Ictraining.net
Infection prevention/control and management
guidelines for patients with Middle East Respiratory
Syndrome Coronavirus (MERS-CoV) infection
2nd Edition
8 December 2014
Scientific Advisory Council
Ministry of Health
Saudi Arabia
8 December 2014 Page 1
TABLE OF CONTENTS
NO. TITLE
Case definition and surveillance guidance
Algorithm for managing patients with suspected MERS-CoV
General infection prevention and control precautions
Triage for rapid identification of patients with acute respiratory illness
Infection prevention and control precautions when caring for patients
with suspected, probable, or confirmed MERS-CoV infection
Fit test and seal check
VIII Infection prevention and control precautions for aerosol-generating
Admission criteria
Management of health care workers who had contacts with patients with
MERS-CoV infection
Management of household contacts of patients with MERS-CoV
XIII Duration of isolation precautions for MERS-CoV infection
XIV Managing bodies in the mortuary
General outlines of management
XVI MERS-designated hospitals
XVII Guidance for MERS sampling packaging and shipment
XIX Members of the Scientific Advisory Council who contributed to the
8 December 2014 Page 2
Preamble
The Scientific Advisory Council formed by His Excellency the acting Minister of Health, Engineer Adel Fakeih developed guidelines dated 24 June 2014 (1st Edition) to meet the urgent need for up-to-date information and evidence-based recommendations for the safe care of adult and pediatric patients with suspected, probable, or confirmed Middle East Respiratory Syndrome Coronavirus (MERS-CoV) infection [1,2]. The main bulk of these guidelines was adapted from previous guidelines produced by the World Health Organization (WHO)[3] and the Centers for Disease Control and Prevention (CDC)[4]. Council members revised these two documents and made important modifications based on the current epidemiological evidence and the members' clinical experience in Infectious Diseases, Infection Control, Emergency Medicine, Intensive Care, and management of patients with MERS-CoV. The council revised the case definition based on the latest epidemiological and clinical features observed in patients reported in Jeddah.
Recently, it was confirmed that dromedary camels are primary sources of human infection [5,6]. Additionally, several publications reported detection of high loads of MERS-CoV nucleic acid in nasal swabs from dromedary camels using RT-PCR and also recovery of live virus through culture [7-9]. High seroprevalance of MERS-like CoV in dromedary camels but not in other domestic animals has also been reported frequently [10-16]. Therefore, history of contact with camels in the 14 days before the onset of illness is an important epidemiological clue to suspect MERS-CoV infection. Such contact may be either direct ie the patient him/herself having the history of contact with camels, or indirect, ie the patient had contact with another healthy person who had the history of contact with camels. Human-to-human transmission has also been well documented and account for the vast majority of cases [17]. Therefore, history of contact with an ill patient with an acute respiratory illness in the community or healthcare setting in the 14 days before the onset of illness is another important clue to suspect MERS-CoV infection.
In addition to standard and contact precautions, the previous guidelines (1st Edition) developed by the Scientific Advisory Council on 24 June 2014 recommended droplet precautions in general for patients with suspected or confirmed MERS-CoV infection, and airborne precautions to be followed only for those patients requiring aerosol-generating procedures or those who are critically ill. In this version (2nd Edition), dated 8 December 2014, the council has made further revision to upgrade the isolation precautions to airborne precautions for all categories of patients. When negative pressure rooms are not available, patients should be placed in adequately ventilated single rooms with a portable HEPA filter, turned on to the maximum power, placed at the head side of the patient's bed. A fit-tested, seal checked N-95 mask should always be worn upon entering a room housing a suspected MERS-CoV
8 December 2014 Page 3
patient. Upgrading infection control precautions from standard/contact/droplet to standard/contact/airborne is based on several reasons: 1. Some patients, mostly health care workers, in hospitals housing MERS-CoV patients were infected without direct exposure to the MERS-CoV patients. In addition to the possibility of airborne transmission in these instances, contact with contaminated environment and/or droplet transmission from asymptomatically infected personnel are other possible alternative routes of transmission; 2. A recent study confirming detection of MERS-CoV RNA in air samples collected from a barn of camels infected with MERS-CoV [18]; 3. Preliminary results of another study conducted by the council that is not yet published showing that healthcare workers who used N95 respirators were three times more protected than those who used surgical masks when handling patients with MERS-CoV; 4. The consistently high morbidity and mortality rate associated with this infection ranging from 30-40%; 5. Unknown modes of human to human transmission; 6. Lack of vaccine or chemopropylaxis; 7. Many MERS-CoV patients require aerosol-generating procedures. The current revised guidelines also include sections on the minimum distance that has to be maintained between beds in various hospital units (page 10), respirator (N95) fit test and seal check (page 15-16), outlines of management including indications and contraindications for extra-corporeal membrane oxygenation (ECMO) (pages 24-25), list of MERS-designated centers (page 26), and guidelines for MERS sampling packaging and shipment (pages 27-33). All revisions and additions made in this edition have been highlighted in yellow. As more information becomes available, these guidelines will be re-evaluated and updated as needed.
Tariq A. Madani
Chairman, Scientific Advisory Council
8 December 2014 Page 4
II. Case definition and surveillance guidance [2]
Suspect case (patients who should be tested for MERS-CoV)1,2
I. A person with fever and community-acquired pneumonia or acute respiratory
distress syndrome based on clinical or radiological evidence.3
OR
II. A hospitalized patient with healthcare associated pneumonia based on clinical and
radiological evidence.3
OR
III. A person with 1) acute febrile (≥38°C) illness, AND 2) body aches, headache,
diarrhea, or nausea/vomiting, with or without respiratory symptoms, AND 3)
unexplained
(WBC<3.5x109/L)
thrombocytopenia
(platelets<150x109/L)4.
OR
IV. A person (including health care workers) who had protected or unprotected
exposure5 to a confirmed or probable case of MERS-CoV infection and who presents with upper6 or lower7 respiratory illness within 2 weeks after exposure.8
Probable case
A probable case is a patient in category I or II above with absent or inconclusive laboratory results for MERS-CoV and other possible pathogens who is a close contact9 of a laboratory-confirmed MERS-CoV case or who works in a hospital where MERS-CoV cases are cared for.
Confirmed case
A confirmed case is a suspect case with laboratory confirmation10 of MERS-CoV infection.
1Important epidemiological clues to MERS-CoV infection include: A. History of contact with camels in the 14 days before the onset of illness. Such contact may either be direct ie the patient him/herself having the history of contact with camels, or indirect, ie the patient had contact with another healthy person who had had contact with camels; B. History of contact with an ill patient suffering from an acute respiratory illness in the community or healthcare setting in the 14 days before the onset of illness.
8 December 2014 Page 5
2All suspected cases should have nasopharyngeal swabs, and, when intubated, lower respiratory secretions samples collected for MERS-CoV testing. 3Patients who meet the criteria for category I or II above should also be evaluated for common causes of community-acquired pneumonia (such as influenza A and B, respiratory syncytial virus, Streptococcus pneumoniae, Hemophilus influenzae, Staphylococcus aureus, and Legionella pneumophila). This evaluation should be based on clinical presentation and epidemiologic and surveillance information. Testing for MERS-CoV and other respiratory pathogens can be done simultaneously. Positive results for another respiratory pathogen (e.g H1N1 and other influenza viruses) should not necessarily preclude testing for MERS-CoV because co-infection can occur. 4Laboratory tests to exclude other causes of this clinical presentation (e.g., dengue, Alkhumra hemorrhagic fever virus, CMV, EBV, typhoid fever, and malaria) should be simultaneously performed if clinically and epidemiologically indicated. 5Protected exposure is defined as contact within 1.5 meters with a patient with confirmed or probable MERS-CoV infection while wearing all personal protective equipment (Surgical or N95 mask, gloves, and gowns, and, when indicated, goggles). Unprotected exposure is defined as contact within 1.5 meters with a patient with confirmed or probable MERS-CoV infection without wearing all personal protective equipment (Surgical or N95 mask, gloves, and gowns, and, when indicated, goggles). 6Rhinorrhea, sore throat, and/or cough 7Shortness of breath, hypoxemia, or pneumonic infiltration evident on chest x-ray. 8Testing asymptomatic contacts is generally not recommended. Under certain circumstances e.g. investigation of a hospital or community outbreak, such testing may be considered in consultation with an Infectious Diseases/Infection Control consultant. 9Close contact is defined as a) any person who provided care for the patient, including a healthcare worker or family member, or had similarly close physical contact; or b) any person who stayed at the same place (e.g. lived with, visited) as the patient while the patient was ill.
10Confirmatory laboratory testing requires a positive PCR on at least two specific genomic targets (upE and ORF1a) OR a single positive target (upE) with sequencing of a second target (RdRpSeq or NSeq). It is strongly advised that lower respiratory specimens such as sputum, endotracheal aspirate, or bronchoalveolar lavage should be used when possible. If patients do not have signs or symptoms of lower respiratory tract infection or lower tract specimens are not possible or clinically indicated, both nasopharyngeal and oropharyngeal specimens should be collected and combined in a single collection container and tested together. If initial testing
8 December 2014 Page 6
of a nasopharyngeal swab is negative in a patient who is strongly suspected to have MERS-CoV infection, patients should be retested using a lower respiratory specimen or, if not possible, a repeat nasopharyngeal and oropharyngeal specimen. For patients in whom adequate lower respiratory samples are not possible, investigators may also want to consider other types of auxiliary testing such as nasopharyngeal wash for MERS-CoV PCR and paired acute and convalescent sera for serological tests. Collection of additional specimens such as stool, urine, and serum for MERS-CoV PCR is also recommended as the virus has also been demonstrated in these body fluids.
III. Algorithm for managing patients with suspected
MERS-CoV [2]
8 December 2014 Page 7
IV. General infection prevention and control precautions
Standard Precautions
– Standard Precautions, a cornerstone for providing safe health care and reducing
the risk of further infection, should always be applied in all health-care settings for all patients.
– Standard Precautions include
HCWs should apply "My 5 moments for hand hygiene": before
touching a patient, before any clean or aseptic procedure, after body fluid exposure, after touching a patient, and after touching a patient's surroundings, including contaminated items or surfaces.
Hand hygiene includes either washing hands with antiseptic soap and
water or the use of an alcohol-based waterless hand sanitizer (waterless hand rub).
Wash hands with antiseptic soap and water when they are visibly soiled. The use of gloves does not eliminate the need for hand hygiene. Hand
hygiene is necessary after taking off gloves and other personal protective equipment (PPE).
o Respiratory Hygiene and Cough Etiquette
To prevent the transmission of all respiratory infections in healthcare settings, including MERS-CoV and influenza, the following infection control measures should be implemented at the first point of contact with a potentially infected person. They should be incorporated into infection control practices as one component of Standard Precautions.
1. Visual Alerts
Post visual alerts (in appropriate languages) at the entrance to outpatient facilities (e.g., emergency rooms and clinics) instructing patients and persons who accompany them (e.g., family, friends) to inform healthcare personnel of symptoms of acute respiratory illness (including fever with cough, sore throat, rhinorrhea, sneezing, shortness of breath, and/or wheezing) when they first register for care and to practice the following Respiratory Hygiene/Cough Etiquette. Cover your mouth and nose with a tissue when coughing or sneezing; Dispose of the tissue in the nearest waste receptacle right after use;
8 December 2014 Page 8
Perform hand hygiene (e.g., hand washing with non-antimicrobial soap
and water, alcohol-based hand sanitizer, or antiseptic handwash) after having contact with respiratory secretions and contaminated objects/materials.
2. Masking and Separation of Persons with Respiratory Symptoms Offer regular (surgical) masks to persons who are coughing. Regular
(surgical) masks may be used to contain respiratory secretions (N-95 masks are not necessary for this purpose).
When space and chair availability permit, encourage coughing persons
to sit at least 1 meter away from others in common waiting areas.
Healthcare facilities should ensure the availability of materials for
adhering to Respiratory Hygiene/Cough Etiquette in waiting areas for patients and visitors.
Provide tissues and no-touch receptacles for used tissue disposal. Provide conveniently located dispensers of alcohol-based hand
Where sinks are available, ensure that supplies for hand washing (i.e.,
antiseptic soap and disposable towels) are consistently available.
– Prevention of overcrowding in clinical areas is essential to prevent cross
infection. The following table shows the minimum distance that should be maintained between patients' beds in general wards and intensive care, hemodialysis and emergency units as recommended by the Ministry of Health, the American Institute of Architects (AIA) Academy of Architecture for Health and the International Federation of Infection Control:
8 December 2014 Page 9
Table. The minimum distance that should be maintained between patients' beds
in selected clinical units as recommended by the Ministry of Health (MoH), the
American Institute of Architects (AIA) Academy of Architecture for Health [19],
and the International Federation of Infection Control (IFIC) [20-22].
Distance between beds recommended by:
A minimum A minimum of 1.22 meters Basic: 1 meter.
(4 feet) between beds.
Standard: 2 meters.
Minimum of 9.29 square
Ideal: 2 meters.
meters (100 square feet) of
clear floor per bed.
Minimum 2.44 meters (8
Basic: 1.5 meters.
feet) between beds for both Standard: 2 meters.
pediatric and adult ICUs
Ideal: 2 meters.
Minimum of 18.58 square
meter (200 square feet) of
clear floor area per bed.
A minimum A minimum of 1.22 meters
(4 feet) between beds and/or No recommendation
A minimum 7.43 square
meters (80 square feet) of
clear floor area per patient
A minimum A minimum of 1.22 meters Standard: 1.5 meters.
(4 feet) between
Ideal: 2 meters.
A minimum 7.43 square
meters (80 square feet) of
clear floor area per patient
*IFIC recommendations are given in three levels:
• Basic – Even with severely limited resources, this is what you should do as a minimum.
• Standard – this is what you should aim for in less wealthy countries.
• Ideal – if you have the resources, this is what you could do.
8 December 2014 Page 10
– Environmental ventilation in all areas within a health-care facility.
– Environmental cleaning.
– Prevention of needle-stick or sharps injury.
– Safe waste management.
– Follow standard procedures, per hospital policy and manufacturers'
instructions, for cleaning and/or disinfection of:
• Environmental surfaces and equipment
• Textiles and laundry
• Food utensils and dishware
– Follow standard procedures for cleaning and/or disinfection of environmental
surfaces and patient-care equipment, linen, stretcher (trolley), and bed. For equipment that requires sterilization, follow routine sterilization procedures.
– Ensure that cleaning and disinfection procedures are followed consistently and
correctly. Cleaning environmental surfaces with water and detergent and applying commonly used disinfectants (such as hypochlorite diluted 10 times) is an effective and sufficient procedure. Manage laundry, food service utensils and medical waste in accordance with routine procedures.
– Policies and procedures for all facets of occupational health, with emphasis on
surveillance of acute respiratory illnesses (ARIs) among HCWs and the importance of seeking medical care
– Monitoring of compliance, along with mechanisms for improvement as needed.
V. Triage for rapid identification of patients with acute
respiratory illness (ARI).
o Clinical triage should be used for early identification of all patients with
ARI in the Emergency Rooms and the Clinics.
o Rapid identification of patients with ARI and patients suspected of MERS-
CoV infection is key to prevent healthcare associated transmission of MERS-CoV or other respiratory viruses. Appropriate infection control precautions and respiratory etiquette (described above) for source control should be promptly applied.
o Identified ARI patients should be asked to wear a surgical mask. They
should be evaluated immediately in an area separate from other patients.
8 December 2014 Page 11
Infection control and prevention precautions should be promptly implemented.
o If ARI patients can not be evaluated immediately, they should wait in a
waiting area dedicated for the ARI patients with spatial separation of at least 1 m between each ARI patient and others.
o Clinical and epidemiological aspects of the cases should be evaluated as
soon as possible and the investigation should be complemented by laboratory evaluation.
VI. Infection prevention and control precautions when
caring for patients with suspected, probable, or
confirmed MERS-CoV infection
o Standard, contact, and, airborne precautions are recommended for
management of patients with suspected, probable, or confirmed MERS-CoV infection particularly for patients who are critically ill (e.g. pneumonia with respiratory distress or hypoxemia) and when performing aerosol-generating procedures which may be associated with an increased risk of infection transmission including both elective procedures such as bronchoscopy, sputum induction, elective intubation and extubation, as well as emergency procedures such as cardiopulmonary resuscitation, emergency intubation, open suctioning of airways, manual ventilation via umbo bagging through a mask before intubation, and initiation of non-invasive ventilation (e.g. Bilevel Positive Airway Pressure - BiPAP) which is not recommended in MERS-CoV infected patients because of the high risk of generating infectious aerosols and lack of evidence for efficacy over elective endotracheal intubation and mechanical ventilation for patients with pneumonia.
o Selected components of recommended precautions for prevention of
MERS-CoV transmission
Placement:
Place patients with suspected, probable, or confirmed MERS-CoV
infection in Airborne Infection Isolation rooms (Negative Pressure Rooms).
When negative pressure rooms are not available, place the patients
in adequately ventilated single rooms. When available, a portable HEPA filter, turned on to the maximum power, should be placed at the head side of the patient's bed.
8 December 2014 Page 12
When single rooms are not available, place patients with the same
diagnosis together (cohorting). If this is not possible, place patient beds at least 1.2 meters apart.
Avoid the movement and transport of patients out of the isolation
room or area unless medically necessary. The use of designated portable X-ray, ultrasound, echocardiogram, and other important diagnostic machines is recommended when possible.
If transport is required:
Patients should wear a surgical mask to contain secretions Use routes of transport that minimize exposures of staff, other
patients, and visitors.
Notify the receiving area of the patient's diagnosis and
necessary precautions as soon as possible before the patient's arrival.
Ensure that healthcare workers (HCWs) who are transporting
patients wear appropriate PPE and perform hand hygiene afterwards.
Personal Protective Equipment (PPE) for Healthcare Workers (HCWs)
The following PPE should be worn by HCWs upon entry into patient
rooms or care areas:
Gowns (clean, non-sterile, long-sleeved disposable gown) Gloves Eye protection (goggles or face shield) A fit-tested, seal checked N-95 mask. For those who failed
the fit testing of N95 masks (e.g those with beards), an alternative respirator, such as a powered air-purifying respirator, should be used.
Upon exit from the patient room or care area, PPE should be
removed and discarded.
Except for N95 masks, remove PPE at doorway or in
anteroom. Remove N95 mask after leaving patient room and closing door.
Remove PPE in the following sequence: 1. Gloves, 2.
Goggles or face shield, 3. Gown, and 4. N95 mask.
You should note and observe the following:
8 December 2014 Page 13
1. Gloves
Outside of gloves is contaminated Grasp outside of glove with opposite gloved hand; peel off Hold removed glove in gloved hand Slide fingers of ungloved hand under remaining glove at wrist Peel glove off over first glove Discard gloves in waste container
2. Goggles or face shield
Outside of goggles or face shield is contaminated To remove, handle by head band or ear pieces Place in designated receptacle for reprocessing or in waste
Gown front and sleeves are contaminated Unfasten ties Pull away from neck and shoulders, touching inside of gown
Turn gown inside out Fold or roll into a bundle and discard
4. N95 masks
Front of mask is contaminated -DO NOT TOUCH Grasp bottom, then top ties or elastics and remove Discard in waste container
Never wear a surgical mask under the N95 mask as this prevents
proper fitting and sealing of the N95 mask thus decreasing its efficacy.
For female staff who wear veils, the N95 mask should always be
placed directly on the face behind the veil and not over the veil. In this instance, a face-shield should also be used along with the mask to protect the veil from droplet sprays.
Perform hand hygiene before and after contact with the patient or
his/her surroundings and immediately after removal of PPE.
If possible, use either disposable equipment or dedicated equipment
(e.g. stethoscopes, blood pressure cuffs and thermometers).
If equipment needs to be shared among patients, clean and disinfect
it after each patient use.
HCWs should refrain from touching their eyes, nose or mouth with
potentially contaminated gloved or ungloved hands.
8 December 2014 Page 14
Environmental Infection Control
Follow standard procedures, per hospital policy and manufacturers'
instructions, for cleaning and/or disinfection of:
Environmental surfaces and equipment Textiles and laundry Food utensils and dishware
Clean and disinfect patient-contact surfaces (e.g. bed and machines)
Limit the number of HCWs, family members and visitors in contact with
a patient with probable or confirmed MERS-CoV infection.
To the extent possible, assign probable or confirmed cases to be cared
for exclusively by a group of skilled HCWs and housekeepers both for continuity of care and to reduce opportunities for inadvertent infection control breaches that could result in unprotected exposure.
Family members and visitors in contact with a patient should be limited
to those essential for patient support and should be trained on the risk of transmission and on the use of the same infection control precautions as HCWs who are providing routine care. Further training may be needed in settings where hospitalized patients are often cared for by family members (sitters).
VII. Fit test and seal check
What is a respirator (N95) fit test?
o A fit test is a test protocol conducted to verify that a respirator (N95 mask)
is both comfortable and correctly fits the user.
o Fit testing uses a test agent, either qualitatively detected by the wearer's
sense of taste, smell or involuntary cough (irritant smoke) or quantitatively measured by an instrument, to verify the respirator's fit.
o The benefits of this testing include better protection for the employee and
verification that the employee is wearing a correctly-fitting model and size of respirator.
o MOH requires a respirator fit test to confirm the fit of any respirator that
forms a tight seal on the wearer's face before it is to be used in the workplace.
8 December 2014 Page 15
o MOH prohibits tight fitting respirators to be worn by workers who have
facial hair that comes between the sealing surface of the facepiece and the face of the wearer. In this case, a Powered Air Purifying Respirator (PAPR) should be used instead.
o Because each brand, model, and size of particulate facepiece respirators
will fit slightly differently, a user should engage in a fit test every time a new model, manufacture type/brand, or size is worn. Also, if weight fluctuates or facial/dental alterations occur, a fit test should be done again to ensure the respirator remains effective. Otherwise, fit testing should be completed at least annually to ensure continued adequate fit.
o A fit test only qualifies the user to put on (don) the specific
brand/make/model of respirator with which an acceptable fit testing result was achieved. Users should only wear the specific brand, model, and size respirators that he or she wore during successful fit tests. Respirator sizing is variable and not standardized across models or brands. For example a medium in one model may not offer the same fit as a different manufacturer's medium model.
What is a respirator (N95) user seal check?
o It is a procedure conducted by the wearer of a respirator to determine if the
respirator is properly seated to the face. A user seal check is sometimes referred to as a fit check.
o Once a fit test has been done to determine the best model and size of
respirator for a particular user, a user seal check should be done by the user
every time the respirator is to be worn to ensure an adequate seal is
achieved.
o A user seal check may be accomplished by using the procedures
recommended by the manufacturer of the respirator. This information can be found on the box or individual respirator packaging. There are positive and negative pressure seal checks and not every respirator can be checked using both. You should refer to the manufacturer's instructions for conducting user seal checks on any specific respirator.
o The user seal check can be either a positive pressure or negative pressure
check. The following positive and negative user seal check procedures for filtering facepiece respirators are provided as examples of how to perform these procedures. Positive pressure check –Once the particulate respirator is properly put
on (donned), your hands over the facepiece, covering as much surface area as possible. Exhale gently into the facepiece. The face fit is
8 December 2014 Page 16
considered satisfactory if a slight positive pressure is being built up inside the facepiece without any evidence of outward leakage of air at the seal. Examples of such evidence would be the feeling of air trickling onto the your face along the seal of the facepiece, fogging of your glasses, or a lack of pressure being built up inside the facepiece. If the particulate respirator has an exhalation valve, then performing a positive pressure check may be impossible. If so, then do a negative pressure check.
Negative pressure check – Negative pressure seal checks are conducted
on particulate respirators that have exhalation valves. To conduct a negative pressure user seal check, cover the filter surface with your hands as much as possible and then inhale. The facepiece should collapse on your face and you should not feel air passing between your face and the facepiece.
VIII. Infection prevention and control precautions for
aerosol-generating procedures
o An aerosol-generating procedure is defined as any medical procedure that
can induce the production of aerosols of various sizes, including small (< 5 micron) particles.
o Aerosol-generating procedures that may be associated with an increased
risk of infection transmission includes both elective procedures such as bronchoscopy, sputum induction, elective intubation and extubation, as well as emergency procedures such as cardiopulmonary resuscitation, emergency intubation, open suctioning of airways, manual ventilation via umbo bagging through a mask before intubation, and initiation of non-invasive ventilation (e.g. Bilevel Positive Airway Pressure - BiPAP) which is not recommended in MERS-CoV infected patients should be avoided in patients with suspected MERS-CoV pneumonia because of the high risk of generating infectious aerosols and lack of evidence for efficacy over elective endotracheal intubation and mechanical ventilation for patients with pneumonia.
o Additional precautions should be observed when performing aerosol-
generating procedures, which may be associated with an increased risk of infection transmission.
8 December 2014 Page 17
o Additional precautions when performing aerosol-generating procedures:
Wear N95 masks –Every healthcare worker should wear a fit tested N95
mask (or an alternative respirator if fit testing failed). Additionally, when putting on N95 mask, always check the seal.
Wear eye protection (i.e. goggles or a face shield). Wear a clean, non-sterile, long-sleeved gown and gloves (some of these
procedures require sterile gloves).
Wear an impermeable apron for some procedures with expected high
fluid volumes that might penetrate the gown;
Perform procedures in a negative pressure room. Limit the number of persons present in the room to the absolute
minimum required for the patient's care and support;
Perform hand hygiene before and after contact with the patient and his
or her surroundings and after PPE removal.
IX. Admission criteria
o Not all suspected MERS-CoV patients should be admitted to health-care
facilities (please refer to section III. Algorithm for managing patients with suspected MERS-CoV).
o Patients suspected to have MERS-CoV infection who have shortness of
breath, hypoxemia, and/or clinical or radiological evidence of pneumonia should be hospitalized.
o Patients with suspected MERS-CoV who have no shortness of breath,
hypoxemia, or evidence of pneumonia may be cared for and isolated in their home when suitable.
X. Home isolation
o is defined as the separation or restriction of activities of an ill
person with a contagious disease from those who are well.
o Before the ill person is isolated at home a healthcare professional should:
Assess whether the home is suitable and appropriate for isolating the
ill person. You can conduct this assessment by phone or direct observation.
o The home should have a functioning bathroom. If there are multiple
bathrooms, one should be designated solely for the ill person.
8 December 2014 Page 18
o The ill person should have his or her own bed and preferably a
private room for sleeping.
o Basic amenities, such as heat, electricity, potable and hot water,
sewer, and telephone access, should be available.
o There should be a primary caregiver who can follow the healthcare
provider's instructions for medications and care. The caregiver should help the ill person with basic needs in the home and help with obtaining groceries, prescriptions, and other personal needs.
o If the home is suitable and appropriate for home care and isolation you
should give the patient, the caregiver, and household members the following instructions:
For the patient
Separate yourself from other people in your home
As much as possible, you should stay in a different room from other people in your home. Also, you should use a separate bathroom, if available.
Call ahead before visiting your doctor
Before your medical appointment, call the healthcare provider and tell him or her that you may have MERS-CoV infection. This will help the healthcare provider's office take steps to keep other people from getting infected.
Wear a surgical mask
You should wear a surgical mask when you are in the same room with other people and when you visit a healthcare provider. If you cannot wear a surgical mask, the people who live with you should wear one while they are in the same room with you.
Cover your coughs and sneezes
Cover your mouth and nose with a tissue when you cough or sneeze, or you can cough or sneeze into your sleeve. Throw used tissues in a lined trash can, and immediately wash your hands with soap and water or disinfect it with waterless alcohol-based hand sanitizer.
Wash your hands
Wash your hands often and thoroughly with antiseptic soap and water. You can use an alcohol-based hand sanitizer if antiseptic soap and water are not available and if your hands are not visibly dirty. Avoid touching your eyes, nose, and mouth with unwashed hands.
8 December 2014 Page 19
Avoid sharing household items
You should not share dishes, drinking glasses, cups, eating utensils, towels, bedding, or other items with other people in your home. After using these items, you should wash them thoroughly with soap and warm water.
For caregivers and household members
If you live with or care for someone at home who is ill and being evaluated for MERS-CoV infection, you should:
Make sure that you understand and can help the ill person follow the
healthcare provider's instructions for medication and care. You should help the ill person with basic needs in the home and provide support for getting groceries, prescriptions, and other personal needs.
Have only people in the home who are essential for providing care for the
ill person. o Other household members should stay in another home or place of
residence. If this is not possible, they should stay in another room, or be separated from the ill person as much as possible. Use a separate bathroom, if available.
o Restrict visitors who do not have an essential need to be in the home. o Keep elderly people and those who have compromised immune systems
or specific health conditions away from the ill person. This includes people with chronic heart, lung or kidney diseases, and diabetes.
Make sure that shared spaces in the home have good air flow, such as by
air-conditioner or an opened window.
Wear a disposable surgical mask, gown, and gloves when you touch or
have contact with the ill person's blood, body fluids and/or secretions, such as sweat, saliva, sputum, nasal mucous, vomit, urine, or diarrhea. o Throw out disposable surgical masks, gowns, and gloves after using
them. Do not reuse.
o Wash your hands immediately after removing your surgical mask,
gown, and gloves.
Wash your hands often and thoroughly with soap and water. You can use
an alcohol-based hand sanitizer if soap and water are not available and if your hands are not visibly dirty. Avoid touching your eyes, nose, and mouth with unwashed hands.
Avoid sharing household items. You should not share dishes, drinking
glasses, cups, eating utensils, towels, bedding, or other items with an ill person who is being evaluated for MERS-CoV infection. After the ill person uses these items, you should wash them thoroughly with soap and warm water.
8 December 2014 Page 20
Clean all "high-touch" surfaces, such as counters, tabletops, doorknobs,
bathroom fixtures, toilets, and bedside tables, every day. Also, clean any surfaces that may have blood, body fluids and/or secretions on them. o Wear disposable gloves and gown while cleaning surfaces. o Use a diluted bleach solution or a household disinfectant. To make a
bleach solution at home, add 1 tablespoon of bleach to 4 cups of water. For a larger supply, add ¼ cup of bleach to 16 cups of water.
Wash laundry thoroughly.
o Immediately remove and wash clothes or bedding that have blood, body
fluids and/or secretions on them.
o Wear disposable gloves while handling soiled items. Wash your hands
immediately after removing your gloves.
o Wash the items with detergent and warm water at the maximum
available cycle length then machine dry them.
Place all used gloves, gowns, surgical masks, and other contaminated
items in a lined container before disposing them with other household waste. Wash your hands immediately after handling these items.
Follow the guidance fbelow.
For close contacts including health care workers
If you have had close contact with someone who is ill and being evaluated for MERS-CoV infection, you should:
Monitor your health for 14 days, starting from the day you were last
exposed to the ill person. Watch for these symptoms:
o Fever (38° C, or higher). Take your temperature twice a day. o Coughing. o Shortness of breath. o Other early symptoms to watch for are chills, body aches, sore throat,
headache, diarrhea, nausea/vomiting, and runny nose.
If you develop symptoms, follow described above,
and call your healthcare provider as soon as possible. Before your medical appointment, call the healthcare provider and tell him or her about your possible exposure to MERS-CoV. This will help the healthcare provider's office take steps to keep other people from getting infected. Ask your healthcare provider to call the MOH.
If you do not have any of the symptoms, you can continue with your daily
activities, such as going to work, school, or other public areas.
Provide "Ministry of Health's
brochure to the ill person, the caregiver, and household members. This brochure is available in
8 December 2014 Page 21
common languages (Arabic, English, Urdu, Pilipino, Indonesian, Bangladeshi, Somalian, and Ethiopian (see appendix).
XI. Management of health care workers who had contacts
with patients with MERS-CoV infection.
o Health care facilities should trace all health care workers who had protected
or unprotected contacts with patients with suspected, probable, or confirmed MERS-CoV infection.
o Contacts should not be routinely tested for MERS-CoV unless they develop
upper or lower respiratory illness.
o Contacts should continue to work in the hospital unless they develop upper
or lower respiratory illness.
o The infection control unit of the facility or equivalent thereof should
proactively call by phone all contacts to assess their health on a daily basis for a total of 14 days. Contacts should also be instructed to report immediately to the Staff Health Clinic or Emergency Room if they develop upper or lower respiratory illness.
o The Infection Control unit should be notified of all contacts who develop a
respiratory illness.
o Symptomatic contacts should be assessed clinically. Nasopharyngeal swabs
should be collected and tested for MERS-CoV PCR.
o Symptomatic contacts should be managed as suspected cases using the
same protocol described in the MERS-CoV management algorithm in section III above.
XII. Management of household contacts of patients with
MERS-CoV infection.
o The Department of Public Health in the local Ministry of Health Directorate
Office should trace all household or other contacts of patients with suspected, probable, or confirmed MERS-CoV infection.
o Contacts should not be routinely tested for MERS-CoV unless they develop
upper or lower respiratory illness.
8 December 2014 Page 22
o The Department of Public Health should proactively call by phone all
contacts to assess their health on a daily basis for a total of 14 days. Contacts should also be instructed to report immediately to the nearest hospital if they develop upper or lower respiratory illness.
o Symptomatic contacts should be assessed clinically. Nasopharyngeal swabs
should be collected and tested for MERS-CoV PCR.
o Symptomatic contacts should be managed as suspected cases using the
same protocol described in the MERS-CoV management algorithm in section III above.
XIII. Duration of isolation precautions for MERS-CoV
infection
o Since the duration of infectivity for MERS-CoV infection is unknown,
nasopharyngeal swab should be repeated every 3 days for in-patients and every week for home-isolated patients with confirmed MERS-CoV infection to test for viral shedding to assist the decision making particularly in regard to when to stop isolation in the hospital or the home setting.
o While standard precautions should continue to be applied always,
additional isolation precautions should be used during the duration of symptomatic illness and continued until 48 hours after the resolution of symptoms; AND At least one nasopharyngeal sample is negative for MERS-CoV RNA.
o If the sample is still positive, and the patient is well enough to go home,
he/she can be allowed to go home with instruction to isolate him/herself at home and come wearing a surgical mask to the clinic for follow up every week to have nasopharyngeal swab repeated until it is proven to be negative.
o Note that additional infection prevention precautions or considerations may
be needed if a MERS-CoV patient has other conditions or illnesses that warrant specific measures (e.g., tuberculosis, Clostridium difficile, multi-drug resistant organisms).
XIV. Managing bodies in the mortuary
o Deceased bodies may pose a potential risk of infections when handled by
either family members or body washers.
o Body washing must be done in the hospital
o If family members wish to perform the body washing, they must strictly
adhere to standard precautions and use PPE
8 December 2014 Page 23
o When washing the body, wear gloves, N95 mask, a face shield (visor) or
goggles, impermeable protective gown, and shoe cover. Observe hand hygiene. For transfer to the cemetery, use MoH approved body bag.
XV. General outlines of management
o Call MOH hotline "937" to report any suspected MERS patient or to
arrange for transfer of the patient to a MERS-designated center.
o MOH recommends transferring MERS patients admitted to any MOH or
private hospitals to one of the 20 MOH MERS-designated centers. MERS patients admitted to non-MOH governmental hospitals (e.g. Armed Forces, National Guard, Security Force, King Faisal Specialist Hospital, and Universities) may be managed in the hospitals they are admitted to. However, when required, MOH will accept transfer of such patients to MOH MERS-designated centers.
o Patients admitted with suspected MERS-CoV pneumonia should be
initiated on empiric antimicrobials to cover alternative causes of pneumonia.
o For community acquired pneumonia, a 3rd generation cephalosporin (e.g
ceftriaxone) to cover Streptococcus pneumoniae and a macrolide (e.g. erythromycin, clarithromycin, or azithromycin) to cover atypical organisms (e.g. Mycoplasma pneumoniae and Chlamydophila pneumonia) should be initiated. The use of respiratory quinolones (e.g. levofloxacin or moxifloxacin) is NOT advisable because of their valuable anti-tuberculosis (TB) activity and the fact that TB is common in our community. Many patients hospitalized with what is believed to be community-acquired pneumonia turn out to have pulmonary TB in which case respiratory quinolones will partially treat them and mask the diagnosis of TB and lead to emergence of resistance to these important second line anti-TB therapy.
o Oseltamivir (Tamiflu) should also be empirically added when viral
pneumonia is suspected (e.g. a patient whose illness started with an influenza like illness for a few days followed by pneumonia).
o For hospital-acquired pneumonia, Gram-negative bacteria should be
primarily covered. A third generation cephalosporin effective against Pseudomonas aeruginosa (e.g. ceftazidime), anti-psuedomonal penicillin (e.g. Pipaeracillin/tazobactam), or a carbapenem (e.g. imipenem or meropenem) should be used for empiric treatment and subsequently modified according to the respiratory and blood culture results. If the patient is known to be colonized with methicillin-resistant Staphylococcus aureus, vancomycin should also be added to the anti-Gram negative coverage.
8 December 2014 Page 24
o The use of non-invasive ventilation (e.g. Bilevel Positive Airway Pressure
- BiPAP) should be avoided in patients with suspected MERS-CoV pneumonia because of the high risk of generating infectious aerosols and lack of evidence for efficacy over elective endotracheal intubation and mechanical ventilation for patients with pneumonia.
o Supportive care is paramount to decrease mortality from MERS-CoV
infection. This includes conservative fluid resuscitation and when necessary ionotropic support for hypotensive patients, mechanical ventilation for patients with respiratory failure, and renal-replacement therapy for patients with renal failure.
o Extra-corporeal membrane oxygenation (ECMO) available in Riyadh,
Jeddah, and Dammam MERS-designated centers should be considered in patients with acute severe hypoxemic respiratory failure with a ratio of arterial oxygen tension to fraction of inspired oxygen (PaO2/FiO2) of <80 mmHg despite optimization of the ventilator settings, including the Fraction of Inspired Oxygen (FiO2), positive end-expiratory pressure (PEEP), and inspiratory to expiratory (I:E) ratio.
o The following are the usual indications for veno-venous ECMO:
Age < 60 years with a potentially reversible lung pathology PaO2/FiO2 of <80 on 100% FiO2 Respiratory acidosis (PH < 7.2) Positive End Expiratory Pressure (PEEP) > 15 cm H2O with a plateau
pressure (Pplat) > 35 cm H2O.
o Relative contraindications for ECMO include:
if anticoagulation is contraindicated (eg, bleeding, recent surgery, recent
intracranial injury).
if the cause of the respiratory failure is irreversible. if the patient has been mechanically ventilated for longer than seven
days because outcomes may be poor in this population.
If the patient has hemodialysis-dependent end stage renal disease. End stage liver, lung, and heart disease. Other characteristics that may exclude some patients from receiving
ECMO include advanced age, morbid obesity (Body Mass Index-BMI > 45 kg/m2), neurologic dysfunction, or poor preexisting functional status.
8 December 2014 Page 25
XVI. MERS-designated hospitals
Hospital
Prince Mohammed bin Abdul-Aziz Hospital
Al-Noor Hospital
King Fahd Hospital
King Faisal Hospital
Dammam Medical Complex
King Fahd General Hospital in Hafoof
King Khaled General Hospital
Buraidah Central Hospital
King Fahd Hospital
King Khaled Hospital
Prince Meteb Bin Abdul-Aziz Hospital
Qurayyat General Hospital
Asir Central Hospital
King Abdullah Central Hospital
King Fahd Hospital
Abu-Areesh General Hospital
King Khalid Hospital
Al-Qunfudah General Hospital
Northern Borders
Arar Central Hospital
8 December 2014 Page 26
XVII. Guidance for MERS sampling, packaging, and
shipment
o Before collecting and handling specimens for Middle East Respiratory
Syndrome Coronavirus (MERS-CoV) testing, determine whether the person meets the current case definition for a Suspect, Probable or Confirmed case.
o All specimens should be regarded as potentially infectious, and HCWs who
collect or transport clinical specimens should adhere rigorously to standard precautions to minimize the possibility of exposure to pathogens.
o Ensure that HCWs who collect specimens wear appropriate PPE.
o Ensure that personnel who transport specimens are trained in safe handling
practices and spill decontamination procedures.
o Place specimens for transport in leak-proof specimen bags (secondary
container) that have a separate sealable pocket for the specimen (i.e. a plastic biohazard specimen bag), with the patient's label on the specimen container (primary container), and a clearly written request form.
o Ensure that health-care facility laboratories adhere to appropriate biosafety
practices and transport requirements according to the type of organism being handled.
o Deliver all specimens by hand whenever possible. Do not use pneumatic-
tube systems to transport specimens.
o State the name of the suspected ARI of potential concern clearly on the
accompanying request form. Notify the laboratory as soon as possible that the specimen is being transported.
o For further information on specimen handling in the laboratory and
laboratory testing for MERS-CoV, see CDC and WHO Laboratory bio-risk management [23,24], and the Laboratory testing for MERS-CoV [25,26], and CDC and WHO laboratory biosafety manuals [27,28].
Specimen type and priority
Little is known about pathogenic potential and transmission dynamics of MERS-CoV. To increase the likelihood of detecting infection, lower respiratory specimens (sputum, endotracheal secretions, or bronchoalveolar lavage) are preferred based on current data they are the most likely to provide positive results. When a lower respiratory specimen is not possible, combined nasopharyngeal and oropharyngeal (NP/OP) specimen is recommended. Additional specimens
8 December 2014 Page 27
such as blood, and serum should be collected on presentation and when convalescent. Collection of stool and urine is also recommended.
Points to consider when collecting specimens from a patient under investigation for MERS include:
Maintain proper infection control when collecting specimens Use approved collection methods and equipment when collecting specimens Handle, store, and ship specimens following appropriate protocols
Respiratory specimens should be collected as soon as possible after symptoms begin – ideally within 7 days and before antiviral medications are administered. However, if more than a week has passed since onset of illness and the patient is still symptomatic, respiratory samples should still be collected, especially lower respiratory specimens since respiratory viruses can still be detected by rRT-PCR.
General guidelines
Samples should be stored in hospital for less than 4 hours before collection
by FedEx. ONLY FedEx delivery is allowed for MERS-CoV samples. Pick up
MUST be requested at the following number (800 6149999).
Label each specimen container with the unique MERS number, patient's hospital ID number, specimen type and the date the sample was collected.
1. Diagnostic samples
A. Respiratory samples
a. Lower respiratory tract
Bronchoalveolar lavage, tracheal aspirate, pleural fluid should be collected whenever clinically appropriate: Collect 2-3 ml into a sterile, leak-proof, screw-cap sputum collection cup or sterile dry container. Refrigerate specimen at 2-8°C up to 72 hours; if exceeding 72 hours, freeze at -70°C and ship on dry ice.
Sputum: Have the patient rinse the mouth with water and then expectorate deep cough sputum directly into a sterile, leak-proof, screw-cap sputum collection cup or sterile dry container. Refrigerate specimen at 2-8°C up to 72 hours; if exceeding 72 hours, freeze at -70°C and ship on dry ice.
8 December 2014 Page 28
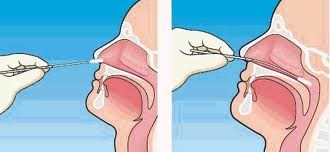
b. Upper respiratory tract
Nasopharyngeal AND oropharyngeal swabs (NP/OP swabs) MUST BE TAKEN TOGETHER. Use only synthetic fiber swabs with plastic shafts. Do not use calcium alginate swabs or swabs with wooden shafts, as they may contain substances that inactivate some viruses and inhibit PCR testing. Place swabs immediately into sterile tubes containing 2-3 ml of viral transport media. NP/OP specimens MUST BE combined, placing both swabs in the same vial. Refrigerate specimen at 2-8°C up to 72 hours; if exceeding 72 hours, freeze at -70°C and ship on dry ice.
Nasopharyngeal swabs: Insert a swab into the nostril parallel to the hard
palate. Leave the swab in place for a few seconds to absorb secretions. Swab both nasopharyngeal areas.
Figure 1: Correct technique for taking a nasopharyngeal swab
For more information see NEJM Procedure: Collection of Nasopharyngeal Specimens with the Swab Technique:
Oropharyngeal swabs: Swab the posterior pharynx, avoiding the tongue. Nasopharyngeal wash/aspirate or nasal aspirates: Collect 2-3 ml into a
sterile, leak-proof, screw-cap sputum collection cup or sterile dry container. Refrigerate specimen at 2-8°C up to 72 hours; if exceeding 72 hours, freeze at -70°C and ship on dry ice.
8 December 2014 Page 29
B. Blood samples
a. Serum for serologic testing
For serum antibody testing: Serum specimens should be collected during the acute stage of the disease, preferably during the first week after onset of illness, and again during convalescence, ≥ 3 weeks after the acute sample was collected. However, since we do not want to delay detection at this time, a single serum sample collected 14 or more days after symptom onset may be beneficial. Serologic testing is NOT currently available but will be implemented within the next 2 months at key regional laboratories.
Please be aware that the MERS-CoV serologic test is currently under investigation and is for research/surveillance purposes and not yet for diagnostic purposes - it is a tool developed in response to the MERS-CoV outbreak. for consultation and approval if serologic testing is being considered.
b. Serum for rRT-PCR testing
For rRT-PCR testing (i.e., detection of the virus and not antibodies), a single serum specimen collected optimally during the first week after symptom onset, preferably within 3-4 days, after symptom onset, may be also be beneficial.
Note: These time frames are based on SARS-CoV studies. The kinetics of MERS-CoV infection in humans is not well understood and may differ from SARS-CoV. Once additional data become available, these recommendations will be updated as needed. Children and adults. Collect 1 tube (5-10 ml) of whole blood in a serum
separator tube. Allow the blood to clot, centrifuge briefly, and separate sera into sterile tube container. The minimum amount of serum required for testing is 200 µl. Refrigerate the specimen at 2-8°C and ship on ice- pack; freezing and shipment on dry ice is permissible.
Infants. A minimum of 1 ml of whole blood is needed for testing of
paediatric patients. If possible, collect 1 ml in an EDTA tube and in a serum separator tube. If only 1 ml can be obtained, use a serum separator tube.
c. EDTA blood (plasma) Collect 1 tube (10 ml) of heparinized (green-top) or EDTA (purple-top) blood. Refrigerate specimen at 2-8°C and ship on ice-pack; do not freeze.
8 December 2014 Page 30
C. Stool samples
Collect 2-5 grams of stool specimen (formed or liquid) in sterile, leak-proof, screw-cap sputum collection cup or sterile dry container. Refrigerate specimen at 2-8°C up to 72 hours; if exceeding 72 hours, freeze at -70°C and ship on dry ice.
2. Packaging
Diagnostic and clinical specimens must be triple-packaged and compliant with IATA
Packing Instructions 650 are detailed in Figure 2. The maximum quantity for a primary receptacle is 500 ml or 500 g and outer packaging must not contain more than 4 L or 4 kg.
Figure 2 Packing instruction 650
8 December 2014 Page 31
Packing containers
1. Packages must be of good quality, strong enough to withstand the rigors of transport
2. Triple packaging consisting of leak proof primary receptacles (for liquid shipments), silt-proof primary receptacles (for solid shipments), leak-proof secondary packaging, outer packaging of sufficient strength to meet the design type test (1.2 meter drop test)
3. For liquid shipments, primary receptacle or secondary packaging capable of withstanding a 95Kpa internal pressure differential
4. Absorbent material sufficient to absorb the entire contents of the shipment
5. An itemized list of contents must be included between the secondary and outer packaging
6. "Biological Substance, Category B" must appear on the package
7. Minimum dimension 100 mm
Samples containing multiple samples will be packaged so that the samples are organized in numerical order of patient hospital ID. Patient Data Sheets and an Itemized List of Contents will accompany the package. The paperwork will be packaged inside the outer package NOT the secondary container.
3. Labeling
The outer container of all diagnostic/clinical specimen packages must display the following on two opposite sides:
Sender's name and address Recipient's name and address The words "Biological Substance, Category B" UN 3373 label Class 9 label, including UN 1845, and net weight if packaged with dry ice
All specimens must be pre-packed to prevent breakage and spillage. Specimen containers should be sealed with Parafilm® and placed in ziplock bags. Place enough absorbent material to absorb the entire contents of the Secondary Container (containing Primary Container) and separate the Primary Containers (containing specimen) to prevent breakage. Send specimens with cold packs or other refrigerant blocks that are self-contained, not actual wet ice. This prevents leaking and the appearance of a spill. When large numbers of specimens are being shipped, they should be organized in a sequential manner in boxes with separate compartments for each specimen.
8 December 2014 Page 32
For additional information, consultation, or the appropriate shipping address, contact tor the Regional MERS Laboratory.
4. Shipping
Any human or animal material including, but not limited to, excreta, secreta, blood and its components, tissue and tissue fluids, being transported for diagnostic or investigational purposed, but excluding live infected animals.
Specimens from suspected MERS-CoV cases must be packaged, shipped, and transported according to the current edition of the International Air Transport Association (IATA) Dangerous Goods Regulations. At present MERS-CoV diagnostic specimens must be assigned to UN3373 and must be packaged as Category B infectious substances.
Category B infectious substances should have the proper shipping name "Biological Substance, Category B" and the identification number UN 3373.
5. Rejection of packages and samples
Samples and packages will be rejected if:
Samples are not packaged according to packing instruction P650 as UN3373
Diagnostic Specimens.
An itemized list of samples organized by hospital patient ID number is NOT
included inside the outer package.
The patient data sheets are incomplete, missing or incorrectly filled out. If the primary container has leaked If dry ice is placed in the "Primary Container" or "Secondary Container",
foam envelopes, ziplock bags, cryovial boxes, or hermetically sealed containers.
If the Primary Containers sideways or upside down in ziplock bags. Primary containers must be packaged securely in an upright position and in
the numerical order used on the Itemized List of contents
If red top Secondary Containers for Category A Infectious Substances are
If any paperwork in the Secondary Containers or ziplock bags, so as not to
damage the paperwork.
If biohazard/autoclave bags to prepack your materials due to the inadequate
seal of these bags.
8 December 2014 Page 33
XVIII. References
1. Madani TA, Althaqafi AO, Alraddadi BSaudi Med Journal 2014 Aug;35(8):897-913. 2. Madani Lancet Infectious Diseases 2014 Oct;14(10):911-3. 3. Infection prevention and control during health care for probable or confirmed cases of novel coronavirus (nCoV) infection. Interim Guidance. World Health Organization (WHO). 6 May 2013. Available at: 4. Interim Infection Prevention and Control Recommendations for Hospitalized Patients with Middle East Respiratory Syndrome Coronavirus (MERS-CoV). Centers for Disease Control and prevention (CDC). Available at: 5. Azhar EI, El-Kafrawy SA, Farraj SA, Hassan AM, Al-Saeed MS, Hashem AM, Madani TANew England Journal of Medicine 2014 Jun 26;370(26):2499-505. 6. Madani TA, Azhar EI, Hashem New England Journal of Medicine 2014 Oct 2;371(14): 1360. 7. Alagaili AN, Briese T, Mishra N, Kapoor V, Sameroff SC, Burbelo PD, de Wit E, Munster VJ, Hensley LE, Zalmout IS, Kapoor A, Epstein JH, Karesh WB, Daszak P, Mohammed OB, Lipkin WI. Middle East respiratory syndrome coronavirus infection in dromedary camels in Saudi Arabia. mBio 2014;5(2):e00884-14. 8ddle East Respiratory Syndrome Coronavirus Quasispecies That Include Homologues of Human Isolates Revealed through Whole-Genome Analysis and Virus Cultured from Dromedary Camels in Saudi Arabia. mBio. 2014 May-Jun; 5(3): e01146-14.
8 December 2014 Page 34
9 MERS Coronavirus in Dromedary Camel Herd, Saudi Arabia. Emerging Infectious Diseases Jul 2014;20(7):1231–1234.
10. Reusken CB, Haagmans BL, Müller MA, Gutierrez C, Godeke GJ, Meyer B, Muth D, Raj VS, Smits-De Vries L, Corman VM, Drexler JF, Smits SL, El Tahir YE, De Sousa R, van Beek J, Nowotny N, van Maanen K, Hidalgo-Hermoso E, Bosch BJ, Rottier P, Osterhaus A, Gortázar-Schmidt C, Drosten C, Koopmans MP. Middle East respiratory syndrome coronavirus neutralising serum antibodies in dromedary camels: a comparative serological study. Lancet Infectious Diseases 2013;13:859–866.
11. Perera RA, Wang P, Gomaa MR, El-Shesheny R, Kandeil A, Bagato O, Siu LY, Shehata MM, Kayed AS, Moatasim Y, Li M, Poon LL, Guan Y, Webby RJ, Ali MA, Peiris JS, Kayali G. Seroepidemiology for MERS coronavirus using microneutralisation and pseudoparticle virus neutralisation assays reveal a high prevalence of antibody in dromedary camels in Egypt, June 2013. Euro Surveillance 2013;18:20574.
12. Hemida MG, Perera RA, Wang P, Alhammadi MA, Siu LY, Li M, Poon LL, Saif L, Alnaeem A, Peiris M. Middle East respiratory syndrome (MERS) coronavirus seroprevalence in domestic livestock in Saudi Arabia, 2010 to 2013. Euro Surveillance 2013;18:20659.
13. Reusken C, Ababneh M, Raj V, Meyer B, Eljarah A, Abutarbush S, Godeke G, Bestebroer T, Zutt I, Muller M, Bosch B, Rottier P, Osterhaus A, Drosten C, Haagmans B, Koopmans M. Middle East respiratory syndrome coronavirus (MERS-CoV) serology in major livestock species in an affected region in Jordan, June to September 2013. Euro Surveillance 2013;18:20662.
14. Haagmans BL, Al Dhahiry SH, Reusken CB, Raj VS, Galiano M, Myers R, Godeke GJ, Jonges M, Farag E, Diab A, Ghobashy H, Alhajri F, Al-Thani M, Al-Marri SA, Al Romaihi HE, Al Khal A, Bermingham A, Osterhaus AD, AlHajri MM, Koopmans MP. Middle East respiratory syndrome coronavirus in dromedary camels: an outbreak investigation. Lancet Infectious Diseases 2014;14:140–145.
15. Alexandersen S, Kobinger GP, Soule G, Wernery U. Middle East respiratory syndrome coronavirus antibody reactors among camels in Dubai, United Arab Emirates, in 2005. Transboundary Emerging Diseases 2014;61:105–108.
8 December 2014 Page 35
16. Meyer B, Müller MA, Corman VM, Reusken CB, Ritz D, Godeke GJ, Lattwein E, Kallies S, Siemens A, van Beek J, Drexler JF, Muth D, Bosch BJ, Wernery U, Koopmans MP, Wernery R, Drosten C. Antibodies against MERS coronavirus in dromedary camels, United Arab Emirates, 2003 and 2013. Emerging Infectious Diseases 2014;20:552–559. 17. Oboho IK, Tomczyk SM, Al-Asmari AM, Banjar AA, Al-Mugti H, Aloraini MS, Alkhaldi KZ, Almohammadi EL, Alraddadi BM, Gerber SI, Swerdlow DL, Watson JT, Madani TA. 2014 Outbreak of MERS Coronavirus in Jeddah: A Link to Healthcare Facilities. New England Journal of Medicine 2014, in press. 18. Azhar EI, Hashem AM, El-Kafrawy SA, Sohrab SS, Aburizaiza AS, Farraj SA, Hassan AM, Al-Saeed MS, Jamjoom GA, Madani TAmBio 2014 Jul 22;5(4):e01450-14. 19. The American Institute of Architects Academy of Architecture for Health. Health and Human Services Guidelines for Design and Construction of Hospital and Healthcare Facilities, 2001 Edition. 20. Walter Popp, Peter Hoffman, Judene Bartley. Design of a general ward (Version 3). International Federation of Infection Control (IFIC) Construction, Design and Renovation Interest Group. 1 February 2010: page 1. Available at: 21. Ulrika Ransjö, Walter Popp, Peter Hoffman. Design of Intensive care units (Version 1). International Federation of Infection Control (IFIC) Construction, Design and Renovation Interest Group. 15 January 2011, page 2. Available at: 22. Céline Drolet, SIG business meeting 2009. Emergency Unit (Version 1). International Federation of Infection Control (IFIC) Construction, Design and Renovation Interest Group. 1 February 2010, page 3. Available at: 23. Interim Guidelines for Collecting, Handling, and Testing Clinical Specimens from Patients Under Investigation (PUIs) for Middle East Respiratory Syndrome Coronavirus (MERS-CoV) – Version 2. Centers for Disease Control and prevention (CDC). 9 January 2014. Available at:
8 December 2014 Page 36
24. Laboratory testing for novel coronavirus: Interim recommendations. World Health Organization (WHO). 21 Dec 2012. Available at: 25. Interim Laboratory Biosafety Guidelines for Handling and Processing Specimens Associated with Middle East Respiratory Syndrome Coronavirus (MERS-CoV). Centers for Disease Control and prevention (CDC). Available at: 26. Laboratory biorisk management for laboratories handling human specimens suspected or confirmed to contain novel coronavirus: Interim recommendations. World Health Organization (WHO). 19 Feb 2013. Available at:
27. Biosafety in Microbiological and Biomedical Laboratories (BMBL) 5th Edition. Dec 2009. Centers for Disease Control and prevention (CDC). Available at:
28. Laboratory Biosafety Manual - Third Edition. World Health Organization (WHO). 2004. Available at:
8 December 2014 Page 37
XIX. Members of the Scientific Advisory Council who
contributed to the guidelines
1. Prof. Tariq Ahmed Madani, Professor of Internal Medicine & Infectious Diseases,
Advisor to His Excellency the Minister of Health, and Chairman of the Scientific Advisory Council, Ministry of Health, Department of Medicine, Faculty of Medicine, King Abdulaziz University, Jeddah; email:
2. Prof Eltayeb Abuelzein, Professor of Virology, Scientific Chair for Viral
Hemorrhagic fever, Special Infectious Agents Unit, King Fahd Medical Research Center, King Abdulaziz University, Jeddah, ema
3. Dr Esam Ibraheem Azhar, Associate professor of Molecular Virology,
Department of Medical Laboratory Technology, Faculty of Applied Medical Sciences, and Special Infectious Agents Unit, King Fahd Medical Research Center, King Abdulaziz University, Jeddah; email:
4. Dr Anees Sendi, Assistant Professor of Pulmonary and Critical Care Medicine,
Department of Anesthesia and Critical care, Faculty of Medicine, King Abdulaziz University, Jeddah; ema
5. Dr Abdulhakeem Okab Althaqafi, Assistant Professor of Internal Medicine &
Infectious Diseases, King Saud Bin Abdulaziz University for Health Sciences, Ministry of National Guard, Jeddah, em
6. Dr Mohammad Qutb, Consultant Laboratory Medicine, King Faisal Specialist
Hospital & Research Center, Jeddah; em
7. Dr Basem Alraddadi, Consultant, Internal Medicine & Infectious Diseases, King
8. Dr Ali Sharaf Alshareef, Assistant Professor of Emergency Medicine & Intensive
Care, King Saud Bin Abdulaziz University for Health Sciences, Ministry of National Guard, Jeddah,
9. Dr Steven M. Jones, Adjunct Professor of Immunology and Medical
Microbiology, Head of Immunopathology, Head of Emerging Bacterial Diseases, Special Pathogens Program National Laboratory of Zoonotic Diseases and Special Pathogens, Faculty of Medicine, University of Manitoba, Canada, ema
8 December 2014 Page 38
XX. Acknowledgment
The Scientific Advisory Council would like to thank Dr Abdullah M. Assiri,
Consultant, Internal Medicine & Infectious Diseases, Assistant Deputy Minister
for Preventive Health, Ministry of Health, Riyadh, Dr Ali Albarrak, Consultant,
Internal Medicine & Infectious Diseases, Prince Sultan Military Medical City,
Riyadh, Dr John Jernigan, Consultant, Internal Medicine & Infectious Diseases,
Centers for Disease Control and Prevention, Atlanta, USA, and Dr David Kuhar,
Consultant, Internal Medicine & Infectious Diseases, Centers for Disease Control
and Prevention, Atlanta, USA, and Dr Wail A. Tashkandi, Consultant of Surgery
and Critical Care, King Abdulaziz University Hospital, Jeddah, and Dr Ahmad
M. Wazzan, Consultant Emergency Medicine and Trauma, Ministry of Health,
for their critical review of the guidelines.
8 December 2014 Page 39
Source: http://www.ictraining.net/index.php/ar/business/2012-09-06-06-34-22?download=24:mers-cov-updated-infection-control-guidelines-december-2014
An executive summary for tackling global challenges HIV/AIDS in the Context of Other Global Challenges Special Report for the UN High-Level Meeting on AIDS, 8-10 June 2011 HIV/AIDS in the Context of Other Global Challenges Special Report for the UN High-Level Meeting on AIDS Global2015 e. V. is an independent, non-profit and non-partisan association, registered under German law.Its mission is to provide a comprehensive analysis and survey of the most urgent global challenges for human needs and life, and to encourage further action in tackling challenges such as world nutri-tion, climate change, and epidemics.
Etwa 32 Mil ionen Deutsche leiden unter einer Venenschwäche. Krampfadern (Vari-zen) sind die am häufigsten vorkommende Beschwerdeart: Bei ungefähr der Hälfte al er Mitteleuropäer im Alter zwischen 25 und 74 Jahren treten sie insbesondere an den Beinen auf. Meist erschlaffen hierbei die Venenwände in Folge einer Bindege-websschwäche, die Venenklappen können nicht dicht schließen und das Blut fließt als Folge nicht mehr ausreichend ab. Stattdessen staut es sich in den oberflächlichen Venen an, welche sich durch die dauerhafte Erweiterung deutlich sichtbar bläulich bis lila unter der Haut entlang schlängeln. Im Gegensatz zu Besenreisern – kleinsten erweiterten Venen, die ähnlich der Form eines Reisigbesens durch die Haut schimmern (lesen Sie hierzu auch unseren Patientenratgeber zum Thema) – stel en Krampfadern nicht nur ein ästhetisches Problem dar, sondern können auch gesundheitliche Beschwerden wie etwa geschwollene Füße und schwere, schmerzende Beine verursachen. Es ist daher sinnvol , bei entsprechend auftretenden Symptomen möglichst frühzei-tig medizinischen Rat einzuholen. Schwerwiegenderen Komplikationen wie dem Entstehen von Venen-entzündungen, Geschwüren oder Blutgerinnseln kann so vorgebeugt werden.


