removing this
15 m x 0.10 mm I.D., 0.10 µm
key matrix interference prior to analysis, detection limits of
20 m x 0.18 mm I.D., 0.36 µm
30 m x 0.25 mm I.D., 0.25 µm
less than 0.5 ppm are readily achieved.
30 m x 0.53 mm I.D., 0.50 µm
30 m x 0.53 mm I.D., 1.0 µm
SPB-608 Capillary GC Columns
30 m x 0.25 mm I.D., 0.25 µm
1. Copland et al. Environ. Sci. Technol. 1982, 16, 121-124
30 m x 0.53 mm I.D., 0.50 µm
2. Solid Phase Extraction of PCBs from Transformer Oil and Waste Oil and Analysis By
Equity-1701 Capillary GC Columns
Capil ary GC, Supelco Application Note 67,1998, T395067A
15 m x 0.10 mm I.D., 0.10 µm
3. Storr-Hansen et al. Chemosphere 1992, 24, 323-333
30 m x 0.25 mm I.D., 0.25 µm
4. Larsen et al. Chemosphere 1991, 23, 1077-1084
30 m x 0.53 mm I.D., 0.50 µm
30 m x 0.53 mm I.D., 1.0 µm
5. Numata et al. Anal. Chem. 2003, 75, 1450-1457
! Related Information
For more information, please request the Supelclean Sulfoxide Data/Instruction Sheet, T707009, and Analysis of PCBs in Transformer Oil with a Sulfoxide Bonded SPE Phase and GC-MS, T408040, on the attached post card. These publications are available in electronic form only. Be sure to include your email address on the request form.
For a complete listing of our PCB standards and related reagents, please visit sigma-aldrich.com/pcb-standards
DSD-DNPH Diffusive Sampler:
The Right Choice for Indoor Air Sampling of Carbonyls
Kristen L. Schultz
The DSD-DNPH is comprised of a porous
polyethylene tube, which acts as the diffusive
The DSD-DNPH diffusive sampler was first introduced in
membrane, to which is attached a small
Japan and was an integral device for monitoring carbonyls in
polypropylene syringe used for the elution of
indoor air, specifical y related to "sick building syndrome".
the analytes from the adsorbent (1).
Sick building syndrome results from exposure to building
Because the diffusive membrane is round, it
materials that emit VOC's such as formaldehyde. Symptoms
permits exposure from all sides, making it
of formaldehyde sickness include coughing, burning eyes,
unique compared to other diffusive samplers.
nose bleeds, and sinus infections. Common building materi-
Silica gel coated with 2,4-dinitrophenylhydra-
als known to emit formaldehyde are: adhesives, paints,
zine (DNPH) acts as the adsorbent and moves
plywood, particle board, and wal paper. Under hot, humid
from the diffusive end during sample col ection
conditions, formaldehyde lets off toxic fumes which are
to the syringe end for sample extraction, by
especial y harmful to children with young lungs. The
inverting the device. Aldehydes and ketones
National Institute of Health Sciences (NIHS) in Japan conduct-
diffuse through the membrane reacting with
ed a study using the DSD-DNPH diffusive sampler from April
DNPH to form stable derivatives. The DNPH-
2000 to March 2004. Now this unique device is available in
derivatives are then eluted with acetonitrile and
the US and offers the fol owing benefits:
analyzed by high performance liquid chroma-
tography (HPLC).
l Specified in OSHA 1007 Method for
Determination of Aldehydes
Comparison to Active Sampling
l Col ection and analysis of carbonyls without
(US and Canada only)
transfer of the adsorbent, which minimizes
Sampling Rate: Paral el measurements were made with
the risk of contamination
the DSD-DNPH cartridges (28221-U) and active sampling
l High-purity adsorbent provides col ection
of ppb levels of a wide range of carbonyls
cartridges using EPA Method IP-6A/TO-11, widely used as
in a convenient, easy-to-use configuration
a conventional method for active sampling of carbonyl
l Excel ent uptake rates-faster, stable for
compounds in environmental air. It is possible to obtain the
wind, temperature and humidity
sampling rate of the DSD-DNPH sampler from the compari-
l Stable blank data – important for LOQ
son with the known sampling rate of the active sampling.
Active sampling was conducted using high precision
l Versatile – use for indoor air, personal
sampling, and ambient air
apparatus (100 mL/min) composed of a mass flow control er
(continued on page 20)
Figure 1. Relationship Between Diffusive Sampling Method (DSD-DNPH) and Active Sampling Method on Formaldehyde, Acetaldehyde and 2-butanone (µg/m3); Sampling Period = 24 hours, N=188
/ 814-359-3441 technical service: 800-359-3041
y = 0.99x – 0.��
Diffusive Sampling (DSD-DNPH)
Diffusive Sampling (DSD-DNPH)
Diffusive Sampling (DSD-DNPH)
Relationship between 2�-hour and 7-day monitoring data. Data collected using a 2�–hour monitoring period are presented as mean values for 7 days and are plotted as ordinate. Seven-day monitoring data are plotted as abscissa.
dering: 800-247-6628 or
(continued from page 19)
Effect of Face Velocity
(model SEC-400 MARK3; STEC inc., Kyoto Japan), a wet gas
The effect of air velocity on the sampling rate was studied
meter (WS D-1A; Shinagawa Co., Tokyo), Supelco LpDNPH
by moving the DSD-DNPH device in indoor air. Twenty DSD-
S10L (505358) cartridges and Ozone scrubber (505285) to
samplers were fixed at intervals of 10 cm on a 2 meter rod
col ect carbonyl compounds. Samples were simultaneously
(Figure 2), and then rotated by an electric motor at 48 rpm
col ected in indoor and outdoor air for 24 hours. Col ected
for 24 hours. A sampler, which was fixed 1 meter from the
amounts were measured by HPLC.
central pivot of the rod corresponded to a face velocity of
When one specific sampling rate of carbonyl compound
5.0 m/s. Wind velocity demonstrated little influence on the
(e.g. formaldehyde) is determined from experimental data,
DSD-DNPH method. The DSD-DNPH sampler indicated little
the sampling rates of various other carbonyl compounds
susceptibility to the face velocity because the rounded tube
can be calculated from the ratio of diffusion coefficients,
structure is omni-directional.
because the sampling rate is proportional to the diffusion
The sampling amounts of formaldehyde and acetalde-
coefficient. The diffusion coefficients can be obtained from
hyde increased slightly depending on face velocity. RSD
Ful er's equation or Graham's Law.
values for formaldehyde and acetaldehyde concentrations
The values by diffusive sampling were in fair agreement
were 5.5% and 8.6% respectively with a face velocity from
with the values by active sampling. Figure 1 (pg 19) shows
0 to 5.0 m/s1.
the comparisons between the two methods concerning
formaldehyde, acetaldehyde and 2-butanone at 188 data points. The coefficients of determination for formaldehyde,
Is daily monitoring of indoor aldehydes necessary? The
acetaldehyde and 2-butanone were 0.970, 0.961 and 0.971
DSD-DNPH demonstrates that it is sufficient to measure
respectively. The slopes of the regression line were 0.95,
formaldehyde every 7 days. Continuous sampling was performed for 24 hours and 7 days in 24 homes and results
0.96 and 0.99 respectively. It is thought that the sampling rate calculated from Graham's law is reasonable for the
demonstrate that daily changes of formaldehyde during the
DSD-DNPH method because good agreement was found
measurement period for 7 days showed very large variation
between the results obtained from diffusive and active
and ranged from 16-170 µg/m3 (mean 86 µg/m3). Concen-
sampling. Table 1 demonstrates these findings.
trations of formaldehyde estimated by the seven-day sampling method were nearly equal to the mean value
The concentrations in Table 1 were mean values obtained
calculated from the 24-hour sampling period measured
from paral el measurements made with active sampling
over 7 days. This confirmed that the concentration of
compared to the diffusive sampling using the DSD-DNPH
formaldehyde could be precisely monitored by 7 day
device of compounds in indoor air of 188 houses through-
continuous sampling. (2)
out Japan from November 2001 to March 2002.
Table 1. Diffusion Coefficient (D), Sampling Rate (R), and Mean Concentration (C) of Carbonyl Compounds Calculated from Those Constant Values (n=188)
ACTIVE SAMPLING
DIFFUSIVE SAMPLING
Compound
(µgm-3)
(cm2 S-1) (mL min-1) (µg m-3)
(cm2 S-1) (mL min-1) (µg m-3)
m,p-Tolualdehyde
Figure 2. The Measurement of the Effect of Face Velocity
+ Featured Products
Description
Pkg. Size
DSD-DNPH Diffusive Sampling Device
+ Related Products
Description
Pkg. Size
Accessories
Perforated Holder
Female Luer Fitting to Tubing 5/32"
Filtration Column w/o frit, 6 mL
Plastic color-coded cap insert
Visiprep™-DL vacuum manifold
Figure 3. Relation Between Face Velocity and the
Visi-1™ Sample Processor
Amount Collected in the Sampler
TO11/IP-6A Aldehyde/Ketone-DNPH Mix
Formaldehyde-DNPH, 1 mL
Acetaldehyde-DNPH, 1 mL
Acetone-DNPH, 1 mL
Acrolein-DNPH, 1 mL
Propionaldehyde, 1 mL
Discovery® RP-Amide, 25 cm x 4.6 mm I.D., 5µm
! Related Information
For more information, request the DSD-DNPH Product Flyer
T408065 (KIX) and A High Efficiency Diffusive Sampler for the
Determination of Aldehydes and Ketones in Ambient and Indoor
(US and Canada only)
Face Velocity (m/s1)
Air, T400128 (DIC) on the attached postcard. These publications are available in electronic form only. Be sure to include your
email address on the request form.
Since implementing the use of the DSD-DNPH diffusive
sampler as one of the devices to effectively monitor indoor air quality in reference to carbonyl compounds in Japan; the Japanese government instituted regulation
changes. These changes lowered acceptable levels of carbonyl compounds for residential housing. In addition,
guidelines are provided for construction and use of related building materials (3).
References
1. S. Uchiyama and S Hasegawa, "A Reactive Sensitive Diffusion Sampler for the De-
termination of Aldehydes and Ketones in Ambient Air", Atmospheric Environment, 1999, 33, 1999-2005.
2. S. Uchiyama, S Aoyagi, ad Ando, Masanori, "Evaluation of a Diffusive Sampler for
Measurement of Carbonyl Compounds in Air", Atmospheric Environment, 2004, 38, 6319-6326.
/ 814-359-3441 technical service: 800-359-3041
3. Building Guidance Division, Housing Bureau: Ministry of Land, Infrastructure and
Transport The amended Building Standard law on Sick House Issues. Japan, July 1,2003
"Instructions Regarding the Building Standard Law on Sick-House issues"
"Overview of Countermeasures Regarding Sick House Issues under the Amended
Building Standard Law"
TRADEMARKS: Agilent - Agilent Technologies; Ascentis, CHIROBIOTIC, CHROMASOLV, CYCLOBOND, Discovery, Fluka, SP, Supelclean, Supelco, SupelMIP, Visi-1, Visiprep – Sigma-Al-
drich Biotechnology LP; AutoSystem, PerkinElmer - PerkinElmer Corp.; Carbowax - Union Carbide Chemicals & Plastics Technology Corp.; Florisil - US Silica Company; FocusLiner
- SGE International Pty Ltd.; Shimadzu - Shimadzu Corp.; Varian - Varian Associates Corp.; Waters - Waters Associates, Inc.
dering: 800-247-6628 or
New! EPA Method 8270 LCS Mixes with Improved Stability
Steve Cecil, Jim Walbridge, Vicki Yearick
l Both LCS Spiking solutions are special y
formulated to increase analyte stability including
anilines and benzidines, while still providing
Low spike recoveries for EPA Method 8270 are often
for a water-soluble matrix.
seen for aniline and benzidine compounds in the LCS*
l Our chemists have also addressed temperature,
mixes. Sigma-Aldrich laboratory studies of these low
light and oxygen stability issues.
recoveries have determined the cause to be interactions
l Temperature has been determined in the
of aniline and benzidines with other mix components
Supelco laboratory studies to be the single largest
contributor to shelf-life degradation. To ensure
and/or the sample matrix. In addition to reactive
the integrity of our spiking mixes, we ship them
stability, our studies show low recoveries are also seen
on dry ice. End-users should store the solutions in
the freezer at -15 °C or colder, as noted on the
for these compounds with increased exposure to oxygen,
spike mixes documentation.
light and temperature.
l Reactivity with oxygen is avoided by blanketing
Sigma-Aldrich chemists have designed two new
the mixes with an inert gas when preparing and
ampulizing the new mixes.
Supelco brand 8270 LCS Spike mix formulations to better
l UV light degradation is minimized through the
meet the spike-recovery requirements when performing
use of non-UV emitting lights during production
semi-volatile assays utilizing SW-846 methodologies.
and the use of amber glass for storage.
These new 78-component LCS spiking standards are
l Both spike mixes are offered in convenient 25 mL
engineered for the improved stability need in today's
volumes and include detailed lot specific mix
preparation and analytical testing results.
Description
EPA 8270 LCS Spike Mix
100 µg/mL each component in methanol:dichloromethane:benzene (90:9.4:0.6)
EPA HC 8270 LCS Spike Mix
200 µg/mL each component in methanol:dichloromethane:benzene (80:18.75:1.25)
Azobenzene
Benzoic acid
Dibutyl phthalate
Benzyl alcohol
Benzyl butyl phthalate
Diethyl phthalate
* LCS (Laboratory Control Sample/Blank Spike) Spikes are typical y required when sample matrix spike recoveries are determined to be outside the control limits. LCS standards are
prepared by spiking known concentrations of target analytes into clean sample matrixes. The spiked LCS mix is then subjected to the same sample preparation and analysis protocols as the sample. LCS spike recoveries are calculated and used to determine the analytical accuracy of the method.
Did you know.?
A safe and inexpensive method for
removing the top from a 2 mL glass ampul is to use a Sigma-Aldrich ampul breaker (Z122904). Simply insert the top of the ampul into the breaker and snap off the top. The ampul top is retained in the breaker for safe disposal.
Prescreened, In-Stock Chemicals:
B100 Biodiesel, Epigallocatechin, Butyl Mercaptan, Tetrabutyltin and More
What do these compounds have in common? They are exam-
ples of the thousands of prescreened, in stock chemicals available through the Sigma-Aldrich custom standards group. We can formulate, test, and package custom standard solutions to meet your needs for all your chromatographic applications. Our custom standard chemists will gladly discuss stability and solubility concerns with you, and make suggestions where needed to improve the quality of your purchase.
You can rely on Sigma-Aldrich custom standard solutions to include: l raw materials and solvents screened for identity and purity
l your choice of gravimetric, qualitative, and quantitative testing
l packaging choices from ampuls to bottles
l manufacturing processes fol owing the guidelines of ISO 9001/2000
l proper handling of light- and/or oxygen-sensitive chemicals
l documentation and Material Safety Data Sheets
l free technical support
l strict adherence to all shipping regulations
! Related Information
If you are interested in a customized standard. please email us at [email protected], or complete the on-line custom standards
(US and Canada only)
quote request form, available 24 hours a day – 7 days a week, at our website sigma-aldrich.com/standards
Are Your Vials & Inserts Compatible?
Ron Shawley
Table 1. Differing Specifications for US & European Vials & Inserts
The I.D. of 2 mL vials can vary by as much as
US Manufacturer
0.3 mm. As a result of this variation, your vial
insert may not properly fit, costing you time,
money and causing frustration.
Standard I.D. (mm) 5.04–5.06 4.58-4.68
5.18-5.22 4.95-5.00
Due to a lack of worldwide standards, this
5.94–6.08 5.67-5.77
6.18–6.21 5.98-6.00
problem is magnified when comparing products manufactured in the US and Europe.
Compatible Vials & Inserts from US and European Manufacturers
These differences are shown in Table 1.
Description
/ 814-359-3441 technical service: 800-359-3041
To ensure these parts fit together properly,
US Crimp Neck Vial, Large Opening, 2 mL, 12 x 32 mL
we recommend that your vials and inserts be
purchased from the same manufacturer.
US Glass inserts
Sigma-Aldrich offers a variety of compatible
0.2 mL, 6 mm x 29 mm with bottom spring
vial and insert products. For help with product
European Crimp Neck Vial, Large Opening, 1.5 mL, 11.6 x 32 mm
selection, email Sigma-Aldrich Technical Service
at [email protected] or visit our website
European Glass inserts
0.1 mL, 5.7 x 29 mm with bottom spring
dering: 800-247-6628 or
SIGMA-ALDRICH DE ARGENTINA S.A.
SIGMA-ALDRICH CHIMIE S.à.r.l.
SIGMA-ALDRICH JAPAN K.K.
SIGMA-ALDRICH RUS, LLC
Free Tel: 0810 888 7446
Free Tel: 0800 211 408
Tel: (+81) 3 5796 7300
Tel: +7 (495) 621 6037
Tel: (+54) 11 4556 1472
Free Fax: 0800 031 052
+7 (495) 621 5828
Fax: (+81) 3 5796 7315
Fax: (+54) 11 4552 1698
Fax: +7 (495) 621 5923
Tel: (+33) 474 82 28 00 Fax: (+33) 474 95 68 08
SIGMA-ALDRICH KOREA
SIGMA-ALDRICH PTE. LTD.
SIGMA-ALDRICH PTY LTD.
Tel: (+65) 6779 1200
Free Tel: 1800 800 097
Free Tel: (+82) 80 023 7111
SIGMA-ALDRICH CHEMIE GmbH
Fax: (+65) 6779 1822
Free Fax: 1800 800 096
Free Tel: 0800 51 55 000
Free Fax: (+82) 80 023 8111
Tel: (+61) 2 9841 0555
Free Fax: 0800 64 90 000
Tel: (+82) 31 329 9000
South Africa
Fax: (+61) 2 9841 0500
Tel: (+49) 89 6513 0
Fax: (+82) 31 329 9090
Fax: (+49) 89 6513 1160
SOUTH AFRICA (PTY) LTD.
Free Tel: 0800 1100 75
SIGMA-ALDRICH HANDELS GmbH
Free Fax: 0800 1100 79
SIGMA-ALDRICH (M) SDN. BHD
Tel: (+43) 1 605 81 10
Tel: (+27) 11 979 1188
SIGMA-ALDRICH (O.M.) LTD.
Tel: (+60) 3 5635 3321
Fax: (+27) 11 979 1119
Fax: (+43) 1 605 81 20
Tel: (+30) 210 994 8010
Fax: (+60) 3 5635 4116
Fax: (+30) 210 994 3831
SIGMA-ALDRICH QUÍMICA, S.A.
Free Tel: 900 101 376
Free Tel: 0800 14747
SIGMA-ALDRICH QUÍMICA, S.A. de C.V.
SIGMA-ALDRICH Kft
Free Fax: 900 102 028
Free Fax: 0800 14745
Free Tel: 01 800 007 5300
Ingyenes zöld telefon: 06 80 355 355
Tel: (+34) 91 661 99 77
Tel: (+32) 3 899 13 01
Free Fax: 01 800 712 9920
Fax: (+34) 91 661 96 42
Ingyenes zöld fax: 06 80 344 344
Fax: (+32) 3 899 13 11
Tel: (+36) 1 235 9055
Tel: 52 722 276 1600
Fax: (+36) 1 235 9050
Fax: 52 722 276 1601
SIGMA-ALDRICH SWEDEN AB
SIGMA-ALDRICH BRASIL LTDA.
Tel: (+46) 8 742 4200
Fax: (+46) 8 742 4243
Free Tel: 0800 701 7425
SIGMA-ALDRICH CHEMICALS
SIGMA-ALDRICH CHEMIE BV
Tel: (+55) 11 3732 3100
Free Tel: 0800 022 9088
Fax: (+55) 11 5522 9895
Free Fax: 0800 022 9089
SIGMA-ALDRICH CHEMIE GmbH
Bangalore: (+91) 80 6621 9600
Free Tel: 0800 80 00 80
Tel: (+31) 78 620 5411
New Delhi: (+91) 11 4165 4255
Free Fax: 0800 80 00 81
SIGMA-ALDRICH CANADA LTD.
Fax: (+31) 78 620 5421
Tel: (+41) 81 755 2828
Mumbai: (+91) 22 2570 2364
Free Tel: 1800 565 1400
Fax: (+41) 81 755 2815
Free Fax: 1800 265 3858
Hyderabad: (+91) 40 4015 5488
New Zealand
Tel: (+1) 905 829 9500
SIGMA-ALDRICH NEW ZEALAND LTD.
Fax: (+1) 905 829 9292
Bangalore: (+91) 80 6621 9650
Free Tel: 0800 936 666
SIGMA-ALDRICH COMPANY LTD.
New Delhi: (+91) 11 4165 4266
Free Tel: 0800 717 181
Free Fax: 0800 937 777
Free Fax: 0800 378 785
Mumbai: (+91) 22 2579 7589
Tel: (+61) 2 9841 0555
Tel: (+44) 1747 833 000
SIGMA-ALDRICH (SHANGHAI)
Hyderabad: (+91) 40 4015 5466
Fax: (+61) 2 9841 0500
Fax: (+44) 1747 833 313
TRADING CO. LTD.
SAFC (UK) Free Tel: 01202 712305
Free Tel: 800 819 3336
Tel: (+86) 21 6141 5566
SIGMA-ALDRICH IRELAND LTD.
Fax: (+86) 21 6141 5567
Free Tel: 1800 200 888
SIGMA-ALDRICH NORWAY AS
Free Fax: 1800 600 222
Tel: (+47) 23 17 60 60
Tel: (+353) 1 404 1900
Fax: (+47) 23 17 60 50
St. Louis, Missouri 63178
SIGMA-ALDRICH spol. s r. o.
Fax: (+353) 1 404 1910
Toll-Free: 800 325 3010
Tel: (+420) 246 003 200
Toll-Free Fax: 800 325 5052 Call Collect: (+1) 314 771 5750
Fax: (+420) 246 003 291
SIGMA-ALDRICH Sp. z o.o.
SIGMA-ALDRICH ISRAEL LTD.
Tel: (+1) 314 771 5765
Tel: (+48) 61 829 01 00
Fax: (+1) 314 771 5757
Free Tel: 1 800 70 2222
Fax: (+48) 61 829 01 20
SIGMA-ALDRICH DENMARK A/S
Tel: (+972) 8 948 4100
Internet
Tel: (+45) 43 56 59 10
Fax: (+972) 8 948 4200
Fax: (+45) 43 56 59 05
SIGMA-ALDRICH QUÍMICA, S.A.
SIGMA-ALDRICH S.r.l.
Free Tel: 800 202 180
SIGMA-ALDRICH FINLAND OY
Numero Verde: 800 827018
Free Fax: 800 202 178
Tel: (+358) 9 350 9250
Tel: (+39) 02 3341 7310
Tel: (+351) 21 924 2555
Fax: (+358) 9 350 92555
Fax: (+39) 02 3801 0737
Fax: (+351) 21 924 2610
Printed on Recycled Paper —A Worldwide Operation with a Global Commitment
Order/Customer Service (800) 325-3010 • Fax (800) 325-5052
3050 Spruce St., St. Louis, MO 63103
Technical Service (800) 325-5832 • sigma-aldrich.com/techservice
Development/Bulk Manufacturing Inquiries
2008 Sigma-Aldrich Co. Al rights reserved. SIGMA, SAFC,
, SIGMA-ALDRICH, ALDRICH, FLUKA, and SUPELCO, are trademarks belonging to Sigma-Aldrich Co.
and its affiliate Sigma-Aldrich Biotechnology, L.P. Sigma brand products are sold through Sigma-Aldrich, Inc. Sigma-Aldrich, Inc. warrants that its products conform to the information contained in this and other Sigma-Aldrich publications. Purchaser must determine the suitability of the product(s) for their particular use. Additional terms and conditions may apply. Please see reverse side of the invoice or packing slip.
Accelerating Customers' Success through Leadership in Life Science, High Technology and Service
Source: http://www.chemshow.cn/UploadFile/datum/1004/sigmaaldrich_2008922112417804368.pdf
PROGETTO UNIVA 2013 Journal Club Pietro Gareri, MD, PhD Geriatra ASP Catanzaro Lamezia Terme 3 Luglio 2013 Drug-induced parkinsonism (DIP) was recognized in the early 1950s as a commoncomplication of antipsychotic therapy; initially considered to be present in 4 - 40%of patients treated with the first neuroleptics
The National Defense Council Foundation ARTEMISININ NEW HOPE FOR MALARIA VICTIMS THE SCOURGE OF MALARIA Malaria is one of the triumvirate of diseases that has devastated the developing world. Along with AIDS and Tuberculosis, it has reached pandemic proportions in Asia and Africa with some 120 million clinical cases reported annually. Although the vast majority








