Levitra enthält Vardenafil, das eine kürzere Wirkdauer als Tadalafil hat, dafür aber schnell einsetzt. Männer, die diskret bestellen möchten, suchen häufig nach levitra kaufen ohne rezept. Dabei spielt die rechtliche Lage in der Schweiz eine wichtige Rolle.
An in vitro ischemic penumbral mimic perfusate increases nadph oxidase-mediated superoxide production in cultured hippocampal neurons
An in vitro ischemic penumbral mimic perfusate increasesNADPH oxidase-mediated superoxide production in culturedhippocampal neurons
Matthew E. PameSameh S. Qingbo , J. Cameron ,Xiang Q. , Laura L. , Gabriel G.
aDepartment of Pediatrics, Division of Respiratory Medicine, University of California San Diego, La Jolla, CA 92093, USAbDepartment of Anesthesiology, University of California San Diego, La Jolla, CA 92093, USAcSchool of Medicine, Department of Geriatric Medicine, University of California San Diego, La Jolla, CA 92093, USAdDepartment of Neuroscience, University of California San Diego, La Jolla, CA 92093, USAeThe Rady Children's Hospital-San Diego, San Diego, CA 92123, USA
The currently accepted scheme for reactive oxygen species production during ischemia/
Accepted 1 March 2012
reperfusion injury is characterized by a deleterious mitochondria-derived burst of radical
Available online 9 March 2012
generation during reperfusion; however, recent examination of the penumbra suggests acentral role for NADPH-oxidase (Nox)-mediated radical generation during the ischemic pe-
riod. Therefore, we utilized a novel in vitro model of the penumbra to examine the free rad-
ical profile of ischemic murine hippocampal neurons using electron paramagnetic
Membrane permeability
resonance spectroscopy, and also the role of Nox in this generation and in cell fate. We re-
Two-photon microscopy
port that free radical production increased 75% at 2 h of ischemia, and this increase was
abolished by: (1) scavenging of extracellular free radicals with superoxide dismutase(SOD), (2) a general anion channel antagonist, or (3) the Nox inhibitor apocynin. Similarly,at 24 h of ischemia, [ATP] decreased >95% and vital dye uptake increased 6-fold relative tocontrols; whereas apocynin, the Cl− channel antagonist 5-nitro-2-(3-phenylpropylamino)-benzoate (NPPB), or the free radical scavenger N-acetyl cysteine (NAC) each provided mod-erate neuroprotection, ameliorating 13–32% of [ATP]-depletion and 19–56% of vital dye up-take at 24 h. Our results support a cytotoxic role for Nox-mediated free radical productionfrom penumbral neurons during the ischemic period.
Published by Elsevier B.V.
⁎ Corresponding author at: Department of Pediatrics, University of California San Diego, 9500 Gilman Dr., La Jolla, CA 92093–0735, USA.
Fax: +1 858 534 6972.
E-mail address: (M.E. Pamenter).
Abbreviations: AMPAR, 2-amino-3-(5-methyl-3-oxo-1,2-oxazol-4-yl)propanoic acid receptor; Apo, apocynin; APV, (2R)-amino-5-phos-
phonovaleric acid; BBB, blood brain barrier; CNQX, 6-cyano-7-nitroquinioxaline-2,3-dione; CrOx, chromium oxalate; DIDS, 4,4-diisothio-cyanatostilbenedisulphonic acid; EPR, electron paramagnetic resonance; IS, ischemic solution; MCAO, middle cerebral artery occlusion;NAC, N-acetyl cysteine; NMDAR, N-methyl-D-aspartic acid receptor; Nox, nicotinamide adenine dinucleotide phosphate H oxidase; NPPB,5-nitro-1-(3-phenylpropylamino)benzoic acid; O•2, superoxide anion; PI, propidium iodide; ROS, reactive oxygen species; SOD, superoxidedismutase; Z-VAD-FMK, carbobenzoxy-valyl-alanyl-aspartyl-[O-methyl]-fluoromethylketone
1 These authors contributed equally to this work.
0006-8993/$ – see front matter. Published by Elsevier B.V.
doi:
that mimic the infarct core, where blood flow is occluded andcells are anoxic ). Conversely, blood flow to
Reactive oxygen species (ROS) are important second messen-
the penumbral region is hypoperfused, but not blocked; and
gers that regulate a myriad of intra- and extracellular signaling
thus penumbral cells are hypoxic, and likely exhibit a different
pathways In ischemic stroke pathol-
free radical profile (Due to technical limita-
ogy neuronal ROS production spikes during reperfusion, which
tions, ROS generation from penumbral neurons cannot be
damages DNA locally, and activates deleterious immune and
measured in vivo (therefore, we used an
cell death pathways in surrounding tissue
electron paramagnetic resonance (EPR) spin-trapping assay
). In addition, ROS
to determine the effect(s) of a penumbral mimic (ischemic so-
generated during ischemia are a key contributor to the perme-
lution: IS )) on ROS production in vitro. In addi-
abilization of the blood brain barrier (BBB), which leads to
tion, we tested the role of anion channels in ischemic ROS
edema and contributes to the formation and expansion of
efflux using 4,4-diisothiocyanatostilbenedisulphonic acid
the penumbra following stroke (). The penumbra
(DIDS); since it is widely used as a general antagonist of
is the hypoperfused region surrounding the ischemic core,
anion channels ), and because there is ev-
and the relatively slow propagation of cell death in the penum-
idence that DIDS provides cytoprotection against ischemic in-
bra makes this region an attractive target for clinical rescue,
sults via regulation of anion channels (
particularly as the majority of stroke-related morbidity and
Finally, we examined the cytoprotective effects of
mortality is attributable to progressive expansion of the ische-
limiting plasmalemmal ROS generation during ischemic insult
mic core into the penumbra (). The mechanism(s) of
by anion channel or Nox antagonism, or by scavenging ROS
cell death here are poorly understood, but are likely initiated
with N-acetyl cysteine (NAC).
by deleterious alterations of the local perfusate following therelease of cytoplasmic contents from ruptured core cells (Early BBB breakdown following ische-
mic stroke is a key factor in the development of the penumbrasince this event contributes significantly to edema and cell
Ischemia increases extracellular ROS accumulation
swelling, which in turn underlies cell rupture Therefore, ischemic ROS generation plays a pivotal role in
ROS generation in IS-challenged neurons increased 75% rel-
the initiation and spread of cell death via a variety of cellular
ative to controls (EPR intensity (a.u.): 744.0 ± 82.0 vs. 427.6
and intercellular interactions.
± 27.0, F(2,35) = 7.8, A and B), and computer simulations in-
Historically it has been suggested that the primary source
dicated that the observed signal was due to spin trapping of
of ROS generation is mitochondrial superoxide (O•
both hydroxyl and O2. To determine the relative contribution
). Another important free radical source is plasma
to the ischemic change in total radical production of intracel-
membrane-bound proteins such as the NADPH oxidase
lular ROS accumulation compared to extracellular ROS efflux,
(Nox), which is a principle enzyme for the production of O•2
we treated samples with chromium oxalate (CrOx, 1.25 μg/μl)
critical to intracellular signaling and cell death during ische-
or superoxide dismutase (SOD, 100 U/ml), which dissipate
mic stress, and which underlies ROS-mediated BBB break-
only the resonance signal of, or scavenge, extracellular ROS,
down following stroke (
respectively In control experiments, CrOx
). Indeed, neuronal Nox mRNA and protein expres-
or SOD decreased ROS generation 30–35%, indicating that at
sion are increased following middle-cerebral artery occlusion
rest, intracellular versus extracellular ROS accumulation oc-
(MCAO), whereas BBB and cellular permeability, infarct
curs at a 2:1 ratio (CrOx: 263.3 ± 44.4, F(2,26) = 4.7; SOD: 284.3
spread, and neuronal apoptosis are all reduced or abolished
± 12.6, F(2,23) = 4.18, ). Conversely, either compound entire-
by Nox inhibition with apocynin, or by knockout of Nox1,
ly abolished the ischemic increase in ROS generation (IS
Nox4, or the Nox family subunit gp91phox
+ CrOx: 273.3 ± 12.6, F(2,24) = 8.7, IS + SOD: 272.3 ± 35.7, F(2,22)
= 4.8), and total ROS generation when CrOx or SOD was used
to ablate the extracellular ROS signal was not different in IS
Regardless of the source of generation, intracellularly de-
relative to controls; indicating that the change in ROS genera-
rived ROS gain access to the extracellular space primarily via
tion during ischemia is attributable to extracellular accumula-
plasmalemmal anion channels and
tion of ROS.
since elevated extracellular ROS can induce cell injury anddeath, it is reasonable to assume that preventing ROS efflux
Anion channel-mediated efflux of Nox-derived
would limit the spread of cell death following insult. Indeed,
radicals underlies ischemic ROS production
numerous studies in a variety of pathological models havedemonstrated protective effects of anion channel inhibition
In controls, DIDS (400 μM) reduced ROS generation to the same
against ROS-mediated inflammation
degree as CrOx or SOD (290.0 ± 40.0, F(2,28) = 5.5, A and B),
). Therefore, regulation of ROS transmission
and DIDS in the presence of CrOx or SOD did not reduce ROS
via blockade of anion channels is an attractive target for clin-
generation beyond the effect of any of these compounds
ical intervention and may help limit the spread of cell death in
alone (DIDS + CrOx: 279.6 ± 14.7, F(2,26) = 4.8; DIDS + SOD: 253.0
the ischemic penumbra.
± 3.5, F(2,22) = 4.2,). Similarly, during ischemia, DIDS-treatment
Previous examinations of ROS generation in ischemic brain
reduced total radical generation to control levels (396.3 ± 26.7,
have predominantly utilized acute models of focal ischemia
F(2,31) = 7.9); however, generation remained greater than in
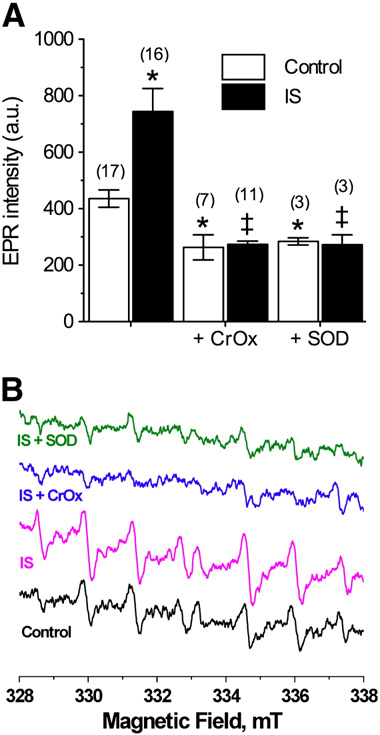
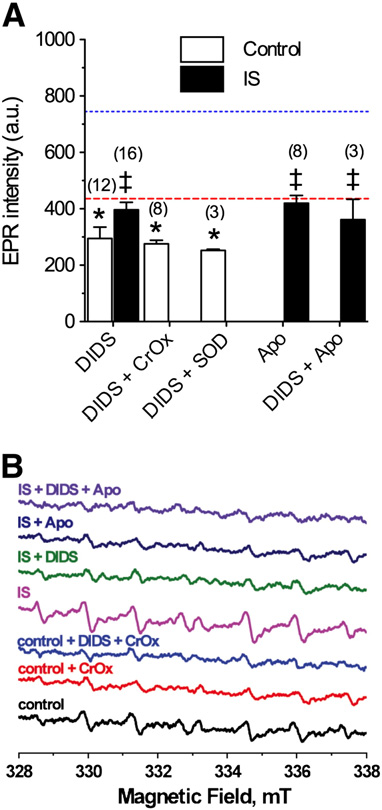
Fig. 1 – IS increases free radical production from neurons. (A)Summary of EPR-revealed changes in superoxide productionfrom neurons treated as indicated for 2 h. (B) Raw EPR traces
Fig. 2 – DIDS or Apo prevents IS-mediated increase of ROS
from (A). Parentheses ( ) indicate n values. Data are mean
generation. (A) Summary of EPR-revealed changes in superoxide
± SEM. Asterisks (*) indicate significant difference from
production from neurons treated as indicated for 2 h. (B) Raw
control; double daggers indicate significant difference from
EPR traces from (A). Parentheses ( ) indicate n values. Data are
IS-alone (p < 0.05).
mean±SEM. Dashed and dotted lines indicate normoxic andIS controls from A, respectively. Asterisks (*) indicatesignificant difference from control A); double daggers
normoxic DIDS-treated neurons. Therefore during ischemia,
indicate significant difference from IS-alone p<0.05).
and with anion channels blocked, ROS accumulate in the cy-tosol. Similarly, the general Nox inhibitor apocynin (Apo:10 μM) reduced the IS-mediated increase in ROS generationto control levels (420.0 ± 27.7, F(2,23) = 3.6, and B), while
Nox inhibition or ROS scavenging provide moderate
co-treatment of DIDS with Apo did not further reduce ROS
neuroprotection against ischemic insult
(362.0 ± 72.0).
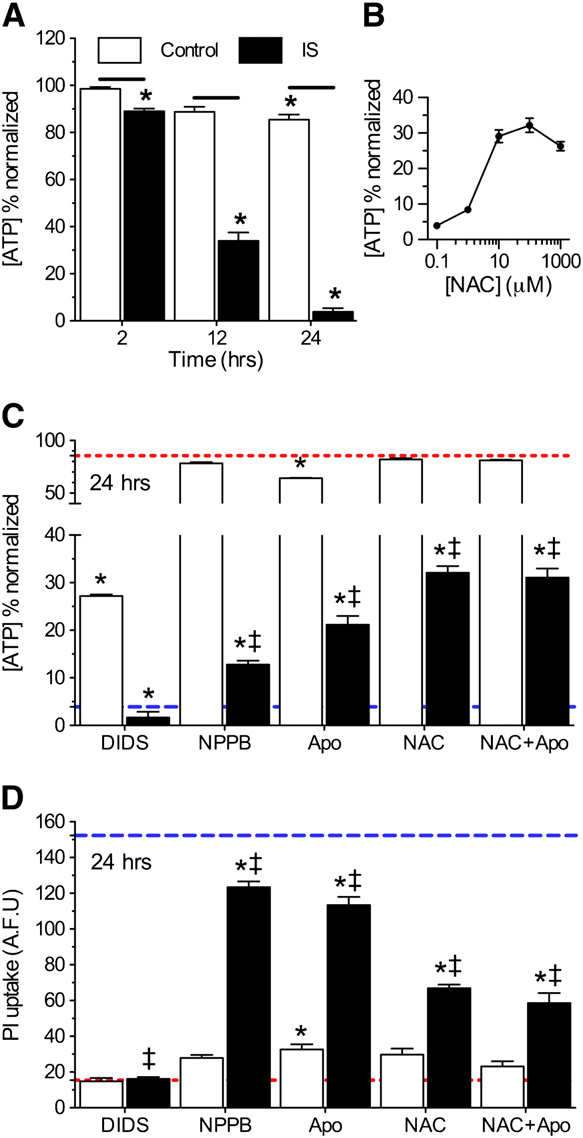
Next, we assessed the effects of IS ± DIDS ± Apo on neuronal vi-
DIDS does not permeate intact neurons
ability. Relative to experimental onset (t = 0 h), control cellsmaintained >85% of their [ATP] through 24 h; whereas [ATP]
DIDS blocks ROS transmission across multiple lipid mem-
dropped progressively to 89.2 ± 0.1, 34.0 ± 3.5, and 3.9 ± 2.0% fol-
lowing 2-, 12-, and 24-h IS-treatment, respectively (
therefore, to assess the penetration of DIDS into neurons,
n = 8–10 for each treatment). DIDS did not preserve [ATP] in
we employed 2-photon confocal microscopy and took advan-
IS-treated neurons at 24 h (n = 10 for each), likely due
tage of the natural fluorescence of DIDS molecules
to secondary intracellular actions of DIDS on cells with dam-
In control experiments DIDS did not permeate
aged membranes at this time-point (However, another
plasma membranes through 2 h (n = 4 for each),
general Cl− channel antagonist, 5-nitro-1-(3-phenylpropyla-
whereas in IS, DIDS fluorescence was observed in some
mino)benzoic acid (NPPB, 100 μM) increased [ATP] 3-fold rela-
cells. To determine whether this penetration was due to
tive to IS-alone and decreased IS-mediated PI uptake 18.8%
membrane degradation, we also measured propidium iodide
B and C and D, n = 16 for each). Similarly, Apo or the
(PI) uptake. At 2 h, 15% of neurons took up PI, and this up-
ROS scavenger NAC had minimal effects on [ATP] in control
take coincided with cellular DIDS fluorescence (
experiments, but in ischemia increased [ATP] 7- and 10-fold,
n = 10–20 for each). Therefore, the accumulation of DIDS in
respectively, and ameliorated 25.7 ± 3.2 and 56.1 ± 4.5% of IS-
some cells at 2 h is due to membrane degradation, and
mediated PI uptake relative to IS-alone –D, n = 3–10 for
DIDS-mediated inhibition of ROS production from intact
each [NAC]). When ischemic neurons were treated with both
cells at this time-point is likely due to extracellular actions
Apo and NAC simultaneously, their neuroprotective effects
were not additive.
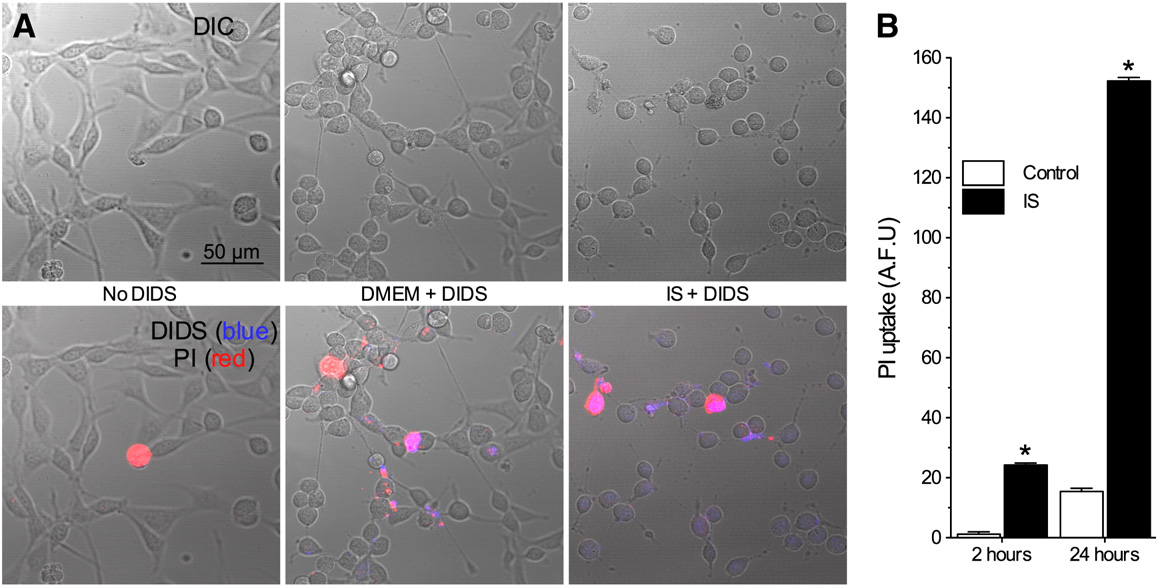
Fig. 3 – DIDS penetrates damaged, but not intact neuronal membranes. (A) Two-photon confocal DIC and Z-stack projection images(2 microns total) of neurons treated without DIDS, or with DIDS during normoxia or IS. Upper panel: DIC images. Lower panel:DIC images (grey) overlaid with PI (red) and DIDS (blue) fluorescent Z-stack projection images. Images are representative of 4separate experiments for each treatment. (B) Summary of PI uptake. Data are mean ± SEM from 10–20 replicates per treatment.
Asterisks (*) indicate significant difference from control (p < 0.05).
Ischemic solution induces mixed modes of cell death in
this increase was abolished by co-treatment with Z-VAD-FMK
B and C, n = 3).
Finally, since glutamate receptor activation and associated
In the infarct core, cell death proceeds primarily via necrosis
Ca2+ influx has been linked to ROS and reactive nitrogen spe-
due to excitotoxicity, mediated by over-activation of excitatory
cies production we examined the effect of
AMPAR and NMDAR inhibition on ROS production. In nor-
panoic acid and N-methyl-D-aspartic acid receptors (AMPARs,
moxia, application of CNQX + APV (50 μM each) increased
NMDARs) (Conversely, in the penum-
ROS generation 14% from 466.5 ± 34.2 to 545.0 ± 28.7 at 2 h,
bra, cell death is thought to occur via a mixture of cell death mo-
while during IS-perfusion, CNQX + APV similarly increased
dalities, including necrosis, apoptosis, and autophagy
ROS generation 23%, from 655.2 ± 18.2 to 854.2 ± 48.1 (a.u.,
In our 2-photon experi-
n = 4 for each).
ments, we observed moderate vital dye uptake at 2 h and B), consistent with the occurrence of rapid necrosis insome cells treated with this mimic solution. Therefore, we
next examined the role of necrotic cell death in the IS modelby antagonizing AMPARs and NMDARs with 6-cyano-7-nitro-
We demonstrate that deleterious ROS generation is increased
quinioxaline-2,3-dione (CNQX) and (2R)-amino-5-phosphono-
in hippocampal neurons treated with an in vitro ischemic pen-
valeric acid (APV), respectively; and also of apoptosis using the
umbral mimic perfusate, and that this increase is primarily
due to efflux of Nox-derived ROS via plasma membrane anion
channels. This conclusion is supported by our observations
In vital dye exclusion assays, none of these treatments
that anion channel or Nox antagonism, or ROS scavenging,
had a significant effect on cell viability during control ex-
each: (1) prevent IS-mediated increases in ROS generation,
periments, whereas during IS-treatment, higher concentra-
and (2) partially ameliorate IS-mediated neurotoxicity, in non-
tions of CNQX + APV (50 μM each) reduced PI uptake 20%,
additive manners. In neurons, Nox subunits are found at vari-
Z-VAD-FMK (5 or 50 μM) ameliorated approximately one-
ous lumen membranes, and following prolonged ischemia,
third of IS-mediated PI uptake at 24 h (n = 16–32 for
are translocated to plasma, mitochondrial, and endoplasmic
each). To confirm the occurrence of apoptosis in this model,
reticulum membranes ), and therefore contrib-
we next examined the expression of Lamin A, a commonly
ute to both cytosolic and extracellular ROS generation. Our re-
studied marker of apoptosis whose cleavage is indicative of cas-
sults suggest a predominant role for cytosolic Nox-derived
pase 6 activation ). In Western blot
ROS production in the penumbra since co-antagonism of
analysis of Lamin A expression, we observed that IS treatment
anion channels and Nox did not reduce EPR spectra beyond ei-
(6 h) increased Lamin A 15-fold relative to controls, and that
ther treatment alone, as would be expected if ROS were
ROS generation from penumbral neurons cannot presently
be measured in vivo because tracers do not reach the ischemictissue (however, our results are consistentwith ex vivo measurements from mouse brain followingMCAO, where Nox-mediated O•2 production was increased inthe penumbral region, but not in the core ).
Furthermore, our data are also consistent with measurementsfrom penumbral epithelial cells and arteries in brain in whichROS production increases markedly during ischemia via aNox-dependant mechanism Conversely, ROS production does not increase in ische-mic core arteries. These results from three penumbral tissuesfurther demonstrate that the profile of ROS generation in thepenumbral region is markedly different from the infarctcore, and in particular support a dominant contribution fromNox. Based on our present findings and these recent examina-tions, we conclude that Nox-mediated O•2 production is a keyregulator of deleterious ROS signaling in the penumbral re-gion, and that neuronal Nox contribute to this mechanism.
In the penumbra, oxidative phosphorylation persists due
to a steady, albeit restricted supply of oxygen. This persistentreduced level of oxygen availability likely partially preservescells' ability to generate ATP, and thus opposes excitotoxic de-polarization and early rapid necrotic cell death (). However, this temporary preservation of ATP pro-duction allows for the induction of apoptosis and autophagyduring prolonged insult ), as occurs in thepenumbra. As a result, a variety of cell death mechanisms ap-pear to be concomitantly activated in this model present report), and this ex-plains why cell viability was only partially preserved in our ex-periments by ROS scavenging, or by glutamate receptor orcaspase antagonism.
In conclusion, we have used the present gold standard for
free radical measurements (EPR), to record ROS productionfrom live neurons treated with a novel ischemic penumbralmimic perfusate for the first time. We conclude that: (1) neu-
Fig. 4 – Nox inhibition or ROS scavenging is moderately protective
ronal ROS production is increased during IS and this increase
against prolonged ischemic insult. (A) Summary of [ATP]
is manifested extracellularly, (2) this production is due to in-
changes. (B) Dose–response curve of [ATP] vs. [NAC] at 24 h.
creased Nox-derived superoxide production, (3) the efflux of
(C) Summary of [ATP] changes from neurons treated as
Nox-derived superoxide occurs via anion channels, (4) Nox in-
indicated for 24 h. Red and blue dashed lines represent
hibition provides mild protection against IS insult in neurons,
baseline and ischemic controls, respectively, from Fig. 4A. (D)
and (5) neuronal cell death is mediated by a variety of path-
Summary of PI uptake by neurons treated as indicated for 24 h.
ways during IS insult.
Red and blue dashed lines represent baseline and ischemiccontrols, respectively, from B. Data are mean ± SEM from8 to 10 replicates per treatment. Asterisks (*) indicate
Experimental Procedures
significant difference from control (Fig. 4A); double daggersindicate significant difference from IS (Fig. 4A; p < 0.05).
HT22 mouse hippocampal neurons (a generous gift from Dr.
Pam Maher, The Salk Institute, La Jolla, CA) were cultured
produced by plasma membrane-bound Nox. Therefore, unlike
in Dulbecco's Modified Eagle Medium (DMEM, ATCC) sup-
in anoxic infarct core cells where ROS production is low during
plemented with 10% bovine calf serum (Hyclone, Santa
ischemia and cytotoxicity is predominately mediated by a del-
Clara, CA) and 100 U/ml penicillin/streptomycin (Invitrogen,
eterious burst of ROS during reperfusion
Carlsbad, CA) and grown at 37 °C in a 5% CO2 incubator.
hypoperfused penumbral neurons maintain elevation of the
Cells were grown for 5–8 passages and split when they
generation, efflux, and extracellular accumulation of ROS,
reached 60–80% confluence. For experiments, cells were
which are known to contribute to the spread of cell injury and
seeded into 96-well microplates (Corning, Lowell, MA),
death in the penumbra ).
glass-bottom 35-mm culture dishes (MatTek, Ashland, MA),
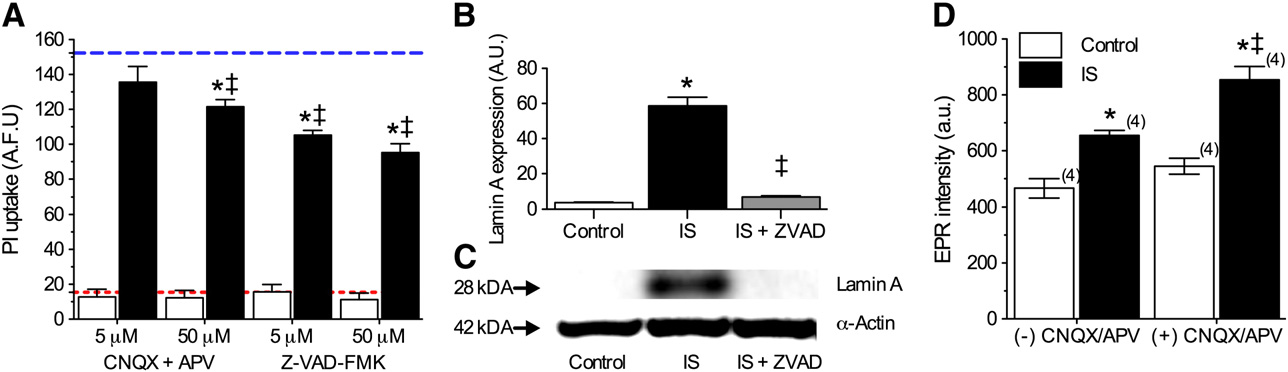
Fig. 5 – Multiple cell death pathways are activated in IS-treated neurons. (A) Summary of PI uptake by neurons treated as indicatedfor 24 h. Red and blue dashed lines represent baseline and ischemic controls, respectively, from B. Data are mean ± SEMfrom 16–32 replicates per treatment. (B) Summary of Lamin A expression in neurons treated as indicated for 6 h. Data arenormalized to expression of α-actin on the same blot. (C) Sample paired Western blot images of Lamin A α-actin expressionfrom (B). Images are representative of 3 separate experiments. (D) Summary of EPR-revealed changes in superoxide productionfrom neurons treated as indicated for 2 h. Parentheses ( ) indicate n values. Asterisks (*) indicate significant difference fromcontrol; double daggers indicate significant difference from IS (p < 0.05).
or poly-L-lysine coated custom designed plastic coverslips pre-
was designed to incorporate and mimic key changes previous-
cisely cut to the dimensions of the EPR tissue culture chamber
ly described in the literature from in vivo examinations of the
(Fisher, Pittsburgh, PA). Cells were allowed to grow to 80% con-
penumbral rim (the region of tissue immediately adjacent to
fluence before experimentation and samples were treated as
the infarct core, and thus most effected by local cell rupture),
specified in the experimental design section (below). To reduce
and the individual effects of each of these alterations on cell
shear stress, cells seeded into 96-well microplates were gently
viability have been carefully examined elsewhere
washed with a TECAN PW96/384 Washer (TECAN, San Jose,
CA) and then examined visually to ensure cells had not beenwashed away.
Determination of superoxide production in live neuronal
cultures by electron paramagnetic resonance (EPR) spectroscopy
Experimental design
Measurement of O•2 production in live neuronal cultures was
Samples were treated for 2, 6, 12 or 24 h (as indicated) in two
carried out by EPR spin trapping method using 70 mM of the
treatment groups: cell death-negative control (DMEM/F12
spin trap agent DEPMPO (5-(diethylphosphoryl)-5-methyl-1-
media (Invitrogen), pH 7.4, gassed with 21% O2, 5% CO2, bal-
pyrroline-N-oxide). DEPMPO readily permeates mouse heart,
ance N2), or an ischemic penumbral perfusate mimic (IS, in
liver, and brain tissues in vivo and is evenly distributed within
mM: K+ 64, Na+ 51, Cl− 77.5, Ca2+ 0.13, Mg2+ 1.5, glucose 3, glu-
15 min (); and DEPMPO-spin adducts diffuse
tamate 0.1, [315 mOsM, pH 6.5, 1.5% O2, 15% CO2, balance N2])
through lipid bilayers effectively (Samples
(). APV (5 or 50 μM), CNQX (5 or 50 μM),
were treated for 2 h before transferring the coverslip to the
CrOx (1.25 μg/μl), NAC (0.1–1000 μM), and SOD (100 U/ml) were
EPR tissue culture chamber containing the spin trap agent in
dissolved in water. SOD does not penetrate the cell membrane
40 μL of normoxic or IS gas-equilibrated media. The EPR cell
and therefore specifically scavenges extracellular superoxide
was then tightly covered to avoid media leakage and inserted
(Apo (10 mM), DIDS (400 μM),
in the EPR cavity of a MiniScope MS200 Benchtop spectrome-
NPPB (100 μM), and Z-VAD-FMK (5 or 50 μM) were dissolved
ter maintained at 37 °C. EPR spectra were recorded using the
in DMSO to a final bath [DMSO] < 0.01%, and all solutions
following spectral settings: microwave frequency, 9.45 GHz;
were made fresh daily. Chemicals were purchased from
microwave power, 5 mW; modulation amplitude, 0.2 mT;
Sigma unless otherwise indicated (Sigma-Aldrich, St. Louis,
modulation frequency, 100 kHz; sweep width, 15 mT centered
MO). It is important to note that atmospheric [O2] (21%) is
at 335 mT; scan rate, 0.75 mT s−1 and each spectrum was the
hyperoxic relative to in situ tissue pO2. Nonetheless, 21% [O2]
average of 10 scans.
is commonly used as "normoxic" control in in vitro experimen-
EPR traces were quantified by measuring the relative signal
tation due to convenience and cost-related complications,
amplitudes detected automatically using the Magnettech
and therefore we utilized 21% as our baseline control [O2] in
Analysis Software (Version 2.02; Berlin, Germany). The average
order to be consistent with the majority of in vitro experiments
amplitudes of the largest four peaks in each spectrum were
previously published. Also, the in vivo ischemic penumbra is a
computed to minimize the random noise effects on the indi-
heterogeneous milieu in which ionic, pH, metabolic substrate,
vidual peaks. Computer simulation was carried out to identify
and gaseous derangements vary considerably depending on
the radical species enveloped in the signals using published
the severity of insult and the time since insult-onset. There-
hyperfine coupling constants (
fore, this region is difficult to exactly duplicate in vitro. IS
hyperfine parameters of DEPMPO-OOH and DEPMPO-OH radi-
Protein extraction and Western blotting
cal adducts are inserted into the WinSim 2002 program
Samples grown in 150 cm2 culture flasks were treated as indi-
) including initial guess of the percentage contribu-
cated in the results section, and then rinsed twice with PBS
tion from each species, and the program is allowed to produce
and detached from the matrix with a cell scrapper into ice-
the resultant spectrum, or to fit the experimental spectrum
cold PBS. The resulting cell suspensions were centrifuged at
with the calculated one. This procedure was used to confirm
250 ×g for 5 min at 4 °C, the supernatant was aspirated away,
the assignment of the radical species observed via representa-
and cells were re-suspended in cell lysis buffer. Samples
tive spectra.
were then homogenized by vortexing for 60 s and proteinswere extracted by incubation in lysis buffer with mixing at
DIDS permeation imaging
4 °C for 45 min, followed by centrifugation for 10 min at14,000 ×g at 4 °C. Supernatants were taken as whole cell ly-
Samples were grown in glass-bottom 35-mm culture dishes.
sates and protein concentration was measured using a
Prior to experimentation, cells were rinsed once in serum-
bicinchoninic acid kit, according to the manufacturer's in-
free DMEM and then incubated for 2 h with 0.2% DMSO or
structions (Sigma).
DIDS in DMEM or IS, and then rinsed once with DMEM or
Equal amounts of protein (40 μg/well) were separated on
IS. DIDS fluorescence (Ex/Em: 342/418 nm in water, with an
4–12% precast NuPAGE bis-Tris SDS-PAGE gels (Invitrogen)
emission Red-shift to > 450 nM when bound to protein
and transferred to polyvinylidene difluoride membranes
(was visualized by a Zeiss LSM510
(Immobilin-P; Millipore, Bedford, MA). Western blots were per-
META confocal laser scanning 2-photon microscope using a
formed with antibodies against α actin and cleaved Lamin A
40 X C-Apochromat (NA 1.2) water immersion objective. Exci-
(1:2000, Cell Signaling, Danvers, MA). Specific bands were vi-
tation was achieved by a Coherent Mira 900 laser tuning to
sualized after incubation with the appropriate secondary anti-
800 nm. Fluorescence shorter than 490 nm was reflected by
bodies (Invitrogen) using enhanced chemiluminescence (GE
a long pass filter (NFT 490), and then filtered by a short
Healthcare/Amersham Biosciences, Buckinghamshire, UK).
pass filter with cutoff at 685 nm (KP685). After taking DIDS
Densitometry of Western blots from each experimental
and DIC images, 10 μg/ml of propidium iodide (PI, Sigma,
group was obtained (n = 3 for each), and absolute values were
Ex/Em: 514/590 nm) was added, and images from the same
normalized to α-actin. Results were analyzed in arbitrary
view were taken 5 to 8 min afterwards (excited by laser line
units, comparing each value with that obtained from each re-
514 nm, and filtered by a long pass filter (LP560). Z-stacks
spective α-actin measurement on each blot.
were taken at 0.4 μm intervals. Z-projections from 4 to 5 op-tical sections were created by averaging pixel intensity at
Vital dye exclusion membrane viability assay
each pixel position using Image J (NIH). Composite pictureswere generated using Image J from DIC (grey), DIDS (blue),
Membrane viability was assessed as the ability of cells to ex-
and PI (red).
clude the vital dye propidium iodide (PI). The dose-dependent response of DIDS on IS-induced PI uptake was
ATP luciferase assay
assessed using a high-throughput 96-well microplate-basedassay. PI uptake was assessed immediately following experi-
Total ATP content [ATP] was assessed in solid-bottom, black
mental treatment on a Bio-Tek PowerWave 340 microplate
96-well microplates (Corning) using PerkinElmer ATPlite Lu-
spectrophotometer (Bio-Tek, Winooski, VT, Ex/Em: 485/
minescence Assay System kits as specified by the manufac-
630 nm), and analyzed using Gen 5 software (Bio-Tek). Micro-
turers protocol (PerkinElmer, MA, USA) and a Bio-Tek
plate PI experiments were repeated 3–5 times and each plate
PowerWave 340 microplate spectrophotometer (Bio-Tek, Wi-
contained 16 replicate wells each of treatment groups. Blank
nooski, VT). Equal numbers of cells were seeded into each
wells and cell-free wells containing each treatment perfusate
well and standard curves were generated using serial dilu-
with PI were also included on each plate, and the final data is
tions of a known ATP standard provided in each kit. The
corrected for these factors.
sensitivity of the detector was calibrated to the lumines-cence of the highest [ATP] standard in each experiment.
DIDS quenched luminescence in a dose-dependent fashionand this effect was quantified in a separate experiment by
Data were analyzed using one-way analysis of variance
adding serial dilutions of DIDS to serial dilutions of the
(ANOVA), followed by Dunnet's post-test. Significances were
ATP standard and subtracting the resulting luminescence
indicated if P < 0.05 assuming two groups had an equal vari-
from ATP standard luminescence measurements in the ab-
ance. Statistical analysis was performed using Prism software
sence of DIDS on the same plate. Results were corrected
(GraphPad, San Diego, CA, USA).
for this factor and then normalized to ATP luminescencerecorded from control cells assayed at t = 0 h. MicroplateATP luciferase experiments were repeated 10 times and
each plate contained 16 replicate wells of each treatmentgroup. Blank wells and cell-free wells containing each treat-
This work was supported in part by NIH 5P01HD032573 to
ment perfusate were also included on each plate, and the
GGH, NIA 1K25AG026379 to SSA, and a National Sciences and
final data is corrected for these factors.
Engineering Research Council of Canada fellowship to MEP.
We would like to thank Orit Gavriolov and Jacinta Lucero for
Liu, K.J., Miyake, M., Panz, T., Swartz, H., 1999. Evaluation of
DEPMPO as a spin trapping agent in biological systems. FreeRadic. Biol. Med. 26, 714–721.
Liu, Y., Fiskum, G., Schubert, D., 2002. Generation of reactive
oxygen species by the mitochondrial electron transport chain.
J. Neurochem. 80, 780–787.
Lo, E.H., 2008. A new penumbra: transitioning from injury into
Akaike, A., Katsuki, H., Kume, T., Maeda, T., 1999. Reactive oxygen
repair after stroke. Nat. Med. 14, 497–500.
species in NMDA receptor-mediated glutamate neurotoxicity.
Mao, G.D., Poznansky, M.J., 1992. Electron spin resonance study on
Parkinsonism Relat. Disord. 5, 203–207.
the permeability of superoxide radicals in lipid bilayers and
Anzai, K., Aikawa, T., Furukawa, Y., Matsushima, Y., Urano, S.,
biological membranes. FEBS Lett. 305, 233–236.
Ozawa, T., 2003. ESR measurement of rapid penetration of
Miller, A.A., Drummond, G.R., De Silva, T.M., Mast, A.E., Hickey, H.,
DMPO and DEPMPO spin traps through lipid bilayer
Williams, J.P., Broughton, B.R., Sobey, C.G., 2009. NADPH oxidase
membranes. Arch. Biochem. Biophys. 415, 251–256.
activity is higher in cerebral versus systemic arteries of four
Barzilai, A., 2007. The contribution of the DNA damage response to
animal species: role of Nox2. Am. J. Physiol. Heart Circ. Physiol.
neuronal viability. Antioxid. Redox Signal. 9, 211–218.
296, H220–H225.
Beavis, A.D., Davatol-Hag, H., 1996. The mitochondrial inner
Oberhammer, F.A., Hochegger, K., Froschl, G., Tiefenbacher, R.,
membrane anion channel is inhibited by DIDS. J. Bioenerg.
Pavelka, M., 1994. Chromatin condensation during apoptosis is
Biomembr. 28, 207–214.
accompanied by degradation of lamin A+B, without enhanced
Chalier, F., Tordo, P., 2002.
activation of cdc2 kinase. J. Cell Biol. 126, 827–837.
Rami, A., 2008. Upregulation of Beclin 1 in the ischemic penumbra.
DIPPMPO, a crystalline analog of the nitrone DEPMPO:
Autophagy 4, 227–229.
synthesis and spin trapping properties. J. Chem. Soc. Perkin
Rami, A., Kogel, D., 2008. Apoptosis meets autophagy-like cell
Trans. 2 (12), 2110–2117.
death in the ischemic penumbra: two sides of the same coin?
Choi, D.W., Rothman, S.M., 1990. The role of glutamate
Autophagy 4, 422–426.
neurotoxicity in hypoxic-ischemic neuronal death. Annu. Rev.
Shi, H., Liu, K.J., 2007. Cerebral tissue oxygenation and oxidative
Neurosci. 13, 171–182.
brain injury during ischemia and reperfusion. Front. Biosci. 12,
Eisinger, J., Flores, J., Salhany, J.M., 1982. Association of cytosol
hemoglobin with the membrane in intact erythrocytes. Proc.
Shimizu, T., Numata, T., Okada, Y., 2004. A role of reactive oxygen
Natl. Acad. Sci. U.S.A. 79, 408–412.
species in apoptotic activation of volume-sensitive Cl(−)
Flamm, E.S., Demopoulos, H.B., Seligman, M.L., Poser, R.G.,
channel. Proc. Natl. Acad. Sci. U.S.A. 101, 6770–6773.
Ransohoff, J., 1978. Free radicals in cerebral ischemia. Stroke 9,
Stolze, K., Udilova, N., Nohl, H., 2000a. Spin trapping of lipid
radicals with DEPMPO-derived spin traps: detection of
Fraser, P.A., 2011. The role of free radical generation in increasing
superoxide, alkyl and alkoxyl radicals in aqueous and lipid
cerebrovascular permeability. Free Radic. Biol. Med. 51, 967–977.
phase. Free Radic. Biol. Med. 29, 1005–1014.
Frejaville, C., Karoui, H., Tuccio, B., Le Moigne, F., Culcasi, M.,
Stolze, K., Udilova, N., Nohl, H., 2000b. Lipid radicals: properties
Pietri, S., Lauricella, R., Tordo, P., 1995.
and detection by spin trapping. Acta Biochim. Pol. 47, 923–930.
5-(Diethoxyphosphoryl)-5-methyl-1-pyrroline N-oxide: a new
Tang, X.N., Cairns, B., Cairns, N., Yenari, M.A., 2008. Apocynin
efficient phosphorylated nitrone for the in vitro and in vivo spin
improves outcome in experimental stroke with a narrow dose
trapping of oxygen-centered radicals. J. Med. Chem. 38, 258–265.
range. Neuroscience 154, 556–562.
Han, D., Williams, E., Cadenas, E., 2001. Mitochondrial respiratory
Vallet, P., Charnay, Y., Steger, K., Ogier-Denis, E., Kovari, E.,
chain-dependent generation of superoxide anion and its release
Herrmann, F., Michel, J.P., Szanto, I., 2005. Neuronal expression
into the intermembrane space. Biochem. J. 353, 411–416.
of the NADPH oxidase NOX4, and its regulation in mouse
Hawkins, B.J., Madesh, M., Kirkpatrick, C.J., Fisher, A.B., 2007.
experimental brain ischemia. Neuroscience 132, 233–238.
Superoxide flux in endothelial cells via the chloride channel-3
Walder, C.E., Green, S.P., Darbonne, W.C., Mathias, J., Rae, J.,
mediates intracellular signaling. Mol. Biol. Cell 18, 2002–2012.
Dinauer, M.C., Curnutte, J.T., Thomas, G.R., 1997. Ischemic
Hochachka, P.W., Buck, L.T., Doll, C.J., Land, S.C., 1996. Unifying
stroke injury is reduced in mice lacking a functional NADPH
theory of hypoxia tolerance: molecular/metabolic defense and
oxidase. Stroke 28, 2252–2258.
rescue mechanisms for surviving oxygen lack. Proc. Natl. Acad.
Wang, X., Takahashi, N., Uramoto, H., Okada, Y., 2005. Chloride
Sci. U.S.A. 93, 9493–9498.
channel inhibition prevents ROS-dependent apoptosis
Hong, H., Zeng, J.S., Kreulen, D.L., Kaufman, D.I., Chen, A.F., 2006.
induced by ischemia-reperfusion in mouse cardiomyocytes.
Atorvastatin protects against cerebral infarction via inhibition
Cell. Physiol. Biochem. 16, 147–154.
of NADPH oxidase-derived superoxide in ischemic stroke. Am.
Wang, Q., Tompkins, K.D., Simonyi, A., Korthuis, R.J., Sun, A.Y.,
J. Physiol. Heart Circ. Physiol. 291, H2210–H2215.
Sun, G.Y., 2006. Apocynin protects against global cerebral
Kahles, T., Luedike, P., Endres, M., Galla, H.J., Steinmetz, H., Busse,
ischemia-reperfusion-induced oxidative stress and injury in
R., Neumann-Haefelin, T., Brandes, R.P., 2007. NADPH oxidase
the gerbil hippocampus. Brain Res. 1090, 182–189.
plays a central role in blood–brain barrier damage in
Yao, H., Shu, Y., Wang, J., Brinkman, B.C., Haddad, G.G., 2007a.
experimental stroke. Stroke 38, 3000–3006.
Factors influencing cell fate in the infarct rim. J. Neurochem.
Kahles, T., Kohnen, A., Heumueller, S., Rappert, A., Bechmann, I.,
100, 1224–1233.
Liebner, S., Wittko, I.M., Neumann-Haefelin, T., Steinmetz, H.,
Yao, H., Sun, X., Gu, X., Wang, J., Haddad, G.G., 2007b. Cell death
Schroeder, K., Brandes, R.P., 2010. NADPH oxidase Nox1
in an ischemic infarct rim model. J. Neurochem. 103,
contributes to ischemic injury in experimental stroke in mice.
Neurobiol. Dis. 40, 185–192.
Yao, H., Felfly, H., Wang, J., Zhou, D., Haddad, G.G., 2009. DIDS
Kakkar, P., Singh, B.K., 2007. Mitochondria: a hub of redox activities
protects against neuronal injury by blocking Toll-like receptor
and cellular distress control. Mol. Cell. Biochem. 305, 235–253.
2 activated-mechanisms. J. Neurochem. 108, 835–846.
Leist, M., Single, B., Castoldi, A.F., Kuhnle, S., Nicotera, P., 1997.
Zhu, Y., Fenik, P., Zhan, G., Mazza, E., Kelz, M., Aston-Jones, G.,
Intracellular adenosine triphosphate (ATP) concentration: a
Veasey, S.C., 2007. Selective loss of catecholaminergic wake
switch in the decision between apoptosis and necrosis. J. Exp.
active neurons in a murine sleep apnea model. J. Neurosci. 27,
Med. 185, 1481–1486.
Source: http://pamenterlab.ca/wp-content/docs/1-s2.0-S0006899312004404-main.pdf
Controls and features Seating and safety restraints Starting and driving Maintenance and care Capacities and specifications Reporting safety defects All rights reserved. Reproduction by any means, electronic or mechanical includingphotocopying, recording or by any information storage and retrieval system or translationin whole or part is not permitted without written authorization from Ford Motor Company.Ford may change the contents without notice and without incurring obligation.
CRCP/ DCS 9:201X DRAFT NO. 3 CARICOM REGIONAL CODE OF PRACTICE Code of practice for organically produced foods DCS/ CRCP 9:201X Caricom Regional Organisation for Standards and Quality, CROSQ 2ND Floor Nicholas House 29 & 30 Broad Street Bridgetown, St Michael Barbados Telephone: 246-622-7677 Fax: 246-622-6778 Email: [email protected] Website: http://www.crosq.org





